Abstract
Surgical site infections (SSIs) are a well-known potential complication of surgery. They are assocaited with preoperative malnutrition and lead to increased medical costs and longer hospital stays. Therefore, surgeons should appropriately identify patients who are at a high risk. The geriatric nutritional risk index (GNRI) is a tool, increasingly utilized to assess the degree of malnutrition, particularly in elderly patients. Therefore, the present study attempted to validate whether GNRI could predict the risk of SSI in patients following pancreaticoduodenectomy (PD). A cohort study was retrospectively conducted on 106 patients in the Department of Digestive Surgery, Kawaguchi Municipal Medical Center, Japan from January 2007 to December 2017. All patients were subjected to nutritional screening using GNRI and followed up for the occurrence of postoperative complications, including SSI post PD. Additionally, risk factors for developing SSI, and the patient's height, body mass index and preoperative laboratory values were documented. Patients were divided into SSI (n=15) and non-SSI (n=91) groups with a determined incidence of 14.2% (15/106) for SSI. The results revealed that the SSI group had GNRI values that were significantly reduced compared with the non-SSI group (P<0.001). Receiver operating characteristic curve analysis was performed to determine the cut-off value of GNRI that conferred an increased risk of SSI; it was determined as 94 (sensitivity 80.0%, specificity 83.5%). Univariate analysis confirmed that a GNRI <94 was significantly associated with SSI (P<0.001), whereas multivariate logistic regression analysis revealed that a GNRI <94 was independently associated with SSI following PD (relative risk=1.73, 95% confidence interval=1.23-2.43; P<0.001). Therefore, a GNRI <94 is a potential predictive marker for SSI risk following PD.
Keywords: pancreaticoduodenectomy, geriatric nutritional risk index, surgical site infection
Introduction
Pancreaticoduodenectomy (PD) is the standard treatment method for malignant hepatobiliary pancreatic tumors. However, the perioperative mortality rate of PD has remained at 5% over the last few decades, despite improved techniques and advances in surgical assist devices (1,2). In addition, perioperative morbidity rates have been reported to range from 30 to 60% (3,4). Among the potential complications of PD, the most common are surgical site infections (SSIs), delayed gastric emptying and postoperative pancreatic fistula (POPF). Especially, POPF is an independent risk factor for SSIs such as intra-abdominal abscesses (5). SSIs are well-known factors, which lead to increased medical costs and prolonged hospital stays. Therfore, in order to reduce those medical costs and hospital stays, necessitating prompt identification and prevention of SSIs are clinically very important. More recently, the geriatric nutritional risk index (GNRI) has gained favor in assessing a patient's nutritional status and in predicting the clinical outcomes of elderly patients, particularly those with chronic kidney disease and heart failure (6–8). More importantly, GNRI is easily and inexpensively attainable, as it only requires body weight, height and serum albumin levels. Due to the intimate relationship between preoperative nutritional status and SSI, the authors hypothesized that GNRI could be utilized as a novel tool to predict the SSI in patients who would undergo PD. Thus, a retrospective study was performed to assess this association between GNRI and SSI. The aim of the present study was to evaluate the GNRI and SSI in patents who underwent PD. The identification of predictive markers for SSI may help identify patients who are at a high risk of developing them in the future.
Patients and methods
Patients
A total of 106 patients who underwent PD for malignant hepatobiliary pancreatic tumors between January 2008 and December 2017 were retrospectively analyzed at Kawaguchi Municipal Medical Center for SSI. This protocol was reviewed and approved at the Kawaguchi Municipal Medical Center in 2016. All participants including retrospectively registered patients or their guardians verbally consented to the use of their medical information for scientific research (no. KMMC2017-27).
Clinicopathological data
Medical records were analyzed to determine SSI rates and evaluate the role of other potential risk factors for SSI. Demographic variables (sex and age), anthropometric parameters [height, weight, and body mass index (BMI)], comorbidities, history of smoking and alcohol use, American society of anesthesiologist (ASA)'s physical status classification, estimated blood loss, operation time, and laboratory data (albumin) were collected from individual medical records.
Nutritional assessment using GNRI
Nutritional status was determined according to GNRI [GNRI=(14.89×serum albumin (g/l)) + (41.7×present/ideal body weight (kg))]. Ideal body weight was defined using patient height and a BMI of 22. When present body weight was higher than the ideal body weight, present/ideal body weight ratio was set to 1.
Analytic method
All statistical analyses were performed using Graphpad Prism v5.0 (Graphpad Software Inc., La Jolla, CA, USA) and StatView (Abacus Concepts, Inc., Berkeley, CA, USA). Differences between the SSI and non-SSI groups were compared using the Fisher's exact test or Chi-squared test. The optimal cut-off value of GNRI was determined using a receiver operating characteristic (ROC) curve. Potential risk factors for SSI were evaluated using univariate and multivariate analyses. The Chi-squared test or Fisher's exact test as univariate analyses was performed for the SSI group. Independent risk factors for SSI were identified by univariate analysis using a logistic regression. The probability of P<0.05 was considered statistically significant.
Results
Patient characteristics
The male-to-female ratio was 1:1 (53/53). The mean age was 70.2±8.7 years. Among them, 15 patients had wound infection complications (15.4%) and 17 (16.0%) developed POPFs.
Univariate analysis for SSI risk following PD
Patients were divided into two groups according to the presence or absence of SSI. Clinical and demographic data from each group are summarized in Table I. No statistically significant difference in sex, age, smoking habit, ASA classification, BMI, alcohol abuse, preoperative biliary drainage, estimated blood loss, operation time, blood transfusions, and postoperative hospital stays were observed between SSI and non-SSI groups. However, BMI was higher in the SSI group than in the non-SSI group (24± 4.4 vs. 21.8 ±3.0, P=0.05). Statistically significant differences were observed for preoperative albumin (P < 0.001), GNRI values (P < 0.001), postoperative hospital stays (P < 0.001), and pancreatic fistulization (P < 0.001). POPF occurred in 17 patients (16.0%), 6 of whom (40.0%) developed SSIs. Moreover, majority of SSIs were caused at the surface (n=12) in Table II.
Table I.
Background of patients with or without SSI.
| Characteristics | SSI group (n=15) | Non-SSI group (n=91) | P-value |
|---|---|---|---|
| Sex | |||
| Male/female | 6/9 | 47/44 | 0.58 |
| Age (years) | 70.9±8.5 | 70.1±8.7 | 0.72 |
| Smoking | |||
| Yes (%) | 2 (13.3) | 15 (16.5) | 0.76 |
| ASA classification | |||
| 1 | 0 (0.0) | 3 (3.3) | 0.48 |
| 2 or 3 | 15 (100.0) | 88 (96.7) | |
| Body mass index | 24.0±4.4 | 21.8±3.0 | 0.05 |
| Alcohol abuse | |||
| Yes (%) | 3 (20.0) | 36 (39.6) | 0.25 |
| Diabetes mellitus | |||
| Yes (%) | 4 (26.7) | 35 (38.5) | 0.56 |
| Preoperative biliary drainage | |||
| Yes (%) | 13 (86.7) | 66 (72.5) | 0.34 |
| Preoperative albumin (g/l) | 3.2±0.5 | 4.0±0.4 | <0.001 |
| Geriatric nutritional risk index | 87.9±7.5 | 100.0±7.6 | <0.001 |
| <94 | 12 (80.0) | 15 (16.5) | <0.001 |
| ≥94 | 3 (20.0) | 76 (83.5) | |
| Time of operation (min) | 458.0±83.8 | 476.2±104.5 | 0.52 |
| Estimated blood loss (ml) | 1,461.5±1,002.1 | 1,447.4±1,432.1 | 0.97 |
| Blood transfusion | |||
| Yes (%) | 11 (73.3) | 64 (70.3) | 0.81 |
| Postoperative pancreatic fistula | |||
| Yes (%) | 8 (53.3) | 9 (9.9) | <0.001 |
| Postoperative hospital stays (day) | 58.5±15.0 | 27.2±2.0 | <0.001 |
The analysis revealed significantly higher incidence of SSI in geriatric nutritional risk index <94 patients. SSI, Surgical site infection.
Table II.
Type of SSI and disease.
| Type of SSI (n=15) | Type of disease (n=15) |
|---|---|
| Surface: 12 (overlapped data) | Pancreatic head cancer 20.0% (n=3) |
| Deep: 5 (overlapped data) | Bile duct cancer 40.0% (n=6) |
| Organ/space: 5 (overlapped data) | Cancer of the ampulla of vater 40.0% (n=6) |
The type of SSI was mainly surface (12/15). SSI, surgical site infection.
Determination of the optimal GNRI cut-off value
The cut-off value was identified using ROC curve analysis (Fig. 1). Area under the curve was 0.87. A GNRI value of 94 was determined as the most appropriate cut-off value, rendering a sensitivity of 80.0% and a specificity of 83.5%. Patients were then divided into two groups: Group A (GNRI ≥94, n=79) and group B (GNRI <94, n=27) using the established GNRI cut-off value of 94. The observed SSI rate was 3.8% in group A and 44.4% in group B.
Figure 1.
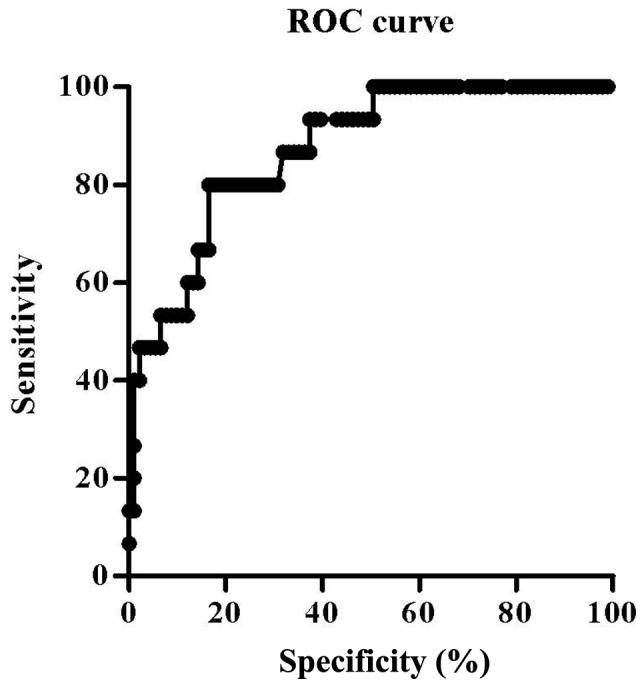
ROC curve analysis. Geriatric nutritional risk index was chosen by 94 as an optimal cut-off value with sensitivity 80.0% and specificity 83.5%. ROC, receiver operating characteristic.
Univariate analysis of a GNRI <94 for SSI risk following PD
Univariate analysis was performed to identify factors predicting SSI risk after PD. The incidence of SSI was significantly higher in group B than in group A (P<0.001).
Multivariable with logistic regression analyses
Logistic regression analysis revealed that a GNRI value <94 (P<0.001) and POPF occurrence (P<0.006) were independent predictors for SSI as outlined in Table III.
Table III.
Multivariate analysis by logistical regression.
| Characteristics | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Body mass index | 0.85 | 0.72-1.00 | 0.05 |
| Preoperative albumin (g/l) | 0.36 | 0.12-1.10 | 0.07 |
| Geriatric nutritional risk index <94 | 0.05 | 0.01-0.20 | <0.001 |
| Postoperative pancreatic fistula | 9.39 | 1.91-46.12 | <0.006 |
GNRI <94 was independent risk factor to predict surgical site infections following pancreaticoduodenectomy.
Discussion
SSIs remain one of the most common postoperative complications (9), with an estimated incidence of 10-33% of patients after PD (10–12). In general, risk factors for postsurgical wound complications following abdominal procedures include the ASA score, obesity, diabetes, age, operation time, estimated blood loss, and poor nutrition (13,14). Among them, malnutrition is by far the most common risk factor for developing SSIs (15,16), and Bozzetti et al found that hypoalbuminemia was an independent predictor (17). As SSIs are associated with prolonged hospital stay, ultimately leading to higher medical costs, surgeons should promptly identify at-risk patients prior to elective procedures. In this regard, several nutritional assessment tools have been developed and validated: The nutrition risk index (NRI), malnutrition inflammation score, malnutrition universal screening tool, prognostic nutritional index (PNI), and GNRI (18–21). Among them, recent reports revealed that GNRI has been identified as a prognostic indicator in patients with heart failure and chronic renal disease (6–8). GNRI was originally developed to evaluate malnutrition and related morbidity and mortality in elderly patients (22). Therefore, we aimed to determine whether GNRI can predict the incidence of SSI following PD. Several studies have reported the use of nutritional assessment tools in predicting the incidence of postoperative complications, such as SSI. Hu et al reported that preoperative PNI is a useful predictor of SSI in gastrointestinal surgery (23). Additionally, Yamana et al showed that GNRI is useful in predicting the development of respiratory complications in patients with esophageal malignancies (24). In contrast, Shinkawa et al found that NRI is an independent predictive factor for the risk of SSI after PD (12). Likewise, Kitagawa et al reported that GNRI could be used to evaluate postoperative nutritional status after PD (25).
In the present study, 15.4% of the patients developed SSI following PD in our center. Among them, a low GNRI value (<94) was strongly associated with a higher risk of SSI, supporting the use of nutritional assessment prior to an elective procedure. In addition, the development of POPFs is also a potential predictor of SSI, although they are intimately related to SSI, particularly space/organ infections. Parikh et al reported that 55% of POPFs contributed to the occurrence of intra-abdominal abscess (26). In our data, SSIs were observed in 40% of patients with POPFs. Therefore, low GNRI might be a possible marker of POPF and SSI.
Although our data are consistent with previously published NRI data (12), we are the first to demonstrate the relationship between GNRI and SSI. However, our study has several limitations. The most important limitation is the lack of statistical power due to small sample size. The second is that our data were collected at a single center. Therefore, a more comprehensive prospective study should be conducted in the future to validate the present findings.
In conclusion, the results of the present study suggest that a GNRI score of <94 is a probable candidate for predicting SSI in patients who undergo PD.
Acknowledgements
The authors would like to thank Dr Curtis Lacy (Department of Internal Medicine, Mayo Clinic, USA) for his advice and assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
NF performed the experimental studies and wrote the manuscript. NF and YN participated in the design of the study. NF, YN, TI and KK performed the surgeries. YN and TI collected the patient data. NF conceived the study and performed the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, Wargo JA, Ferrone CR, Thayer SP, Lillemoe KD, Warshaw AL. Evolution of the whipple procedure at the Massachusetts general hospital. Surgery. 2012;152(3 Suppl 1):S56–S63. doi: 10.1016/j.surg.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: The 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259:773–780. doi: 10.1097/SLA.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, Patel SH, Bentram DJ, Weber SM, Cho CS, et al. Factors influencing readmission after pancreaticoduodenectomy: A multi-institutional study of 1302 patients. Ann Surg. 2012;256:529–537. doi: 10.1097/SLA.0b013e318265ef0b. [DOI] [PubMed] [Google Scholar]
- 4.Hill JS, Zhou Z, Simons JP, Ng SC, McDade TP, Whalen GF, Tseng JF. A simple risk score to predict in-hospital mortality after pancreatic resection for cancer. Ann Surg Oncol. 2010;17:1802–1807. doi: 10.1245/s10434-010-0947-x. [DOI] [PubMed] [Google Scholar]
- 5.DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–939. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumura T, Mitani Y, Oki Y, Fujimoto Y, Ohira M, Kaneko H, Kawashima T, Nishio M, Ishikawa A. Comparison of geriatric nutritional risk index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung. 2015;44:534–538. doi: 10.1016/j.hrtlng.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu M, Okazaki M, Tsuchiya K, Kawaguchi H, Nitta K. Geriatric nutritional risk index is a simple predictor of mortality in chronic hemodialysis patients. Blood Purif. 2015;39:281–287. doi: 10.1159/000381798. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko H, Suzuki S, Goto M, Yuzawa Y, Arita T, Yagi N, Murata N, Kato Y, Kano H, Matsuno S, et al. Geriatric nutritional risk index in hospitalized heart failure patients. Int J Cardiol. 2015;181:213–215. doi: 10.1016/j.ijcard.2014.11.167. [DOI] [PubMed] [Google Scholar]
- 9.Walz JM, Paterson CA, Seligowski JM, Heard SO. Surgical site infection following bowel surgery: A retrospective analysis of 1446 patients. Arch Surg. 2006;141:1014–1018. doi: 10.1001/archsurg.141.10.1014. [DOI] [PubMed] [Google Scholar]
- 10.Pisters PW, Hudec WA, Hess KR, Lee JE, Vauthey JN, Lahoti S, Raijman I, Evans DB. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47–55. doi: 10.1097/00000658-200107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poruk KE, Lin JA, Cooper MA, He J, Makary MA, Hirose K, Cameron JL, Pawlik TM, Wolfgang CL, Eckhauser F, Weiss MJ. A novel, validated risk score to predict surgical site infection after pancreaticoduodenectomy. HPB (Oxford) 2016;18:893–899. doi: 10.1016/j.hpb.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinkawa H, Takemura S, Uenishi T, Sakae M, Ohata K, Urata Y, Kaneda K, Nozawa A, Kubo S. Nutritional risk index as an independent predictive factor for the development of surgical site infection after pancreaticoduodenectomy. Surg Today. 2013;43:276–283. doi: 10.1007/s00595-012-0350-2. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Miyata H, Gotoh M, Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Kitagawa Y, Sugihara K, Mori M. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg. 2014;260:1034–1039. doi: 10.1097/SLA.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 14.Takagi K, Yoshida R, Yagi T, Umeda Y, Nobuoka D, Kuise T, Fujiwara T. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg. 2017;17:64. doi: 10.1186/s12893-017-0261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzu MA, Terzioğlu H, Genç V, Erkek AB, Ozban M, Sonyürek P, Elhan AH, Torun N. Preoperative nutritional risk assessment in predicting postoperative outcome in patients undergoing major surgery. World J Surg. 2006;30:378–390. doi: 10.1007/s00268-005-0163-1. [DOI] [PubMed] [Google Scholar]
- 16.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: The joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26:698–709. doi: 10.1016/j.clnu.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Putwatana P, Reodecha P, Sirapo-ngam Y, Lertsithichai P, Sumboonnanonda K. Nutrition screening tools and the prediction of postoperative infectious and wound complications: Comparison of methods in presence of risk adjustment. Nutrition. 2005;21:691–697. doi: 10.1016/j.nut.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Sharma Y, Thompson C, Kaambwa B, Shahi R, Miller M. Validity of the malnutrition universal screening tool (MUST) in Australian hospitalized acutely unwell elderly patients. Asia Pac J Clin Nutr. 2017;26:994–1000. doi: 10.6133/apjcn.022017.15. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K, Nagahara H, Shibutani M, Otani H, Sakurai K, Toyokawa T, Tanaka H, Kubo N, Muguruma K, Kamata N, et al. A preoperative low nutritional prognostic index correlates with the incidence of incisional surgical site infections after bowel resection in patients with Crohn's disease. Surg Today. 2015;45:1366–1372. doi: 10.1007/s00595-014-1044-8. [DOI] [PubMed] [Google Scholar]
- 22.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 23.Hu Q, Wang G, Ren J, Ren H, Li G, Wu X, Gu G, Li R, Guo K, Deng Y, et al. Preoperative prognostic nutritional index predicts postoperative surgical site infections in gastrointestinal fistula patients undergoing bowel resections. Medicine (Baltimore) 2016;95:e4084. doi: 10.1097/MD.0000000000004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamana I, Takeno S, Shibata R, Shiwaku H, Maki K, Hashimoto T, Shiraishi T, Iwasaki A, Yamashita Y. Is the geriatric nutritional risk index a significant predictor of postoperative complications in patients with esophageal cancer undergoing esophagectomy? Eur Surg Res. 2015;55:35–42. doi: 10.1159/000376610. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa Y, Kawabata Y, Fujishiro K, Fukata S. Postoperative nutritional evaluation using geriatric nutritional risk index (GNRI) for aged patients with pancreatocoduodenectomy. J Geriatr Oncol. 2014;5(Suppl 1):S12–S13. doi: 10.1016/j.jgo.2014.06.029. [DOI] [Google Scholar]
- 26.Parikh JA, Beane JD, Kilbane EM, Milgrom DP, Pitt HA. Is American college of surgeons NSQIP organ space infection a surrogate for pancreatic fistula? J Am Coll Surg. 2014;219:1111–1116. doi: 10.1016/j.jamcollsurg.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


