Abstract
Prostate cancer is one of the most common cancer types, affecting millions of individuals worldwide. The present study reported two cases of metastatic prostate cancer presenting with newly diagnosed monoclonal gammopathy of undetermined significance (MGUS). To the best of our knowledge, prostate cancer leading to MGUS has not been documented previously. MGUS is generally thought to be benign and has been demonstrated to convert into multiple myeloma (MM), as well as other lymphoproliferative disorders. Due to the high mortality rate associated with MM, further studies are required to confirm and clarify the association between prostate cancer and MGUS. Additionally, patients can be counseled on the requirement for follow up studies following a diagnosis of prostate cancer.
Keywords: MGUS, prostate cancer, multiple myeloma, lymphoproliferative disorders
Introduction
Prostate cancer is the second most common cancer in men following lung cancer, and the fourth most common cancer type worldwide (1). Prostate cancer frequently metastasizes to bone and can present as osteolytic or osteoblastic lesions (2). Monoclonal gammopathy of undetermined significance (MGUS) is a condition where an abnormal protein is produced by plasma cells. The diagnostic criteria for MGUS is M protein <3 g/dl, clonal plasma cell population in bone marrow >10% and no end organ damage (3). MGUS has been documented to transform into multiple myeloma (MM) or similar lymphoproliferative disorders at a rate of 1-3% per year, or 17, 34 and 39% at 10, 20 and 25 years, respectively (4,5). In a prospective study performed in 2009, all or nearly all cases of MM were preceded by MGUS (6). The 5 year survival rate for individuals with MM is ~45%. Therefore, it is essential that any condition, which increases the risk of developing MM be addressed. The present study reported two cases where it is hypothesized that metastatic prostate cancer may have led to the condition of MGUS.
Case 1
This case study was approved by the Bioethics Committee of the Raritan Bay Medical Center (Perth Amboy, NJ, USA). A 76-year-old Caucasian male without significant past medical history presented to the emergency department of the Raritan Bay Medical Center with intractable lower back pain. The patient started developing back pain with increasing severity, which was aggravated by movements and decreased with rest, 2 weeks prior to admission. The patient complained of obstructive urinary symptoms, including hesitancy and urgency, which started 6 months previously. Physical examination findings were normal, other than the patient resisting movements of his lower extremities for fear of aggravating his back pain, and his vital signs were: Blood pressure 129/88 mmHg, respiratory rate of 19/min and a temperature of 98.6°F. Laboratory examination revealed hemoglobin (Hb) levels of 12.6 g/dl, hematocrit (hct) 37.3%, white blood cells (WBC) 9.1 K/µl and platelets 263 K/µl. A lumbar spine computed tomography (CT) scan, without contrast, revealed a mild compression fracture, superior endplate L2 without soft tissue involvement. Heterogeneous appearance of osseous elements and a lytic lesion involving the cortex of the visualized portion of the right iliac bone raised suspicion of osseous metastases or myeloma. Additionally, the levels of prostate-specific antigen (PSA) were determined to be 681.4 ng/ml. Based on imaging, a serum protein electrophoresis (SPE) was performed to rule out MM. The results revealed: Serum albumin, 2.70 l, α 1 globulin, 0.3, α 2 globulin, 0.8, β globulin, 0.7, γ globulin, 1.0 and M-spike, 0.5 (Fig. 1). A bone marrow aspirate revealed <10% plasma cells. These results failed to meet the criteria for MM. The patient was subsequently diagnosed with MGUS and metastatic prostate cancer. The patient was started on bicalutamide treatment and referred to a cancer center for further management.
Figure 1.
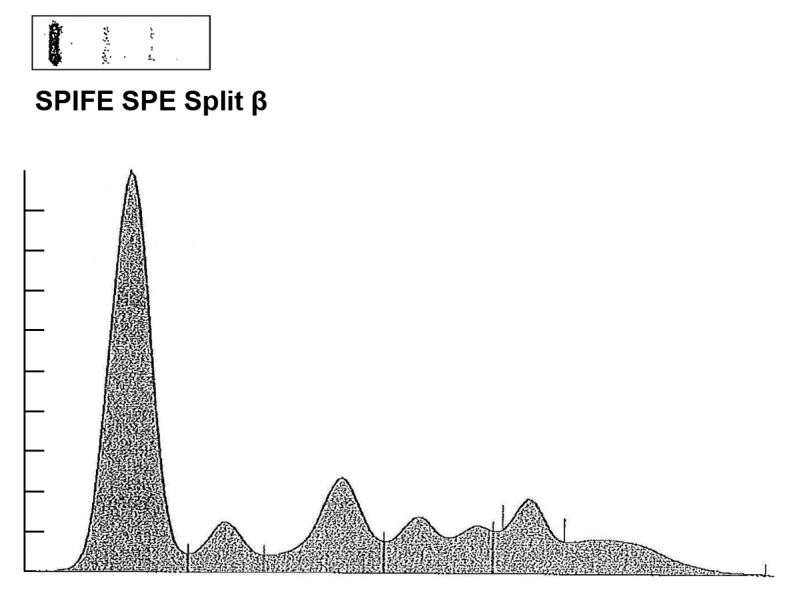
SPE was performed to detect the levels of serum albumin, 2.70 g/dl (normal, 3.2-5.6 g/dl); α 1, 0.3 g/dl (normal, 0.1-0.4 g/dl); α 2, 0.8 g/dl (Normal 0.4-1.2 g/dl); β, 0.7 g/dl (Normal 0.6-1.3 g/dl); γ, 1.0 g/dl (normal, 0.5-1.6 g/dl); M-spike, 0.5 g/dl. SPE, serum protein electrophoresis.
Case 2
An 83-year-old Hispanic male, with a past medical history significant for prostatic adenocarcinoma, presented with altered mental status. The patient began to look very weak and lethargic 2 days prior to admission. The family stated that the patient had difficulty passing urine and appeared disoriented. Upon physical examination, vital signs were a pulse rate of 101 beats/minute, blood pressure of 158/96 mmHg, temperature of 98.3°F, respiratory rate was 18/min and the remainder of the physical exam was unremarkable, with the exception that the patient was lethargic. Laboratory analysis revealed: Hb 10.1 g/dl, Hct 30%, WBC 13.4 K/µl, platelets 119,000 K/µl, sodium 131 mmol/l, potassium 6.5 mmol/l, chloride 95 mmol/l, blood urea nitrogen 76 mg/dl, creatinine 8.4 mg/dl and glucose 41 mg/dl. An electrocardiogram revealed sinus tachycardia. A chest x-ray was within the normal limits and a CT scan of the head revealed no acute intracranial hemorrhage or significant mass effect. The patient was admitted to the intensive care unit for altered mental status, secondary to uremia, acute kidney injury and hyperkalemia. A CT urogram revealed an enlarged and lobulated prostate gland, measuring 4.2×6.3 cm, with consequent bilateral hydronephrosis along with small sclerotic lesions scattered throughout the lower thoracic spine, lower lumbosacral spine, iliac bones, left superior pubic ramus, bilateral inferior pubic rami and left proximal femur. recom bilateral nephrostomy tube was placed and the patient underwent dialysis, which improved his renal function. A whole body bone scan confirmed the findings of the CT urogram and demonstrated focal areas of radioisotope uptake within the upper right ribs peripherally and mid right ribs posteriorly. SPE revealed an M spike of 0.3 (Fig. 2). Based on the M spike and findings on imaging, the patient was diagnosed with MGUS and metastatic prostatic adenocar cinoma upon biopsy. The patient was started on bicalutamide and Leuprolide treatment for metastatic prostate cancer, and was referred to a cancer center for further management.
Figure 2.
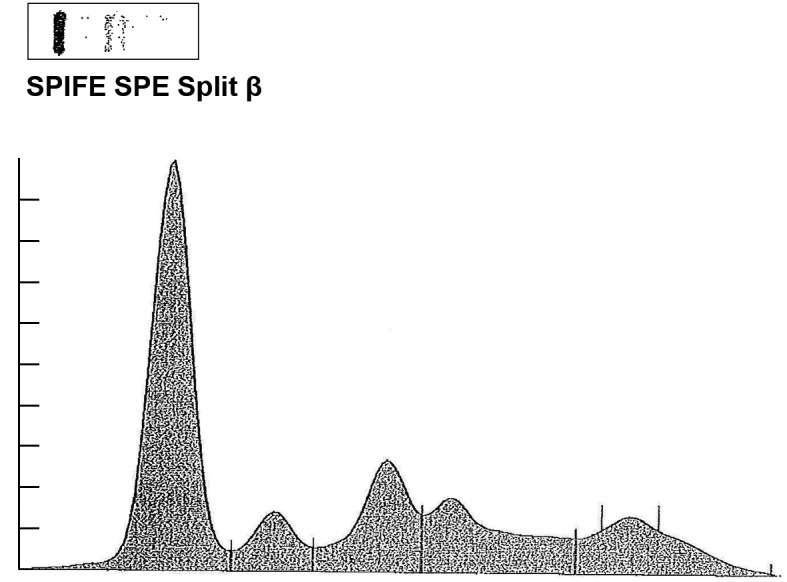
SPE was performed to detect the levels of serum albumin, 2.6 g/dl (normal 3.2-5.6 g/dl); α 1, 0.4 g/dl (normal, 0.1-0.4 g/dl); α 2, 0.9 g/dl (normal, 0.4-1.2 g/dl); β, 0.9 g/dl (normal, 0.6-1.3 g/dl); γ, 0.7 g/dl (normal, 0.5-1.6 g/dl); M spike of 0.3 g/dl. SPE, serum protein electrophoresis.
Discussion
In 2012, >1 million cases of prostate cancer were diagnosed worldwide and >2/3 of those occurred in developed nations (1). According to the American Cancer Society, in the United States alone, 233,000 novel cases of prostate cancer were predicted in 2014 and ~30,000 men are expected to succumb to prostate cancer. Although the risk factors remain to be completely understood, diet, ethnicity, age and genetics are important. A diet consisting of red meat and large quantities of dairy puts an individual at a greater risk of developing prostate cancer (2). In addition, African American men are at an increased risk of developing prostate cancer (2,7).
Patients with prostate cancer often present with obstructive symptoms, pain/discomfort in the lower back, hips or thighs, and bone pain. In the majority of cases, prostate cancer metastasizes to the bone causing osteoblastic legions, though rarely, osteolytic lesions can be observed (2,8,9). However, since there are a large number of prostate cancer cases, it is easy to identify a case, which may present itself differently.
Patients with MGUS have been previously shown to have a higher risk of developing MM, as well as Waldenstrom macroglobulinemia, light-chain amyloidosis, or associated disorders (10).
In the initial case in the present study, the patient was observed to exhibit prostate cancer from the classical signs of difficulty voiding, back pain and a high levels of PSA. However, since the predominant complaint was the lower back pain, the patient was initially hypothesized to exhibit metastatic spread to the lumbar spine. The lumbar CT revealed osteolytic lesions, which are classical for multiple myeloma, however, further studies, including protein electrophoresis and bone marrow aspirate ruled out MM. This raised a question about the possible scenario of prostate cancer leading to MGUS.
A few cases have been documented in the past two decades noting MGUS presenting with prostate cancer (3). Other cases of prostate cancer presenting with MM have been previously observed (11). However, the present study noted that no sufficient studies suggesting the possibility for MGUS forming from prostate cancer existed. Although the mechanism remains to be elucidated, chronic inflammation, in this case from cancer, is believed to stimulate excessive growth of a single clone of a plasma cell (3). This may lead to MGUS and quite possibly other hematologic cancer types.
Since the patient in case 1 exhibited a high PSA level and had trouble voiding for the past 6 months, it was hypothesized that the prostate cancer may have been present for at least 6-7 months. During that 6-7 months, the constant inflammation and proliferation of the plasma cells may have resulted in MGUS. Furthermore, it is likely that the osteolytic lesions are from prostate cancer since the patient does not fit into any other characteristics of MM.
The patient in case 2 presented with a history of prostate cancer, diagnosed 13 years previously, which has now recurred along with being newly diagnosed with MGUS. Based on the levels of M protein and the exponential increase in the risk of MGUS turning into MM or other lymphoproliferative disorders, the present study hypothesized that his MGUS is in fact a novel development likely to be caused by his prostate cancer.
Currently, recommendations for evaluating patients with high risk MGUS are using baseline bone marrow examination with cytogenetics and fluorescence in situ hybridization (12). Additionally it is recommended that these patients undergo bone imaging studies, and SPE every 6 months for the first year, followed by an annual SPE (13). Although MGUS is generally a benign condition, the possibility of MGUS becoming MM warrants the requirement for careful observation and annual studies. The present study recommend that patients with prostate cancer have more frequent clinical examinations, as well as using SPE, in order to diagnose lymphoproliferative disorders at an earlier stage.
Glossary
Abbreviations
- CT
computed tomography
- MGUS
monoclonal gammopathy of undetermined significance
- MM
multiple myeloma
- SPE
serum protein electrophoresis
References
- 1.Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME. Prostate cancer and the influence of dietary factors and supplements: A systematic review. NutrMetab (Lond) 2014;11:30. doi: 10.1186/1743-7075-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajendiran G, Green L, Chhabra G. A rare presentation of prostate cancer with diffuse osteolytic metastases and PSA of 7242 ng/ml. International Journal of Case Reports and Images. 2011;2:16–20. doi: 10.5348/ijcri-2011-09-55-CR-5. [DOI] [Google Scholar]
- 3.Tsutsumi M, Hara T, Fukasawa R, Koiso K. Prostatic cancer presenting monoclonal gammopathy: Report of two cases. Hinyokika Kiyo. 1993;39:569–571. [PubMed] [Google Scholar]
- 4.Bladé J. Clinical practice. Monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;355:2765–2770. doi: 10.1056/NEJMcp052790. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 6.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts R. From bench to bedside: The realities of reducingglobalprostatecancerdisparity in black men. Ecancermedicalscience. 2014;28:458. doi: 10.3332/ecancer.2014.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinjamoori AH, Jagannathan JP, Shinagare AB, Taplin ME, Oh WK, Van den Abbeele AD, Ramaiya NH. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR AM J Roentgenology. 2012;199:367–372. doi: 10.2214/AJR.11.7533. [DOI] [PubMed] [Google Scholar]
- 9.Migita T, Maeda K, Ogata N. A case of prostate cancer associated with osteolytic bone metastases. Hinyokika Kiyo. 1999;45:371–374. (In Japanese) [PubMed] [Google Scholar]
- 10.Kyle RA. Monoclonal gammopathy of undetermined significance: Natural history in 241 cases. Am J Med. 1978;64:814–826. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 11.Pérez López ME, Garcia Mata, Garcia Gómez J, Salgado Fernández M, Fírvida Pérez JL. Prostate adenocarcinoma and synchronous multiple myeloma: A case report. ActasUrol Esp. 2007;31:157–159. doi: 10.1016/S0210-4806(07)73614-8. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 12.Kyle RA, Buadi F, Rajkumar SV. Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) Oncology (Williston Park) 2011;7:578–586. [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Int Agency Res Canc. 2010 [Google Scholar]


