Abstract
Complement plays a crucial role in microbial defense and clearance of apoptotic cells. Emerging evidence suggests complement is an important contributor to alcoholic liver disease. While complement component 1, Q subcomponent (C1q)-dependent complement activation contributes to ethanol-induced liver injury, the role of the alternative pathway in ethanol-induced injury is unknown. Activation of complement via the classical and alternative pathways was detected in alcoholic hepatitis patients. Female C57BL/6J [wild type (WT)], C1q-deficient (C1qa−/−, lacking classical pathway activation), complement protein 4-deficient (C4−/−, lacking classical and lectin pathway activation), complement factor D-deficient (FD−/−, lacking alternative pathway activation), and C1qa/FD−/− (lacking classical and alternative pathway activation) mice were fed an ethanol-containing liquid diet or pair-fed control diet for 4 or 25 days. Following chronic ethanol exposure, liver injury, steatosis, and proinflammatory cytokine expression were increased in WT but not C1qa−/−, C4−/−, or C1qa/FD−/− mice. In contrast, liver injury, steatosis, and proinflammatory mediators were robustly increased in ethanol-fed FD−/− mice compared with WT mice. Complement activation, assessed by hepatic accumulation of C1q and complement protein 3 (C3) cleavage products (C3b/iC3b/C3c), was evident in livers of WT mice in response to both short-term and chronic ethanol. While C1q accumulated in ethanol-fed FD−/− mice (short term and chronic), C3 cleavage products were detected after short-term but not chronic ethanol. Consistent with impaired complement activation, chronic ethanol induced the accumulation of apoptotic cells and fibrogenic responses in the liver of FD−/− mice. These data highlight the protective role of complement factor D (FD) and suggest that FD-dependent amplification of complement is an adaptive response that promotes hepatic healing and recovery in response to chronic ethanol.
NEW & NOTEWORTHY Complement, a component of the innate immune system, is an important pathophysiological contributor to ethanol-induced liver injury. We have identified a novel role for factor D, a component of the alternative pathway, in protecting the liver from ethanol-induced inflammation, accumulation of apoptotic hepatocytes, and profibrotic responses. These data indicate a dual role of complement with regard to inflammatory and protective responses and suggest that accumulation of apoptotic cells impairs hepatic healing/recovery during alcoholic liver disease.
Keywords: alcoholic liver disease, apoptosis, steatosis
INTRODUCTION
Alcohol is a leading cause of preventable morbidity and mortality worldwide (11, 30). Chronic alcohol abuse underlies the pathogenesis of alcoholic liver disease (ALD), encompassed by a spectrum of pathologies, ranging from steatosis to more severe forms of liver injury, including alcoholic hepatitis (AH), fibrosis, and cirrhosis. AH, an inflammatory condition characterized by infiltration of leukocytes and hepatocellular injury, remains an important contributor to mortality from ALD (16). Twenty-eight-day mortality rates for severe AH remain between 25 and 35%, and there remains no consensus regarding the preferred first-line treatment option for AH (22). Moreover, ~70% of individuals who develop AH will progress to cirrhosis (44). While current therapies focus on suppressing inflammation, they are relatively ineffective in patients with severe AH, and outcomes remain poor (11, 19, 57).
The innate immune system is an important contributor to the pathogenesis of ALD. Multiple components of the innate immune response, including hepatic macrophages and cytokine/chemokine networks, are impacted by alcohol and contribute to a dysregulated innate immune environment (3, 12). Persistent activation of the innate immune system by alcohol in turn contributes to excessive cell death of hepatocytes, by both apoptosis and necroptosis, and drives further hepatic tissue injury. Repeated cycles of cell damage and further stimulation of the innate immune response contributes to nonresolving inflammation in patients with AH (28).
Recent studies have also implicated complement as an important pathophysiological contributor to ethanol-induced liver injury. Complement, a component of the innate and adaptive immune system, plays a crucial role in microbial defense and clearance of apoptotic cells, but aberrant uncontrolled activation of complement causes extensive tissue injury (17, 37). Complement is activated by three pathways, the classical, lectin, and alternative pathways. Classical pathway activation is initiated upon the binding of complement component 1, Q subcomponent (C1q), the recognition subunit of the first component (C1), to antigen-antibody complexes while lectin pathway activation occurs via pathogenic recognition by mannose-binding lectin and ficolin (31). Both the classical and lectin pathways converge at complement protein 4 (C4), leading to downstream cleavage of complement protein 3 (C3). The alternative pathway provides amplification and low-level continuous formation of a soluble C3 convertase that associates with factor B (FB); factor D (FD) serves to activate this self-amplification loop, termed the “tick-over” pathway of complement activation, providing a state of complement “alertness” (29, 37).
Understanding the role of complement in the development of ALD has been a central research focus in the last decade. Complement is activated in mouse models of ALD (mALD); accumulation of C1q and C3 cleavage products (C3b/iC3b/C3c) can be detected in the liver (9, 40, 47), and serum levels of the anaphylatoxin fragment of C3 (C3a) are elevated (33). Expression of C1q, C3, C5, and anaphylatoxin fragment of C5 (C5a) receptor (C5aR) is elevated in livers of AH patients (46). In murine models of ALD, C5−/− mice are protected from ethanol-induced elevations of plasma alanine transaminase (ALT) and hepatic cytokine production while C3−/− mice are protected from steatosis following chronic ethanol feeding (5, 33).
The interaction between different pathways of complement activation and tissue injury is poorly understood. We have previously shown that C1q-dependent complement activation via the classical pathway contributes to the pathogenesis of ethanol-induced liver injury (9); however, the relative contribution of the alternative pathway in ALD is unknown. While Bykov et al. reported that chronic ethanol feeding in mice reduces the expression of FD and other soluble regulators of the alternative pathway, including factor H and C4bp, these studies only provided associative evidence for a role of the alternative pathway in ethanol-induced liver injury (6). In carbon tetrachloride-induced hepatotoxity, FD is involved in the clearance of apoptotic cells from the site of injury (10). Complement inactivation impairs the clearance of dying cells, leading to prolonged exposure to self-antigens and immune-mediated host responses (25).
Complement is activated in liver from AH patients due in part to the alternative pathway; therefore, we tested the hypothesis that FD contributes to the maintenance of tissue homeostasis during chronic ethanol by facilitating the clearance of apoptotic cells. Making use of C57BL/6J [wild type (WT)], C1q-deficient (C1qa−/−, lacking classical pathway activation), complement protein 4-deficient (C4−/−, lacking both the classical and lectin pathways), complement factor D-deficient-deficient (FD−/−, lacking alternative pathway activation), and C1qa/FD−/− (lacking both the classical and alternative pathways) mice, we have identified a novel role for FD in protecting the liver from ethanol-induced steatosis, inflammation, accumulation of apoptotic hepatocytes, and initiation of profibrotic responses. These data demonstrate for the first time that FD, a key component of the alternative pathway, contributes to complement activation in response to chronic ethanol feeding and that FD acts as a protective factor involved in the resolution of ethanol-induced tissue injury.
MATERIALS AND METHODS
Animals
Female 8- to 10-wk-old C57BL/6J WT and knockout (KO) mice were used for all experiments. C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Complement knockout mice were obtained from the following sources: C4−/− (14) and C1qa−/− (4) were provided by Dr. Michael Carroll (Harvard Medical School, Boston, MA); FD−/− (56) and C1qa/FD−/− (53) were created by Dr. Gregory Stahl (Harvard Medical School). All KO animals have been backcrossed to a C57BL/6 background and were bred in-house at the Cleveland Clinic. Genotyping was routinely performed in complement KO mice using DNA extracted from tails with a DNeasy Blood & Tissue Kit per the manufacturer’s instructions (Qiagen, Germantown, MD); primer sequences used for genotyping were: C1qa−/− WT forward 5′-GGG GCC TGT GAT CCA GAC AG-3′, common reverse 5′-ACC AAT CGC TTC TCA GGA CC-3′, and Mut forward 5′-GGG GAT CGG CAA TAA AAA GAC-3′; C4−/− WT forward 5′-ATA ACC TGG GTC GGA CTT TGG-3′, common reverse 5′-TCT TCC GAA ACT GCT GGA TCC-3′, forward (NEO) 5′-TAC CTG GGT ACT GCG GAA TGC-3′, reverse (NEO) 5′-AAG CCG GTC TTG TCG ATC AG-3′; FD−/−: common forward 5′-GAC GTG GAT CTG AGA TGC-3′, reverse (NEO) 5′-GGC CGA TCC CAT ATT GGC-3′, and reverse 5′-GGT TGC TCT CTG CAC ACA T-3′.
Ethanol Feeding Trials
All animal procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Female mice (C57BL/6J WT, C1qa−/−, C4−/−, FD−/−, and C1qa/FD−/−) were housed in standard microisolator cages (2 animals/cage). Age- and weight-matched mice were randomized into pair- and ethanol-fed groups, adapted to control liquid diet for 2 days, and fed increasing concentrations of ethanol for 4 or 25 days as previously described (9). For short-term studies, the ethanol-fed animals were acclimated to increasing concentrations of ethanol, 1% vol/vol (5.5% kcal) for 2 days and 2% vol/vol (11% kcal) for 2 days, and is denoted as 11%, day 4. For chronic studies, the ethanol-fed mice were acclimated to ethanol as follows: 1% vol/vol for 2 days, 2% vol/vol for 2 days, 4% vol/vol (22% kcal) for 1 wk, 5% vol/vol (27% kcal) for 1 wk, and last 6% vol/vol (32% kcal) for 1 wk and is denoted as 32%, day 25. Ethanol-fed mice were allowed ad libitum access to liquid diet. Control mice were pair fed a diet that received isocalorically substituted maltose dextrins for ethanol. Plasma ethanol concentrations were measured in samples collected 3 h into the feeding/dark cycle in C57BL/6J mice. Ethanol and pair-fed mice increased their body mass over the course of the short-term (data not shown) and chronic (Table 1) studies. Lieber-DeCarli high-fat ethanol and control diets were purchased from Dyets (catalog no.710260; Bethlehem, PA).
Table 1.
Body weights and food intakes for mice allowed free access to chronic ethanol-containing diets for 25 days (1% ethanol for 2 days, 2% ethanol for 2 days, 4% ethanol for 1 wk, 5% ethanol for 1 wk, and 6% ethanol for 1 wk)
| Chronic Ethanol Model (32%, day 25) | |||||
|---|---|---|---|---|---|
| Pair fed |
EtOH fed |
EtOH fed | |||
| Genotype | Initial body wt, g | Final body wt, g | Initial body wt, g | Final body wt, g | Average daily food intake, ml/cage |
| C57BL/6J* | 18.10 ± 0.27 | 22.58 ± 0.62a† | 18.36 ± 0.29 | 21.87 ± 0.50a† | 21.83 ± 1.25a |
| C4−/−§ | 16.90 ± 0.68 | 19.05 ± 1.29b† | 17.10 ± 0.48 | 19.62 ± 0.52b† | 19.31 ± 1.13b |
| FD−/−§ | 17.11 ± 0.51 | 20.05 ± 0.88b† | 17.60 ± 0.32 | 20.03 ± 0.43b† | 18.88 ± 1.03b |
| C1qa/FD−/−§ | 16.32 ± 0.65 | 20.31 ± 0.89b† | 17.18 ± 0.53 | 20.10 ± 0.64b† | 21.16 ± 1.29a,b |
Values are means ± SE. C4−/−, complement protein 4-deficient; FD−/−, complement factor FD-deficient; C1qa/FD−/−, complement component 1, Q subcomponent FD deficient. Body weights and diet intakes after chronic feeding (day 25) for C1qa-deficient mice have been previously reported (8).
Data for the wild type are combined values from 2 independent feeding trials, n = 8–12 animals/group.
Data are from one independent feeding trial, n = 4–6 animals/group. Values with different superscript letters are significantly different from each other between ethanol-fed genotypes (P < 0.05).
Values are significantly different from initial body weights within each genotype (P < 0.05).
Sample Collection
At the conclusion of these experiments, mice were anesthetized, blood was taken from the posterior vena cava, and livers were perfused with 0.9% saline via the hepatic portal vein. Livers were excised, and portions were stored in RNAlater (Ambion, Austin, TX), fixed in formalin, or frozen in optimal-cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) for histological analysis or snap-frozen in liquid nitrogen and stored at −80°C for later analysis. Blood was transferred into EDTA-containing microtainer tubes (BD Biosciences), and plasma was isolated following centrifugation and stored at −80°C until further analysis.
Acute Ethanol Exposure (Gavage) and Plasma Ethanol Measurements
Male mice were given a 6 g/kg dose of ethanol via an intragastric gavage. After the gavage (90 min), blood was collected via tail nick in heparinized microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, PA) and centrifuged in a microcentrifuge with a microhematocrit rotor for 3 min. Plasma samples were assayed for ethanol using a commercially available enzymatic assay kit (Sekisui Diagnostics, Lexington, MA). Mice were then euthanized by inhalation of CO2 and cervical dislocation.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling Staining
Apoptotic DNA fragmentation was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) using the ApopTag Plus fluorescence in situ apoptosis detection kit (Millipore, Temecula, CA) on paraffin-embedded liver sections according to the manufacturer’s protocol. Fluorescent images were captured on an upright confocal microscope (Leica Microsystems, Buffalo Grove, IL). Percent TUNEL-positive nuclei of total nuclei was determined by counting TUNEL-positive cells and 4,6-diamidino-2-phenylindole staining from three different fields per slide using ImagePro Plus software (Media Cybernetics, Silver Spring, MD).
Liver Histology, Immunohistochemistry, and Immunofluorescence
For histological analysis, formalin-fixed tissues were paraffin embedded, sectioned, and stained with hematoxylin and eosin. Paraffin-embedded liver sections were deparaffinized and stained with antibodies against tumor necrosis factor-α (TNF-α, catalog no. 70R-TR008; Fitzgerald Industries, Acton, MA) (27, 39); M30, a caspase-dependent cleavage product of cytokeratin 18 (M30CytoDEATH staining kit, catalog no.12140322001; Roche) (55), 4-hydroxynonenal (4-HNE) protein adducts (kindly provided by Dr. Dennis Petersen, University of Colorado Anschutz Medical Campus) (48); and PicroSirus Red (39). Frozen liver sections were mounted on glass slides, fixed with paraformaldehyde, and stained for C3b/iC3b/C3c (catalog no. HM1065; Hycult Biotech, Plymouth Meeting, PA) and C1q (catalog no. HM1044; Hycult Biotech) or Oil Red O as previously described (41, 47). Fluorescent images were acquired using an upright confocal microscope (Leica Microsystems). Formalin and OCT samples are coded at the time of collection for a blinded analysis; at least three images were acquired per tissue section, and semiquantification of positive staining was performed using ImagePro Plus software. No specific immunostaining was seen in sections incubated with PBS rather than the primary antibody (data not shown).
Biochemical Assays
Plasma samples were assayed for ALT and aspartate aminotransferase (AST) activity using the Sekisui Diagnostic enzymatic assay kit according to the manufacturers protocol. Flash-frozen liver samples were used to quantify hepatic triglyceride accumulation using a commercially available kit from Pointe Scientific (Lincoln Park, MI) per the manufacturer’s protocol.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
RNA was isolated from liver stored in RNAlater using RNeasy Mini kits per the manufacturer’s instructions (Qiagen, Germantown, MD). Liver RNA (4 μg) was reverse transcribed and analyzed with PowerSYBR Quantitative Real-Time Polymerase Chain Reaction kits (ThermoFisher, Grand Island, NY) on an Mx3000P analyzer (Stratagene, La Jolla, CA). Relative mRNA expression was determined using the following gene-specific primers: human C1qR: forward 5′-AGT GCC TGG ACC CTA GTC TG-3′, reverse 5′-GCT TGG AGA TGC ACG AGT TC-3′; human C3a receptor (C3aR): forward 5′-CCC TAC GGC AGG TTC CTA TG-3′, reverse 5′-GAC AGC GAT CCA GGC TAA TGG-3′; human C5aR: 5′-TCC TTC AAT TAT ACC ACC CCT GA-3′, reverse 5′-ACG CAG CGT GTT AGA AGT TTT AT-3′; human C5a receptor-like 2 (C5L2): forward 5′-CTG TAT GCC GCC ATC TTC CT-3′, reverse 5′-TGG CAT ACA TGG TCA GCA GG-3′; human 18S: 5′-CGG CTA CCA CAT CCA AGG AA-3′, reverse 5′-GCT GGA ATT ACC GCG GCT-3′; mouse TNF-α: forward 5′-CCC TCA CAC TCA GAT CAT CTT CT-3′, reverse 5′-GCT ACG ACG TGG GCT ACA G; mouse macrophage chemoattractant protein-1 (MCP-1/CCL2): forward 5′-AGG TCC CTG TCA TGC TTC TG-3′, reverse 5′-TCT GGA CCC ATT CCT TCT TG-3′; mouse α-smooth muscle actin (α-SMA): forward 5′-GTC CCA GAC ATC AGG GAG TAA-3′, reverse 5′-TCG GAT ACT TCA GCG TCA GGA-3′; mouse collagen type I α1-chain (Col1A1): forward 5′-ATG TTC AGC TTT GTG GAC CTC-3′, reverse 5′-CAG AAA GCA CAG CAC TCG C-3′; mouse plasminogen activator inhibitor-1 (PAI-1): forward 5′-TTC AGC CCT TGC TTG CCT C-3′, reverse 5′-ACA CTT TTA CTC CGA AGT CGG T-3′; mouse transforming growth factor-β1 (TGF-β): forward 5′-TGA CGT CAC TGG AGT TGT ACG G-3′, reverse 5′-GGT TCA TGT CAT GGA TGG TGC-3′; mouse 18S: forward 5′-ACG GAA GGG CAC CAC CAG GA-3′, reverse 5′-CAC CAC CAC CCA CGG AAT CG-3′. Statistical analyses were performed on the ∆Ct values (average Ct of gene of interest – average Ct of 18S).
Patients
Early transplant tissue repository.
Liver tissue samples were provided by the National Institute on Alcohol Abuse and Alcoholism-supported Clinical Resource for Alcoholic Hepatitis Investigations at Johns Hopkins University (R24AA025017); all studies have been approved by Institutional Review Boards at Johns Hopkins Medical Institutions. Tissue samples from explanted livers in patients with severe AH during liver transplantation or wedge biopsies from the donor livers (healthy control) were snap-frozen in liquid nitrogen and stored at −80°C. Biochemical/clinical data for these samples have been reported previously (21). All samples are deidentified and coded for blinded analysis.
Barcelona cohort.
All procedures conformed to the ethical guidelines of the 1975 Declaration of Helsinki and were approved by the Ethics Committee of the Hospital Clinic of Barcelona; only patients with signed informed consent were included. The study included patients admitted to the Liver Unit of the Hospice Clinic, Barcelona (2007–2009), with clinical, analytical, and histological features of AH. Inclusion criteria for AH patients encompassed 1) active alcohol consumption (>60 g/day) for at least 3 mo before admission, 2) increased aminotransferase levels (AST > ALT), high γ-glutamyltranspeptidase and bilirubin serum levels, and 3) histological diagnosis of AH characterized by the presence of hepatocellular damage, inflammatory infiltrate, and pericellular fibrosis as previously described (1, 24). A total of 36 patients with severe AH, indicated by Model for End-Stage Liver Disease scores >20, were included in this study.
Serum from healthy control patients was obtained from the Northeast Ohio Alcohol Center at the Cleveland Clinic; all procedures were approved by the Cleveland Clinic Institutional Review Board.
Liver Homogenates and Western Blotting
Protein lysates were made from frozen liver in lysis buffer containing: 0.5% Triton X-100, 20 mM HEPES (pH 7.4), 150 mM MgCl2, 2 mM EGTA, 10 mM NaF, 1 mM PMSF, 1 mM Na3(VO3)4, 12.5 mM β-glycerol phosphate, 2 mM DTT, and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Protein concentrations were determined by the DC Protein Assay (Bio-Rad, Hercules, CA), and samples were denatured at 95°C in Laemmli buffer. Samples were separated on SDS-PAGE gels, transferred to polyvinylidene fluoride membranes with a semidry transfer apparatus (Bio-Rad), and blocked in 3% bovine serum albumin. Membranes were probed with antibodies specific for CYP2E1 (catalog no. AB1252; EMD Millipore, Billerica, MA), heat shock cognate 71 kDa protein (HSC70, catalog no. sc-7298; Santa Cruz Biotechnologies, Carlsbad, CA), C3 (catalog no. 55033; MP Biomedicals, Solon, OH), and complement factor B (catalog no. AF2739; R&D Systems, Minneapolis, MN). Horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were applied, and membranes were developed using Immobilon Western Developing reagents (Millipore). Chemiluminescence was visualized using an Eastman Kodak Image Station 4000R (Rochester, NY). Arbitrary density of immunopositive bands was quantified using ImageJ software.
Enzyme-Linked Immunosorbant Assay
Anaphylatoxin fragment of C5 (C5a) enzyme-linked immunosorbant assay (ELISA) was performed in patient serum according to the manufacturer’s protocol (human C5a ELISA; Hycult Biotech).
Statistical Analysis
Values reported are means ± SE. Data were collected from several different feeding trials. The data were analyzed by the general linear models procedure (SAS, Carey, IN) followed by least-square means analysis of differences between groups, blocking for experiment effects when data from more than one experiment were used in any given dataset. Data were log transformed to obtain a normal distribution, if necessary.
RESULTS
Complement is Activated in Patients with Severe AH
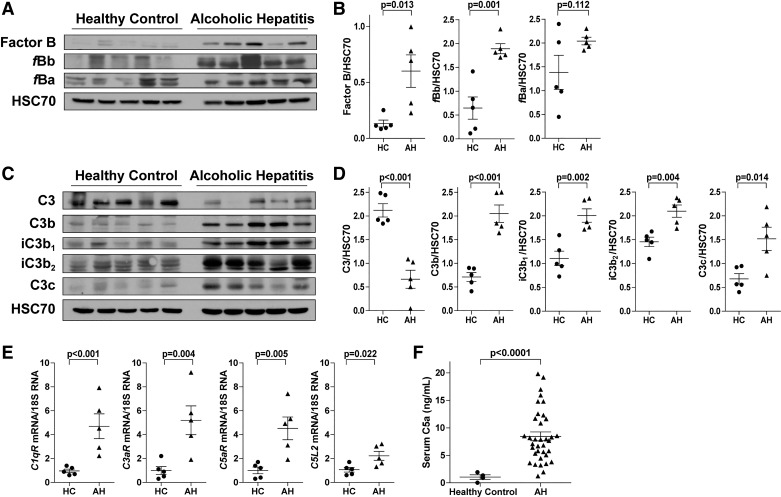
Activation of complement contributes to mALD; however, complement activation in patients with ALD has not been extensively studied. Complement activation products were measured in liver explants from AH patients or wedge biopsies from control livers (Fig. 1). In liver, expression of FB and the active cleavage fragment fBb, but not fBa, was increased in AH patients compared with controls (Fig. 1, A and B). Cleavage of C3 into C3b, iC3b, and C3c fragments was increased in AH liver (Fig. 1, C and D). Expression of C1q receptor (C1qR) and the anaphylatoxin receptors C3aR, C5aR, and C5L2 was increased in AH liver (Fig. 1E). Furthermore, circulating anaphylatoxin C5a was increased in patients with AH compared with healthy controls (Fig. 1F). Taken together, these data provide evidence for activation of complement via the classical and alternative pathways in livers of AH patients.
Fig. 1.
Complement is activated in liver explants and peripheral serum of patients with alcoholic hepatitis (AH). Liver lysates were prepared from liver explants from AH patients or wedge biopsies from control livers [healthy controls (HC)], and proteins were separated by SDS-PAGE. Factor B and activation fragments fBb and fBa (A and B) and complement protein 3 (C3), C3 cleavage fragments (C3b, iC3b, C3c), and heat shock cognate 71 kDa protein (HSC70, loading control) (C and D) were measured by Western blot. Relative expression is denoted as arbitrary units of density. Values represent means ± SE; n = 5 mice/group. E: expression of complement component 1, Q subcomponent receptor (C1qR), anaphylatoxin fragment of C3 (C3a) receptor (C3aR), anaphylatoxin fragment of C5 (C5a) receptor (C5aR), and C5a receptor-like 2 (C5L2) was detected in AH liver or wedge biopsies from HC by Real-Time Polymerase Chain Reaction. Data are relative fold change over wedge biopsies from HC. Values represent means ± SE; n = 5/group. F: circulating C5a was measured in plasma. Values represent means ± SE; n = 5 HC and n = 36 AH.
FD−/− Mice Have Increased Chronic Ethanol-Induced Liver Injury
C1q, C3, and C5 are required for the progression of ethanol-induced liver injury in mice (5, 9, 33, 40). While aberrant complement activation can contribute to tissue injury, failure to mount an appropriate activation response is known to negatively affect tissue homeostasis and maintenance. Therefore, in an effort to better understand the involvement of specific pathways of complement activation and the progression of ethanol-induced liver injury, C57BL6/J WT, C1qa−/−, C4−/−, FD−/−, and C1qa/FD−/− mice were fed ethanol-containing diets for 25 days (32%, day 25). Diet consumption was monitored throughout the study; C4−/− and FD−/− consumed 10–12% less diet compared with WT, C1qa−/−, and C1qa/FD−/− mice (Table 1). Plasma ethanol levels in WT mice increased when the diet contained 4% ethanol (vol/vol) or higher, reaching ~50 mM after 6% (vol/vol) ethanol (Fig. 2A). After 25 days, plasma ALT and AST activities were increased in WT mice compared with pair-fed controls. Chronic ethanol-induced increases in ALT/AST activities were lower in C4−/− and C1qa/FD−/− mice than in WT mice (Fig. 2, B and C), similar to the protection previously reported in C1qa−/− mice (9). In contrast, increases in ALT and AST were higher in FD−/− mice compared with WT mice (Fig. 2, B and C). Hepatic triglycerides were increased in ethanol-fed WT mice compared with pair-fed controls (Fig. 2D). C4−/− and C1qa/FD−/− mice were protected from chronic ethanol-induced increases in hepatic triglyceride accumulation, congruent with C1qa−/− mice (9). Hepatic triglycerides were increased in FD−/− compared with WT mice (Fig. 2D). Oil Red O staining of neutral lipid was consistent with the biochemical assessment of triglyceride (Fig. 2E). Lipid accumulated in the periportal (zone 1) and midzonal (zone 2) regions of WT mice liver after chronic ethanol feeding (Fig. 2F). In C1qa−/−, C4−/−, and C1qa/FD−/− mice, there was minimal lipid accumulation. FD−/− mice had extensive steatosis in both the periportal and midzonal regions of the lobule after ethanol feeding for 25 days. Taken together, these data demonstrate that FD protects against hepatic lipid accumulation and hepatocyte injury following chronic ethanol feeding.
Fig. 2.
Complement factor D-deficient (FD−/−) mice had pronounced liver injury and steatosis after chronic ethanol (EtOH) feeding. Wild-type (WT), complement component 1, Q subcomponent-deficient (C1qa−/−), complement protein 4-deficient (C4−/−), FD−/−, and C1qa/FD−/− were allowed free access to EtOH [32%, day (d) 25] or pair-fed control diets. A: plasma EtOH concentrations were measured 3 h into the feeding/dark cycle from WT mice. Values represent means ± SE; n = 3 for pair-fed and 6–10 for EtOH-fed mice. Values with different symbols are significantly different from each other. Alanine transaminase (ALT, B) and aspartate aminotransferase (AST, C) activity was measured in plasma. D: hepatic triglyceride content was measured in whole liver tissue. E: frozen liver sections were stained with Oil Red O. Images were acquired using a ×20 objective. F: paraffin-embedded liver sections were deparaffinized followed by staining with hematoxylin and eosin (H&E). Images were acquired using ×10 and ×20 objectives. Periportal (zone 1), midzonal (zone 2), and centrilobular (zone 3) regions are indicated in ×20 images. Values represent means ± SE; data for WT are combined values from 2 independent feeding trials, n = 8–12 animals/group. C1qa−/−, C4−/−, FD−/−, and C1qa/FD−/− are from one independent feeding trial; n = 4–6 animals/group. Values with different superscripts are significantly different from each other (P < 0.05). Biochemical measures of injury for C1qa−/− mice have been previously reported (9).
Ethanol Metabolism Was Not Different Between Genotypes
To ensure that differences in susceptibility to ethanol-induced injury were not because of differences in ethanol metabolism, plasma ethanol was quantified following an acute gavage of ethanol. Plasma ethanol levels were measured 90 min after the gavage and ranged from 104 to 112 mM (Table 2); no differences between WT, C1qa−/− (9), C4−/−, FD−/−, or C1qa/FD−/− mice were observed. Mice that received saline as a control had no detectable levels of ethanol (data not shown). These data suggest that any differences in liver injury between genotypes are independent of differences in ethanol metabolism.
Table 2.
Plasma ethanol concentration in WT, C4−/−, FD−/−, and C1qa/FD−/− mice 90 min after a 6 g/kg oral gavage
| Genotype: | C57BL/6J | C4−/− | FD−/− | C1qa/FD−/− |
|---|---|---|---|---|
| Plasma ethanol, mM | 104.7 ± 5.1 | 107.4 ± 7.1 | 112.2 ± 14.5 | 104.5 ± 8.6 |
Data are means ± SE; n = 3–5 animals per group. C4−/−, complement protein 4-deficient; FD−/−, complement factor FD-deficient; C1qa/FD−/−, complement component 1, Q subcomponent/FD deficient. Plasma ethanol concentrations after acute gavage from C1qa-deficient mice have been previously reported (8).
C1qa−/− and C4−/− Mice Are Protected From, While FD−/− Mice Have Increased, Hepatic Inflammation After Chronic Feeding
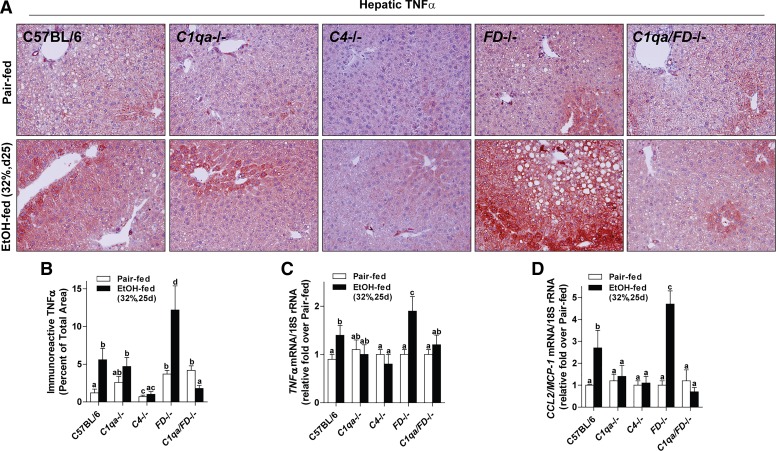
Ethanol activates cellular components of the innate immune system, including Kupffer cells, to secrete cytokines/chemokines; TNF-α is a key proinflammatory cytokine that contributes to liver injury during ethanol exposure. In pair-fed WT, C1qa−/−, and C4−/− mice, TNF-α expression assessed by immunohistochemistry was low and localized near the portal vein (Fig. 3, A and B). FD−/− and C1qa/FD−/− pair-fed mice had slightly higher expression of TNF-α in the liver, in both the periportal and centrilobular regions in the liver. In WT mice, ethanol feeding increased TNF-α expression throughout the lobule, with punctate expression along the sinusoids and dispersed expression near the centrilobular region (zone 3) in cells with the morphological appearance of hepatocytes (Fig. 3A). In contrast, TNF-α expression was lower in C1qa−/−, C4−/−, and C1qa/FD−/− mice. TNF-α expression was more pronounced in ethanol-fed FD−/− mice, with marked deposition near the centrilobular region. Consistent with increased expression of immunoreactive TNF-α, expression of TNF-α and MCP-1/CCL2 mRNA was increased in liver of WT mice after chronic ethanol feeding compared with pair-fed controls (Fig. 3, C and D). C1qa−/−, C4−/−, and C1qa/FD−/− mice were protected from ethanol-induced increases in TNF-α and MCP-1 mRNA. In contrast, FD−/− mice had increased expression of proinflammatory cytokines after ethanol feeding compared with WT mice (Fig. 2, C and D).
Fig. 3.
Complement component 1, Q subcomponent (C1q) and complement protein 4 (C4) contributed to, while complement factor D (FD) protected from, chronic ethanol (EtOH)-induced hepatic inflammation. Wild-type (WT), C1q-deficient (C1qa−/−), complement protein 4-deficient (C4−/−), complement factor D-deficient (FD−/−), and C1q/FD−/− were allowed free access to EtOH [32%, day (d) 25] or pair-fed control diets. A: paraffin-embedded liver sections were deparaffinized followed by immunodetection of tumor necrosis factor-α (TNF-α). Nuclei were counterstained with hematoxylin. All images were acquired using a ×20 objective. B: TNF-α-stained areas were quantified from at least three images per slide using Image-Pro Plus software. Expression of TNF-α (C) and macrophage chemoattractant protein-1 (MCP-1/CCR2, D) mRNA was detected in mouse livers using Quantitative Real-Time Polymerase Chain Reaction. Values with different alphabetical superscripts were significantly different from each other (P < 0.05). Values represent means ± SE; n = 4–8 pair-fed and 6–12 EtOH-fed mice.
Hepatic 4-HNE Protein Adducts Were Increased in FD−/− Mice After Chronic Ethanol Feeding
Lipid peroxidation is characteristic of ethanol-induced liver injury; oxidative stress is induced both because of ethanol metabolism via CYP2E1 and increased expression of inflammatory mediators (49). The accumulation of 4-HNE adducts, a well-characterized by-product of lipid peroxidation and indicator of cellular oxidative stress, was increased in WT mice after chronic ethanol feeding compared with pair-fed controls (Fig. 4, A and B). Ethanol-fed FD−/− mice had increased hepatic 4-HNE adducts compared with WT mice (Fig. 4, A and B). The expression of CYP2E1 protein was increased in WT and FD−/− mice after chronic ethanol feeding, with no difference between genotypes (Fig. 4C). These data suggest that increased oxidative stress was likely because of an increased inflammatory milieu in FD−/− mice rather than an increased rate of ethanol metabolism via CYP2E1.
Fig. 4.
Complement factor D-deficient (FD−/−) mice were more susceptible to oxidative stress. Wild-type (WT) and FD−/− mice were allowed free access to ethanol [EtOH, 32%, day (d) 25] or pair-fed control diets. A: paraffin-embedded liver sections were deparaffinized, and 4-hydroxynonenal adducts were detected by immunohistochemistry. B: positive staining was quantified in ×10 images from at least three images per slide using Image-Pro Plus software. C: liver lysates were prepared and proteins were separated by SDS-PAGE. CYP2E1 and heat shock cognate 71 kDa protein (HSC70, loading control) were measured by Western blot. Relative expression is denoted as arbitrary units of density. Values represent means ± SE; values with different superscripts were significantly different from each other (P < 0.05); n = 4 pair-fed and 3–6 EtOH-fed mice.
Complement Activation Was Impaired in FD−/− Mice After Chronic, But Not Short-Term, Ethanol Feeding
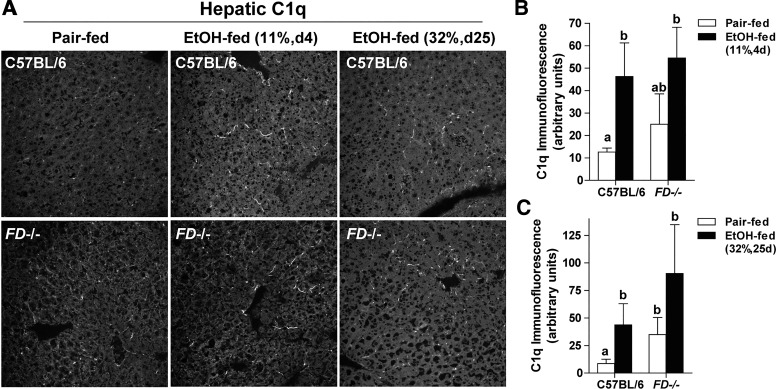
C1q accumulates in the liver after both short-term and chronic ethanol feeding, consistent with a role of C1qa in chronic ethanol-induced liver injury (9). Here we find that FD was not required for C1q accumulation in liver in response to ethanol feeding. After short-term ethanol feeding, immunoreactive C1q increased in the sinusoids of WT and FD−/− mice compared with WT pair-fed controls (Fig. 5, A and B). C1q deposition was also increased in ethanol-fed WT and FD−/− mice compared with WT pair-fed controls after chronic ethanol (Fig. 5, A and C).
Fig. 5.
Hepatic complement component 1, Q subcomponent (C1q) deposition was increased in wild-type (WT) and complement factor D-deficient (FD−/−) mice after short-term and chronic ethanol (EtOH) feeding. WT and FD−/− mice were allowed free access to EtOH [11%, day (d) 4 and 32%, day 25] or pair-fed control diets. A: frozen liver sections were immunostained for C1q deposition. Representative images for C1q deposition in pair-fed controls for chronic 25-day studies were similar to 4 days (data not shown). All images were acquired using a ×40 oil immersion objective. Positive immunofluorescence after short-term (B) and chronic (C) EtOH feeding was quantified from at least three images per slide using Image-Pro Plus software. Values represent means ± SE; values with different superscripts were significantly different from each other (P < 0.05); n = 4 pair-fed and 6 EtOH-fed mice.
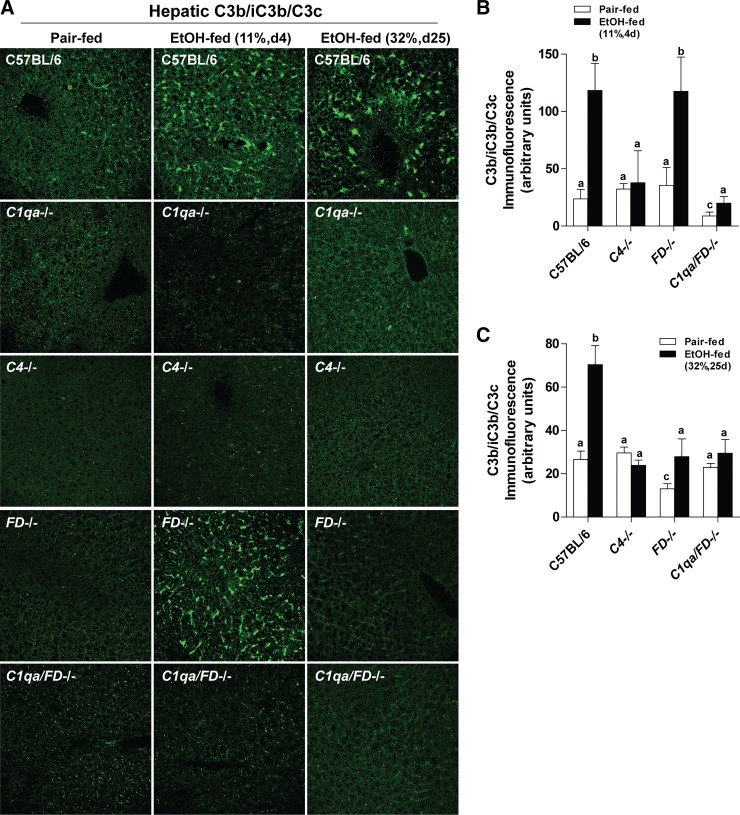
Cleavage of C3 in the liver was assessed by immunofluorescence with an antibody directed against the neoepitopes of C3b/iC3b/C3c (referred to as C3b) that are revealed once C3 is cleaved (26). After short-term ethanol feeding, deposition of C3b increased in the hepatic sinusoids of WT and FD−/− mice, but not C1qa−/− (9), C4−/−, or C1qa/FD−/− mice (Fig. 6, A and B) compared with pair-fed controls. After chronic ethanol, C3b deposition shifted to the parenchyma in WT mice but was absent in all other genotypes (9) (Fig. 6, A and C). These data suggest that, while early ethanol-induced activation of complement is C1q dependent (9), complement activation after chronic/high-dose ethanol feeding is both C1q (9) and FD dependent, with FD likely serving to amplify the activation via C1q.
Fig. 6.
Hepatic C3b deposition was impaired in complement factor D-deficient (FD−/−) mice after chronic, but not short-term, ethanol (EtOH) feeding. WT, complement component 1, Q subcomponent-deficient (C1qa−/−), complement protein 4-deficient (C4−/−), FD−/−, and C1qa/FD−/− mice were allowed free access to EtOH [11%, day (d) 4 and 32%, day 25] or pair-fed control diets. A: frozen liver sections were immunostained for C3b/iC3b/C3c deposition. Representative images for C3b/iC3b/C3c deposition in pair-fed controls for chronic 25-day studies were similar to 4 days (data not shown). All images were acquired using a ×40 oil immersion objective. Positive immunofluorescence after short-term (B) and chronic (C) EtOH feeding was quantified for C4−/−, FD−/−, and C1qa/FD−/− mice from at least three images per slide using Image-Pro Plus software. Positive immunofluorescence for C1qa−/− has been previously reported (9). Values represent means ± SE; values with different superscripts were significantly different from each other (P < 0.05); n = 4–8 pair-fed and 5–12 EtOH-fed mice.
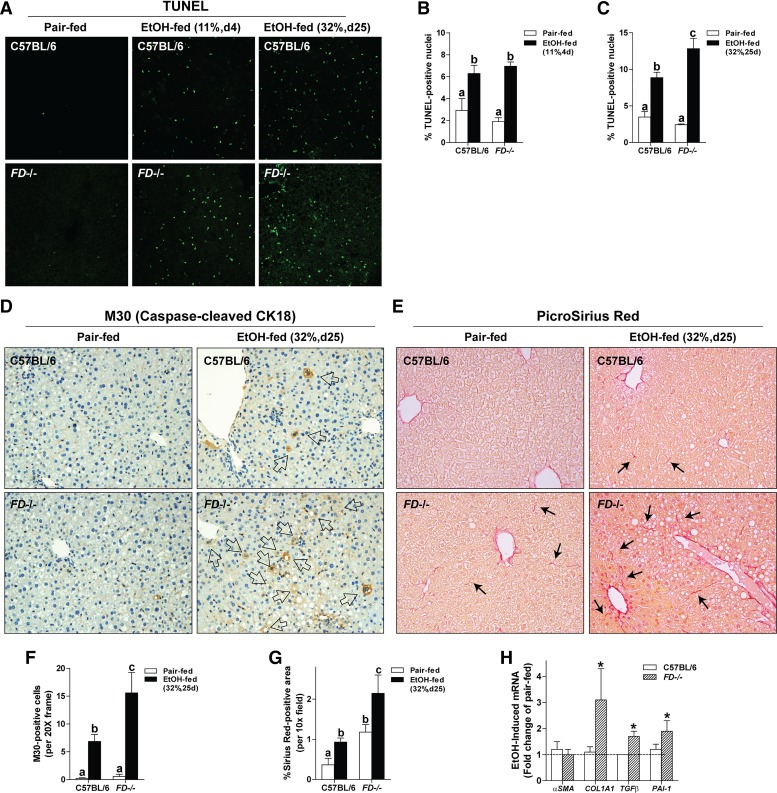
FD−/− Mice Accumulated More Apoptotic Hepatocytes After Chronic Ethanol Feeding
Hepatocyte apoptosis is associated with the progression of ethanol-induced liver injury, driven, at least in part, by increased inflammatory mediators and oxidant stress within the parenchyma. Accumulation of apoptotic cell bodies in the liver occurs via increased apoptosis and/or impaired clearance (2, 10). Given the enhanced inflammation and oxidative stress evident in the FD−/− mice, as well as the decreased deposition of C3b, an important opsonin for the clearance of apoptotic cells, we predicted that FD−/− mice would accumulate more apoptotic hepatocytes in response to chronic ethanol. After short-term ethanol feeding, WT and FD−/− mice accumulated more TUNEL-positive nuclei in the liver compared with pair-fed controls (Fig. 7, A and B). After chronic ethanol feeding, FD−/− had even more TUNEL-positive cells than WT mice (Fig. 7, A and C). M30 recognizes the caspase-cleavage product of cytokeratin-18, a marker of hepatocyte apoptosis (55) and component of Mallory-Denk bodies (42). M30-positive hepatocytes were increased in ethanol-fed WT mice and further increased in FD−/− mice (Fig. 7, D and F).
Fig. 7.
Complement factor D-deficient (FD−/−) mice had increased accumulation of apoptotic cells and initiation of profibrotic responses after chronic ethanol (EtOH) feeding. Wild-type and FD−/− mice were allowed free access to EtOH [11%, day (d) 4 and 32%, day 25] or pair-fed control diets. Paraffin-embedded livers were deparaffinized followed by immunohistochemistry. All images were quantified from at least three images per slide. A: apoptotic cells were visualized in paraffin-embedded liver sections. B and C: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells were enumerated and expressed as a percent of the total 4,6-diamidino-2-phenylindole (DAPI) nuclei/×40 field. D: apoptotic hepatocytes were visualized with a caspase-dependent cleavage product of cytokeratin 18. F: M30-positive cells, indicated by open arrows, were counted and represented as M30-positive cells/×20 field. E: extracellular matrix was visualized with PicroSirius Red. Black arrows indicate positive staining within the parenchyma. G: PicroSirius Red-positive staining was quantified using Image-Pro Plus software and normalized to the total area per ×10 field. H: expression of α-smooth muscle actin (α-SMA), collagen type I α1-chain (Col1A1), transforming growth factor-β1 (TGF-β), and plasminogen activator inhibitor-1 (PAI-1) was detected in mouse livers using Quantitative Real-Time Polymerase Chain Reaction. Values represent means ± SE; values with different superscripts were significantly different from each other (P < 0.05); n = 4 pair-fed and 3–6 EtOH-fed mice. *Significantly different from genotype pair-fed controls (P < 0.05).
Fibrotic Responses Were Induced In Ethanol-Fed FD−/− Mice
Because of their proximity to hepatocytes, hepatic stellate cells (HSCs) can engulf apoptotic bodies, stimulating fibrogenic activity (7). Because FD−/− mice had increased accumulation of apoptotic hepatocytes after chronic ethanol feeding, combined with the failure to activate complement, an important pathway for clearance of apoptotic cells (52), we predicted that FD−/− mice have an increased activation of HSCs with associated expression of profibrogenic markers in the liver after chronic ethanol feeding. PicroSirius Red staining was modestly increased in ethanol-fed WT mice, without increases in the expression of Col1A1, TGF-β, PAI-1, or α-SMA mRNA (Fig. 7, E, G, and H). More robust chicken-wire fibrosis was observed in ethanol-fed FD−/− mice that coincided with an upregulation of Col1A1, TGF-β, and PAI-1, but not α-SMA, mRNA (Fig. 7, E, G, and H). Taken together, these data indicate that FD−/− mice have increased accumulation of apoptotic cells and/or impaired clearance of cellular debris that was associated with fibrogenic responses in the liver after chronic ethanol feeding.
DISCUSSION
Complement plays a fundamental role in both innate and adaptive immunity primarily as a defense mechanism against infections. Consistent with this function, deficiencies/defects in the complement cascade can result in increased susceptibility to infection and/or complement-mediated tissue damage (8). Complement is implicated as a major driver of alcohol-induced liver injury in mice (5, 9, 27, 33, 40, 47). Although C1q-mediated complement activation contributes to ethanol-induced liver injury in mice (9), the role of other pathways of complement activation has not yet been explored in the context of ALD. Here, for the first time, we identified a crucial role of FD, a key component of the alternative pathway, in chronic ethanol-induced complement activation in mice. Importantly, FD is a protective factor that is involved in hepatocyte injury (ALT/AST/triglyceride) and hepatic inflammation. Moreover, the ability of FD to amplify complement is necessary for appropriate clearance of apoptotic cells and maintenance of tissue homeostasis after chronic ethanol feeding (Fig. 8).
Fig. 8.
Complement activation and involvement during alcoholic liver disease. In mouse models of ALD, ethanol (EtOH) induces the activation of complement via complement component 1, Q subcomponent (C1q), leading to the cleavage of complement protein 3 (C3) into the anaphylatoxin fragment of C3 (C3a) and C3b. The potent anaphylatoxins C3a and C5a act on cognate receptors (C3aR and C5aR) on resident hepatic macrophages (Kupffer cells) to initiate the transcription of proinflammatory mediators. During chronic EtOH, amplification of complement via the alternative pathway is required to provide essential opsonins (C3b/iC3b/C3c) on the surface of damaged cells in the liver, facilitating clearance of debris and allowing for resolution and repair. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MCP-1, macrophage chemoattractant protein-1; FB, complement factor B; FD, complement factor D; C4, complement protein 4; C2, complement protein 2; C4b, 2a, C3 convertase; fBb, FB activation fragment b; fBa, FB activation fragment a. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2018. All Rights Reserved.
While complement activation contributes to mALD, less is known about the patterns of complement activation in ALD patients. Our data reveal that complement is activated in explants from liver of AH patients, due in part to the alternative pathway, as evidenced by the cleavage of FB. Our data are consistent with Shen et al. who observed increased immunoreactivity of C1q, as well as C3, C5, and C5aR, in liver biopsies from AH patients (46). Collectively, these data indicate that both the classical and alternative pathways of complement contribute to local activation of complement in the liver in ALD. Our data in mALD suggest that classically vs. alternatively activated complement may have differential impact on progression of ALD whereby the classical pathway contributes to nonresolving inflammation and the alternative pathway promotes resolution/wound healing via the clearance of damaged tissue/cells (Fig. 8). Because the alternative pathway is protective in mALD, we hypothesize that increased activation of complement via the alternative pathway is an adaptive mechanism to promote resolution of tissue injury; however, during end-stage liver disease, FD-dependent amplification of complement is not sufficient to encourage recovery. Understanding patterns of complement activation may be a powerful diagnostic tool and therapeutic target in patents with ALD.
The interaction between classical and alternative pathway activation in response to tissue injury is complex and poorly understood. Although complement activation can occur via the classical, lectin, and alternative pathways, engagement of the alternative pathway is required to cause tissue injury in vivo in several disease models (51). For example, in models of systemic lupus erythematosus (SLE), FB−/− and FD−/− mice, lacking the alternative pathway, are protected from C3 glomerulonephritis (13), whereas C4−/− mice, deficient in both classical and lectin pathways, are more diseased (34). This protective role for C4 and proinjury role for the alternative pathway also holds true in models of rheumatoid arthritis and renal ischemia-reperfusion injury (51). The influence of the alternative pathway is likely related to its ability to amplify complement activation; amplification by the alternative pathway may account for up to 80% of the C5a and membrane attack complex generated by classical pathway activation (18, 51).
In contrast to the injurious role of FD and protection via C4 described in SLE, rheumatoid arthritis, and renal injury, here we find that the contributions of FD and C4 are reversed in chronic ethanol-induced injury. FD−/− mice had greater ethanol-induced injury, and C4−/− mice, deficient in both the classical and lectin pathways, were protected from ethanol-induced liver injury. The protection in C4−/− mice is consistent with the protection of C1qa−/− mice from ethanol-induced liver injury (9). Together, the impact of C4 and C1qa deficiency implicates an important role for the classical pathway in ethanol-induced liver injury. Interestingly, C1qa/FD−/− mice were protected from injury, indicating that C1q is required for ethanol-induced liver injury in FD−/− mice.
It is interesting to speculate that the differential contributions of C4 and FD to inflammation may be tissue specific, with a protective role for FD in the liver compared with other diseases. In patients with nonalcoholic steatohepatitis (NASH), activation of complement is associated with the accumulation of both classical and lectin components (35), as well as activation of the alternative pathway (45). Hepatic properdin, a positive regulator of the alternative pathway, and C3 activation fragments were positively correlated with lobular inflammation in NASH, whereas hepatic factor H, a negative regulator of alternative activation, mRNA was downregulated (45). It will be important for future studies to investigate C4−/− and FD−/− mice in high-fat diet-induced models of NASH to more clearly define the role of FD in either promotion or protection from metabolic liver injury.
The dynamics of complement activation in the liver were complex, differing after the dose and duration of ethanol exposure. Hepatic C1q and C3b deposition occurs after short-term low-dose ethanol feeding in mice; this early complement activation is C1q dependent (5, 9), but FD independent, since C3b accumulated in the liver of FD−/− mice after short-term ethanol feeding. In contrast, C3b deposition after chronic ethanol exposure was both C1q and FD dependent (Ref. 9 and Fig. 6), suggesting that FD-dependent amplification of complement is necessary, but not sufficient, for C3 activation after chronic/high-dose ethanol; C1q is not sufficient to sustain complement activation.
Another characteristic of FD−/− mice was elevated steatosis; the mechanism for this is not known. Complement is implicated in the cross talk between the immune system and metabolism in adipose tissue and liver (31), both significant targets of alcohol (54). FD, also known as adipsin, is mainly produced by adipocytes and is an adipokine with both direct and indirect immunometabolic properties (23). Acylation-stimulating protein (ASP/C3adesArg), a downstream degradation product of C3, can stimulate triglyceride synthesis and glucose transport in adipocytes through the actions of the C3a receptor C5L2 (20, 32). Indeed, FD is sufficient to induce the expression of PPARγ, a transcription factor involved in assimilation of lipid, in preadipocytes (50). Modulation of the adipose-liver axis is one potential mechanism by which FD could protect from ethanol-induced steatosis.
A major hallmark of ethanol-induced liver injury is apoptosis. Complement contributes to the resolution of inflammation by promoting clearance of apoptotic cells and immune complexes through the cooperative actions of its soluble pattern recognition molecules, like C1q, opsonins, and receptors (15, 36, 52). Following injury and activation of complement, C3b is released and deposited on both apoptotic and necrotic cells, facilitating phagocytosis and clearance of the damaged cells (52). Because FD−/− mice had decreased deposition of C3b/iC3b/C3c in the liver after chronic ethanol feeding in mice, they likely had impaired opsonization of apoptotic bodies in the parenchyma. Cells that undergo apoptosis initially maintain membrane integrity, ensuring that cellular contents are confined and properly ingested by phagocytes. However, if clearance of apoptotic bodies is impaired or delayed, these cells can undergo a process known as secondary necrosis, an event that leads to membrane permeability and eventual leakage of proinflammatory macromolecules (38, 43). Multiple pathways contribute to the clearance of apoptotic/necrotic debris from the liver in response to carbon tetrachloride-induced injury, including FD and plasminogen activators; the absence of these clearance pathways contributes to a continued exacerbation of inflammation and injury (2, 10). Increased accumulation of apoptotic cells within the parenchyma of FD−/− mice after chronic ethanol feeding is one potential contributing factor to elevated inflammation, liver injury, and upregulation of profibrotic responses observed in FD−/− mice.
Whether FD−/− mice have increased apoptosis or impaired clearance of apoptotic cells after chronic ethanol feeding remains unclear. Indeed, inflammatory mediators contribute to hepatocyte dysfunction, including apoptosis and necroptosis, to perpetuate tissue injury. Failure to activate complement is linked to increased sensitization of hepatocytes to ethanol; signaling via C5aR on hepatocytes is cytoprotective from ethanol/TNF-α-induced cytotoxicity (27). Because FD−/− mice had increased cytokine expression and elevated 4-HNE adducts, as well as impaired alternative pathway-driven complement activation after chronic ethanol feeding, it is likely that both increased apoptosis and impaired clearance is occurring.
In summary, complement plays a critical role in the development of alcohol-induced liver injury. In patients with severe AH, complement is activated in liver, at least in part via the alternative pathway. Our findings in mALD suggest FD promotes the clearance of apoptotic cells during the development of alcohol-induced liver injury. Moreover, this work suggests that FD is protective in maintaining tissue homeostasis via its opsonization function after ethanol feeding in mice, in contrast to driving pathological complement activation via its potent amplification loop. Finally, these data highlight the twofold purpose of complement in inflammatory and protective responses and suggest that inappropriate complement activation likely delays the clearance of apoptotic cells and impairs healing/recovery in the liver in response to chronic ethanol.
GRANTS
This work was supported in part by National Institutes of Health Grants P50-AA-024333, U01-AA-020821, and R37-AA-011876 to L. E. Nagy; T32-DK-00731935 and K99-AA-025386 to R. L. McCullough; contributions from the Case Western Reserve University/Cleveland Clinic (Clinical and Translational Science Award UL1-RR-024989); and Shared Instrumentation Grant 1S10-RR-026820.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L.M., M.R.M., M.T.P., and L.E.N. conceived and designed research; R.L.M., M.R.M., M.M.S., K.L.P., S.R., D.J.C., and M.T.P. performed experiments; R.L.M., S.R., D.J.C., and M.T.P. analyzed data; R.L.M. and L.E.N. interpreted results of experiments; R.L.M. prepared figures; R.L.M. drafted manuscript; R.L.M. and L.E.N. edited and revised manuscript; R.L.M., M.R.M., K.L.P., S.R., D.J.C., M.T.P., J.C., and L.E.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Michael Carroll and Gregory Stahl for providing the complement-deficient mice used in this publication. We also thank David Schumick, Center for Medical Art and Photography at the Cleveland Clinic, for our summary figure.
REFERENCES
- 1.Affò S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millán C, Loaeza-del-Castillo A, Altamirano J, García-Pagán JC, Arroyo V, Ginès P, Caballería J, Schwabe RF, Bataller R. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62: 452–460, 2013. doi: 10.1136/gutjnl-2011-301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezerra JA, Currier AR, Melin-Aldana H, Sabla G, Bugge TH, Kombrinck KW, Degen JL. Plasminogen activators direct reorganization of the liver lobule after acute injury. Am J Pathol 158: 921–929, 2001. doi: 10.1016/S0002-9440(10)64039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieghs V, Trautwein C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol 34: 446–452, 2013. doi: 10.1016/j.it.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet 19: 56–59, 1998. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 5.Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med 38: 280–286, 2006. doi: 10.1080/07853890600664608. [DOI] [PubMed] [Google Scholar]
- 6.Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Effect of chronic ethanol consumption on the expression of complement components and acute-phase proteins in liver. Clin Immunol 124: 213–220, 2007. doi: 10.1016/j.clim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology 39: 273–278, 2004. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 8.Carter AM. Complement activation: an emerging player in the pathogenesis of cardiovascular disease. Scientifica (Cairo) 2012: 1–14 2012. doi: 10.6064/2012/402783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology 139: 664–674, 2010. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cresci GA, Allende D, McMullen MR, Nagy LE. Alternative complement pathway component Factor D contributes to efficient clearance of tissue debris following acute CCl4-induced injury. Mol Immunol 64: 9–17, 2015. doi: 10.1016/j.molimm.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasarathy S, Brown JM. Alcoholic liver disease on the rise: interorgan cross talk driving liver injury. Alcohol Clin Exp Res 41: 880–882, 2017. doi: 10.1111/acer.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol 3: 785–797, 2013. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int 65: 129–138, 2004. doi: 10.1111/j.1523-1755.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer MB, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard RG, Rothstein TL, Kremmer E, Rosen FS, Carroll MC. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol 157: 549–556, 1996. [PubMed] [Google Scholar]
- 15.Galvan MD, Greenlee-Wacker MC, Bohlson SS. C1q and phagocytosis: the perfect complement to a good meal. J Leukoc Biol 92: 489–497, 2012. doi: 10.1189/jlb.0212099. [DOI] [PubMed] [Google Scholar]
- 16.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 300: G516–G525, 2011. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol 138: 439–446, 2004. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi-Barve S, Kirpich I, Cave MC, Marsano LS, McClain CJ. Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: mechanistic similarities and differences. Cell Mol Gastroenterol Hepatol 1: 356–367, 2015. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalant D, MacLaren R, Cui W, Samanta R, Monk PN, Laporte SA, Cianflone K. C5L2 is a functional receptor for acylation-stimulating protein. J Biol Chem 280: 23936–23944, 2005. doi: 10.1074/jbc.M406921200. [DOI] [PubMed] [Google Scholar]
- 21.Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, Llorente C, Pan SQ, Yang Q, Li Y, Lazaro R, Ansong C, Smith RD, Bataller R, Morgan T, Schnabl B, Tsukamoto H. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis. Hepatology 67: 1737–1753, 2018. doi: 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang R, Liu A, Perumpail RB, Wong RJ, Ahmed A. Advances in alcoholic liver disease: An update on alcoholic hepatitis. World J Gastroenterol 21: 11893–11903, 2015. doi: 10.3748/wjg.v21.i42.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P, Banks AS, Khandekar MJ, Dietrich A, Flier JS, Cinti S, Blüher M, Danial NN, Berggren PO, Spiegelman BM. Adipsin is an adipokine that improves β cell function in diabetes. Cell 158: 41–53, 2014. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marin V, Poulsen K, Odena G, McMullen MR, Altamirano J, Sancho-Bru P, Tiribelli C, Caballeria J, Rosso N, Bataller R, Nagy LE. Hepatocyte-derived macrophage migration inhibitory factor mediates alcohol-induced liver injury in mice and patients. J Hepatol 67: 1018–1025, 2017. doi: 10.1016/j.jhep.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 171: 715–727, 2007. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastellos D, Prechl J, László G, Papp K, Oláh E, Argyropoulos E, Franchini S, Tudoran R, Markiewski M, Lambris JD, Erdei A. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol 40: 1213–1221, 2004. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 27.McCullough RL, McMullen MR, Das D, Roychowdhury S, Strainic MG, Medof ME, Nagy LE. Differential contribution of complement receptor C5aR in myeloid and non-myeloid cells in chronic ethanol-induced liver injury in mice. Mol Immunol 75: 122–132, 2016. doi: 10.1016/j.molimm.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy LE. The role of innate immunity in alcoholic liver disease. Alcohol Res 37: 237–250, 2015. [PMC free article] [PubMed] [Google Scholar]
- 29.Nesargikar PN, Spiller B, Chavez R. The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol (Bp) 2: 103–111, 2012. doi: 10.1556/EuJMI.2.2012.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol 28, Suppl 1: 77–84, 2013. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol 25: 47–53, 2013. doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poursharifi P, Lapointe M, Fisette A, Lu H, Roy C, Munkonda MN, Fairlie DP, Cianflone K. C5aR and C5L2 act in concert to balance immunometabolism in adipose tissue. Mol Cell Endocrinol 382: 325–333, 2014. doi: 10.1016/j.mce.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Edward Medof M, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology 132: 1117–1126, 2007. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity 9: 721–731, 1998. doi: 10.1016/S1074-7613(00)80669-X. [DOI] [PubMed] [Google Scholar]
- 35.Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, Greve JW, Buurman WA. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology 50: 1809–1817, 2009. doi: 10.1002/hep.23228. [DOI] [PubMed] [Google Scholar]
- 36.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797, 2010. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12: 383–401, 2016. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol 3: 99–126, 2008. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roychowdhury S, McCullough RL, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, Pollard KA, McMullen MR, Croniger CM, Nagy LE. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology 64: 1518–1533, 2016. doi: 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, Stavitsky AB, Nagy LE. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology 49: 1326–1334, 2009. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saikia P, Bellos D, McMullen MR, Pollard KA, de la Motte C, Nagy LE. MicroRNA 181b-3p and its target importin α5 regulate toll-like receptor 4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology 66: 602–615, 2017. doi: 10.1002/hep.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakhuja P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J Gastroenterol 20: 16474–16479, 2014. doi: 10.3748/wjg.v20.i44.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2: 965–975, 2002. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis 16: 659–666, 2012. doi: 10.1016/j.cld.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Segers FM, Verdam FJ, de Jonge C, Boonen B, Driessen A, Shiri-Sverdlov R, Bouvy ND, Greve JW, Buurman WA, Rensen SS. Complement alternative pathway activation in human nonalcoholic steatohepatitis. PLoS One 9: e110053, 2014. doi: 10.1371/journal.pone.0110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen H, French BA, Liu H, Tillman BC, French SW. Increased activity of the complement system in the liver of patients with alcoholic hepatitis. Exp Mol Pathol 97: 338–344, 2014. doi: 10.1016/j.yexmp.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smathers RL, Chiang DJ, McMullen MR, Feldstein AE, Roychowdhury S, Nagy LE. Soluble IgM links apoptosis to complement activation in early alcoholic liver disease in mice. Mol Immunol 72: 9–18, 2016. doi: 10.1016/j.molimm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smathers RL, Galligan JJ, Shearn CT, Fritz KS, Mercer K, Ronis M, Orlicky DJ, Davidson NO, Petersen DR. Susceptibility of L-FABP-/- mice to oxidative stress in early-stage alcoholic liver. J Lipid Res 54: 1335–1345, 2013. doi: 10.1194/jlr.M034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact 192: 107–112, 2011. doi: 10.1016/j.cbi.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song NJ, Kim S, Jang BH, Chang SH, Yun UJ, Park KM, Waki H, Li DY, Tontonoz P, Park KW. small molecule-induced complement factor d (adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS One 11: e0162228, 2016. doi: 10.1371/journal.pone.0162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol 176: 1305–1310, 2006. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 52.Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol 45: 1199–1207, 2008. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol 175: 541–546, 2005. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 54.Wang ZG, Dou XB, Zhou ZX, Song ZY. Adipose tissue-liver axis in alcoholic liver disease. World J Gastrointest Pathophysiol 7: 17–26, 2016. doi: 10.4291/wjgp.v7.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 44: 27–33, 2006. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 56.Xu Y, Ma M, Ippolito GC, Schroeder HW Jr, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci USA 98: 14577–14582, 2001. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeluru A, Cuthbert JA, Casey L, Mitchell MC. Alcoholic hepatitis: risk factors, pathogenesis, and approach to treatment. Alcohol Clin Exp Res 40: 246–255, 2016. doi: 10.1111/acer.12956. [DOI] [PubMed] [Google Scholar]