Abstract
Elderly patients with endometrial carcinoma (EMC) are considered to have a poor clinical outcome. The present study included 79 patients aged ≥70 years with EMC stage I or II according to the International Federation of Gynecology and Obstetrics classification, and it was conducted to analyse the clinicopathological significance of histological type (I or II), depth of myometrial invasion (<1/2 or ≥1/2), lymphovascular invasion (+ or -) and immunohistochemical profile. The aim of these analyses was to determine whether these factors may adversely affect the patient outcome and the underlying mechanisms. The immunohistochemical markers used were estrogen receptor (ER), Ki-67 and p53. The expression of these markers was evaluated as high (+) or low (−). Accordingly, the patients were divided into groups as follows: 54 cases type I vs. 25 cases type II; 48 cases with myometrial invasion <1/2 vs. 31 cases without myometrial invasion ≥1/2; 63 cases with lymphovascular invasion vs. 16 cases without lymphovascular invasion; 57 cases with ER (+) vs. 22 cases with ER (−); 24 cases with Ki-67 (+) vs. 55 cases with Ki-67 (−); and 29 cases with p53 (+) vs. 50 cases with p53 (−). In conclusion, close attention must be paid to elderly patients with EMC due to the tumor's intrinsic aggressiveness, which may include the ER (−) and p53 (+) pattern as an independent poor prognostic factor.
Keywords: endometrial carcinoma, elderly women, estrogen receptor, p53
Introduction
Endometrial carcinoma (EMC) is a common malignancy in developed countries and its mortality is approaching that of cervical cancer. The age of patients with EMC ranges widely, from young adults to elderly women, but the overall population is currently aging. The majority of data in the literature indicate that advanced age is a predictor of poor outcome in patients with EMC (1–5). Focusing on elderly women (aged ≥70 years), EMC specific to this subpopulation may be of particular interest. The number of EMC patients aged ≥70 years has gradually increased in Japan as well, recently reaching ~20% of all EMC cases (6).
The prognosis of patients with EMC is associated with variable pathological factors, including histological type, tumor grade (7), depth of myometrial invasion and lymphovascular invasion (8), as well as clinicopathological stage as determined by the International Federation of Gynecology and Obstetrics (FIGO) classification (9), which is a critical determinant of the prognosis of EMC, irrespective of its histological type. Tumor grade is essentially prognostic for endometrioid carcinoma, but not for type II EMC, as this type has the potential to behave aggressively (10). Type II EMC, which comprises serous carcinoma and clear cell carcinoma, accounts for the higher proportion of cases among post-menopausal rather than pre-menopausal women. Advancing age is associated with an increasing tendency toward high-grade endometrioid carcinoma and a likelihood of type II EMC, but the histological characteristics do not appear to be able to fully explain the unfavorable prognosis of elderly EMC patients. An additional factor may be that a relative lack of immune competence may be more prevalent among elderly patients (11).
Estrogen receptor (ER) is highly expressed in endometrioid carcinoma, particularly G1/2, showing a strong dependency on estrogenic growth. However, ER expression in type II EMC, i.e., high-grade carcinoma, such as endometrioid carcinoma G3, serous carcinoma, clear cell carcinoma and carcinosarcoma, is negative or mild, if present (12). The expression of p53 tends to be enhanced in type II EMC, represented by serous carcinoma (13,14). The immunohistochemical ‘ER (+) and p53 (−)’ staining pattern is an indicator of endometrioid carcinoma G1/2, whereas the reverse pattern favors type II EMC. Ki-67 is an excellent marker for defining the proliferation status of tumor cells (15), but the prognostic value of Ki-67 expression in EMC also remains controversial (16–20).
In the present study, attention was focused on age specificity (≥70 years) in early-stage EMC (FIGO I or II), and an attempt was made to divide elderly EMC patients into subpopulations based on outcome.
Patients and methods
Patient selection
The cutoff age for elderly women with EMC was defined as ≥70 years. These elderly patients were selected from the archives of all EMC patients (n=1,171) who had been treated at Kanagawa Cancer Center (Yokohama, Japan) between 1985 and 2011. Among patients aged ≥70 years, 79 were preoperatively staged as FIGO I or II, and the clinicopathological factors were postoperatively confirmed after total abdominal hysterectomy and salpingoophorectomy, with or without regional lymphadenectomy. Neither preoperative chemotherapy nor irradiation had been performed. The study protocol was approved by the Institutional Review Board of the Kanagawa Cancer Center, and informed consent was obtained from all patients.
Endometrioid carcinoma G1/2 and mucinous carcinoma were categorized as type I, whereas other types of carcinomas, including serous carcinoma, clear cell carcinoma, undifferentiated carcinoma and carcinosarcoma, were categorized as type II EMC.
Immunohistochemistry
Representative formalin-fixed and paraffin-embedded tissue blocks were cut into 4-μm sections for the immunohistochemical staining of ER, Ki-67 and p53. The sections were deparaffinized, and endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide. Heat-induced antigen retrieval was applied using an autoclave in citrate buffer (10 mM, pH 6.0) at 121°C for 15 min. The sections were incubated with the following primary antibodies: ER (rabbit monoclonal, clone SP1, 1:1, Ventana, Tucson, AZ, USA); Ki-67 (mouse monoclonal, clone MIB-1, 1:50, Dako, Glostrup, Denmark), and p53 (mouse monoclonal, clone DO-7, 1:50, Dako). After rinsing in phosphate-buffered saline (10 mM/l, pH 7.2), the sections were incubated with the Envision Kit (Dako) for 30 min at room temperature. The reaction products were visualized with diaminobenzidine tetrahydrochloride.
The immunoexpression of ER, Ki-67 and p53 was semi-quantitatively evaluated as low (−) or high (+) as follows: ER (−) <30% vs. (+) ≥30%; Ki-67 (−) <30% vs. (+) ≥30%; and p53 (−) <80% vs. (+) ≥80%.
Statistical analysis
The Fisher's exact test was used to analyse the association between outcome and pathological factors, namely histological type, myometrial invasion depth and vascular invasion. The outcome was determined using disease-free survival (DFS) and overall survival (OS) rates. DFS was defined as the time from the date of surgery to the date of first recurrence, which was detected by imaging examinations. OS was calculated from the date of surgery to the date of death. OS and DFS were estimated using the Kaplan-Meier method, and the differences between survival rates were compared by the log-rank test. Data from survivors were censored at the last follow-up. Multivariate analyses applying the Cox proportional hazards regression model were used to assess an independency as a prognostic factor. Differences with P<0.05 were considered as statistically significant. All statistical analyses were performed using SPSS ver. 20 (IBM Corp., Armonk, NY, USA).
Results
Pathological presentations
According to the histological diagnosis of EMC, the 79 patients were grouped into 54 cases with type I EMC (52 endometrioid carcinomas G1/2 and 2 mucinous carcinomas) and 25 cases with type II EMC (11 endometrioid carcinomas G3, 8 serous carcinomas, 3 clear cell carcinomas, 2 carcinosarcomas and 1 undifferentiated carcinoma). If an EMC was mixed (type I and type II), it was categorized as type II EMC (Table I). In the univariate log-rank analysis, the histological type tended to adversely affect the outcome, but a critical correlation between the two was not statistically confirmed (Table II). Neither myometrial invasion depth nor lymphovascular invasion were apparently correlated with the outcome (Table II).
Table I.
Histological and immunohistochemical profile of patients with endometrial carcinoma aged ≥70 years.
| ER | Ki-67 | p53 | |||||
|---|---|---|---|---|---|---|---|
| Type of cancer | n=79 | (+) | (−) | (+) | (−) | (+) | (−) |
| Endometrioid Ca | 63 | 50 | 13 | 16 | 47 | 16 | 47 |
| G1 | 31 | 26 | 5 | 5 | 26 | 5 | 26 |
| G2 | 21 | 18 | 3 | 7 | 14 | 8 | 13 |
| G3 | 11 | 6 | 5 | 4 | 7 | 3 | 8 |
| Mucinous Ca | 2 | 2 | 0 | 2 | 0 | 1 | 1 |
| Serous Ca | 8 | 5 | 3 | 5 | 3 | 8 | 0 |
| Clear cell Ca | 3 | 0 | 3 | 1 | 2 | 2 | 1 |
| Undifferentiated Ca | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Carcinosarcoma | 2 | 0 | 2 | 0 | 2 | 1 | 1 |
ER, estrogen receptor; Ca, carcinoma.
Table II.
Univariate and multivariate analyses.
| Variables | HR (95% CI) | P-value |
|---|---|---|
| Univariate analysis | ||
| Histological type | 4.836 (0.885-26.43) | 0.069 |
| Depth of myometrial invasion | 2.000 (0.365-10.95) | 0.424 |
| Lymphovascular invasion | 0.794 (0.145-4.346) | 0.791 |
| ER | 0.162 (0.300-0.885) | 0.036 |
| p53 | 10.21 (1.191-87.46) | 0.034 |
| Ki-67 | 3.688 (0.940-19.61) | 0.126 |
| Multivariate analysis | ||
| ER | 0.066 (0.006-0.774) | 0.030 |
| p53 | 14.55 (1.280-165.3) | 0.031 |
HR, hazard ratio; CI, confidence interval; ER, estrogen receptor.
Immunohistochemical analysis
According to the semi-quantitative evaluation of immunohistochemical staining (Fig. 1 and Table I), 57 cases had ER (+) vs. 22 cases with ER (−); 24 cases had Ki-67 (+) vs. 55 cases with Ki-67 (−); 29 cases had p53 (+) vs. 50 cases with p53 (−). The percentage of cases with ER (+), Ki-67 (+) and p53 (+) for type I EMC was 85.2, 25.9 and 25.9 %, respectively, and for type II, 44, 40 and 60%, respectively. The univariate log-rank analysis was followed by multivariate Cox regression analysis, in which ER (P=0.03) and p53 (P=0.03) expression were demonstrated to be independent prognosticators for shorter survival.
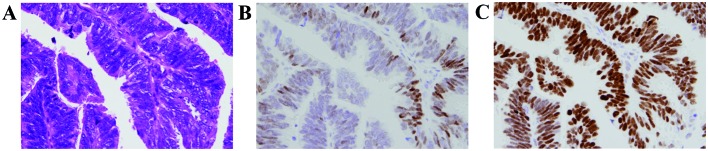
Figure 1.
Endometrioid carcinoma (magnification, ×60). (A) The carcinoma cells are arranged mainly in a papillary pattern, with a scanty vascular core. Despite the structural differentiation, nuclear atypia and stratification favor the diagnosis of endometrioid carcinoma G2 rather than G1, but this is not sufficient to be diagnosed as serous carcinoma (hematoxylin and eosin staining). (B) The percentage of ER-positive carcinoma cells was evaluated as 20%, categorized as low expression (−). (C) The percentage of p53-positive carcinoma cells was evaluated as ~100%, categorized as high expression (+). ER, estrogen receptor.
Kaplan-Meier method
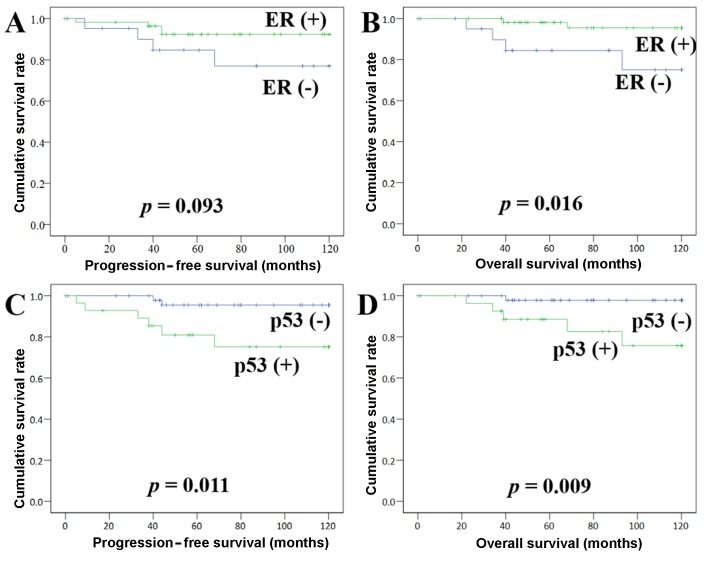
The follow-up period for survivors ranged from 0 to 120 months, with a median of 87 months. At the last follow-up, 92.4% of the patients were still alive, 10.1% experienced recurrence, and 7.6% had succumbed to their disease. Kaplan-Meier survival curves demonstrated that ER is associated with DFS: (+) vs. (−) = 113.4 vs. 101.9 months, respectively (P=0.09), and that ER (−) is significantly associated with shorter OS: (+) vs. (−) = 117.1 vs. 103.8 months, respectively (P=0.01) (Fig. 2). p53 (+) was found to be closely associated with shorter DFS: (+) vs. (−) = 99.2 vs. 116.5 months, respectively (P=0.01), as well as a shorter OS: (+) vs. (−) = 105.0 vs. 118.3 months, respectively (P=0.01) (Fig. 2). Ki-67 (+) was found to be associated with shorter DFS: (+) vs. (−) = 102.8 vs. 113.6 months, respectively (P=0.09), but not with OS: (+) vs. (−) = 102.8 vs. 115.8 months, respectively (P=0.10).
Figure 2.
(A and B) Disease-free survival (DFS) and overall survival (OS) by the Kaplan-Meier method. (B) Patients with ER (+) had a favorable outcome compared with those with ER (−), with a statistically significant difference in OS. (C and D) Patients with p53 (+) had an unfavorable outcome compared with those with p53 (−), with a statistically significant difference in (C) DFS and (D) OS.
Discussion
It was reported that poor outcome is correlated with lymphovascular invasion (8), aggressive histology and cervical invasion in early (stage I or II) EMC in the multivariate analysis (1). In addition, elderly EMC patients (≥70 years) appear to have worse outcomes compared with younger patients, regardless of other poor prognostic factors, and EMC also appears to be intrinsically more aggressive in elderly patients. The cutoff age of 70 years is arbitrary, but it has been used extensively in the medical literature when comparing elderly and younger patients (1). Since the greater majority of patients with EMC are in the seventh decade of life or older and have acquired comorbidities, it is a reasonable hypothesis that overall survival will be age-dependent (1,21–25). However, the significance of age as a prognostic factor remains controversial when risk-adjusted for the higher prevalence of adverse prognostic factors in elderly EMC patients (21).
Among EMCs arising in elderly women, type II EMC has a higher incidence compared with that in younger women (25). In our experience, type II EMC, including mixed carcinoma, accounts for ~40% of the cases. Serous carcinoma with an aggressive behavior (10,26), representing type II EMC, is often found to have an extrauterine spread (stage IV), irrespective of the absence of myometrial invasion or lymphovascular invasion (7,27–29). In the present study, the examined EMC patients aged ≥70 years were confined to stage I or II, and advanced cases with extrauterine spread were not included. Our preliminary study with stage I or II EMC, arising in patients in their fifth (368 cases) and sixth (251 cases) decades of life, indicated that histological type and lymphovascular invasion are associated with outcome (data not shown). Subsequently, an association of myometrial invasion depth with outcome was noted in patients in the fifth decade, but not in those in the sixth decade of life. In patients aged ≥70 years, no statistically significant correlations were demonstrated for these factors (Table II).
The FIGO grading system of endometrioid carcinoma relies first and foremost on the glandular architecture. The architecture usually corresponds well to nuclear grade, but nuclear grade is often a more reliable indicator of prognosis (30,31). The majority of endometrioid carcinomas G1/2 react with ER on immunohistochemical staining, and staining for ER and progesterone receptor (PgR) may prove to be of some value in determining which patients may be suitable for hormonal therapy (32). p53 mutations are found in 5-10% of all endometrioid carcinomas, but occur in 50% of endometrioid carcinomas G3, and even more frequently (90%) in serous carcinoma (12–14,33–35). On the contrary, ER and PgR expression is absent or only weak in serous carcinomas (28).
As shown in the Kaplan Meier curves, the ‘ER (−) and p53 (+)’ pattern is considered a promising prognostic indicator for predicting an unfavorable clinical outcome in elderly EMC patients (Fig. 2). However, this pattern is usually an indicator of serous carcinoma with the risk of an aggressive clinical course. Some of endometrioid carcinomas G1 exhibit high-grade nuclear atypia without solid growth (identical to grade 1 architecture), which is the basis for raising the grade from 1 to 2 (Fig. 1), particularly in elderly patients. Therefore, it is considered that these cases should be treated as high-grade/type II EMC rather than endometrioid carcinoma G2 (low-grade/type I EMC).
With the aid of immunohistochemical staining (36–38), prognostication of EMC may be achieved to a greater extent. In order to predict EMC with aggressive potential, which appears to increase in association with patient age, the ‘ER (−) and p53 (+)’ pattern may become an easily applicable indicator. Elderly patients with EMC must be closely followed up, as these tumors occasionally exhibit an intrinsic aggressiveness, regardless of low-grade/type I appearance of endometrioid carcinoma.
Acknowledgements
This article is dedicated to the late Dr Hiroki Nakayama (Head of the Department of Gynecology, Kanagawa Cancer Center, Yokohama, Japan) for his authentic and conscientious instructions throughout the course of this study.
Funding
No funding was received.
Availability of data and materials
All datasets generated in this study are available from the corresponding author upon reasonable request.
Authors' contributions
All the authors have read and approved the final version of this manuscript. NO contributed to the conception, design, acquisition, analysis and interpretation of data, and drafting of the manuscript. MY and TK took part in acquisition and analysis of data. SK critically revised the manuscript for important intellectual content. HK, YK, SH, and MY contributed to the conception and design of the manuscript for important intellectual content.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the Kanagawa Cancer Center, and informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests to disclose.
References
- 1.Alektiar KM, Venkatraman E, Abu-Rustum N, Barakat RR. Is endometrial carcinoma intrinsically more aggressive in elderly patients? Cancer. 2003;98:2368–2377. doi: 10.1002/cncr.11830. [DOI] [PubMed] [Google Scholar]
- 2.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: An analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- 3.Zaino RJ, Kurman RJ, Diana KL, Morrow CP. Pathologic models to predict outcome for women with endometrial adenocarcinoma: The importance of the distinction between surgical stage and clinical stage-a Gynecologic Oncology Group study. Cancer. 1996;77:1115–1121. doi: 10.1002/(SICI)1097-0142(19960315)77:6<1115::AID-CNCR17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Abeler VM, Kjørstad KE. Endometrial adenocarcinoma in Norway. A study of a total population. Cancer. 1991;67:3093–3103. doi: 10.1002/1097-0142(19910615)67:12<3093::AID-CNCR2820671226>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Irwin C, Levin W, Fyles A, Pintilie M, Manchul L, Kirkbride P. The role of adjuvant radiotherapy in carcinoma of the endometrium-results in 550 patients with pathologic stage I disease. Gynecol Oncol. 1998;70:247–254. doi: 10.1006/gyno.1998.5064. [DOI] [PubMed] [Google Scholar]
- 6.Saito T, Takahashi F, Katabuchi H. 2016 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology: Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: Patient Annual Report for 2014 and Treatment Annual Report for 2009. J Obstet Gynaecol Res. 2017;43:1667–1677. doi: 10.1111/jog.13450. [DOI] [PubMed] [Google Scholar]
- 7.Carcangiu ML, Chambers JT. Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: comparison of clinicopathologic features and survival. Int J Gynecol Pathol. 1995;14:30–38. doi: 10.1097/00004347-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Sakaki M, Yasuda M, Yano M, Goto Y, Nakamura M, Kayano H, Hasegawa K. The prognostic significance of tumor lymphangiogenesis and lymphatic vessel density in endometrioid carcinoma of the uterine corpus. Oncol Lett. 2017;14:5313–5318. doi: 10.3892/ol.2017.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogane N, Yasuda M, Kato H, Kato T, Yano M, Kameda Y, Kamoshida S. Cleaved caspase-3 expression is a potential prognostic factor for endometrial cancer with positive peritoneal cytology. Cytopathology. 2018;29:254–261. doi: 10.1111/cyt.12550. [DOI] [PubMed] [Google Scholar]
- 10.Carcangiu ML, Chambers JT. Uterine papillary serous carcinoma: A study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinoma. Gynecol Oncol. 1992;47:298–305. doi: 10.1016/0090-8258(92)90130-B. [DOI] [PubMed] [Google Scholar]
- 11.Mutter GL, Prat J. Pathology of the Female Reproductive Tract. 3rd edition. Churchill Livingstone Elsevier; Boston, MA: 2014. Endometrial adenocarcinoma; p. 397. [Google Scholar]
- 12.Kounelis S, Kapranos N, Kouri E, Coppola D, Papadaki H, Jones MW. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Pathol. 2000;13:379–388. doi: 10.1038/modpathol.3880062. [DOI] [PubMed] [Google Scholar]
- 13.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: Evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268–1274. doi: 10.1016/0046-8177(95)90204-X. [DOI] [PubMed] [Google Scholar]
- 14.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: Evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–824. doi: 10.1002/(SICI)1097-0142(20000215)88:4<814::AID-CNCR12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Kallakury BV, Ambros RA, Hayner-Buchan AM, Sheehan CE, Malfetano JH, Ross JS. Cell proliferation-associated proteins in endometrial carcinomas, including papillary serous and endometrioid subtypes. Int J Gynecol Pathol. 1998;17:320–326. doi: 10.1097/00004347-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Salvesen HB, Iversen OE, Akslen LA. Identification of high-risk patients by assessment of nuclear Ki-67 expression in a prospective study of endometrial carcinomas. Clin Cancer Res. 1998;4:2779–2785. [PubMed] [Google Scholar]
- 18.Geisler JP, Geisler HE, Miller GA, Wiemann MC, Zhou Z, Crabtree W. MIB-1 in endometrial carcinoma: Prognostic significance with 5-year follow-up. Gynecol Oncol. 1999;75:432–436. doi: 10.1006/gyno.1999.5615. [DOI] [PubMed] [Google Scholar]
- 19.Semczuk A, Skomra D, Cybulski M, Jakowicki JA. Immunohistochemical analysis of MIB-1 proliferative activity in human endometrial cancer. Correlation with clinicopathological parameters, patient outcome, retinoblastoma immunoreactivity and K-ras codon 12 point mutations. Histochem J. 2001;33:193–200. doi: 10.1023/A:1017996506357. [DOI] [PubMed] [Google Scholar]
- 20.Lundgren C, Auer G, Frankendal B, Moberger B, Nilsson B, Nordstrom B. Nuclear DNA content, proliferative activity, and p53 expression related to clinical and histopathologic features in endometrial carcinoma. Int J Gynecol Pathol. 2002;12:110–118. doi: 10.1046/j.1525-1438.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- 21.AlHilli MM, Bakkum-Gamez JN, Mariani A, Weaver AL, McGree ME, Keeney GL, Jatoi A, Dowdy SC, Podratz KC. Risk-adjusted outcomes in elderly endometrial cancer patients: Implications of the contrasting impact of age on progression-free and cause-specific survival. Gynecol Oncol. 2015;138:133–140. doi: 10.1016/j.ygyno.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Fleming ND, Lentz SE, Cass I, Li AJ, Karlan BY, Walsh CS. Is older age a poor prognostic factor in stage I and II endometrioid endometrial adenocarcinoma? Gynecol Oncol. 2011;120:189–192. doi: 10.1016/j.ygyno.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Farley JH, Nycum LR, Birrer MJ, Park RC, Taylor RR. Age-specific survival of women with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2000;79:86–89. doi: 10.1006/gyno.2000.5934. [DOI] [PubMed] [Google Scholar]
- 24.Jolly S, Vargas CE, Kumar T, Weiner SA, Brabbins DS, Chen PY, Floyd W, Martinez AA. The impact of age on long-term outcome in patients with endometrial cancer treated with postoperative radiation. Gynecol Oncol. 2006;103:87–93. doi: 10.1016/j.ygyno.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Vance S, Yechieli R, Cogan C, Hanna R, Munkarah A, Elshaikh MA. The prognostic significance of age in surgically staged patients with Type II endometrial carcinoma. Gynecol Oncol. 2012;126:16–19. doi: 10.1016/j.ygyno.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Connelly PJ, Alberhasky RC, Christopherson WM. Carcinoma of the endometrium. III. Analysis of 865 cases of adenocarcinoma and adenoacanthoma. Obstet Gynecol. 1982;59:569–575. [PubMed] [Google Scholar]
- 27.Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, Cain JM, Tamimi HK, Figge DC, Greer BE. Uterine papillary serous carcinoma: Patterns of metastatic spread. Gynecol Oncol. 1994;54:264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 28.Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum Pathol. 1998;29:551–558. doi: 10.1016/S0046-8177(98)80002-6. [DOI] [PubMed] [Google Scholar]
- 29.Jia L, Yuan Z, Wang Y, Cragun JM, Kong B, Zheng W. Primary sources of pelvic serous cancer in patients with endometrial intraepithelial carcinoma. Modern pathology : An official journal of the United States and Canadian Academy of Pathology. Inc. 2015;28:118–127. doi: 10.1038/modpathol.2014.76. [DOI] [PubMed] [Google Scholar]
- 30.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Zaino RJ, Kurman RJ, Diana KL, Morrow CP. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer. 1995;75:81–86. doi: 10.1002/1097-0142(19950101)75:1<81::AID-CNCR2820750114>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol. 1998;69:220–225. doi: 10.1006/gyno.1998.5023. [DOI] [PubMed] [Google Scholar]
- 33.Matias-Guiu X, Catasus L, Bussaglia E, Lagarda H, Garcia A, Pons C, Muñoz J, Argüelles R, Machin P, Prat J. Molecular pathology of endometrial hyperplasia and carcinoma. Hum Pathol. 2001;32:569–577. doi: 10.1053/hupa.2001.25929. [DOI] [PubMed] [Google Scholar]
- 34.Berchuck A, Boyd J. Molecular basis of endometrial cancer. Cancer. 1995;76(Suppl):2034–2040. doi: 10.1002/1097-0142(19951115)76:10+<2034::AID-CNCR2820761321>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.An HJ, Logani S, Isacson C, Ellenson LH. Molecular characterization of uterine clear cell carcinoma. Modern pathology : An official journal of the United States and Canadian Academy of Pathology. Inc. 2004;17:530–537. doi: 10.1038/modpathol.3800057. [DOI] [PubMed] [Google Scholar]
- 36.Huvila J, Laajala TD, Edqvist PH, Mardinoglu A, Talve L, Pontén F, Grénman S, Carpén O, Aittokallio T, Auranen A. Combined ASRGL1 and p53 immunohistochemistry as an independent predictor of survival in endometrioid endometrial carcinoma. Gynecol Oncol. 2018;149:173–180. doi: 10.1016/j.ygyno.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Obata T, Nakamura M, Mizumoto Y, Iizuka T, Ono M, Terakawa J, Daikoku T, Fujiwara H. Dual expression of immunoreactive estrogen receptor β and p53 is a potential predictor of regional lymph node metastasis and postoperative recurrence in endometrial endometrioid carcinoma. PLoS One. 2017;12:e0188641. doi: 10.1371/journal.pone.0188641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geels YP, van der Putten LJ, van Tilborg AA, Lurkin I, Zwarthoff EC, Pijnenborg JM, van den Berg-van Erp SH, Snijders MP, Bulten J, Visscher DW, et al. Immunohistochemical and genetic profiles of endometrioid endometrial carcinoma arising from atrophic endometrium. Gynecol Oncol. 2015;137:245–251. doi: 10.1016/j.ygyno.2015.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated in this study are available from the corresponding author upon reasonable request.