Abstract
Patients with chronic obstructive pulmonary disease (COPD) experience a delayed recovery from skeletal muscle fatigue following exhaustive exercise that likely contributes to their progressive loss of mobility. As this phenomenon is not well understood, this study sought to examine postexercise peripheral oxygen (O2) transport and muscle metabolism dynamics in patients with COPD, two important determinants of muscle recovery. Twenty-four subjects, 12 nonhypoxemic patients with COPD and 12 healthy subjects with a sedentary lifestyle, performed dynamic plantar flexion exercise at 40% of the maximal work rate (WRmax) with phosphorus magnetic resonance spectroscopy (31P-MRS), near-infrared spectroscopy (NIRS), and vascular Doppler ultrasound assessments. The mean response time of limb blood flow at the offset of exercise was significantly prolonged in patients with COPD (controls: 56 ± 27 s; COPD: 120 ± 87 s; P < 0.05). In contrast, the postexercise time constant for capillary blood flow was not significantly different between groups (controls: 49 ± 23 s; COPD: 51 ± 21 s; P > 0.05). The initial postexercise convective O2 delivery (controls: 0.15 ± 0.06 l/min; COPD: 0.15 ± 0.06 l/min) and the corresponding oxidative adenosine triphosphate (ATP) demand (controls: 14 ± 6 mM/min; COPD: 14 ± 6 mM/min) in the calf were not significantly different between controls and patients with COPD (P > 0.05). The phosphocreatine resynthesis time constant (controls: 46 ± 20 s; COPD: 49 ± 21 s), peak mitochondrial phosphorylation rate, and initial proton efflux were also not significantly different between groups (P > 0.05). Therefore, despite perturbed peripheral hemodynamics, intracellular O2 availability, proton efflux, and aerobic metabolism recovery in the skeletal muscle of nonhypoxemic patients with COPD are preserved following plantar flexion exercise and thus are unlikely to contribute to the delayed recovery from exercise in this population.
Keywords: 31P-MRS, PCr recovery kinetics, O2 availability, mitochondrial function, muscle fatigue, COPD
exercise intolerance is a frequent complaint, and an important predictor of mortality (55), in patients with COPD. This attenuated exercise capacity, perhaps initiated by a chronic obstructive pulmonary disease (COPD)-driven downregulation of oxidative capacity in skeletal muscle (49), can be so debilitating that patients progressively become unable to perform activities of daily living. Interestingly, this downward spiral appears to be aggravated by a prolonged recovery of functional capacity following exercise (48). For instance, after performance of a knee-extension exercise to the point of exhaustion, both postexercise maximum voluntary contraction and electrically evoked quadriceps twitch force were significantly depressed in patients with COPD compared with healthy controls and remained so even following ~1 h of rest (41). As daily life is characterized by repetitive activities, this delayed recovery is likely an important contributor to the poor exercise tolerance, the dimished physical activity and ultimately the loss of mobility experienced by patients with COPD. Therefore, elucidating the prevailing physiological determinants of impaired muscle recovery from exercise in this population is an important and clinically relevant endeavor.
Immediately after exercise, the rate of skeletal muscle recovery is influenced by mechanisms intrinsic to contractile function (e.g., Ca2+ handling), the restoration of energetic balance, and O2 delivery to muscle. With regard to contractile function, the effects of exhaustive exercise on Ca2+ handling have yet to be investigated as, currently, the effects of COPD on the contractile apparatus during exercise are still unclear in humans (9, 37). In contrast, a prolonged recovery of the muscle energy stores (i.e., phosphocreatine concentration [PCr]) and pH after exercise has already been documented in the lower limb of hypoxemic (75) and nonhypoxemic patients with COPD (51, 57, 62, 65), likely as a consequence of the detrimental effects of both emphysema per se (49) and muscle disuse (37, 51) on muscle oxidative capacity. Finally, studies focused on the adequacy of postexercise O2 supply to the skeletal muscle in patients with COPD are relatively scarce and conflicting. For instance, the pulmonary O2 consumption (V̇o2) time constant at the offset of a cycling exercise has been correlated with microvascular reoxygenation kinetics measured by near-infrared spectroscopy (NIRS) in the quadriceps of patients with COPD (56), suggestive of O2 supply-limited muscle oxidative metabolism. This interpretation has, however, been challenged by the recent evidence that pulmonary and muscle V̇o2 may be dissociated during recovery from exercise (34), making inferences specific to muscle oxidative metabolism from pulmonary V̇o2 postexercise rather tenuous. More recently, slower microvascular deoxygenation recovery kinetics, measured by NIRS, have been reported following neuromuscular electrical stimulation in patients with COPD compared with sedentary controls (2). This finding was interpreted as evidence that impeded recovery of muscle functional capacity is caused by metabolic abnormalities. However, as the NIRS-derived deoxyhemoglobin (HHb) signal reflects the balance between O2 utilization and supply, in the absence of any direct measurements of limb blood flow or oxidative metabolism, an inadequate hemodynamic response should not be ruled out and could account for the slower kinetics in patients with COPD.
Therefore, it is still unclear whether the interaction between postexercise muscle metabolism and peripheral hemodynamics is altered in patients with COPD compared with healthy sedentary controls. Accordingly, this study sought to examine the dynamics of peripheral O2 transport and metabolism following the cessation of plantar flexion exercise. We hypothesized that if contractile dysfunction is the predominant mechanism delaying muscle recovery in patients with COPD, then 1) postexercise limb and capillary hemodynamics would not be different between controls and patients; 2) as a result, both the convective and diffusive components of O2 transport would adequately match the metabolic demand both in controls and patients; and 3) postexercise PCr and pH kinetics would be similar between controls and patients with COPD.
METHODS
Subjects.
After written informed consent was obtained, 12 nonhypoxemic patients with COPD and 12 age-matched sedentary subjects participated in this study. The patients were recruited based on spirometric evidence of COPD, while the controls subjects were recruited based on no evidence of regular physical activity above that required for activities of daily living (assessed by both questionnaire and accelerometry). Exclusion criteria for the study included overt cardiovascular disease, diabetes, obesity, neuromuscular disease, and known cancer. All subjects performed standard pulmonary function tests during an initial visit to the laboratory. The study was approved by the Human Research Protection Programs of both the University of Utah and the Salt Lake City Veterans Affairs Medical Center.
Exercise protocol.
After subjects were familiarized with the equipment, the individual maximal work rate (WRmax) was determined by having them perform incremental dynamic plantar flexion exercise until exhaustion. On two separate occasions designed to be balanced, subjects performed constant-load submaximal plantar flexion at ~40% of WRmax (frequency of 1 Hz). The exercise protocol was performed on two separate days, once in the whole body MRI system (TimTrio, 2.9T; Siemens Medical Systems, Erlangen, Germany) to assess metabolism and then repeated on a separate day in the laboratory to assess limb and capillary blood flow as well as microvascular oxygenation using Doppler ultrasound imaging and NIRS, respectively. Specifically, the exercise protocol entailed 1 min of rest and 4 min of the constant-load submaximal plantar flexion, followed by 5 min of recovery. Before initiation of the hemodynamics protocol, blood samples were collected to assess blood lipids, fasting glucose, and metabolism and to perform a complete blood cell count. All experimental trials were performed by the participants in a thermoneutral environment following an overnight fast, after they had refrained from any physical activity and smoking for 12 h.
Popliteal blood flow.
Measurements of popliteal artery blood velocity and vessel diameter were performed in the popliteal fossa of the exercising leg proximal to the branching of the medial inferior genicular artery with a Logic 7 Doppler ultrasound system (General Electric Medical Systems, Milwaukee, WI). The ultrasound system was equipped with a linear array transducer operating at an imaging frequency of 9 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probes appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and centered within the vessel. Arterial diameter was measured offline every 12 s using automated edge-detection software (Medical Imaging Applications, Coralville, IA), and mean velocity (Vmean) (angle corrected and intensity-weighted area under the curve) was automatically calculated beat by beat (Logic 7). With the use of arterial diameter and Vmean, blood flow in the popliteal artery was calculated as blood flow = Vmean × π (vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. Arterial O2 saturation () was monitored at baseline with a finger probe oximeter (OxiMax N-600x; Nellcor, Pleasanton, CA). Mean arterial pressure, heart rate, stroke volume, and cardiac output were determined with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). Leg vascular conductance was calculated as popliteal artery blood flow divided by mean arterial pressure. Arterial O2 content () was calculated, utilizing baseline and hemoglobin (Hb), as the sum of bound O2 (1.34 × Hb × ) and dissolved O2 (0.003 × Po2), based on a normal Hb association curve (64). O2 delivery was then calculated as the product of and popliteal artery blood flow.
Microvascular oxygenation and capillary blood flow.
Microvascular oxygenation was assessed using the NIRS technique, which provides continuous, noninvasive measurements of oxygenated (HbO2), deoxygenated (HHb), and total (Hbtot) Hb levels as well as a “tissue” oxygenation index (TOI, i.e., HbO2/Hbtot). Because of identical spectral characteristics, Hb and myoglobin (Mb) are not separated using NIRS. However, although still somewhat contentious (14), the signal is usually considered to be derived mainly from Hb (30). Changes in microvascular oxygenation of the calf muscle were continuously monitored at 2 Hz using a near infrared frequency resolved spectroscopy oximeter (Oxiplex TS; ISS, Champaign, IL). The probe was positioned at the level of the largest circumference of the calf and secured with velcro straps and biadhesive tape. This NIRS device uses intensity-modulated light, and the probe consisted of eight infrared light sources (4 emitting at 690 nm and 4 emitting at 830 nm) and one detection channel (interoptode distance = 1.5 to 4.5 cm) including a selected light detector (photomultiplier tube), thus providing a measurement of absorption and the scattering coefficient of the tissues. Measurement of adipose tissue thickness under the NIRS sample site was performed with a Logic 7 Doppler ultrasound system (General Electric Medical Systems). Microvascular Po2 was derived from the tissue oxygen index (4), assuming that the near infrared spectroscopy signal mainly originates from Hb (30) and then computed from the HbO2 dissociation curve (64).
The estimated capillary blood flow response following the offset of exercise was calculated from a modified version of the method proposed by Ferreira et al. (12, 18) using the kinetics of muscle O2 consumption and the HHb data, as previously described (38). Specifically, the PCr resynthesis rate, measured by 31P-MRS, which is derived almost exclusively from oxidative phosphorylation (58), was used as an index of muscle O2 consumption. Then, as the HHb response determined by NIRS is considered to reflect muscle capillary O2 extraction (i.e., −) (30), and based upon the Fick equation, the temporal characteristics of capillary blood flow were estimated using the PCr resynthesis rate-to-HHb ratio.
31P-MRS.
31P-MRS was performed using a clinical 2.9T MRI system (Tim-Trio; Siemens Medical Solutions) operating at 49.9 MHz for 31P-resonance. 31P-MRS data were acquired with a dual-tuned 31P-proton (1H) surface coil with linear polarization (Rapid Biomedical, Rimpar, Germany) positioned under the calf at its maximum diameter. The 31P-single-loop coil diameter was 125 mm surrounding a 110-mm 1H-coil loop. The centering of the coil around the leg was confirmed by T1-weighted 1H-localizing images, and the coil was repositioned if the coil was not actually centered on the calf, as determined by the thickness of the gastrocnemius muscle. After a three-plane scout proton image, advanced localized volume shimming was performed. Before each experiment, two fully relaxed spectra were acquired at rest with three averages per spectrum and a repetition time of 30 s. Then, MRS data acquisition was performed throughout the rest-exercise-recovery protocol using a free-induction-decay pulse sequence with a 2.56-ms adiabatic-half-passage excitation radiofrequency pulse and the following parameters: repetition time (TR) = 2 s; receiver bandwidth = 5 kHz; 1,024 data points; and 3 averages per spectrum. Saturation factors were quantified by the comparison between fully relaxed (TR = 30 s) and partially relaxed spectra (TR = 2 s).
As previously described (36), relative concentrations of PCr ([PCr]), inorganic phosphate ([Pi]), and ATP ([ATP]) were obtained by a time-domain fitting routine using the Advanced Method for Accurate, Robust, and Efficient Spectral (AMARES) fitting algorithm (71) incorporated into the CSIAPO software (40). Intracellular pH was calculated from the chemical shift difference between the Pi and PCr signals. The free cytosolic ADP concentration ([ADP]) was calculated from [PCr] and pH using the creatine kinase equilibrium constant (KCK = 1.66 × 109 M) and assuming that phosphocreatine represents 85% of total creatine content (23). The resting concentrations were calculated from the average peak areas of the three relaxed spectra (TR = 30 s; n = 3) recorded at rest, assuming an 8.2 mM [ATP]. When Pi splitting was evident, the pH corresponding to each Pi pool was calculated separately as pH1 and pH2 on the basis of the chemical shift of each peak relative to PCr. The overall muscle pH was then calculated as pH = pH1 (area Pi1/total Pi area) + pH2 (area Pi2/total Pi area).
Leg volume.
Leg volume was calculated based on lower leg circumference (3 sites: distal, middle, and proximal), leg length, and skinfold measurements (25). This method has recently been confirmed to provide a valid estimate for muscle volume across a spectrum of individuals with normal muscle mass to severe muscle atrophy (39).
Assessment of physical activity.
Physical activity level (PAL) was assessed using both a subjective PAL recall questionnaire and objective accelerometer data. The PAL questionnaire included items regarding the average type, frequency, intensity, and duration of physical activity in any given week. After receiving standardized operating instructions, subjects wore an accelerometer (GT1M; Actigraph, Pensacola, FL) quantifying both the number of steps per day and intensity of movement for seven continuous days, with adherence automatically recorded by the device. According to the manufacturer specification, thresholds for sedentary, light, lifestyle, moderate, vigorous, and very vigorous activity were defined as <99, 100–759, 760–1,951, 1,952–5,724, and >5,725 counts/min, respectively.
Data analysis.
The limb and capillary blood flow, vascular conductance, PCr, HHb, and TOI recovery kinetics were determined by fitting the time-dependent changes during the recovery period to a monoexponential curve described by the following equation:
| (1) |
where Yend is the level of variable measured at end-of-exercise and Yres refers to the amplitude of the blood flow response, PCr resynthesized, or the resaturation during the recovery. Unlike the other variables, there is no time delay (TD) in the resynthesis of PCr, and therefore, TD was fixed to 0 for PCr kinetics. Then, the initial rate of PCr resynthesis from 31P-MRS (ViPCr) was calculated from the derivative of Eq. 1 at time 0:
| (2) |
in which Δ[PCr] represents the amount of PCr resynthesized during the recovery and the rate constant k = 1/τ (27).
The peak rate of oxidative ATP synthesis from 31P-MRS (Vmax in mM/min) was calculated using the initial rate of PCr synthesis (ViPCr) during the recovery period and [ADP] obtained at the end of exercise as previously described (68):
| (3) |
in which Km (the [ADP] at half the highest oxidation rate) is 30 µM in skeletal muscle (27).
During the recovery period, PCr is regenerated throughout the CK reaction as the consequence of oxidative ATP production in mitochondria. Thus H+efflux can be calculated from the rates of proton production from the CK reaction (H+CK, in mM/min) and mitochondrial ATP production (H+Ox, in mM/min) on one side and the rate of pH changes on the other side. At this time, ATP production is exclusively aerobic, and lactate production is considered negligible:
| (4) |
To improve precision, we use a modified version of this calculation (29) in which the total proton disappearance (i.e., ∫Edt) is estimated cumulatively from the start of recovery and then fitted to an exponential function to obtain the initial efflux recovery rate.
Model variables were determined with an iterative process by minimizing the sum of squared residuals between the fitted function and the observed values. Goodness of fit was assessed by visual inspection of the residual plot and the frequency plot distribution of the residuals, χ2-values, and the coefficient of determination (r2), which was calculated as follows:
| (5) |
where SSreg is the sum of squares of the residuals from the fit and SStot is the sum of squares of the residuals from the mean.
Statistical analysis.
The assessment of differences between COPD and controls was performed with either paired t-tests or nonparametric Wilcoxon tests, where appropriate (Statsoft, version 5.5; Statistica, Tulsa, OK). Statistical significance was accepted at P < 0.05. Results are presented as means ± SD in tables and means ± SE in the figures for clarity.
RESULTS
Subject characteristics.
Subject characteristics are presented in Table 1. Patients with COPD exhibited reduced pulmonary function relative to the healthy sedentary controls and blood gas characteristics consistent with COPD. Despite considerable effort in terms of seeking out sedentary controls, there was still a significant difference in physical activity between groups (Table 1). However, based on the number of steps per day and the time spent in the different intensity domains measured by accelerometry, all subjects can still be confidently defined as sedentary to low active (69). This similar sedentary lifestyle between groups was further confirmed by the lack of a significant difference in plantar flexion peak power output and limb muscle volume between groups. Adipose tissue thickness was not significantly different between groups (controls: 0.88 ± 0.21 cm; COPD: 0.73 ± 0.25 cm, P > 0.05). One patient and one control subject were current smokers. Two patients were studied while using supplemental O2 (resting : 94.5 ± 2.1%).
Table 1.
Subject characteristics
| Controls | COPD | |
|---|---|---|
| Sample size | 12 | 12 |
| Age, yr | 68 ± 6 | 66 ± 6 |
| Anthropometric characteristics | ||
| Height, cm | 173 ± 9 | 171 ± 9 |
| Weight, kg | 76 ± 13 | 77 ± 16 |
| BMI | 25 ± 3 | 26 ± 5 |
| Limb muscle volume, dl | 21 ± 5 | 23 ± 4 |
| Functional characteristics | ||
| Steps per day | 6,079 ± 1,775 | 3,021 ± 747* |
| Sedentary physical activity, min | 1,244 ± 61 | 1,303 ± 43* |
| Light physical activity, min | 104 ± 37 | 84 ± 43 |
| Lifestyle physical activity, min | 56 ± 25 | 31 ± 28* |
| Moderate physical activity, min | 24 ± 15 | 9 ± 14* |
| Vigorous and very vigorous, min | 0 ± 0 | 0 ± 0 |
| Gait speed, m/s | 1.5 ± 0.2 | 1.2 ± 0.2* |
| Plantar flexion maximal work rate, W | 9 ± 3 | 7 ± 2 |
| Pulmonary function | ||
| FVC, liter | 4.8 ± 1.4 | 3.2 ± 0.7* |
| FVC, %pred | 117 ± 22 | 80 ± 16* |
| FEV1, liter | 3.4 ± 0.8 | 1.6 ± 0.5* |
| FEV1, %pred | 114 ± 18 | 53 ± 16* |
| FEV1/FVC% | 73 ± 8 | 51 ± 9* |
| Blood characteristics | ||
| Arterial blood saturation, % | 94 ± 1 | 94 ± 2 |
| Glucose, mg/dl | 84 ± 13 | 91 ± 8 |
| Cholesterol, mg/dl | 202 ± 39 | 198 ± 28 |
| Triglycerides, mg/dl | 151 ± 81 | 87 ± 25 |
| HDL, mg/dl | 51 ± 13 | 71 ± 34 |
| LDL, mg/dl | 130 ± 32 | 108 ± 25 |
| WBCs, K/μl | 5.3 ± 1.4 | 7.4 ± 3.0 |
| RBCs, M/μl | 4.9 ± 0.5 | 4.7 ± 0.3 |
| Hemoglobin, g/dl | 15 ± 2 | 14 ± 1 |
| Hematocrit, % | 45 ± 4 | 44 ± 3 |
| Neutrophil, K/μl | 2.9 ± 1.1 | 4.8 ± 3.4 |
| Lymphocyte, K/μl | 1.7 ± 0.5 | 1.8 ± 0.8 |
| Monocyte, K/μl | 0.5 ± 0.2 | 0.6 ± 0.2 |
| Bicarbonate, mM/l | 25 ± 1 | 28 ± 3* |
| Potassium, mM/l | 4.0 ± 0.3 | 3.8 ± 0.3 |
Data are expressed as means ± SD. COPD, chronic obstructive pulmonary disease; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBCs, white blood cells; RBCs, red blood cells.
P < 0.05, significantly different from controls.
Baseline and plantar flexion exercise.
Intracellular metabolite concentrations and pH, in addition to tissue and microvascular oxygenation indexes (Hbtot, HHb, and TOI) at rest and during the last 30 s of exercise in both controls and patients with COPD are summarized in Table 2. Apart from the resting concentrations of Pi and phosphodiester (PDE), which were lower in COPD (P < 0.05), pH, phosphorylated compounds ([PCr], [Pi], and [ADP]), as well as microvascular oxygenation were not significantly different between groups (P > 0.05) at baseline or at the end of exercise.
Table 2.
Metabolic and microvascular oxygenation responses at rest and during steady-state submaximal plantar flexion exercise in control and COPD subjects
| Controls | COPD | |
|---|---|---|
| Baseline | ||
| Phosphorylated compounds and pH | ||
| PCr, mM | 34 ± 5 | 36 ± 8 |
| Pi, mM | 1.9 ± 1.0 | 1.1 ± 0.4* |
| ADP, μM | 8.2 ± 0.3 | 8.0 ± 0.7 |
| pH | 6.97 ± 0.02 | 6.96 ± 0.04 |
| PDE, mM | 2.0 ± 1.4 | 0.6 ± 0.6* |
| Microvascular oxygenation | ||
| Oxygenation index, % | 62 ± 4 | 60 ± 7 |
| Hb total, µM | 55 ± 20 | 56 ± 27 |
| HHb, µM | 21 ± 8 | 23 ± 13 |
| End exercise | ||
| Phosphorylated compounds and pH | ||
| PCr, mM | 22 ± 6 | 21 ± 6 |
| Pi, mM | 10 ± 4 | 10 ± 4 |
| ADP, μM | 43 ± 21 | 47 ± 47 |
| pH | 6.99 ± 0.06 | 6.93 ± 0.09 |
| Microvascular oxygenation | ||
| Oxygenation index, % | 60 ± 4 | 55 ± 9 |
| Hb total, µM | 58 ± 22 | 58 ± 29 |
| HHb, µM | 24 ± 10 | 28 ± 20 |
Values are expressed as means ± SD. PCr, phosphocreatine; Pi, inorganic phosphate; PME, phosphomonoester; ADP, adenosine diphosphate; PDE, phosphodiester; Hb, total hemoglobin; HHb, deoxyhemoglobin.
P < 0.05, significantly different from controls.
Recovery period: peripheral hemodynamics.
Limb blood flow, vascular conductance, and capillary blood flow dynamics during the recovery period in controls and COPD are displayed in Fig. 1. While end-exercise limb blood flow was not significantly different between groups (controls: 741 ± 216 ml/min; COPD: 704 ± 253 ml/min; P > 0.05), the mean response time at the offset of exercise was significantly prolonged in patients with COPD (controls: ~56 s; COPD: ~120 s; Fig. 1; P < 0.05). Similarly, end-exercise vascular conductance was not significantly different between groups (controls: 7.5 ± 1.4 ml·min−1·mmHg−1; COPD: 6.0 ± 2.5 ml·min−1·mmHg−1; P > 0.05). However, the mean response time at the offset of exercise was significantly prolonged in patients with COPD (controls: ~61 s; COPD: ~127 s; Fig. 1; P < 0.05). In contrast, the time constant for capillary blood flow at the offset of exercise was not significantly different between groups (Fig. 1; P > 0.05).
Fig. 1.
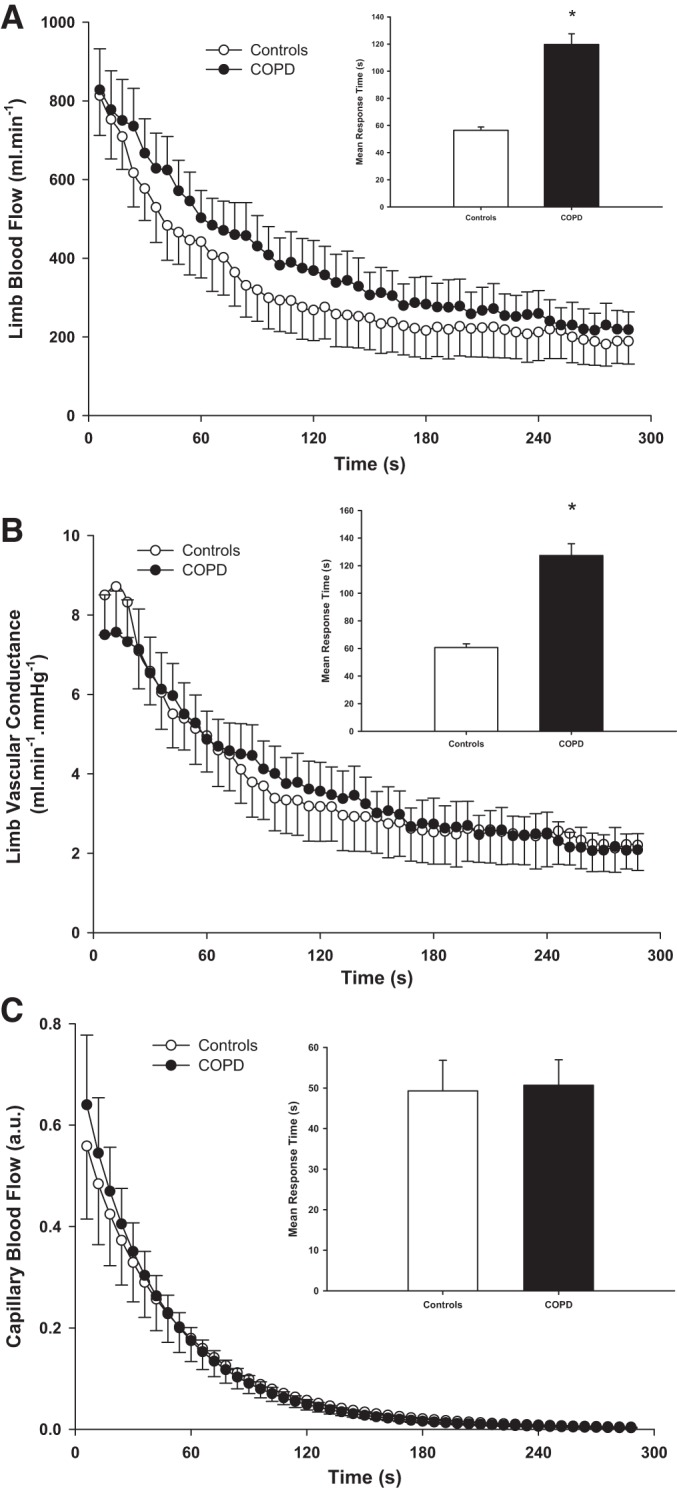
The recovery kinetics of limb blood flow (A), vascular conductance (B) and capillary blood flow (C) following dynamic plantar flexion exercise in controls and patients with chronic obstructive pulmonary disease (COPD). Insets: illustrations of the mean response time. Both for limb blood flow and vascular conductance, the mean response time was significantly slower in patients with COPD compared with controls (P < 0.05). Values are presented as means ± SE; a.u., arbitrary units.
Convective O2 delivery and O2 diffusional conductance.
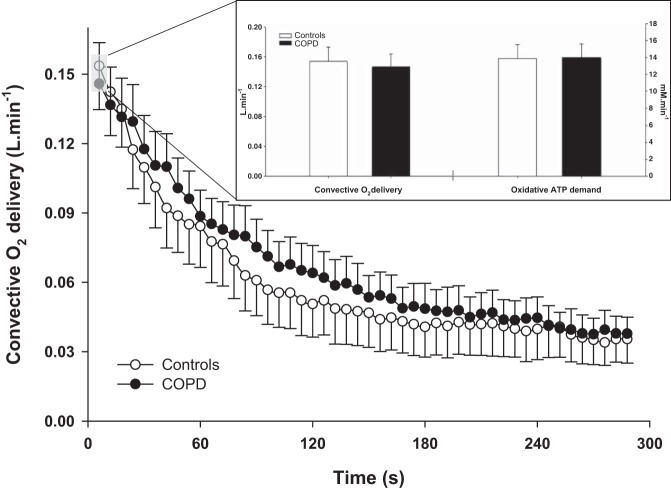
The convective O2 delivery dynamics during the recovery period in controls and patients with COPD is displayed in Fig. 2. The initial postexercise convective O2 delivery and the corresponding oxidative ATP demand were not significantly different between controls and patients with COPD (P > 0.05, Fig. 2). The relationship between microvascular Po2 and initial postexercise PCr resynthesis rate, an index of O2 utilization, during the recovery from plantar flexion exercise in controls and patients with COPD is documented in Fig. 3. The slope of each line from the origin, which reflects O2 diffusional conductance, was not significantly different between groups (controls: 0.47 ± 0.25 mM·mmHg−1·min−1; COPD: 0.55 ± 0.26 mM·mmHg−1·min−1; P > 0.05).
Fig. 2.
Recovery kinetics of convective O2 delivery following dynamic plantar flexion exercise in controls and patients with COPD. Inset: illustration of the immediate postexercise convective O2 delivery and the corresponding oxidative ATP demand in both groups. Neither convective O2 delivery nor oxidative ATP demand were significantly different between controls and patients with COPD (P > 0.05) indicative of a similar matching of O2 supply and demand in both groups. Values are presented as means ± SE.
Fig. 3.
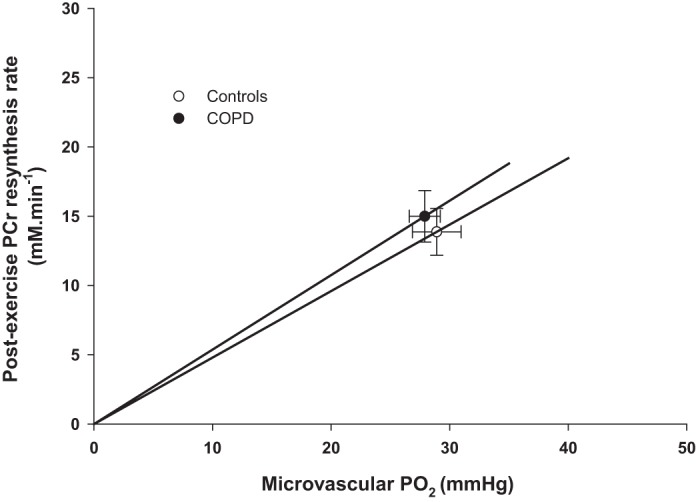
Relationship between microvascular partial pressure of O2 (Po2) and initial postexercise phosphocreatine (PCr) resynthesis rate, an index of O2 utilization, during the recovery from plantar flexion exercise in controls and patients with COPD. The slope of the lines from the origin reflects O2 diffusional conductance according to Fick’s law. Values are presented as means ± SE.
Microvascular oxygenation offset kinetics.
Both the postexercise TOI time constant (controls: 48 ± 74 s; COPD: 53 ± 56 s; P > 0.05) and mean response time (controls: 63 ± 78 s; COPD: 62 ± 59 s; P > 0.05) were not significantly different between groups. Similarly, the HHb recovery time constant (controls: 39 ± 22 s; COPD: 57 ± 46 s; P > 0.05) and mean response time (controls: 75 ± 63 s; COPD: 76 ± 78 s; P > 0.05) were not significantly different between groups.
Metabolic offset kinetics.
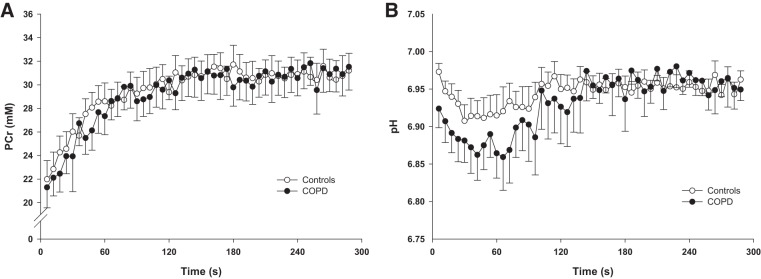
Changes in pH and [PCr] dynamics during the recovery period in controls and patients with COPD are displayed in Fig. 4. Table 3 documents mitochondrial function and proton handling assessed via postexercise metabolic kinetics in both groups. None of these variables were different between controls and patients with COPD.
Fig. 4.
Recovery kinetics of phosphocreatine (A) and pH (B) following dynamic plantar flexion exercise in controls and patients with COPD.
Table 3.
Mitochondrial function and proton handling assessed via postexercise metabolic kinetics in controls and patients with COPD
| Controls | COPD | |
|---|---|---|
| Mitochondrial function | ||
| PCr recovery time constant, s | 46 ± 20 | 49 ± 21 |
| IC 95, s | 16 ± 12 | 15 ± 9 |
| Peak mitochondrial phosphorylation rate, mM/min | 23 ± 10 | 24 ± 10 |
| Proton (H+) handling | ||
| Initial H+ efflux, mM/min | 4.1 ± 3.0 | 4.0 ± 3.8 |
Values are expressed as means ± SD. IC 95, 95% confidence interval.
DISCUSSION
Patients with COPD experience a delayed recovery from skeletal muscle fatigue in the first minutes following exhaustive exercise that likely contributes to their progressive loss of mobility. As this delayed postexercise recovery is not well understood, this study sought to examine the interaction between postexercise muscle metabolism and peripheral hemodynamics following plantar flexion exercise in patients with COPD and healthy sedentary controls. The main findings of this study were that 1) while end-exercise limb blood flow was not significantly different between groups, postexercise vascular conductance and limb blood flow kinetics, but not capillary dynamics, were slower in patients with COPD compared with controls; 2) despite these altered hemodynamics, convective O2 delivery and O2 diffusional conductance appeared to appropriately match muscle metabolic demand both in controls and patients with COPD; and 3) the metabolic recovery and mitochondrial capacity in patients with COPD were not significantly different to the controls. Therefore, in the face of perturbed peripheral hemodynamics, both intracellular O2 availability and metabolic recovery in the skeletal muscle of nonhypoxemic patients with COPD are actually preserved following exercise and thus are unlikely to contribute to the delayed functional recovery from exercise exhibited by this population.
Prolonged postexercise peripheral hemodynamics in patients with COPD.
For a given metabolic demand at the end of the exercise (Fig. 2), blood flow and vascular conductance were not significantly different between sedentary controls and patients with COPD. In contrast, the kinetics of postexercise limb blood flow and vascular conductance were significantly slower in patients with COPD compared with controls (Fig. 1). Similarly, two studies (19, 61) reported no difference in exercise-induced blood flow and vascular conductance during steady-state exercise in patients with COPD compared with healthy age-matched controls. Our results not only extend these findings to the calf muscle but also reveal that, despite no significant difference in the steady-state blood flow response, postexercise hemodynamics are actually prolonged in patients with moderate to very severe COPD. This novel finding confirms the importance of studying not only steady-state response but also postexercise hemodynamics as it provides a sensitive and unique window to examine the regulation of blood flow.
Interestingly, the prolonged recovery dynamics at the macrocirculatory level did not translate into slower capillary hemodynamics within the working muscle, as the mean response time of capillary blood flow in patients with COPD was not significantly different from that of the controls (Fig. 2). While initially somewhat puzzling, this dissociation between macro- and microcirculatory dynamics likely indicates inefficient blood flow redistribution following exercise. Indeed, neither the PCr resynthesis nor pH kinetics were significantly different between groups (Fig. 4), thereby indicating that prolonged limb blood flow recovery did not stem from an elevated ATP demand or metabolic acidosis within the working muscle. Instead, these findings suggest a significant portion of postexercise blood flow was directed toward other areas of the lower limb, with low metabolic activity, in patients with COPD.
Another possible explanation for the different hemodynamics in the conduit artery and capillaries of the working muscle of patients with COPD might stem from substantial heterogeneity in O2 delivery to O2 utilization matching in the exercising muscles (31–33). Specifically, animal studies have revealed that fast-twitch glycolytic fibers rely, to a greater extent, on adjustments in fractional O2 extraction, rather than O2 delivery, to cope with the changes in skeletal muscle O2 demand (33, 50). According to these findings and given the well-established shift in muscle fiber type toward type II glycolytic fibers with COPD (15), it is possible that the region investigated by the NIRS (medial gastrocnemius muscle) might have relied more on alterations in O2 extraction rather than blood flow to match muscle O2 demand compared with other muscles of the lower limb composed of a greater proportion of slow-twitch oxidative fibers.
The potentially abnormal regulation of lower limb hemodynamics in patients with COPD is consistent with disease-related vascular dysfunction in this population. For instance, COPD has been associated with augmented sympathetic activity (20), impaired endothelium-dependent and -independent dilation (8, 10, 22, 53), and alterations in the concentration of circulating vasoactive substances such as nitric oxide (22, 52). Individually or in combination such phenomena have the potential to impair vascular control and peripheral hemodynamics following exercise. Also relevant to the present findings is the previously documented evidence of an attenuated contribution of the muscle metaboreflex to hemodynamic control in the calf muscles of patients with COPD (60). Specifically, the sympathetically mediated vasoconstriction of the calf vasculature following stimulation of the metabosensitive afferents was attributed to the metabolic products of handgrip exercise (60). Such maladaptations can lead to the inefficient coupling between blood flow and metabolic demand, especially at times when metabolic demand varies rapidly, such as the onset and offset of exercise. Combined with the current results, these findings suggest a possible overperfusion of muscle tissue with low metabolic demand after exercise in the lower limb. Although such alterations in blood flow distribution may be of little consequence during a small muscle exercise, this has the potential to result in greater perfusion-metabolism mismatch and/or blood pressure dysregulation at the onset and offset of whole body high-intensity exercise, with the potential to compromise exercise capacity and recovery.
Matching of peripheral O2 supply and utilization in the leg of patients with COPD.
An important finding of this study was that convective O2 delivery to the plantar flexor muscles appeared to be preserved at the offset of exercise in patients with COPD and appeared to match muscle metabolic demand (Fig. 2). In addition, as illustrated in Fig. 3, skeletal muscle O2 diffusional conductance was also not significantly different between sedentary controls and patients with COPD. As a result of this preserved O2 transport system, both the HHb recovery time constant and mean response time were not significantly different between groups, implying that muscle O2 extraction in the plantar flexor muscles of patients with COPD was also not significantly different from that of the controls. In agreement with these findings, Richardson et al. (59) documented a similar relationship between peak muscle O2 consumption and O2 delivery, assessed directly across the exercising muscle, in patients with COPD and controls during single leg knee-extensor exercise (59). It should, however, be noted that in the present study the postexercise HHb response was quite heterogeneous in both groups, which may allude to different determinants of muscle aerobic capacity. However, a discussion of these factors and the existence of different muscle phenotypes in patients with COPD are beyond the scope of the present study. Interestingly, the present functional findings were also supported by morphometric analysis of skeletal muscle fiber capillarization (59). Indeed, according to a series of recent studies, capillary density, capillary-to-fiber ratio, number of capillaries around a fiber, and capillary-to-fiber cross-sectional area are similar between healthy sedentary controls and patients with COPD (11, 14, 59, 73). While not unanimous (24), perhaps due to contrasting levels of physical activity between groups in this latter study, the majority of findings suggest that the size of the capillary-fiber interface, an important site of functional resistance to O2 flux, is likely maintained in patients with COPD. Therefore, in combination, these morphometric and functional findings lend strong support to the concept that the capacity to transport O2 in the periphery is actually well preserved in patients with COPD, especially when employing a small muscle mass exercise paradigm that does not heavily tax central hemodynamics or the pulmonary system (59). This conclusion should, however, be put in perspective with the results from prior studies employing high-intensity cycling exercise, which have consistently documented that O2 transport to limb locomotor muscles was compromised in patients with COPD due to impaired central and peripheral hemodynamics (3, 6, 7, 42, 43, 54), likely caused indirectly by abnormal respiratory mechanics and gas exchange (5, 13, 66, 72).
Metabolic recovery and mitochondrial function in the skeletal muscle of patients with COPD.
Confirming the results from previous work by our group (37), but with a larger sample size, this study has documented that the PCr recovery time constant and the peak rate of mitochondrial phosphorylation of the plantar flexor muscles were not significantly different between sedentary controls and patients with COPD (Table 3). Given the strong dependence of this measurement on the level of muscle activity, these results confirm that despite significant group differences in overall physical activity, measured by accelerometry (due to the extreme inactivity of COPD patients), both groups actually exhibited characteristics of a sedentary lifestyle and thus the same level of muscle disuse. Such metabolic findings add to the growing evidence suggesting that skeletal muscle mitochondrial capacity assessed in vivo is actually preserved with COPD when the level of physical activity is not significantly different between patients and controls (37, 65) and that therefore much of the decline in mitochondrial capacity reported in these patients can likely be attributed to muscle disuse. For instance, 8 wk of supervised endurance and strength training in patients with COPD restored mitochondrial phosphorylation capacity in the quadriceps to that of the controls (51). Furthermore, Shields et al. (65) recently examined the PCr recovery kinetics in the biceps brachial and quadriceps femoris muscles in patients with COPD, with the premise that the upper extremities are less affected by the disease-related reduction in physical activity. This approach allowed the effects of disease and deconditioning on skeletal muscle mitochondrial phosphorylation capacity to be parsed out. Interestingly, using such approach, the authors reported a slower PCr recovery halftime in the quadriceps but not in the biceps brachial, thereby providing additional evidence that muscle disuse may be the predominant factor accounting for the impaired mitochondrial capacity in the skeletal muscle of patients with COPD (65).
It has previously been reported that the initial postexercise H+ efflux rate in the calf muscle was similar between hypoxemic patients with COPD and age-matched controls (67). However, given the contrasting difference in end-exercise pH in this prior study (6.93 in controls and 6.68 in COPD), it is unclear whether this result should, in fact, be interpreted as evidence of impaired H+ clearance. In the current study, for a given metabolic state and pH (Table 2), the initial H+ efflux rate at the offset of the exercise was not significantly different between controls and patients with COPD (Table 3), which translated into similar pH recovery kinetics between groups. This is an important finding as excessive accumulation of lactate and exaggerated metabolic acidosis have previously been reported in patients with COPD during both cycling (42) and plantar flexion exercise (35, 57, 75). In light of the present results, this metabolic acidosis appears not to be related to impaired H+ transport.
One potential explanation in the present study is that preserved limb and capillary blood flow in patients with COPD (Fig. 1) contributed to preserved H+ efflux. During the initial phase of recovery from exercise, H+ transport is also regulated by the monocarboxylate transporters 1 and 4 (MCT1 and MCT4) (17) and the sodium-H+ antiporter (28). Interestingly, and somewhat in contrast with our functional measurement of H+ efflux, a lower MCT4 expression, but not MCT1, has been documented in the vastus lateralis of patients with COPD (16). Considering the severe physical impairment of the patients recruited in the study of Green et al. (26) (: ~90%; V̇o2peak: ~8.6 ml·min−1·kg−1) and the role of physical activity in modulating MCT4 content, here again, it is possible that extreme inactivity in these patients may help to explain the discrepancy between studies. Future studies examining both H+ efflux rate and skeletal muscle MCT content at different stages of the disease across a wide range of exercise intensities, while also controlling for the level of physical activity, will help clarify these divergent results.
Experimental considerations and limitations.
A known limitation of the NIRS technique is that the signal originates from both Hb and Mb, and the relative contribution of Mb to the overall NIRS signal has been a matter of contention (47). Although this may influence our estimation of microvascular Po2 due to different O2 affinity between Hb and Mb, it is unlikely that this issue actually confounded our interpretation of the data. Indeed, most experimental models have demonstrated that the contribution of Mb to the NIRS signal is actually quite minimal, such that the NIRS signal qualitatively reflects blood O2 saturation (30, 44–46, 63, 74). In addition, it has previously been documented that the Mb content in the skeletal muscle of patients with COPD was not significantly different from controls (59). Therefore, the contribution of Mb to the NIRS signal was likely not different between groups in the present study. Along the same lines, adipose tissue thickness, which has the potential to complicate NIRS interpretation, was not significantly different between groups and, therefore, also did not likely confound our interpretation of the data.
This study did not assess the recovery of functional capacity following exercise and, therefore, could not directly investigate the link between muscle recovery and exercise tolerance. However, future studies using an all-out test during small muscle mass exercise to explore the relationships between critical power (and W’) and the determinants of muscle recovery would provide some valuable information on the factors contributing to exercise intolerance in patients with COPD.
Perspective and implications of the delayed skeletal muscle fatigue recovery following exercise.
Overall, the results of this study do not support the hypothesis that the persistent force deficit following exercise experienced by patients with COPD is due to inadequate intracellular O2 availability or prolonged metabolic disturbance and acidosis. Possible alternative explanations for the delayed recovery in skeletal muscle fatigue may involve a reduction in Ca2+ sensitivity of the contractile apparatus (21), depressed Ca2+-ATPase activity (70), or a reduction in Ca2+ release that stem from an impaired coupling between the dihydropyridine receptor and the ryanodine receptor, which releases Ca2+ from the sarcoplasmic reticulum (1). However, whether impaired Ca2+ handling in the skeletal muscle of patients with COPD actually contributes to the persistent postcontractile depression will require further investigations.
Conclusions.
In summary, with the use of an integrative approach, this study revealed altered postexercise peripheral hemodynamics in the plantar flexor of nonhypoxemic patients with COPD, suggesting inefficient blood flow redistribution within the lower limb. Nevertheless, convective O2 delivery and O2 diffusional conductance within the exercising muscle appeared to be appropriately matched with metabolic demand and were associated with preserved aerobic metabolism recovery and proton handling in the calf muscle of these patients. Together, these findings suggest that the persistent muscle force deficit following exercise previously documented in patients with COPD is not likely a consequence of lower intracellular O2 availability or metabolic abnormalities but instead may be linked to impaired Ca2+ handling within the contractile apparatus, at least, in the majority of nonhypoxemic COPD patients, as in this study.
GRANTS
This work was funded in part by a grant from the Flight Attendant Medical Research Institute (FAMRI); National Heart, Lung, and Blood Institute Grants P01-HL-091830 and K99HL125756; Veterans Affairs (VA) Merit Awards E6910-R and E1697-R; VA SPiRe Award E1433-P; and VA Senior Career Scientist Award E9275-L.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.L., J.D.T., Y.L.F., E.-K.J., and R.S.R. conceived and designed research; G.L., C.R.H., J.D.T., O.-S.K., M.J.R., and R.M.B. performed experiments; G.L., C.R.H., J.D.T., and Y.L.F. analyzed data; G.L. and C.R.H. interpreted results of experiments; G.L. prepared figures; G.L. drafted manuscript; G.L., C.R.H., J.D.T., O.-S.K., M.J.R., R.M.B., Y.L.F., E.-K.J., and R.S.R. edited and revised manuscript; G.L., C.R.H., J.D.T., O.-S.K., M.J.R., R.M.B., Y.L.F., E.-K.J., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the subjects in this study for committed participation in this research.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo DP, Medeiros WM, de Freitas FF, Ferreira Amorim C, Gimenes AC, Neder JA, Chiavegato LD. High oxygen extraction and slow recovery of muscle deoxygenation kinetics after neuromuscular electrical stimulation in COPD patients. Eur J Appl Physiol 116: 1899–1910, 2016. doi: 10.1007/s00421-016-3442-7. [DOI] [PubMed] [Google Scholar]
- 3.Borghi-Silva A, Oliveira CC, Carrascosa C, Maia J, Berton DC, Queiroga F Jr, Ferreira EM, Almeida DR, Nery LE, Neder JA. Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax 63: 910–915, 2008. doi: 10.1136/thx.2007.090167. [DOI] [PubMed] [Google Scholar]
- 4.Broxterman RM, Ade CJ, Wilcox SL, Schlup SJ, Craig JC, Barstow TJ. Influence of duty cycle on the power-duration relationship: observations and potential mechanisms. Respir Physiol Neurobiol 192: 102–111, 2014. doi: 10.1016/j.resp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Bruce RM, Turner A, White MJ. Ventilatory responses to muscle metaboreflex activation in chronic obstructive pulmonary disease. J Physiol 594: 6025–6035, 2016. doi: 10.1113/JP272329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiappa GR, Borghi-Silva A, Ferreira LF, Carrascosa C, Oliveira CC, Maia J, Gimenes AC, Queiroga F Jr, Berton D, Ferreira EM, Nery LE, Neder JA. Kinetics of muscle deoxygenation are accelerated at the onset of heavy-intensity exercise in patients with COPD: relationship to central cardiovascular dynamics. J Appl Physiol (1985) 104: 1341–1350, 2008. doi: 10.1152/japplphysiol.01364.2007. [DOI] [PubMed] [Google Scholar]
- 7.Chiappa GR, Queiroga F Jr, Meda E, Ferreira LF, Diefenthaeler F, Nunes M, Vaz MA, Machado MC, Nery LE, Neder JA. Heliox improves oxygen delivery and utilization during dynamic exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179: 1004–1010, 2009. doi: 10.1164/rccm.200811-1793OC. [DOI] [PubMed] [Google Scholar]
- 8.Clarenbach CF, Thurnheer R, Kohler M. Vascular dysfunction in chronic obstructive pulmonary disease: current evidence and perspectives. Expert Rev Respir Med 6: 37–43, 2012. doi: 10.1586/ers.11.82. [DOI] [PubMed] [Google Scholar]
- 9.Debigaré R, Côte CH, Hould FS, LeBlanc P, Maltais F. In vitro and in vivo contractile properties of the vastus lateralis muscle in males with COPD. Eur Respir J 21: 273–278, 2003. doi: 10.1183/09031936.03.00036503. [DOI] [PubMed] [Google Scholar]
- 10.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178: 1211–1218, 2008. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 11.Eliason G, Abdel-Halim SM, Piehl-Aulin K, Kadi F. Alterations in the muscle-to-capillary interface in patients with different degrees of chronic obstructive pulmonary disease. Respir Res 11: 97, 2010. doi: 10.1186/1465-9921-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ. Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol (1985) 98: 1820–1828, 2005. doi: 10.1152/japplphysiol.00907.2004. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 14.Gifford JR, Trinity JD, Layec G, Garten RS, Park SY, Rossman MJ, Larsen S, Dela F, Richardson RS. Quadriceps exercise intolerance in patients with chronic obstructive pulmonary disease: the potential role of altered skeletal muscle mitochondrial respiration. J Appl Physiol (1985) 119: 882–888, 2015. doi: 10.1152/japplphysiol.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosker HR, van Mameren H, van Dijk PJ, Engelen MP, van der Vusse GJ, Wouters EF, Schols AM. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J 19: 617–625, 2002. doi: 10.1183/09031936.02.00762001. [DOI] [PubMed] [Google Scholar]
- 16.Green HJ, Burnett ME, D’Arsigny CL, O’Donnell DE, Ouyang J, Webb KA. Altered metabolic and transporter characteristics of vastus lateralis in chronic obstructive pulmonary disease. J Appl Physiol (1985) 105: 879–886, 2008. doi: 10.1152/japplphysiol.90458.2008. [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343: 281–299, 1999. doi: 10.1042/bj3430281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Matching of blood flow to metabolic rate during recovery from moderate exercise in humans. Exp Physiol 93: 1118–1125, 2008. doi: 10.1113/expphysiol.2008.042895. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann SE, Waltz X, Leigh R, Anderson TJ, Poulin MJ. Blood flow during handgrip rxercise in COPD: effect of vitamin C. Med Sci Sports Exerc 48: 200–209, 2016. doi: 10.1249/MSS.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 20.Heindl S, Lehnert M, Criée CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med 164: 597–601, 2001. doi: 10.1164/ajrccm.164.4.2007085. [DOI] [PubMed] [Google Scholar]
- 21.Howlett RA, Stary CM, Hogan MC. Recovery of force during postcontractile depression in single Xenopus muscle fibers. Am J Physiol Regul Integr Comp Physiol 280: R1469–R1475, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS. Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension 63: 459–467, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeneson JA, Westerhoff HV, Brown TR, Van Echteld CJ, Berger R. Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am J Physiol Cell Physiol 268: C1474–C1484, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA, Simard C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil 18: 432–437, 1998. doi: 10.1097/00008483-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969. [PubMed] [Google Scholar]
- 26.Juel C. Training-induced changes in membrane transport proteins of human skeletal muscle. Eur J Appl Physiol 96: 627–635, 2006. doi: 10.1007/s00421-006-0140-x. [DOI] [PubMed] [Google Scholar]
- 27.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994. [PubMed] [Google Scholar]
- 28.Kemp GJ, Thompson CH, Sanderson AL, Radda GK. pH control in rat skeletal muscle during exercise, recovery from exercise, and acute respiratory acidosis. Magn Reson Med 31: 103–109, 1994. doi: 10.1002/mrm.1910310203. [DOI] [PubMed] [Google Scholar]
- 29.Kemp GJ, Thompson CH, Taylor DJ, Radda GK. Proton efflux in human skeletal muscle during recovery from exercise. Eur J Appl Physiol Occup Physiol 76: 462–471, 1997. doi: 10.1007/s004210050276. [DOI] [PubMed] [Google Scholar]
- 30.Koga S, Kano Y, Barstow TJ, Ferreira LF, Ohmae E, Sudo M, Poole DC. Kinetics of muscle deoxygenation and microvascular Po2 during contractions in rat: comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol (1985) 112: 26–32, 2012. doi: 10.1152/japplphysiol.00925.2011. [DOI] [PubMed] [Google Scholar]
- 31.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol (1985) 103: 2049–2056, 2007. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- 32.Koga S, Poole DC, Fukuoka Y, Ferreira LF, Kondo N, Ohmae E, Barstow TJ. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am J Physiol Regul Integr Comp Physiol 301: R534–R541, 2011. doi: 10.1152/ajpregu.00101.2011. [DOI] [PubMed] [Google Scholar]
- 33.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. doi: 10.1249/MSS.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 34.Krustrup P, Jones AM, Wilkerson DP, Calbet JA, Bangsbo J. Muscular and pulmonary O2 uptake kinetics during moderate- and high-intensity sub-maximal knee-extensor exercise in humans. J Physiol 587: 1843–1856, 2009. doi: 10.1113/jphysiol.2008.166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutsuzawa T, Shioya S, Kurita D, Haida M, Ohta Y, Yamabayashi H. 31P-NMR study of skeletal muscle metabolism in patients with chronic respiratory impairment. Am Rev Respir Dis 146: 1019–1024, 1992. doi: 10.1164/ajrccm/146.4.1019. [DOI] [PubMed] [Google Scholar]
- 36.Layec G, Bringard A, Vilmen C, Micallef JP, Fur YL, Perrey S, Cozzone PJ, Bendahan D. Accurate work-rate measurements during in vivo MRS studies of exercising human quadriceps. MAGMA 21: 227–235, 2008. doi: 10.1007/s10334-008-0117-3. [DOI] [PubMed] [Google Scholar]
- 37.Layec G, Haseler LJ, Hoff J, Richardson RS. Evidence that a higher ATP cost of muscular contraction contributes to the lower mechanical efficiency associated with COPD: preliminary findings. Am J Physiol Regul Integr Comp Physiol 300: R1142–R1147, 2011. doi: 10.1152/ajpregu.00835.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layec G, Haseler LJ, Trinity JD, Hart CR, Liu X, Le Fur Y, Jeong EK, Richardson RS. Mitochondrial function and increased convective O2 transport: implications for the assessment of mitochondrial respiration in vivo. J Appl Physiol (1985) 115: 803–811, 2013. doi: 10.1152/japplphysiol.00257.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: from able-bodied adults to individuals with a spinal cord injury. J Appl Physiol (1985) 116: 1142–1147, 2014. doi: 10.1152/japplphysiol.01120.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ, Kober F. Grid-free interactive and automated data processing for MR chemical shift imaging data. MAGMA 23: 23–30, 2010. doi: 10.1007/s10334-009-0186-y. [DOI] [PubMed] [Google Scholar]
- 41.Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 102–108, 2003. doi: 10.1164/rccm.200202-080OC. [DOI] [PubMed] [Google Scholar]
- 42.Maltais F, Jobin J, Sullivan MJ, Bernard S, Whittom F, Killian KJ, Desmeules M, Bélanger M, LeBlanc P. Metabolic and hemodynamic responses of lower limb during exercise in patients with COPD. J Appl Physiol (1985) 84: 1573–1580, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Maltais F, Simon M, Jobin J, Desmeules M, Sullivan MJ, Bélanger M, Leblanc P. Effects of oxygen on lower limb blood flow and O2 uptake during exercise in COPD. Med Sci Sports Exerc 33: 916–922, 2001. doi: 10.1097/00005768-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Mancini D. Application of near infrared spectroscopy to the evaluation of exercise performance and limitations in patients with heart failure. J Biomed Opt 2: 22–30, 1997. doi: 10.1117/12.263747. [DOI] [PubMed] [Google Scholar]
- 45.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol (1985) 77: 2740–2747, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Mancini DM, Wilson JR, Bolinger L, Li H, Kendrick K, Chance B, Leigh JS. In vivo magnetic resonance spectroscopy measurement of deoxymyoglobin during exercise in patients with heart failure. Demonstration of abnormal muscle metabolism despite adequate oxygenation. Circulation 90: 500–508, 1994. doi: 10.1161/01.CIR.90.1.500. [DOI] [PubMed] [Google Scholar]
- 47.Marcinek DJ, Amara CE, Matz K, Conley KE, Schenkman KA. Wavelength shift analysis: a simple method to determine the contribution of hemoglobin and myoglobin to in vivo optical spectra. Appl Spectrosc 61: 665–669, 2007. doi: 10.1366/000370207781269819. [DOI] [PubMed] [Google Scholar]
- 48.Mattson JP, Martin JC. Emphysema-induced reductions in locomotory skeletal muscle contractile function. Exp Physiol 90: 519–525, 2005. doi: 10.1113/expphysiol.2005.029850. [DOI] [PubMed] [Google Scholar]
- 49.Mattson JP, Poole DC. Pulmonary emphysema decreases hamster skeletal muscle oxidative enzyme capacity. J Appl Physiol (1985) 85: 210–214, 1998. [DOI] [PubMed] [Google Scholar]
- 50.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKeough ZJ, Alison JA, Bye PT, Trenell MI, Sachinwalla T, Thompson CH, Kemp GJ. Exercise capacity and quadriceps muscle metabolism following training in subjects with COPD. Respir Med 100: 1817–1825, 2006. doi: 10.1016/j.rmed.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Montes de Oca M, Torres SH, De Sanctis J, Mata A, Hernández N, Tálamo C. Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J 26: 390–397, 2005. doi: 10.1183/09031936.05.00107404. [DOI] [PubMed] [Google Scholar]
- 53.Moro L, Pedone C, Scarlata S, Malafarina V, Fimognari F, Antonelli-Incalzi R. Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology 59: 357–364, 2008. doi: 10.1177/0003319707306141. [DOI] [PubMed] [Google Scholar]
- 54.Oelberg DA, Kacmarek RM, Pappagianopoulos PP, Ginns LC, Systrom DM. Ventilatory and cardiovascular responses to inspired He-O2 during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 1876–1882, 1998. doi: 10.1164/ajrccm.158.6.9802015. [DOI] [PubMed] [Google Scholar]
- 55.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 167: 544–549, 2003. doi: 10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 56.Okamoto T, Kanazawa H, Hirata K, Yoshikawa J. Evaluation of oxygen uptake kinetics and oxygen kinetics of peripheral skeletal muscle during recovery from exercise in patients with chronic obstructive pulmonary disease. Clin Physiol Funct Imaging 23: 257–262, 2003. doi: 10.1046/j.1475-097X.2003.00500.x. [DOI] [PubMed] [Google Scholar]
- 57.Payen JF, Wuyam B, Levy P, Reutenauer H, Stieglitz P, Paramelle B, Le Bas JF. Muscular metabolism during oxygen supplementation in patients with chronic hypoxemia. Am Rev Respir Dis 147: 592–598, 1993. doi: 10.1164/ajrccm/147.3.592. [DOI] [PubMed] [Google Scholar]
- 58.Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 291: 681–686, 1993. doi: 10.1042/bj2910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 60.Roseguini BT, Alves CN, Chiappa GR, Stein R, Knorst MM, Ribeiro JP. Attenuation of muscle metaboreflex in chronic obstructive pulmonary disease. Med Sci Sports Exerc 40: 9–14, 2008. doi: 10.1249/mss.0b013e3181590bd9. [DOI] [PubMed] [Google Scholar]
- 61.Rossman MJ, Garten RS, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Ascorbate infusion increases skeletal muscle fatigue resistance in patients with chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol 305: R1163–R1170, 2013. doi: 10.1152/ajpregu.00360.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, Barberá JA, Nadal J, de Jover L, Rodriguez-Roisin R, Wagner PD. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 1726–1734, 1999. doi: 10.1164/ajrccm.159.6.9804136. [DOI] [PubMed] [Google Scholar]
- 63.Seiyama A, Hazeki O, Tamura M. Noninvasive quantitative analysis of blood oxygenation in rat skeletal muscle. J Biochem 103: 419–424, 1988. doi: 10.1093/oxfordjournals.jbchem.a122285. [DOI] [PubMed] [Google Scholar]
- 64.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol 46: 599–602, 1979. [DOI] [PubMed] [Google Scholar]
- 65.Shields GS, Coissi GS, Jimenez-Royo P, Gambarota G, Dimber R, Hopkinson NS, Matthews PM, Brown AP, Polkey MI. Bioenergetics and intermuscular fat in chronic obstructive pulmonary disease-associated quadriceps weakness. Muscle Nerve 51: 214–221, 2015. doi: 10.1002/mus.24289. [DOI] [PubMed] [Google Scholar]
- 66.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol (1985) 96: 1920–1927, 2004. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 67.Thompson CH, Davies RJ, Kemp GJ, Taylor DJ, Radda GK, Rajagopalan B. Skeletal muscle metabolism during exercise and recovery in patients with respiratory failure. Thorax 48: 486–490, 1993. doi: 10.1136/thx.48.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trenell MI, Sue CM, Kemp GJ, Sachinwalla T, Thompson CH. Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle Nerve 33: 524–531, 2006. doi: 10.1002/mus.20484. [DOI] [PubMed] [Google Scholar]
- 69.Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, Hatano Y, Inoue S, Matsudo SM, Mutrie N, Oppert JM, Rowe DA, Schmidt MD, Schofield GM, Spence JC, Teixeira PJ, Tully MA, Blair SN. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act 8: 79, 2011. doi: 10.1186/1479-5868-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tupling R, Green H, Grant S, Burnett M, Ranney D. Postcontractile force depression in humans is associated with an impairment in SR Ca2+ pump function. Am J Physiol Regul Integr Comp Physiol 278: R87–R94, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 72.Vogiatzis I, Athanasopoulos D, Habazettl H, Aliverti A, Louvaris Z, Cherouveim E, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 1105–1113, 2010. doi: 10.1164/rccm.201002-0172OC. [DOI] [PubMed] [Google Scholar]
- 73.Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 30: 1467–1474, 1998. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Wilson JR, Mancini DM, McCully K, Ferraro N, Lanoce V, Chance B. Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation 80: 1668–1674, 1989. doi: 10.1161/01.CIR.80.6.1668. [DOI] [PubMed] [Google Scholar]
- 75.Wuyam B, Payen JF, Levy P, Bensaïdane H, Reutenauer H, Le Bas JF, Benabid AL. Metabolism and aerobic capacity of skeletal muscle in chronic respiratory failure related to chronic obstructive pulmonary disease. Eur Respir J 5: 157–162, 1992. [PubMed] [Google Scholar]