Abstract
Microsomal prostaglandin E2 synthase-1 (mPGES-1), an inducible enzyme that converts prostaglandin H2 to prostaglandin E2 (PGE2), plays an important role in a variety of inflammatory diseases. We investigated the contribution of mPGES-1 to renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) for 7 days using wild-type (WT) and mPGES-1 knockout (KO) mice. UUO induced increased mRNA and protein expression of mPGES-1 and cyclooxygenase-2 in WT mice. UUO was associated with increased renal PGE2 content and upregulated PGE2 receptor (EP) 4 expression in obstructed kidneys of both WT and mPGES-1 KO mice; EP4 expression levels were higher in KO mice with UUO than those in WT mice. Protein expression of NLRP3 inflammasome components ASC and interleukin-1β was significantly increased in obstructed kidneys of KO mice compared with that in WT mice. mRNA expression levels of fibronectin, collagen III, and transforming growth factor-β1 (TGF-β1) were significantly higher in obstructed kidneys of KO mice than that in WT mice. In KO mice, protein expression of fibronectin and collagen III was markedly increased in obstructed kidneys compared with WT mice, which was associated with increased phosphorylation of protein kinase B (AKT). EP4 agonist CAY10598 attenuated increased expression of collagen I and fibronectin induced by TGF-β1 in inner medullary collecting duct 3 cells. Moreover, CAY10598 prevented the activation of NLRP3 inflammasomes induced by angiotensin II in human proximal tubule cells (HK2). In conclusion, these findings suggested that mPGES-1 exerts a potentially protective effect against renal fibrosis and inflammation induced by UUO in mice.
Keywords: prostaglandin, fibrosis, AKT, inflammasomes
renal fibrosis and microinflammatory state is a common pathological feature of progressive kidney disease due to a variety of grounds. It is characterized by accumulation of fibroblasts and excessive deposition of extracellular matrix (ECM), and increases of various molecules related to the inflammation and fibrosis, cytokines and TGF-β1, eventually leading to a loss of functional nephrons and end-point renal malfunction. Unilateral ureteral obstruction (UUO) is a classical model of renal fibrosis and inflammation. Accumulating evidence indicates that renal inflammation is associated with activation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasomes, a large cytosolic multiple protein complex regulating proinflammatory cytokine interleukin-1β (IL-1β) production (1). We (46) and others (27) have shown that activation of the NLRP3-ASC-caspase-1-IL-1β axis plays an important role in renal malfunction following UUO. Understanding on pathways of proinflammatory and profibrotic molecules in UUO kidney would translate into the development of anti-inflammation and antifibrosis therapeutic strategies.
Prostaglandin E2 synthase (PGES) terminally converts cyclooxygenase (COX)-derived prostaglandin H2 into prostaglandin E2 (PGE2). Three main mammalian PGES have been cloned including microsomal prostaglandin E synthase-1 (mPGES-1), mPGES-2, and cytosolic PGES (cPGES) (21, 42). In the kidney, mPGES-1 expression is found in macula densa, distal convoluted tubule, collecting duct, and renal medullary interstitial cells, corresponding to the nephron distribution of PGE2 synthesis (8, 17). mPGES-1 is an essential component of PGE2 production during inflammatory reactions as evidenced by the fact that proinflammatory stimuli clearly induced mPGES-1 in vitro and in vivo (19) and mPGES-1 deficiency or inhibition attenuates pain and inflammatory responses (44).
PGE2, a member of the prostanoid family, possesses anti-inflammatory and pro-resolving inflammation properties. PGE2 inhibits the biosynthesis and release of pro-inflammatory mediators [e.g., tumor necrosis factor-α (TNF-α) and IL-1β] during the late phase of inflammation and induces the formation of cytokines (e.g., IL-10) which actively antagonize inflammation (26, 30). In general PGE2 functions through binding the four G protein-coupled receptors EP1-4. EP4 couples to Gs and raises intracellular cAMP concentration. It is also able to activate phosphatidylinositol 3-kinase (PI3K), leading to the activation of protein kinase B (AKT) (3, 5, 45). Recent studies showed controversial roles of the PGE2-EP4 pathway in renal fibrosis. EP4 signaling promotes ECM accumulation in cultured mouse glomerular mesangial cells (47) and exacerbates albuminuria and fibrosis in diabetic kidneys (32), whereas in another study, EP4 was shown to prevent renal fibrosis in obstructed kidneys of mice with UUO (33). mPGES-1-derived PGE2 seems to play a complex role in inflammation and fibrosis, and this role requires further analysis.
In the present study, we examined whether deficiency of mPGES-1 exacerbated renal interstitial fibrosis and promoted activation of NLRP3 inflammasome in mice with UUO. We also investigated whether EP4 activation by agonist protected cellular injury induced by TGF-β1 or ANG II and potential signaling pathways in cultured inner medullary collecting duct cells or proximal tubular cells.
METHODS AND MATERIALS
Materials.
Antibodies to NLRP3 were from Novus, caspase-1 from Abcam, ASC from Santa Cruz Biotechnology, p-AKT (ser437), AKT, and IL-1β from Cell Signaling Technology, fibronectin from Sigma, COX2, α-SMA, collagen I, and collagen III from Boster (Wuhan, China). Recombination human TGF-β1 was purchased from Sigma (St. Louis, MO).
Animals and treatments.
Animal procedures were approved by the Animal Care and Use Committee of Sun Yat-sen University. mPGES-1 mutant mice were originally generated by Trebino et al. (44). This mouse colony was propagated at the Sun Yat-sen University and maintained on a mixed DBA/1lacJxC57/BL6x129/Sv background. Genotypes were identified by PCR (44). All the animals at 3–4 mo of age were maintained on a 12:12-h light/dark cycle at 24°C and received food and water ad libitum before experimentation. Before surgery, the mice were anesthetized with pentobarbital sodium, and during surgery the mice were placed on a heated table to maintain rectal temperature at 37–38°C. UUO were established as previously described (29). In brief, UUO was established through a midline abdominal incision, where the left ureter was exposed. The ureter was then occluded by tightening the tubing with a 5-0 silk suture at the midportion. After 7 days, the mice were euthanized.
Mice were allocated to the groups indicated below. Age- and time-matched sham-operated controls were prepared and observed in parallel with each UUO group in the following groups: WT mice with sham-operated (n = 6), WT mice with UUO for 7 days (n = 7), mPGES-1−/− mice with sham-operated (n = 6), and mPGES-1−/− mice with UUO for 7 days (n = 7). The two kidneys in each animal were removed and separately prepared for protein and mRNA measurement, or for immunohistochemistry.
Blood and urine chemistry.
Urine was collected and clearance studies were performed during 24-h periods throughout the study of UUO. At the end of each protocol, blood samples were collected into heparinized tubes for the determination of serum creatinine and osmolality when the mice were euthanized. The osmolality of urine and serum was determined by freezing-point depression (OM 806, Omometer, Loser, Germany). The serum and urine concentrations of creatinine were determined by using an EIA kit according to the manufacturer’s instructions (BioAssay Systems).
Cell culture and treatment.
The established murine inner medullary collecting duct cell line (IMCD3) was previously provided by Dr. Christopher J. Rivard (37). Cell stocks were maintained as described previously (9). IMCD3 and HK2 cells were cultured in DMEM/F12 containing 10% fetal bovine serum (FBS), 1.20 g/l sodium bicarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. IMCD3 cells were first cultured in free serum DMEM/F12 for 24 h, then the media was changed to fresh DMEM/F12 with free FBS containing TGF-β1 (2 ng/ml) for another 48 h accompanied with EP4 agonist CAY10598 (10 μM) pretreated for 1 h with the cells before TGF-β1 treatment. Human proximal tubular epithelial cells (HK2) were first cultured in free serum DMEM/F12 for 24 h, then the media was changed to fresh DMEM/F12 with free FBS containing ANG II (100 nM) for another 12 h accompanied with EP4 agonist CAY10598 (10 μM) pretreated for 1 h with the cells before ANG II treatment.
Electrophoresis and immunoblotting.
At the day of euthanasia, mice were anesthetized with pentobarbital sodium, and kidneys were frozen in liquid nitrogen immediately after removal. Tissue was minced finely and homogenized in dissecting buffer (0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, pH 7.2, and containing the following protease inhibitors: 8.5 µM leupeptin, 1 mM phenylmethyl sulfonyl fluoride). This homogenate was centrifuged at 4,000 g for 15 min at 4°C. The supernatants were assayed for protein concentration using the method of BCA (Pierce, Rockford, IL). Gel samples were made from this pellet. Samples of membrane fractions were run on 12% polyacrylamide minigels. After transferring by electroelution to PVDF membranes, blots were blocked with 5% milk in PBS-T (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 h and incubated with primary antibodies overnight at 4°C. After being washed with PBS-T, the blots were incubated with horseradish peroxidase-conjugated secondary antibody (Pierce). After a final washing as above, corresponding secondary antibodies were visualized using enhanced chemiluminescence (Pierce). The signals were quantified with a chemiluminescence detector and the accompanying densitometry software (UVP, Upland, CA).
Immunohistochemistry.
The kidneys from UUO and Sham mice were fixed by retrograde perfusion via the left ventricle with 4% paraformaldehyde, in 0.1 M cacodylate buffer (pH 7.4). Kidney blocks containing all kidney zones were dehydrated and embedded in paraffin. For immunoperoxidase microscopy, sections (4-μm thick) cut from paraffin-embedded kidney samples were used for immunostaining of collagen III (28). Briefly, after dewaxing and rehydration, a microwave pretreatment in citrate buffer (pH 6.2) was performed to unmask antigens present in the renal tissue. Tissue sections were then incubated overnight at 4°C with primary antibodies. After rinsing in phosphate-buffered saline, slides were exposed for 1 h to the secondary antibody. The sections were later washed with PBS, followed by incubation with diaminobenzidine for 10 min. The microscopic examination was carried out by using a Leica DM2000 light microscope (Leica, Heidelberg, Germany). The quantitative morphometry was performed by an observed blinded to the protocol.
RNA extraction and quantitative real-time PCR.
Total RNA was extracted from the kidneys according to the manufacturer’s instructions for TRIzol reagent (Invitrogen, CA). Total RNA (500 ng) was used for reverse transcription using a PrimeScript RT reagent Kit Perfect Real Time kit (Takara Bio). The cDNA was used for quantitative real-time PCR analysis (qPCR) using SYBR Premix Ex Taq (Perfect Real Time, Takara Bio). Target mRNA was determined using the comparative cycle threshold method of relative quantitation. The calibrator sample was selected from PBS-treated tissue, and GAPDH was used as an internal control. Primer sequences used are provided in Table 1.
Table 1.
Primer sequences for RT-PCR (mouse)
| Target Gene | Primer Sequence (5′→3′) | Target Gene | Primer Sequence (5′→3′) |
|---|---|---|---|
| mPGES-1 F | AGCACACTGCTGGTCATCAA | NLRP3 F | CGAGACCTCTGGGAAAAAGCT |
| mPGES-1 R | CTCCACATCTGGGTCACTCC | NLRP3 R | GCATACCATAGAGGAATGTGATGTACA |
| mPGES-2 F | GCTGGGGCTGTACCACAC | ASC F | ACTTCTGTGACCCTGGCAATGAG |
| mPGES-2 R | GATTCACCTCCACCACCTGA | ASC R | GCTGAGCAGCTGCAAACGAC |
| cPGES F | GGTAGAGACCGCCGGAGT | Caspase-1 F | GATGGCACATTTCCAGGACTGA |
| cPGES R | TCGTACCACTTTGCAGAAGCA | Caspase-1 R | TGTTGCAGATAATGAGGGCAAGAC |
| COX1 F | TAATCCCAGCACTTGGAAGG | IL-1β F | GAAATGCCACCTTTTGACAGTG |
| COX1 R | TGGCTGGCCTAGAACTCACT | IL-1β R | TGGATGCTCTCATCAGGACAG |
| COX2 F | TGGGTGTGAAGGGAAATAAGG | Fibronectin F | ATGTGGACCCCTCCTGATAGT |
| COX2 R | CATCATATTTGAGCCTTGGGG | Fibronectin R | GCCCAGTGATTTCAGCAAAGG |
| EP1 F | TAACGATGGTCACGCGATGG | α-SMA F | CCCAACTGGGACCACATGG |
| EP1 R | ATGCAGTAGTGGGCTTAGGG | α-SMA R | TACATGCGGGGGACATTGAAG |
| EP2 F | ATGCTCCTGCTGCTTATCGT | Collagen III F | CTGTAACATGGAAACTGGGGAAA |
| EP2 R | AGGGCCTCTTAGGCTACTGC | Collagen III R | CCATAGCTGAACTGAAAACCACC |
| EP3 F | TTGGGCTTGCTGGCTCTG | TGF-β F | GCAACATGTGGAACTCTACCAGA |
| EP3 R | CGTCTCCGTGGTGATTCTGC | TGF-β R | GACGTCAAAAGACAGCCACTCA |
| EP4 F | GTTCCGAGACAGCAAAAGC | GAPDH F | TGACCTCAACTACATGGTCTACA |
| EP4 R | CACCCCGAAGATGAACATCAC | GAPDH R | CTTCCCATTCTCGGCCTTG |
mPGES, microsomal prostaglandin E2 synthase; COX, cyclooxygenase; cPGES, cytosolic PGES; EP, prostaglandin E receptor; NLRP3, Nod-like receptor pyrin domain containing 3; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; IL-1β, interleukin-1β; IL-18, interleukin-18; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor-β; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis.
Results are presented as means ± SE. Data were analyzed by ANOVA and the Student-Newman-Keuls test for multiple comparisons. Comparisons between two groups were made by unpaired t-test. Two-way ANOVA was used to check possible interactions followed by the post hoc analysis by Bonferroni’s multiple comparison (GraphPad InStat 3 and Prism 5.0 software). Statistical significance was accepted at the P < 0.05 level. Values represent means ± SE of three independent sets of experiments in cell culture studies.
RESULTS
Expression of mPGES-1 protein and mRNA was increased in obstructed kidneys of WT mice with UUO.
There were no significant changes of urine output or urine osmolality between WT and mPGES-1 KO mice. With the compensation of nonobstructed kidneys, no significant difference was observed in renal function in both WT and mPGES-1 KO mice between Sham and UUO groups (Table 2).
Table 2.
Metabolic data of WT and mPGES-1 KO mice
| WT-Sham | WT-7UUO | KO-Sham | KO-7UUO | |
|---|---|---|---|---|
| UO, μl·24 h−1·g BW−1 | 50.2 ± 6.4 | 48.3 ± 4.8 | 50.3 ± 3.6 | 61.0 ± 4.0 |
| UOsm, mOsm/kgH2O | 2,132 ± 175 | 2,233 ± 387 | 2,013 ± 72 | 2,247 ± 247 |
| CCr, μl·min−1·g BW−1 | 2.19 ± 0.24 | 1.76 ± 0.20 | 2.03 ± 0.08 | 1.80 ± 0.07 |
Values are means ± SE. WT, wild type; KO, knockout; UUO, unilateral ureteral obstruction; UO, urine output; UOsm, urine osmolality; CCr, creatinine clearance.
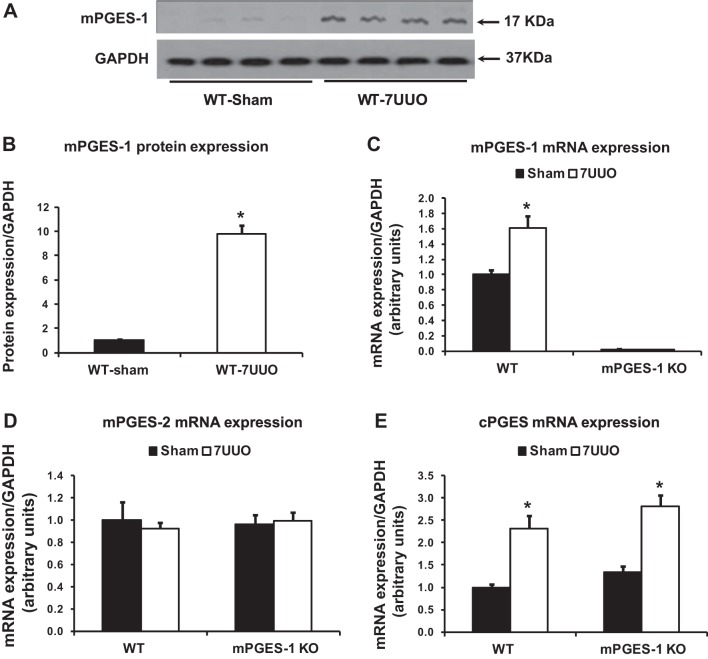
The protein abundance of mPGES-1 in the obstructed kidney showed a 10-fold increase in WT mice with UUO compared with Sham (Fig. 1, A and B); mRNA levels of mPGES-1 were also significantly increased after UUO (Fig. 1C). In contrast, mRNA levels of mPGES-2 did not change in obstructed kidneys from WT and mPGES-1 KO mice with UUO (Fig. 1D). Compared with corresponding Sham groups, cPGES mRNA levels in obstructed kidneys were significantly increased in mPGES-1 WT and KO mice with UUO, but there was no statistical difference in obstructed kidneys of mPGES-1 KO mice compared with that of WT mice (Fig. 1E).
Fig. 1.
mPGES-1 protein expression was dramatically increased after UUO in WT mice. A: Western blot of mPGES-1 protein expression in the kidney of mPGES-1 WT (n = 4) and KO mice (n = 4). B: corresponding densitometric analyses of protein expression levels of mPGES-1 corrected by GAPDH indicated that mPGES-1 protein abundance increased dramatically in obstructed kidneys after UUO compared with controls. C–E: analysis of mRNA expression by quantitative real-time PCR for mPGES-1 (C), mPGES-2 (D), and cPGEs (E) in obstructed kidneys of mPGES-1 WT and KO mice. 7UUO, unilateral ureteral obstruction for 7 days; WT, wild type; KO, mPGES-1 knockout. WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. *P < 0.05 compared with Sham groups.
Expression of COX-2 protein was increased in obstructed kidneys of mPGES-1 KO mice with UUO.
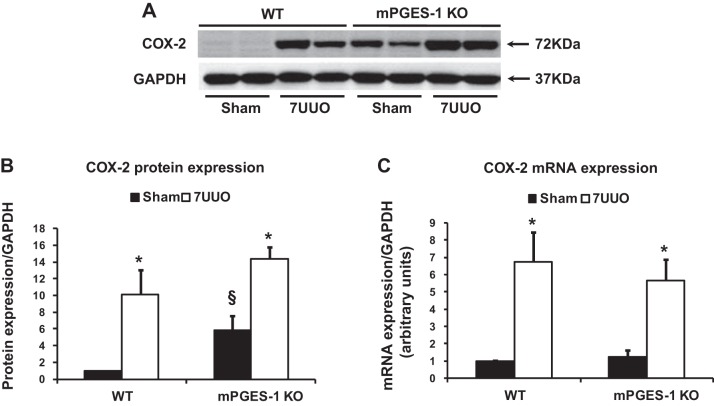
Next, we examined protein expression of COX-2 and COX-1 in the obstructed kidney in mPGES-1 KO mice with 7UUO. Consistent with previous studies (36), protein and mRNA expression of COX-2 was quite low but detectable in the kidney of WT mice, but it was dramatically induced in obstructed kidneys of WT-UUO mice (Fig. 2). In both sham-operated kidneys and obstructed kidneys of mPGES-1 KO mice, protein expressions of COX-2 were significantly upregulated compared with corresponding kidneys of WT mice (Fig. 2, A and B). mRNA levels of COX-2 were markedly increased in obstructed kidneys of mPGES-1 KO mice with UUO (Fig. 2C). In contrast, COX-1 mRNA levels did not show a significant difference in obstructed kidneys of WT (1.30 ± 0.12 in UUO vs. 1.00 ± 0.11 in Sham, P > 0.05) and mPGES-1 KO mice subjected to 7 days of UUO (2.05 ± 0.62 in UUO vs. 1.00 ± 0.51 in Sham, P > 0.05).
Fig. 2.
COX-2 protein expression and mRNA levels were upregulated in obstructed kidneys of mPGES-1 WT and KO mice. A: semiquantitative immunoblots reacted with anti-COX-2 antibodies. GAPDH was used as internal loading control. B: corresponding densitometric analyses of protein expression levels of COX-2 corrected by GAPDH. C: analysis of mRNA expression by quantitative real-time PCR for COX2 in obstructed kidneys of mPGES-1 WT and KO mice. COX-2, cyclooxygenase-2. WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. *P < 0.05 compared with Sham groups. §P < 0.05 compared with WT-Sham.
Expression of EP4 mRNA was increased in obstructed kidneys of mPGES-1 KO mice with UUO.
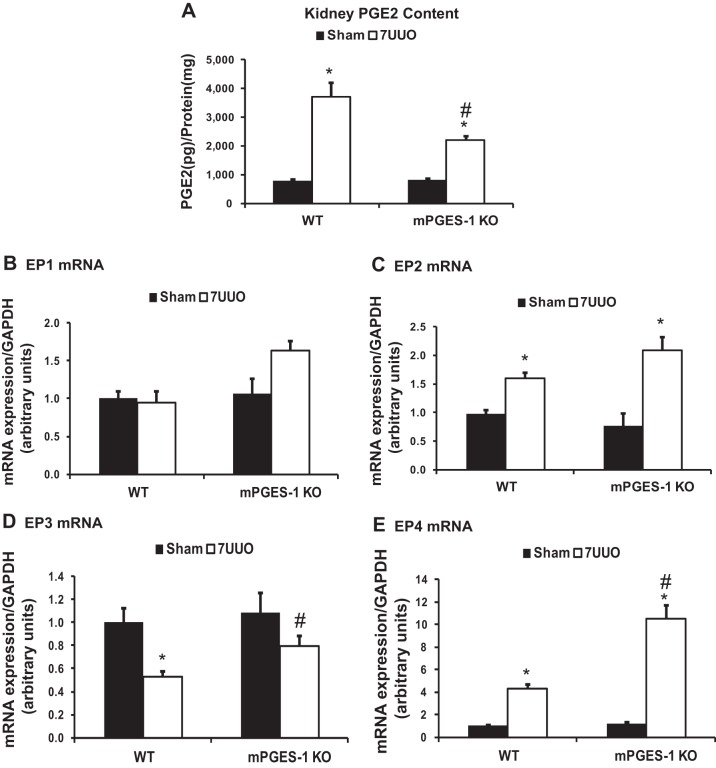
To investigate a potential role of the COX-2/mPGES-1/PGE2 pathway in renal fibrosis, we examined PGE2 production and expression of renal prostanoid receptors in response to UUO. After UUO, renal PGE2 production dramatically increased in WT mice and in mPGES-1 KO mice, indicating that other PGES, not only mPGES-1, also contribute to PGE2 synthesis and secretion in the kidney after obstruction. However, renal PGE2 production from mPGES-1 KO mice was much lower than that of WT mice after UUO (Fig. 3A).
Fig. 3.
Renal PGE2 content and mRNA expression levels of EPs in obstructed kidneys of mPGES-1 WT mice and KO mice. A: renal PGE2 production measured by ELISA. B–E: analysis of mRNA expression by quantitative real-time PCR for EP1 (B), EP2 (C), EP3 (D), and EP4 (E) in obstructed kidneys of mPGES-1 WT and KO mice. WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. *P < 0.05 compared with Sham groups. #P < 0.05 compared with WT-7UUO.
In WT mice after UUO, there was no significant difference in the expression levels of EP1 mRNA (Fig. 3B). The expression level of EP2 and EP4 mRNA increased significantly to levels of up to two- and fourfold (Fig. 3, C and E), whereas EP3 mRNAs decreased significantly in obstructed kidneys compared with Sham (Fig. 3D). In mPGES-1 KO mice, the pattern of EP mRNA expression after UUO was the same as observed in WT mice; however, EP4 mRNA expression levels in obstructed kidneys were strikingly increased to 2.5-fold of that in WT mice. The mRNA expression level of EP3 was also higher in mPGES-1 KO mice than that in WT mice (Fig. 3, D and E).
mPGES-1 deficiency accelerated renal inflammation in obstructed kidneys of mice with 7UUO.
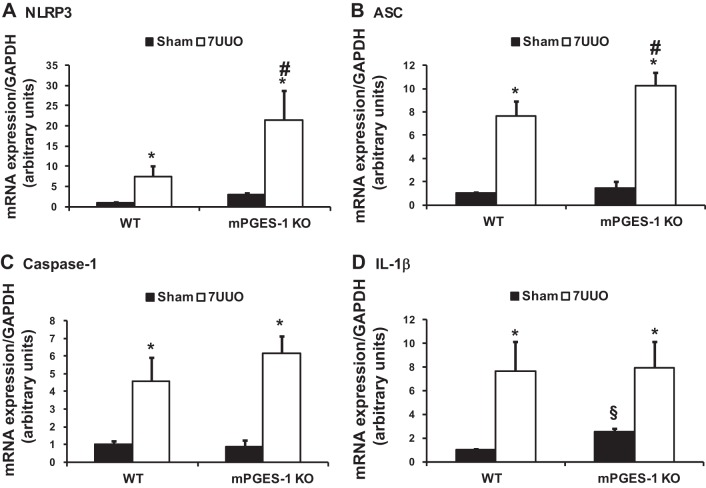
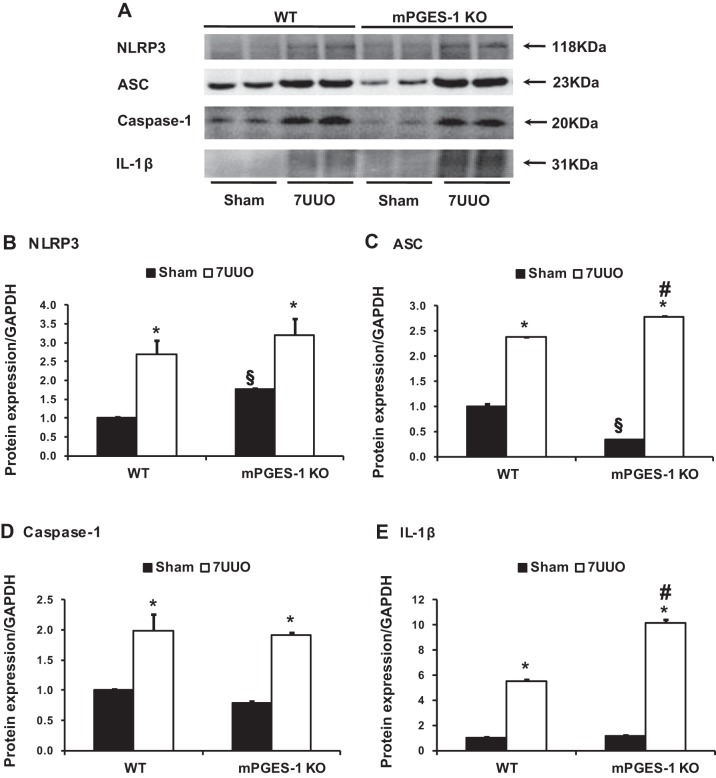
UUO is also associated with inflammatory response and activation of inflammasomes in the kidney (46). mRNA expression levels of NLRP3 inflammasome components (NLRP3, ASC, caspase-1, and IL-1β) were significantly increased in obstructed kidneys of WT mice with UUO (Fig. 4). This increase was also seen in mPGES-1 KO mice. Particularly, the increase of NLRP3, ASC, and caspase-1 mRNA expression was indeed greater in mPGES-1 KO mice than in WT mice (Fig. 4). Protein expression of NLRP3 inflammasome components was markedly unregulated in obstructed kidneys of WT and mPGES-1 KO mice with UUO compared with Sham, with even greater expression of ASC and IL-1β protein in mPGES-1 KO mice. In mPGES-1 KO sham-operated groups, the abundance of NLRP3 protein was increased, whereas expression of ASC was decreased compared with WT Sham mice (Fig. 5).
Fig. 4.
Analysis of mRNA expression levels by quantitative real-time PCR for NLRP3 inflammasome components NLRP3 (A), ASC (B), caspase-1 (C), and IL-1β (D) in obstructed kidneys of mPGES-1 WT and KO mice. NLRP3, Nod-like receptor pyrin domain containing 3; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; IL-1β: interleukin-1β. WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. *P < 0.05 compared with Sham groups. #P < 0.05 compared with WT-7UUO. §P < 0.05 compared with WT-Sham.
Fig. 5.
Western blot analysis of NLRP3 inflammasome components NLRP3, ASC, caspase-1, and IL-1β protein expressions in obstructed kidneys of mPGES-1 WT and KO mice. A: semiquantitative immunoblots reacted with anti-NLRP3, ASC, caspase-1, and IL-1β antibodies. GAPDH was used as internal loading control. Corresponding densitometric analyses of protein expression levels of NLRP3 (B), ASC (C), caspase-1 (D), and IL-1β (E) corrected by GAPDH. WT, wild type; KO, knockout. WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. *P < 0.05 compared with Sham groups. §P < 0.05 compared with WT-Sham. #P < 0.05 compared with WT-7UUO.
mPGES-1 deficiency accelerated renal fibrosis in obstructed kidneys of mice with 7UUO.
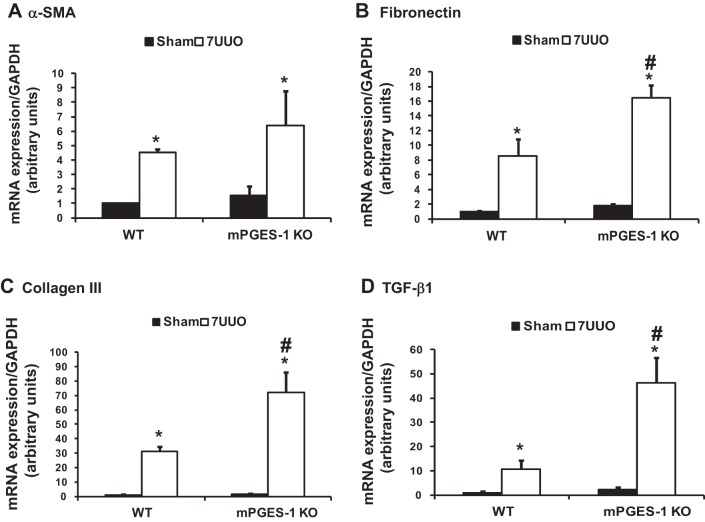
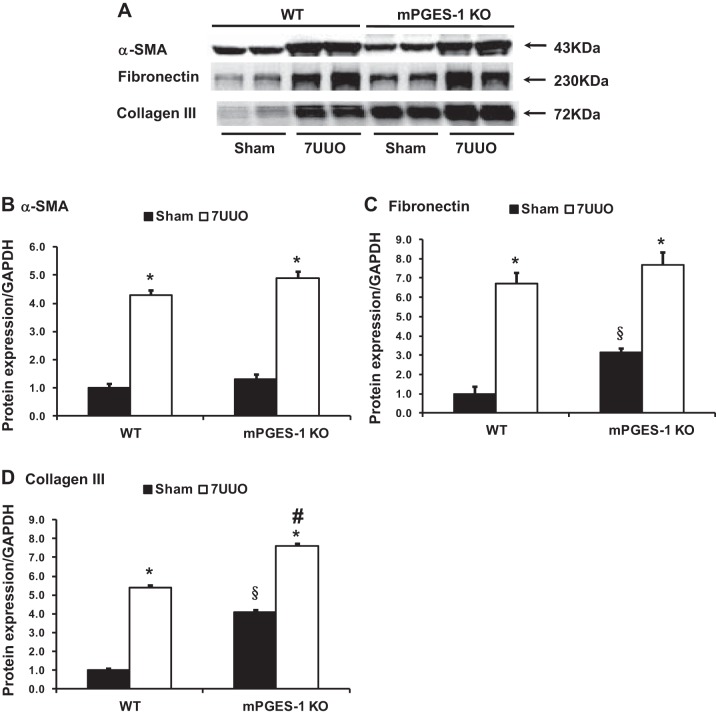
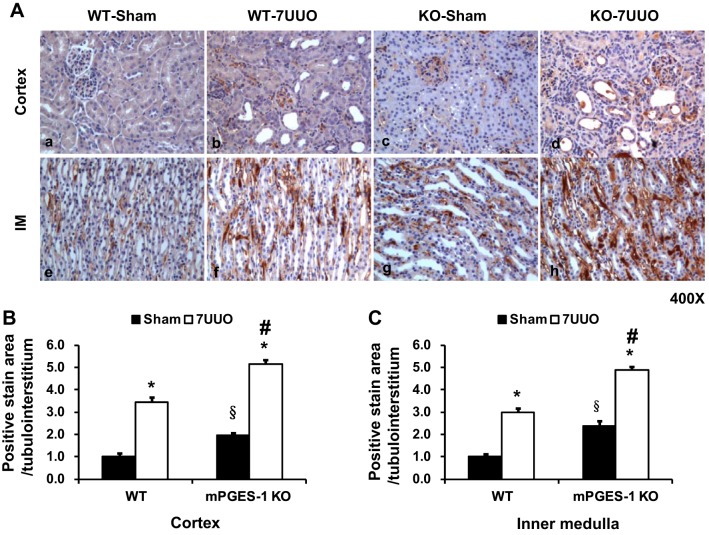
mRNA and protein expression of several profibrotic factors were examined in mPGES-1 KO mice with UUO. mRNA levels of α-SMA, fibronectin, collagen III, and TGF-β were all markedly increased in obstructed kidneys of WT or mPGES-1 KO mice with UUO compared with Sham groups. Moreover, mRNA expression levels of fibronectin, collagen III, and TGF-β in obstructed kidneys of mPGES-1 KO mice were much higher than that in WT mice (Fig. 6). Protein abundance of α-SMA, fibronectin, and collagen III was also increased in obstructed kidneys of mPGES-1 WT or KO mice with UUO compared with Sham, and again this increase was much greater in KO mice (Fig. 7). Interestingly in the basal state, expression levels of fibronectin and collagen III protein in the kidney of mPGES-1 KO mice were higher than that in WT mice (Fig. 7). Immunohistochemistry showed that collagen III staining was more extensive in obstructed kidneys of mPGES KO mice than WT mice (Fig. 8). These data likely indicate involvement of mPGES-1 in development of renal fibrosis after 7UUO.
Fig. 6.
Analysis of mRNA expression by quantitative real-time PCR for α-SMA (A), fibronectin (B), collagen III (C), and TGF-β1 (D) in obstructed kidneys of mPGES-1 WT and KO mice. α-SMA, α-smooth muscle actin; WT, wild type; KO, knockout. WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. *P < 0.05 compared with Sham groups. #P < 0.05 compared with WT-7UUO.
Fig. 7.
Western blot analysis of α-SMA, fibronectin, and collagen III protein expression in obstructed kidneys of mPGES-1 WT and KO mice. A: semiquantitative immunoblots reacted with anti-α-SMA, fibronectin, and collagen III antibodies. GAPDH was used as internal loading control. Corresponding densitometric analyses of protein expression levels of fibronectin (B) and collagen III (C) corrected by GAPDH (B). WT-Sham, n = 6; WT-7UUO, n = 7; KO-Sham, n = 6; KO-7UUO, n = 7. α-SMA, α-smooth muscle actin. *P < 0.05 compared with Sham groups. §P < 0.05 compared with WT-Sham. #P < 0.05 compared with WT-7UUO.
Fig. 8.
mPGES-1 deletion exacerbated UUO-induced increased collagen III staining in kidney cortex and medulla of mPGES-1 WT and KO mice. A: collagen III labeling intensity was significantly increased in obstructed kidneys after UUO. Collagen III labeling in obstructed kidneys of mPGES-1 KO mice with UUO were more extensive and labeling intensity was stronger than WT-7UUO mice. Magnification, ×400. B: quantitative analysis percent of collagen III staining area in mPGES-1 mice on WT-Sham, WT-7UUO, KO-Sham, and KO-7UUO. Statistical analyses were performed by one-way analyses of variance (ANOVA). WT, wild type; KO, knockout. *P < 0.05 compared with Sham groups. §P < 0.05 compared with WT-Sham. #P < 0.05 compared with WT-7UUO.
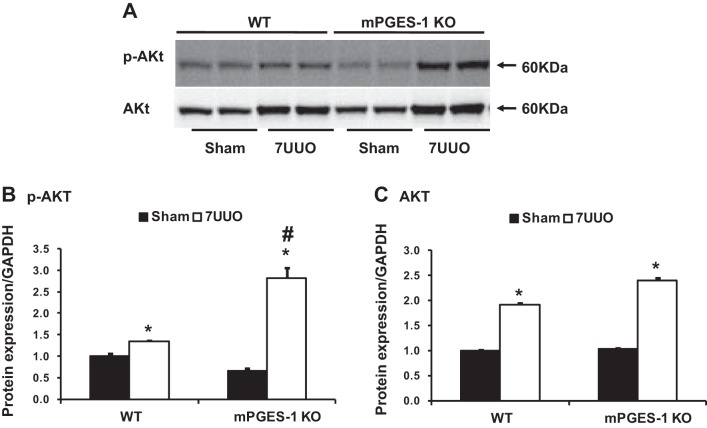
mPGES-1 deficiency promoted AKT phosphorylation in obstructed kidneys of mice with UUO.
Protein kinase B (AKT) plays an important role in cell growth and proliferation (31). To study the potential cellular signaling pathway, we examined active AKT levels in obstructed kidneys. As shown in Fig. 9, A and B, Western blot demonstrated that p-Akt (Ser473) protein was significantly elevated in obstructive kidneys of both mPGES-1 WT and KO mice compared with Sham kidneys, whereas the increase of p-AKT was much greater in mPGES-1 KO mice than that in WT mice. Although expression of AKT protein was also increased in obstructed kidneys, there was no significant difference between mPGES-1 WT and KO mice (Fig. 9C).
Fig. 9.
Western blot analyses of p-AKT and AKT protein expression in obstructed kidneys of mPGES-1 WT and KO mice. A: semiquantitative immunoblots reacted with anti-p-AKT and AKT antibodies. GAPDH was used as internal loading control. Corresponding densitometric analyses of protein expression levels of p-AKT (B) and AKT (C) corrected by GAPDH. WT, wild type; KO, knockout. *P < 0.05 compared with Sham groups. #P < 0.05 compared with WT-7UUO.
EP4 agonist attenuated increased expression of collagen I and fibronectin induced by TGF-β1 in cultured IMCD3 cells.
As EP4 mRNA expression was dramatically increased in the fibrotic kidneys of mPGES-1 KO mice with UUO, we next investigated the potential significance of EP4 activation. Treatment with TGF-β1 significantly increased protein abundance of collagen I and fibronectin in IMCD3 cells (Fig. 10). The EP4 agonist CAY10598 remarkably suppressed TGF-β1-induced increased expression level of collagen I and fibronectin, indicating a protective role of PGE2-EP4 in the development of renal fibrosis (Fig. 10).
Fig. 10.
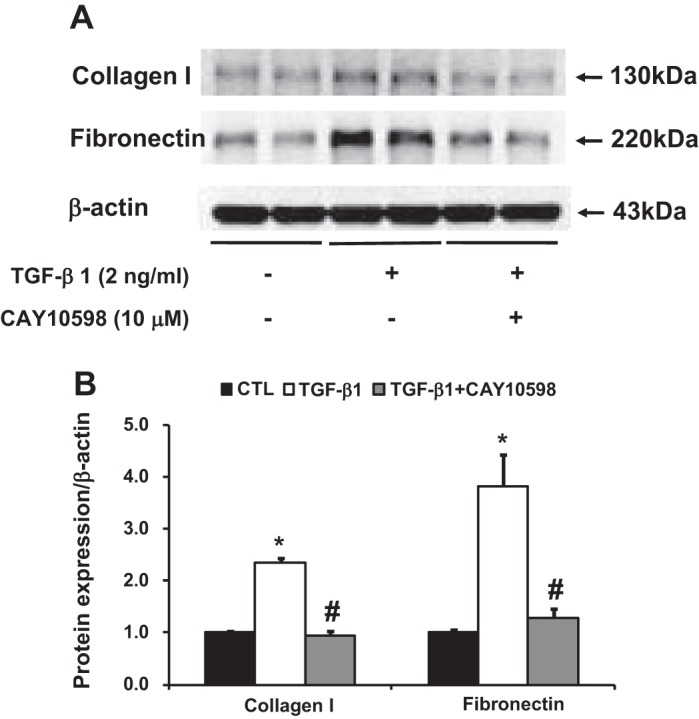
Western blot analysis of collagen I and fibronectin protein expression in IMCD3 cells incubation with TGF-β1 (2 ng/ml) and cotreated with EP4 agonist CAY10598 (10 μM). A: semiquantitative immunoblots reacted with anti- collagen I and fibronectin antibodies. β-Actin was used as internal loading control. Corresponding densitometric analyses of protein expression levels of collagen I and fibronectin corrected by β-actin (B). *P < 0.05 compared with vehicle groups. #P < 0.05 compared with TGF-β1 groups.
EP4 agonist prevented the activation of NLRP3 inflammasomes induced by ANG II in HK2 cells.
ANG II is associated with cell proliferation and inflammation in kidney diseases. It is known that local generation of ANG II was increased in the obstructed kidneys. As NLRP3 inflammasomes were excessively activated in obstructed kidneys of mPGES-1 KO mice after 7UUO, we next investigated whether activation of EP4 receptors suppressed NLRP3 inflammasome induced by ANG II. Treatment with ANGII increased the protein abundance of NLRP3 inflammasome components (ASC, caspase-1, and IL-1β) in HK2 cells. The EP4 agonist CAY10598 significantly decreased the upregulation of ASC, caspase-1, and IL-1β, indicating a protective role of PGE2-EP4 in the development of renal inflammation (Fig. 11).
Fig. 11.
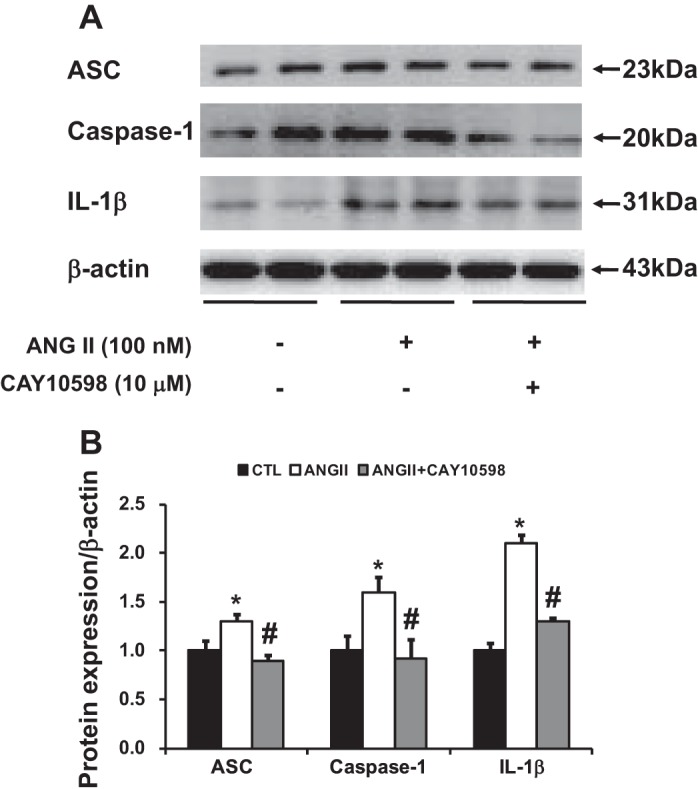
Western blot analysis of NLRP3 inflammasome components in HK2 cells incubated with ANG II (100 nM) and cotreated with EP4 agonist CAY10598 (10 μM). A: semiquantitative immunoblots reacted with anti-ASC, caspase-1, and IL-1β antibodies. β-Actin was used as internal loading control. Corresponding densitometric analyses of protein expression levels of ASC, caspase-1, and IL-1β corrected by β-actin (B). *P < 0.05 compared with vehicle groups. #P < 0.05 compared with ANG II groups.
DISCUSSION
Renal fibrosis is a pathological alteration and end point of most chronic kidney diseases. UUO is a useful and accessible model to identify mechanisms underlying the progression of renal fibrosis. The role of the COX/mPGES-1/PGE2/EP4 cascade in renal fibrosis and inflammation has been recently investigated. The endogenous PGE2–EP4 system in the tubular epithelium was shown to prevent the development of tubulointerstitial fibrosis by suppressing inflammatory responses after UUO (33).
In the current study, we demonstrated that mRNA and protein expressions of mPGES-1 and COX-2 were significantly upregulated in obstructed kidneys of wild-type mice after UUO, which was associated with reduced EP3 and increased EP4 mRNA levels. In obstructed kidneys of mice with mPGES-1 deletion, EP4 expression level was much higher than that in wild-type mice. Renal expression levels of profibrotic factors fibronectin, collagen III, and TGF-β1 were also significantly augmented compared with that in wild-type mice after UUO, indicating a potentially preventive effect of mPGES-1 in the progression of tubulointerstitial fibrosis induced by UUO. Furthermore, mPGES-1 knockout mice exhibited a marked upregulation of NLRP3 inflammasome components ASC in obstructed kidneys, and accordingly caspase-1 activation and subsequent IL-1β processing and release, compared with wild-type mice.
COX-2 was recently reported to play a protective role against fibrosis in UUO kidneys (23). Consistent with previous studies (36), COX-2, an inducible enzyme, was dramatically increased in obstructed kidneys of both mPGES-1 wild-type and knockout mice, and mPGES-1 deficiency was associated with even higher renal COX-2 expression than wild-type groups after UUO, likely suggesting a potential compensation which leads to increased synthesis of prostanoids, including PGE2. Among the three PGESs, mPGES-1 has been identified to have the best PGE2 synthetic property in both in vitro and in vivo conditions (14, 21a). However, increased renal PGE2 was also observed in mPGES-1 knockout mice after UUO (although to a lower level compared with WT mice), indicating that other PGE2 synthases are probably involved when the kidney is under stress. A more than twofold increase of EP4 mRNA expression was observed in the obstructed kidney of mPGES-1 knockout mice compared with that in wild-type mice. Robust increases of renal EP4 in mPGES-1 knockout mice indicate an involvement of the COX2-mPGES-1-PGE2–EP4 axis in UUO-induced kidney injury, tubulointerstitial fibrosis, for example, since the expression of EP4 mRNA was detected mainly in tubular epithelial cells and interstitial cells following UUO (33).
UUO is associated with dramatically elevated profibrotic and proinflammatory factor levels in the injured kidney (e.g., fibronectin, TGF-β1, and IL-1β); among them persistently elevated renal TGF-β1 leads to progressive tissue injury, impaired growth, and eventual loss of kidney function. TGF-β1 and downstream receptor-regulated transcriptional effectors are involved in the development of significant fibrosis in obstructed kidneys. After UUO several profibrotic molecules are significantly upregulated in two types of mice. Notably protein or mRNA expression levels of fibronectin, collagen III, and TGF-β1 were more dramatically increased in mPGES-1 knockout mice compared with wild-type mice after UUO. In IMCD3 cells, EP4 activation by agonist markedly attenuated expression of collagen I and fibronectin induced by TGF-β1, suggesting a potentially protective role of EP4 in development of kidney fibrosis. However, other studies showed that EP4 agonist induces glomerulosclerosis and interstitial fibrosis in diabetic nephropathy (32) and promotes ECM accumulation in cultured glomerular mesangial cells (47). Apparently more studies are therefore warranted to clarify underlying the pathophysiological significance of the mPEGS-1-PGE2-EP axis in renal fibrosis.
EP4 expression in the kidney seems certain. EP4 is prominent in the glomerulus (podocytes and juxtaglomerular apparatus cells) and the afferent arteriole. EP4 is also detected in almost all renal cell types, PT, DCT, CCD, medullary collecting duct, thick ascending limb, and vasa recta (34). PGE2-EP4 is shown to be highly induced in tubular epithelial cells and interstitial cells following obstruction, probably also in macrophages where PGE2 suppress the release of inflammatory chemokines via EP4 (41). In one recent study (39), EP4 mRNA increased remarkably throughout kidneys in UUO rats, which were localized mainly to tubular epithelial cells and to a lesser extent to interstitial cells and glomeruli. Signals for EP4 mRNAs were detected mainly in the structure that coincides with tubular epithelium expressing specific marker E-cadherin.
Increased blood pressure may aggravate renal fibrosis. In the UUO model, systemic blood pressure is usually maintained due to compensation of the nonobstructed kidney (16, 25). However, afferent arteriole pressure may increase to overcome increased capsular pressure caused by obstruction of the ureter. Increased blood flow and pressure in the early time after UUO may potentiate development of fibrosis in obstructed kidneys. EP2 and EP4 receptors mediate the principal vasodepressor actions of PGE2, whereas the vasopressor actions are mediated by activation of the EP1 and EP3 receptors (40). EP2 and EP4 are believed to induce vasodilation in the afferent arteriole (13) and vasa recta. EP2 or EP4 deficiency mice experienced an increase in blood pressure on high-salt diet or a diminished vasodepressor response to PGE2 (4, 24). In our UUO model, mRNA levels of EP1 and EP3 were detected unchanged or decreased, whereas both mRNA levels of EP2 and EP4 were markedly elevated in mPGES-1 deficient mice, which might exert a depressor effect, although total PGE2 production was actually low. The impact of mPGES-1 on blood pressure is still debated. mPGES-1 specific deletion in vascular smooth muscle cell and endothelial cell did not alter blood pressure (10). Deletion of mPGES-1 fails to elevate blood pressure in mice in some studies (11, 15), but in others mPGES-1 deletion augmented the hypertensive response to both salt loading and ANG II infusion (21a). This discrepancy likely attributes to a difference in genetic background among the strains. Nevertheless, a potential depressor effect of PGE2-EP4 may at least partially delay development of fibrosis following UUO.
The other finding in the present study is that deficiency of mPGES-1 exacerbated NLRP3 inflammasome-associated IL-1β production after UUO. We (46) and others (27) have found that activation of NLRP3-ASC inflammasome is involved in renal inflammation and fibrosis after UUO. IL-1β production during kidney injury is known to be regulated by inflammasome, in which ASC recruits and activates caspase-1. Caspase-1 cleaves pro-IL-1β (and pro-IL-18) into mature forms and induces release of IL-1β (and IL-18). Expression of ASC and IL-1β protein in obstructed kidneys was much higher in mPGES-1 knockout mice than that in wild type after UUO. IL-1β has been indicated as a potent stimulator to promote ERK1/2 activation and to induce the expression of TGF-β1 (2). Therefore increased IL-1β seen in mPGES-1 KO mice may execute synergistic effects with other profibrotic factors to exacerbate kidney fibrosis after UUO. mPGES-1 is the terminal synthase in the production of PGE2, and EP4 is supposed the key receptor mediating the effect of PGE2. We found that activation of the NLRP3 inflammasome induced by ANG II was significantly inhibited by the EP4 agonist CAY10598 in cultured kidney proximal tubule cells, which supports the notion that activation of the EP4 signaling pathways attenuated inflammation, although we are unable to present direct evidence showing the protective effect of mPGES-1 depends on reduced inflammasome formation; further studies are therefore warranted.
EP4 receptor is shown to be associated with activation of the PI3K/AKT pathway in several types of cells e.g., macrophages (5), cancer cells (45), and glomerular epithelial cells (3). In our study, mPGES-1 deficient mice demonstrated increased AKT phosphorylation at S473, but the underlining mechanism is unknown. It is noted that PGE2 production was low in mPGES-1 deficient mice, which, theoretically, caused relatively less EP4 activation in obstructed kidneys. The observed increased mRNA levels of EP4 could be considered as a compensatory negative feedback. In this case, whether PGE2-EP4 plays a role in activation of AKT signaling pathway following UUO is undetermined. Nevertheless, mPGES-1 deficiency seems to induce severe kidney fibrosis which was coincidently associated with AKT phosphorylation. Interestingly, other EP receptors are also involved in PGE2-related AKT activation. EP1 receptor inhibition increased AKT phosphorylation, causing MDCK cell growth; in contrast, activation of EP4 mediated the PGE2-induced growth response (43). In one recent study, it was EP1, but not EP4 receptor, which increased fibronectin levels in mouse proximal tubule cells, indicating that EP4 activation was found to be protective. Whether AKT was involved in this process, however, was not reported (35).
In the kidney, mPGES-1 is expressed in the distal nephron including the connecting tubule to the collecting duct (8, 17), where the distribution of mPGES-1 parallels COX expression. This distribution is consistent with the highest capacity for PGE2 generation, abundant EP receptors in the collecting ducts. An involvement of the collecting duct cells in the development of tubulointerstitial fibrosis has been suggested. ANG II has been shown to induce the expression of fibrotic factors (12) and TGF-β causes EMT (20) in the collecting duct cell models. UUO indeed results in loss of the epithelial phenotype and gain of a mesenchymal phenotype in medullary collecting ducts, eventually causes interstitial fibrosis (7). In inner medulla, COX-2 and mPGES-1 are colocalized in medullary interstitial cells (8, 38), and both proteins are coincidently upregulated after UUO. Fibroblasts represent the unique interstitial cell type in the inner medulla (18, 22), and fibroblast differentiation and transformation to myofibroblasts are important in the development of renal interstitial fibrosis; whether COX-2/mPGES-1 play a role in this is still undetermined. Although mPGES-1 location in the proximal tubules is still debated, COX-2 and EP4 protein expression in the cortex was increased in UUO, which is likely a compensatory response as EP4 activation limits development of kidney tubulointerstitial fibrosis (33).
In summary, we demonstrated that UUO induced expression of mPGES-1 and EP4 as well as PGE2 production in the kidney. mPGES-1 deficiency exacerbates renal fibrosis and inflammation induced by UUO. EP4 agonist in vitro attenuates TGF-β1-induced fibrogenesis. Together, these results support renoprotective and antifibrotic roles of the mPGES-1/PGE2/EP4 pathway during obstructive nephropathy.
GRANTS
This work was supported by the National Natural Science Foundation of China (Nos. 81570635, 81370822, 31330037, and 91439205), Natural Science Foundation of Guangdong Province (Nos. S2013010016546, 2014A030313168, and 2014A020212623), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education of China [No.(2013)-1792], National Institute of Diabetes and Digestive and Kidney Diseases RO-1-DK-04072, and Veterans Affairs Merit Review. This work was also supported by the 111 Project, China (No. B13037). T. Yang is a Research Career Scientist in the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.L., Y.K., F.W., X.F., and S.H. performed experiments; R.L., F.W., X.F., and C.L. analyzed data; R.L., T.Y., W.W., and C.L. interpreted results of experiments; R.L., Y.K., and C.L. prepared figures; R.L. and C.L. drafted manuscript; R.L., T.Y., W.W., and C.L. edited and revised manuscript; W.W. and C.L. approved final version of manuscript.
REFERENCES
- 1.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol : 1007–1018, 2011. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Ohnishi H, Hama K, Ishijima T, Satoh Y, Hanatsuka K, Ohashi A, Wada S, Miyata T, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Yasuda H, Mashima H, Sugano K. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol : C1100–C1108, 2006. doi: 10.1152/ajpcell.00465.2005. [DOI] [PubMed] [Google Scholar]
- 3.Aoudjit L, Potapov A, Takano T. Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol Renal Physiol : F1534–F1542, 2006. doi: 10.1152/ajprenal.00267.2005. [DOI] [PubMed] [Google Scholar]
- 4.Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, McNeish JD, Koller BH, Coffman TM. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol Heart Circ Physiol : H924–H930, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab : 492–501, 2008. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt MJ, Tarantal AF, Jimenez DF, Matsell DG. Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction. Kidney Int : 936–944, 2007. doi: 10.1038/sj.ki.5002457. [DOI] [PubMed] [Google Scholar]
- 8.Câmpean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol : F19–F32, 2003. doi: 10.1152/ajprenal.00443.2002. [DOI] [PubMed] [Google Scholar]
- 9.Capasso JM, Rivard CJ, Enomoto LM, Berl T. Chloride, not sodium, stimulates expression of the gamma subunit of Na/K-ATPase and activates JNK in response to hypertonicity in mouse IMCD3 cells. Proc Natl Acad Sci USA : 6428–6433, 2003. doi: 10.1073/pnas.1130871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Yang G, Xu X, Grant G, Lawson JA, Bohlooly-Y M, FitzGerald GA. Cell selective cardiovascular biology of microsomal prostaglandin E synthase-1. Circulation : 233–243, 2013. doi: 10.1161/CIRCULATIONAHA.112.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest : 1391–1399, 2006. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the β-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol : F358–F365, 2015. doi: 10.1152/ajprenal.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards RM. Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Physiol Renal Fluid Electrolyte Physiol : F779–F784, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Facemire CS, Griffiths R, Audoly LP, Koller BH, Coffman TM. The impact of microsomal prostaglandin e synthase 1 on blood pressure is determined by genetic background. Hypertension : 531–538, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francois H, Facemire C, Kumar A, Audoly L, Koller B, Coffman T. Role of microsomal prostaglandin E synthase 1 in the kidney. J Am Soc Nephrol : 1466–1475, 2007. doi: 10.1681/ASN.2006040343. [DOI] [PubMed] [Google Scholar]
- 16.Frokiaer J, Zeidel ML. Urinary tract obstruction, in Brenner and Rector’s The Kidney (Taal MW, editor). (9th ed.). Philadelphia, PA: Elsevier Saunders, 2012, chapt 35, p. 1383–1410. doi: 10.1016/B978-1-4160-6193-9.10037-5 [DOI] [Google Scholar]
- 17.Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol : F558–F564, 2003. doi: 10.1152/ajprenal.00433.2002. [DOI] [PubMed] [Google Scholar]
- 18.Grupp C, Lottermoser J, Cohen DI, Begher M, Franz HE, Müller GA. Transformation of rat inner medullary fibroblasts to myofibroblasts in vitro. Kidney Int : 1279–1290, 1997. doi: 10.1038/ki.1997.453. [DOI] [PubMed] [Google Scholar]
- 19.Hara S, Kamei D, Sasaki Y, Tanemoto A, Nakatani Y, Murakami M. Prostaglandin E synthases: Understanding their pathophysiological roles through mouse genetic models. Biochimie : 651–659, 2010. doi: 10.1016/j.biochi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Ivanova L, Butt MJ, Matsell DG. Mesenchymal transition in kidney collecting duct epithelial cells. Am J Physiol Renal Physiol : F1238–F1248, 2008. doi: 10.1152/ajprenal.00326.2007. [DOI] [PubMed] [Google Scholar]
- 21.Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci USA : 7220–7225, 1999. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res : 1243–1251, 2006. doi: 10.1161/01.RES.0000251306.40546.08. [DOI] [PubMed] [Google Scholar]
- 22.Kaissling B, Le Hir M. Characterization and distribution of interstitial cell types in the renal cortex of rats. Kidney Int : 709–720, 1994. doi: 10.1038/ki.1994.95. [DOI] [PubMed] [Google Scholar]
- 23.Kamata M, Hosono K, Fujita T, Kamata K, Majima M. Role of cyclooxygenase-2 in the development of interstitial fibrosis in kidneys following unilateral ureteral obstruction in mice. Biomed Pharmacother : 174–180, 2015. doi: 10.1016/j.biopha.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med : 217–220, 1999. doi: 10.1038/7426. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Kim SJ, Yoon HE, Chung S, Choi BS, Park CW, Shin SJ. Fimasartan, a novel angiotensin-receptor blocker, protects against renal inflammation and fibrosis in mice with unilateral ureteral obstruction: the possible role of Nrf2. Int J Med Sci : 891–904, 2015. doi: 10.7150/ijms.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koeberle A, Werz O. Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders. Biochem Pharmacol : 1–15, 2015. doi: 10.1016/j.bcp.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Komada T, Usui F, Shirasuna K, Kawashima A, Kimura H, Karasawa T, Nishimura S, Sagara J, Noda T, Taniguchi S, Muto S, Nagata D, Kusano E, Takahashi M. ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am J Pathol : 1287–1298, 2014. doi: 10.1016/j.ajpath.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Wang W, Kwon TH, Isikay L, Wen JG, Marples D, Djurhuus JC, Stockwell A, Knepper MA, Nielsen S, Frøkiaer J. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol : F163–F171, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Wang W, Kwon TH, Knepper MA, Nielsen S, Frøkiaer J. Altered expression of major renal Na transporters in rats with unilateral ureteral obstruction. Am J Physiol Renal Physiol : F155–F166, 2003. doi: 10.1152/ajprenal.00272.2002. [DOI] [PubMed] [Google Scholar]
- 30.Mancini AD, Di Battista JA. The cardinal role of the phospholipase A(2)/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E(2) (PCPP) axis in inflammostasis. Inflamm Res : 1083–1092, 2011. doi: 10.1007/s00011-011-0385-7. [DOI] [PubMed] [Google Scholar]
- 31.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell : 1261–1274, 2007. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed R, Jayakumar C, Ramesh G. Chronic administration of EP4-selective agonist exacerbates albuminuria and fibrosis of the kidney in streptozotocin-induced diabetic mice through IL-6. Lab Invest : 933–945, 2013. doi: 10.1038/labinvest.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa N, Yuhki K, Kawabe J, Fujino T, Takahata O, Kabara M, Abe K, Kojima F, Kashiwagi H, Hasebe N, Kikuchi K, Sugimoto Y, Narumiya S, Ushikubi F. The intrinsic prostaglandin E2-EP4 system of the renal tubular epithelium limits the development of tubulointerstitial fibrosis in mice. Kidney Int : 158–171, 2012. doi: 10.1038/ki.2012.115. [DOI] [PubMed] [Google Scholar]
- 34.Nasrallah R, Hassouneh R, Hébert RL. Chronic kidney disease: targeting prostaglandin E2 receptors. Am J Physiol Renal Physiol : F243–F250, 2014. doi: 10.1152/ajprenal.00224.2014. [DOI] [PubMed] [Google Scholar]
- 35.Nasrallah R, Hassouneh R, Zimpelmann J, Karam AJ, Thibodeau JF, Burger D, Burns KD, Kennedy CR, Hébert RL. Prostaglandin E2 increases proximal tubule fluid reabsorption, and modulates cultured proximal tubule cell responses via EP1 and EP4 receptors. Lab Invest : 1044–1055, 2015. doi: 10.1038/labinvest.2015.79. [DOI] [PubMed] [Google Scholar]
- 36.Nørregaard R, Jensen BL, Li C, Wang W, Knepper MA, Nielsen S, Frøkiaer J. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol : F322–F333, 2005. doi: 10.1152/ajprenal.00061.2005. [DOI] [PubMed] [Google Scholar]
- 37.Rivard CJ, Brown LM, Almeida NE, Maunsbach AB, Pihakaski-Maunsbach K, Andres-Hernando A, Capasso JM, Berl T. Expression of the calcium-binding protein S100A4 is markedly up-regulated by osmotic stress and is involved in the renal osmoadaptive response. J Biol Chem : 6644–6652, 2007. doi: 10.1074/jbc.M609432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider A, Zhang Y, Zhang M, Lu WJ, Rao R, Fan X, Redha R, Davis L, Breyer RM, Harris R, Guan Y, Breyer MD. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int : 1205–1213, 2004. doi: 10.1111/j.1523-1755.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Zhang Y, Zhu Y, Zhang A, Huang S, Yin X, Ding G, Liu M, Jia Z. Inhibition of mitochondrial complex-1 restores the downregulation of aquaporins in obstructive nephropathy. Am J Physiol Renal Physiol : F777–F786, 2016. doi: 10.1152/ajprenal.00215.2015. [DOI] [PubMed] [Google Scholar]
- 40.Swan CE, Breyer RM. Prostaglandin E2 modulation of blood pressure homeostasis: studies in rodent models. Prostaglandins Other Lipid Mediat : 10–13, 2011. doi: 10.1016/j.prostaglandins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama K, García-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem : 44147–44154, 2002. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 42.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem : 32775–32782, 2000. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 43.Taub M, Parker R, Mathivanan P, Ariff MA, Rudra T. Antagonism of the prostaglandin E2 EP1 receptor in MDCK cells increases growth through activation of Akt and the epidermal growth factor receptor. Am J Physiol Renal Physiol : F539–F550, 2014. doi: 10.1152/ajprenal.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA : 9044–9049, 2003. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vo BT, Morton D Jr, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology : 1768–1779, 2013. doi: 10.1210/en.2012-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Luo R, Lin Y, Wang F, Zheng P, Levi M, Yang T, Li C. Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am J Physiol Renal Physiol : F910–F922, 2015. doi: 10.1152/ajprenal.00649.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang GX, Xu YY, Fan YP, Wang J, Chen XL, Zhang YD, Wu JH. A maladaptive role for EP4 receptors in mouse mesangial cells. PLoS One : e104091, 2014. doi: 10.1371/journal.pone.0104091. [DOI] [PMC free article] [PubMed] [Google Scholar]