Abstract
Breast milk lutein is better absorbed by infants than lutein delivered in infant formula. Therefore, we wanted to better understand the possible absorption differences of lutein in breast milk vs. that in infant formula by determining its bioavailability after gastric administration and whether the intestinal absorption of lutein can be improved by using new delivery vehicles. Study 1 compared the intestinal uptake,and the lymphatic and portal transport of lutein in conscious lymph fistula rats. Four groups of lymph- and portal vein-cannulated rats (n = 8–10/group) were randomized to receive via gastric tube increasing doses (10, 20, 40, or 80 mg/kg) of 20% lutein in safflower oil (SO) suspension to assess whether there was a saturable level of lutein that could be absorbed and transported in lymph. Aliquots of hourly portal blood and lymph were taken for lutein and zeaxanthin analyses. The dose-response study showed that 20 mg/kg lutein was the saturable level of lymphatic lutein absorption with no lutein detected in portal circulation at any dosage level tested. Study 2 randomized five groups of lymph fistula rats (n = 4–9/group) to receive 20 mg/kg lutein from either lutein in SO or lutein in four different mono- and diglyceride oils (MDGs). Gastric infusion of lutein suspended in MDG (20 mg/kg) significantly improved (71–211%, P < 0.05) lymphatic lutein output 2–6 h after lipid feeding vs. lutein in SO. Lymphatic zeaxanthin (10% of the lutein fed mixture) transport in both Study 1 and Study 2 followed that of lutein. We conclude that a mixture of MDGs helps solubilize lutein and facilitate gastrointestinal micelle formation, thus improving lymphatic lutein absorption compared with triglyceride oils.
NEW & NOTEWORTHY This paper describes how lutein is digested and absorbed by the gastrointestinal tract by using the conscious lymph fistula rat model. Our dose-response study showed that absorption and lymphatic transport of lutein is a saturable process with no lutein detected in portal circulation at any dosage level tested. Our paper also provides insight into how this process can be improved by modifying the typical lipid mixtures carrying the lutein.
Keywords: absorption, bioavailability, diglycerides, lutein, lymph, monoglycerides
INTRODUCTION
Lutein is a carotenoid that selectively accumulates in the macular region of the retina and protects retinal cells required for vision (17). Humans cannot synthesize lutein and thus must depend on dietary sources. Studies in adults have revealed that dietary or supplemental lutein leads to a variable increase both in serum lutein and in macular pigment (1, 35). Some epidemiological evidence suggests that lutein may reduce the risk of age-related macular degeneration (4).
Lutein may be important well before adulthood. Lutein has been shown recently to be the main carotenoid present in the brain of infants (33) and older adults (9, 16), where it has been suggested to have a beneficial role in brain development and cognitive function. Lutein is present in cord blood at the time of newborn delivery, indicating placental transfer to the fetus, and cord blood concentrations are highly correlated to maternal serum concentrations (36, 37). After birth, breast-fed infants receive lutein from mother’s milk (5). At 1 mo of age, there are differences in serum lutein concentrations between breast-fed and formula-fed infants. Infants fed formula unfortified with lutein have serum lutein concentrations approximately one-sixth those of breast-fed infants in the first few months of life. Previous studies have shown that the blood carotenoid concentration of formula-fed infants was significantly lower than that of breast-fed infants and declined at a greater rate than in breast-fed infants (23, 27). In addition to the differences in blood levels in term infants, there is emerging evidence to suggest that formula-fed infants have a less dense macular pigment density than human milk-fed infants (32). More lutein is needed in infant formula than in human milk to achieve similar serum lutein concentrations among breast-fed and formula-fed infants. Low lutein bioavailability is especially evident with the fact that approximately four times more lutein is needed in infant formula than in human milk to achieve similar lutein concentrations among the breast-fed and formula-fed infants (2). A contributing factor is that a large portion of dietary lutein exists in crystalline form in the intestinal lumen.
Infants who are born before 37-wk gestation often face complications resulting from their prematurity. Preterm infants are more susceptible to increased morbidities that are not common in healthy term infants. Underdeveloped organs such as the lungs, eyes, gastrointestinal (GI) tract, and brain can reveal conditions unique to prematurity, such as chronic lung disease, bronchopulmonary dysplasia, retinopathy of prematurity (ROP), necrotizing enterocolitis, and intraventricular hemorrhage. Infants in the neonatal intensive care unit have increased inflammation and oxidative stress associated with the pathophysiology of prematurity as well as from treatment modalities (11, 22, 25). According to the National Eye Institute (National Institutes of Health), ROP affects ~50% of preterm infants who are born weighing 1,250 g or less. While 90% of infants with ROP experience the milder form of the disease, which requires little or no medical treatment, severe ROP can lead to serious visual impairments or even blindness. Hylander et al. (15) have reported that human milk feeding of preterm infants (<1,500 g birth wt) has been associated with a lower incidence of ROP, possibly driven mainly by the antioxidant content of human milk. Human milk provides a variety of antioxidants to the breast-fed infant, including the carotenoids lutein, zeaxanthin, lycopene, and β-carotene, that appear to be better absorbed compared with infant formula supplemented with carotenoids.
With the growing awareness of the association between increased carotenoid intake and risk reduction in chronic diseases, the absorption of lutein from the diet becomes an important factor in its delivery and physiological action. Lutein is one of the predominant carotenoids in human serum, but factors other than dietary intake can affect its concentration. Various factors have been linked to the absorption of carotenoids. Various carotenoids such as lutein and β-carotene are known to affect the absorption and/or utilization of each other in the human body (18). There are different dietary components that influence the bioavailability and/or serum concentration of lutein, such as dietary fat, fiber, food source, and food preparation (24). The bioavailability of β-carotene, lycopene, and lutein is markedly reduced in the presence of many different kinds of fiber and even differs greatly among vegetables (e.g., spinach, green beans, broccoli) that contain high amounts of carotenoids (6, 24, 31).
Currently, the most frequently used commercially available form of lutein is a crystalline lutein-zeaxanthin mixture suspended in a triglyceride-based oil. The lutein we used in our current study is FloraGLO, 20% suspension in safflower oil from Kemin Industries. The 20% lutein consists of 90% lutein and 10% zeaxanthin. A logical next step in understanding the possible absorption differences of lutein in breast milk vs. infant formula is to shed some light on its bioavailability after oral administration and to investigate quantitatively how lutein is absorbed from the GI tract and whether the absorption is saturable. In addition, our goals were to identify whether improvements in lutein absorption could be achieved by using different combinations of mono- and diglyceride oils (MDG) when similar amounts of lutein are delivered to the stomach. This objective could be achieved by comparing under steady-state conditions the digestion, uptake, and lymphatic and portal transport of lutein by using the well-established lymph fistula rat model (10, 17, 35). The conscious lymph fistula rat with portal vein cannulated model is a method that consists of controlled gastric administration of lipophilic compounds. This is followed by timed lymph and portal vein collections that accurately measure and quantify the absorption of physiological quantities of lipophilic compounds after a test meal.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (300–350 g) were purchased from Harlan (Indianapolis, IN) and used for the study. Rats were allowed at least a couple of weeks in quarantine to acclimate to the animal facilities at the University of Cincinnati before they were used for the study. Meanwhile, they were fed the Harlan-Teklad LM485 chow (Madison, WI). The rats were housed in a room under a 12-h:12-h light-dark cycle and temperature (22 ± 1°C)- and humidity (30–70%)-controlled conditions for the duration of the study. All animals had ad libitum access to food and water.
Lymph Fistula and Portal Vein Rat Model
This study was conducted at the University of Cincinnati-Metabolic Disease Institute (Cincinnati, OH), an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility, in accordance with the animal use protocol approved by the Institutional Animal Care and Use Committee, using guidelines set forth by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Lymph and Portal Vein Absorption Studies
In assessing how lutein is absorbed from the GI tract, we used a well-established conscious rat model with cannulas in both the intestinal lymph duct and the portal vein. There were two separate surgeries performed at two different time points, a portal vein cannulation followed by cannulation of the major mesenteric lymph duct. We performed the portal vein cannulation first, allowed the animals to recover for 3 days, and then performed the lymphatic cannulation. This procedure allowed for better recovery of the animals from portal vein cannulation without negatively affecting the amount of lipid normally transported in the lymph. The amount of lipid transported in this animal is comparable to that observed in many animals that have previously only had the lymph fistula surgery.
Portal Vein Cannulation
Rats were fasted overnight before surgical procedures. The animals were anesthetized with isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane], and surgically prepared (clipped and scrubbed). A ventral midline incision was made to expose the abdominal viscera to allow the placement of a tube in the portal vein via the ileocolic vein, which does not occlude the flow of blood. The cannula used was a Micro-Renathane MRE-033 (OD 0.033 in. and ID 0.014 in.) manufactured by Braintree Scientific (Braintree, MA). The incision was closed in two layers with sutures. The cannula tube was heparinized, tied with a suture, and closed in the peritoneal cavity. It was also exteriorized at the time of lymphatic surgery to allow for blood withdrawal. At that time, the animals were housed in restraining cages to prevent their access to this cannula. Buprenorphine (1 mg/kg) was given to each rat during surgery to alleviate pain. The animals were observed for 3 days and allowed access to chow. Animals with body weight that returned to the approximate level of the presurgical weight were used for lymphatic cannulation.
Lymph Duct Cannulation
Rats were deprived of food overnight before surgical procedures. Under isoflurane anesthesia, the intestinal lymph duct was cannulated according to the method of Bollman et al. (18) for collection of lymph. In addition, a soft silicone infusion tube (1.6 mm OD) was inserted into the fundus of the stomach and secured with a purse-string suture for the purpose of infusion of the test lipids. Postoperatively, the rats were intragastrically infused with a 5% glucose-saline solution containing 145 mM NaCl, 4 mM KCl, and 0.28 M glucose at a rate of 3 ml/h to replenish the loss of fluid and electrolytes due to lymphatic drainage. The animals were allowed to recover overnight in Bollman restraining cages maintained at a temperature of 28°C before lipid infusion.
Experimental Plan And Procedures
Study 1: primary lutein absorption studies.
In this first study, the morning after surgery, rats were fed by the gastric tube a safflower oil (SO) infusion containing different doses of lutein. The lutein we used in our current study is FloraGLO, 20% suspension in safflower oil from Kemin Industries. The 20% lutein consisted of 90% lutein and 10% zeaxanthin. The 20% lutein suspension was combined with safflower oil to obtain final lutein concentrations of 10, 20, 40, and 80 mg/kg. We randomized four groups of rats (n = 8–10/group) to receive increasing doses per kilogram body weight (10, 20, 40, and 80 mg/kg) of a 20% lutein in SO suspension (180 µl) to determine whether there was a saturable level of lutein that could be absorbed from the GI tract. Following the infusion of the 180 µl of lutein containing SO suspension, the tubing was flushed with an additional 180 µl of SO without lutein to ensure the complete delivery of the lutein containing SO. Portal blood and lymph were collected into precooled tubes before lutein infusions. These samples were analyzed as the fasting portal plasma and lymphatic output of lipid. Following the lutein infusions, portal blood was collected at hours 1, 2, 4, and 6, and lymph was collected hourly for 6 h. After the lymph volume was determined, the samples were centrifuged for 20 min at 1,500 g at room temperature to remove lymphocytes and blood cells. The portal blood samples were also centrifuged for the collection of plasma. Aliquots of portal plasma and lymph were taken for the analysis of lutein and zeaxanthin.
Study 2: lutein absorption studies using MDG.
To determine whether the use of MDG improves lutein absorption vs. the standard 20% SO suspension, we randomized five groups of rats (n = 4–9/group) to receive 20 mg/kg lutein from either the 20% lutein SO suspension (control) or one of the four different MDG oils that contained lutein whose composition are shown in Table 1. The morning after surgery, rats were fed by gastric tube a total of 180 µl of oil, that included a specific volume of the lutein suspensions to meet the individual lutein dose plus lutein-free safflower oil or MDG oil to meet a final volume of 180 µl. All gastric infusions were flushed with an additional 180 µl of each specific lutein-free MDG oil to ensure delivery of lutein doses. Portal blood and lymph were collected into precooled tubes before lutein infusions. These samples were analyzed as the fasting portal plasma and lymphatic output of lipid. Following the lutein infusions, portal blood was collected at hours 1, 2, 4, and 6, and lymph was collected hourly for 6 h. After the lymph volume was determined, the samples were centrifuged for 15 min at 700 g at room temperature to remove lymphocytes and red blood cells. Aliquots of portal blood and lymph were taken for the analysis of lutein and zeaxanthin.
Table 1.
Composition of lutein-containing oils
| Fatty Acid | Total Monoglyceride | α1-Monoglyceride | Total Diglyceride | Total Triglyceride |
|---|---|---|---|---|
| Lutein in safflower oil | 100 | |||
| MDG 1 | 31 | 23 | 46 | 23 |
| MDG 2 | 42 | 37 | 33 | 25 |
| MDG 3 | 42 | 37 | 47 | 11 |
| MDG 4 | 35 | 25 | 50 | 15 |
Values are percentages. One mono- and diglyceride (MDG 1) of fatty acids based on high oleic sunflower oil; Kirnol CE 1089. MDG 2 of fatty acids based on high oleic sunflower oil; Oleon (Belgium); FO25. MDG 3 of fatty acids based on high oleic sunflower oil; Abitec (Columbus, OH); Capmul GMO-40. MDG 4 of fatty acids based on corn oil; Cognis.
Analysis of Lutein and Zeaxanthin
Lymph and portal plasma samples for lutein and zeaxanthin analyses were protected from light during collection, processing, and storage. All samples were stored at or below −20°C and shipped to Craft Technologies (Wilson, NC) for carotenoid analysis. Concentrations of lutein and zeaxanthin from fasting and hourly lymph and portal plasma samples were measured using reverse-phase high-performance liquid chromatography with programmed wavelength ultraviolet detection at 450 nm, as previously described (8). We combined cis and trans isomers and report total lutein and zeaxanthin.
Statistical Methods
All values are expressed as means ± SE. A two-way repeated-measures ANOVA was used to determine whether differences existed among groups for each time period of lipid infusion for each dependent variable. If a main effect of group or time was significant, Tukey's studentized range test was carried out to determine where the difference occurred. When a significant interaction was present, a one-way repeated-measures ANOVA was conducted for each group, and a one-way ANOVA was conducted at each time. Significant findings were further analyzed with Tukey's test to determine where the differences occurred. Results were considered statistically significant at P < 0.05.
RESULTS
Study 1: Primary Lutein Absorption Studies
Lymph flow rate.
Gastric infusions of all doses of 20% lutein in SO suspensions were well tolerated by all animals. As shown in Fig. 1, the mean fasting lymph flow for all four groups of rats varied between 1.9 and 2.6 ml/h. In all groups, lymph flow increased significantly after the ingestion of each lutein suspension infusion and reached a maximum output between 3.0 and 3.6 ml/h during the third hour after gastric infusion of the lutein suspension. After peaking at the third hour, lymph flow declined slowly and reached a steady-state output of ~3 ml/h during hours 5 and 6 after lutein infusion. There were no significant differences in the lymph flow rates between the four different lutein dosage groups at any time point of lymph collection with the exception of the second hour. All lutein groups had higher lymph flow than the 10 mg/kg group (P < 0.05). Thus, the data show that the lutein content of the diet overall did not affect the lymph flow rate of the lymph fistula rats.
Fig. 1.
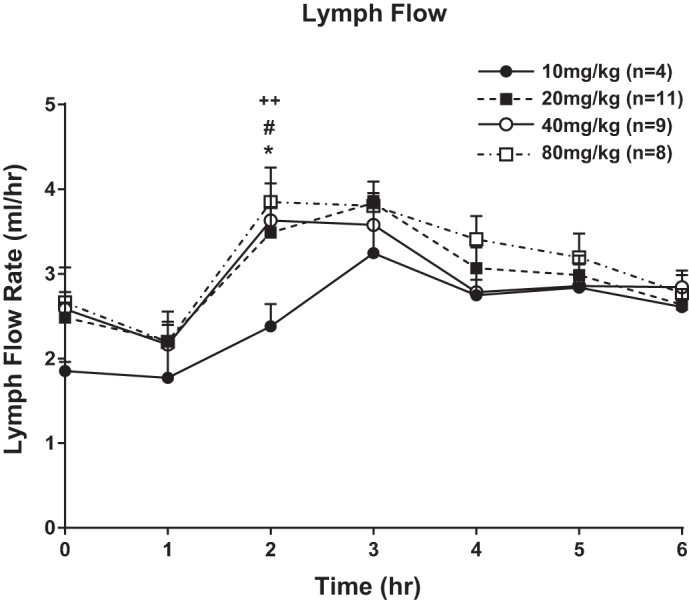
Lymph flow expressed ml/h. Lymph flow was measured 1 h before (fasting) and hourly for 6 h after lipid/lutein infusion. The 4 groups of animals studied included 1) rats infused with 10 mg/kg 20% lutein in safflower oil (SO) suspension, 2) rats infused with 20 mg/kg 20% lutein in SO suspension, 3) rats infused with 40 mg/kg 20% lutein in SO suspension, and 4) rats infused with 80 mg/kg 20% lutein in SO suspension. Values are means + SE. Symbols for statistical difference: *20 vs. 10 mg/kg 20% lutein in SO, #40 vs. 10 mg/kg 20% lutein in SO, and ++80 vs. 10 mg/kg 20% lutein in SO.
Lymphatic lutein output.
The level of lutein output in lymph collected at fasting and during hourly time intervals after gastric infusions of the different lutein doses is shown in Fig. 2A. In all groups, fasting levels of lymph lutein were nondetectable. All dosages of infused lutein had the same pattern of lymphatic transport: peak absorption by hours 3 and 4, followed by a slow decline reaching a steady-state output during hours 5 and 6 after lutein infusion. Gastric infusions of lutein at 20, 40, and 80 mg/kg had higher lymphatic lutein outputs from hours 2 through 6 (Fig. 2A) compared with rats given 10 mg/kg. However, because of the variability and the number of animals used, only the 20 mg/kg group reached statistical significance (P < 0.05) relative to the 10 mg/kg group at the third hour. There were no significant differences in the amount of lutein transported to lymph between the 20, 40, and 80 mg/kg groups for any of the hours between hours 2 and 6 (Fig. 2A). We also summated the total amount of lutein transported over the 6 h as depicted in Fig. 2B, and there were no statistically significant differences between the three groups, the 20, 40, and 80 mg/kg animals. However, the 20, 40, and 80 mg/kg animals had clearly more lutein transported into lymph than the 10 mg/kg animals, but because of the variability and the small sample size, only the 20 mg/kg animals had significantly more lutein transported (P < 0.05) during the 6 h of study than the 10 mg/kg animals. There were no detectable levels of lutein in portal blood for all doses of lutein tested and for all time points of portal blood collection.
Fig. 2.
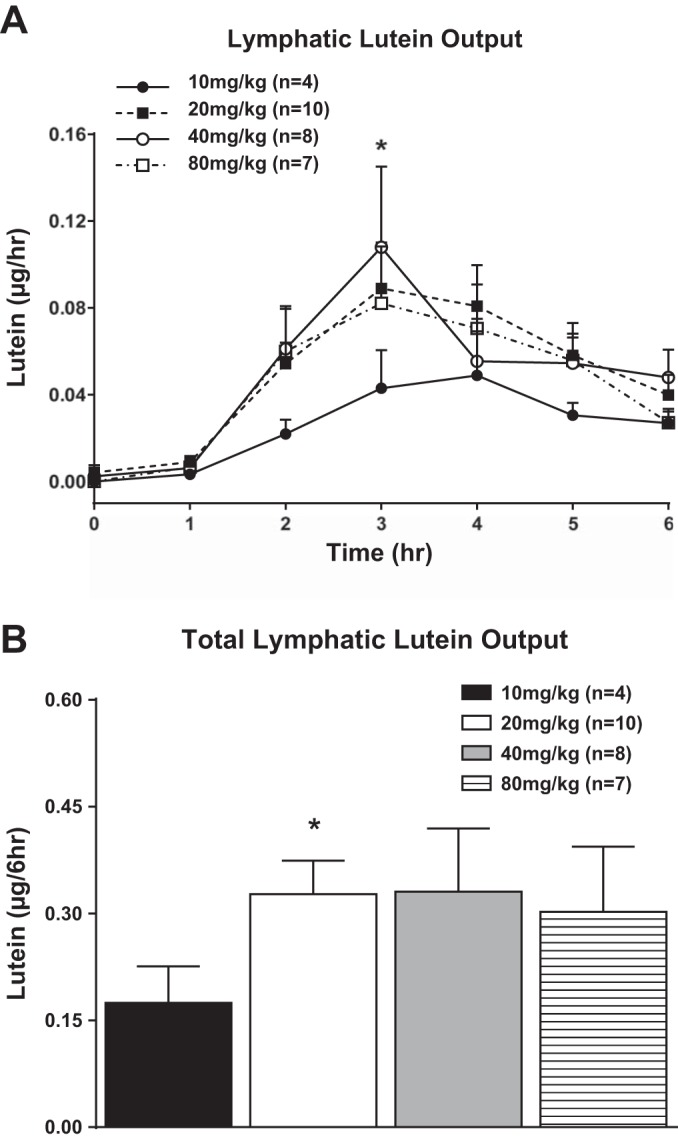
A: total lymphatic lutein (cis and trans) output expressed as μg/h. Cis and trans lutein concentrations were measured in lymph 1 h before (fasting) and hourly for 6 h after lutein infusion. The 4 groups of animals studied are the same as in Fig. 1. B: total lymphatic lutein (cis and trans) output over the 6-h lutein infusion period (area under the curve) for the same 4 groups of animals shown in A. Values are means + SE. *20 vs. 10 mg/kg lutein infusion group, P < 0.05.
Lymphatic zeaxanthin output.
Figure 3A shows the lymphatic zeaxanthin output during the first 6 h after gastric infusion of lutein at dosage levels of 20, 40, and 80 mg/kg. The absorption pattern of zeaxanthin was similar to that of lutein, with nondetectable levels of zeaxanthin in portal blood for all doses of lutein/zeaxanthin containing samples infused for all time points of portal blood collection. Fasting levels of lymph zeaxanthin were nondetectable for all groups of animals. Lymphatic zeaxanthin outputs showed a similar pattern of lymphatic transport as for lutein: peak absorption by hours 3 and 4 followed by a slow decline reaching a steady-state output during hours 5 and 6 after lutein infusion. Rats given gastric infusions of lutein at 20, 40, and 80 mg/kg had higher lymphatic zeaxanthin outputs during the 6 h of infusion. Animals given 40 mg/kg had significantly higher outputs (P < 0.05) than the 10 mg/kg animals at third and fourth hours of infusion. When we compared the total zeaxanthin outputs for the entire 6-h infusion period (Fig. 3B), there were higher numerical differences with the 20, 40, and 80 mg/kg compared with the 10 mg/kg group; however, there were no significant differences between the four groups of animals studied (Fig. 3B). This apparent lack of significant differences may be caused by both the variability and small sample size used in our study.
Fig. 3.
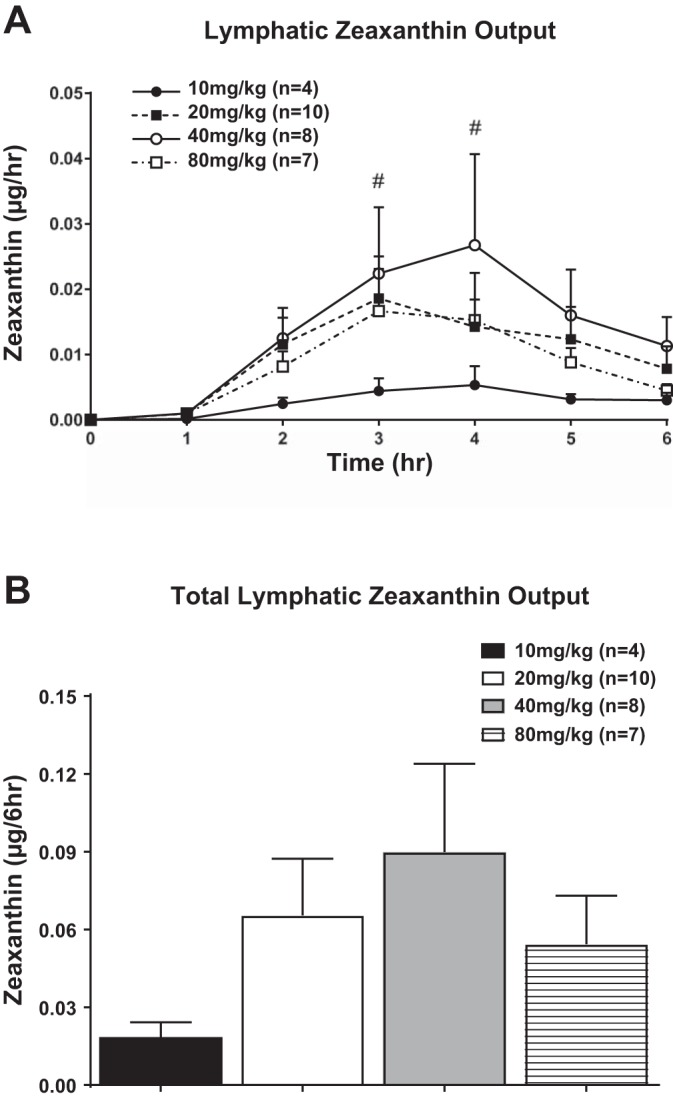
A: total lymphatic zeaxanthin output expressed as μg/h. Zeaxanthin concentrations were measured in lymph 1 h before (fasting) and hourly for 6 h after lutein infusion. The 4 groups of animals studied are the same as in Fig. 1. B: total lymphatic zeaxanthin output over the 6-h lutein infusion period (area under the curve) for the same 4 groups of animals shown in A. Values are means + SE. #40 vs. 10 mg/kg lutein infusion group, P < 0.05.
Study 2: Lutein Absorption Studies Using MDGs
The effect of four different MDGs that contained lutein (Table 1) on lymph flow vs. the standard 20% SO suspension is shown in Fig. 4. All animals received the same dose of lutein, 20 mg/kg body wt, which resulted in saturable level of lymphatic lutein absorption from the dose-response data generated in Study 1. There were no significant effects on lymph flow by any of the MDG oils, as evident by similar changes in lymph flow as previously noted with the 20% lutein SO suspension in Study 1. Briefly, the mean fasting lymph flow for all five groups of rats varied between 2.3 and 2.6 ml/hr. In all groups, lymph flow increased significantly after each MDG oil with lutein infusion and reached a maximum output between 2.6 and 3.6 ml/h and between hours 2 and 3. After peaking at the third hour, lymph flow declined slowly and reached a steady-state output of ~3 ml/h during hours 5 and 6 after lutein infusion. There were no significant differences in the lymph flow rates between the four different MDG lutein groups and the 20% lutein in SO groups at most time points except at 3 h, with the 20% lutein in SO group significantly higher than the most of the MDG groups (P < 0.05).
Fig. 4.
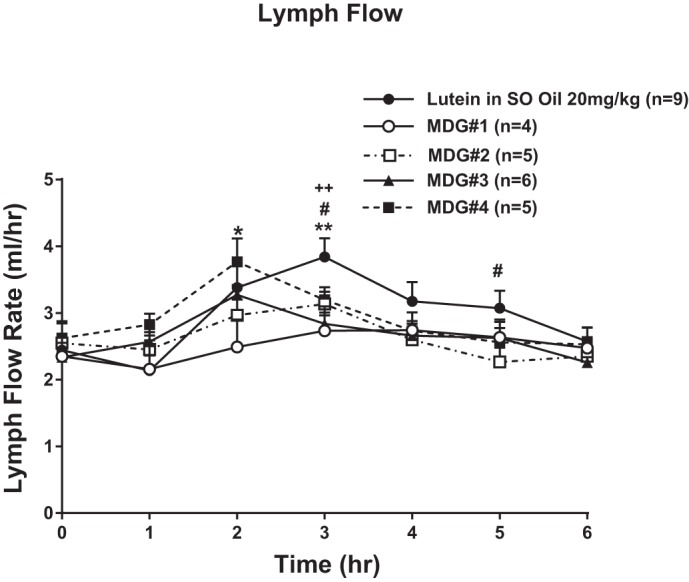
Lymph flow expressed as ml/h. Lymph flow was measured 1 h before (fasting) and hourly for 6 h after lipid/lutein infusion. Five groups of rats (n = 4–9/group) were randomized to receive 20 mg/kg lutein from either lutein in SO or from lutein in 4 different mono-diglyceride oils (MDGs) as outlined in Table 1. Values are means + SE. Single symbol represents P < 0.05 and double symbols represent P < 0.01. *MDG 1 vs. lutein in SO, #MDG 2 vs. lutein in SO, and +MDG 3 vs. lutein in SO.
Figure 5A shows the hourly and Fig. 5B the 6-h overall lymphatic lutein output after gastric infusions of the different MDG lutein combinations compared with 20% lutein in SO. In all groups, fasting levels of lymph lutein were nondetectable. All dosages of infused lutein had the same pattern of lymphatic transport: peak absorption by hours 3 and 4 followed by a slow decline reaching a steady-state output during hours 5 and 6 after lutein infusion. Gastric infusions of lutein with MDGs 1, 3, or 4 significantly improved (P < 0.05) the hourly lutein output from hours 2 through 6 (Fig. 5A) and for the overall 6-h infusion period (Fig. 5B) compared with rats given lutein in SO. Lutein absorption from MDG 2 was significantly lower (P < 0.05) than with MDGs 1, 3, and 4 and not different from the control rats given 20% lutein in SO (P > 0.05).
Fig. 5.
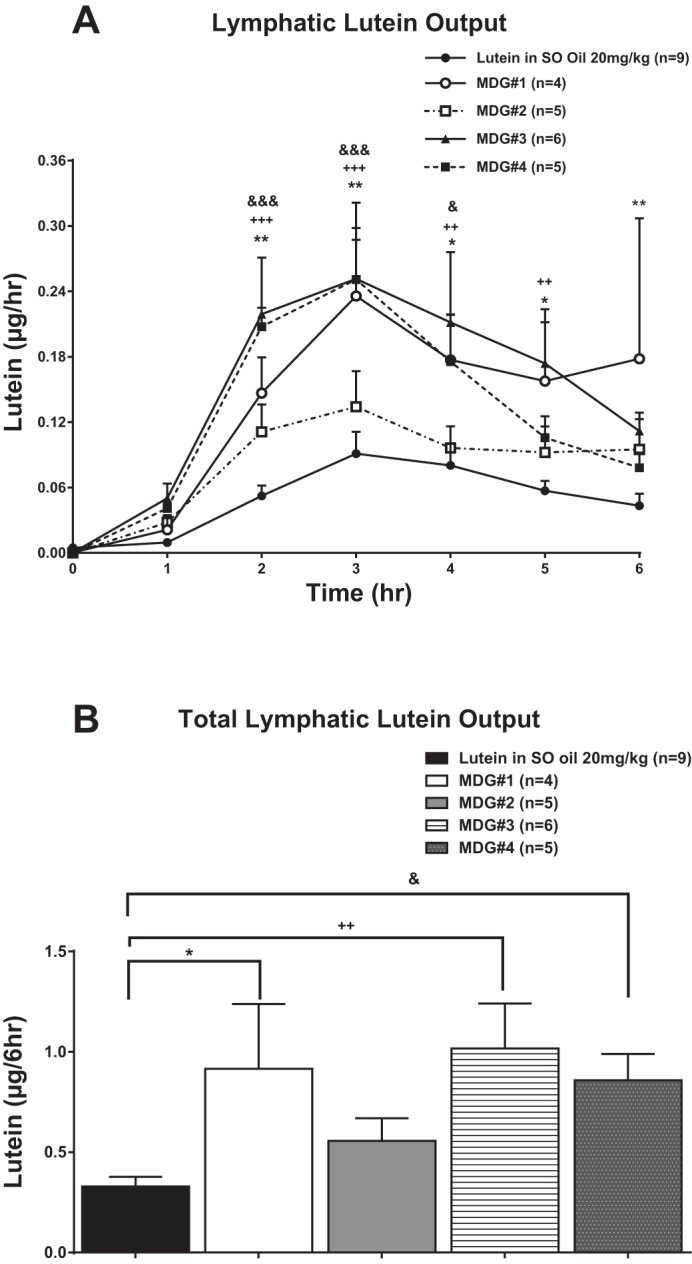
A: total lymphatic lutein (cis and trans) output expressed as as μg/h. Cis and trans lutein concentrations were measured in lymph 1 h before (fasting) and hourly for 6 h after lutein infusion. The 5 groups of animals studied are the same as in Fig. 4. B: total lymphatic lutein (cis and trans) output over the 6-h lutein infusion period (area under the curve) for the same 5 groups of animals shown in A. Values are means + SE. Single symbol represents P < 0.05, double symbols represent P < 0.01, and triple symbols represent P < 0.001. *MDG 1 vs. lutein in SO, +MDG 3 vs. lutein in SO, and &MDG 4 vs. lutein in SO. *P < 0.05 vs. lutein in SO infusion group.
Lymphatic zeaxanthin output during the first 6 h (Fig. 6A) and cumulative 6-h total (Fig. 6B) after gastric infusions of the different MDG lutein combinations are shown in Fig. 6. The absorption pattern of zeaxanthin followed a pattern similar to that of lutein with rats given MDGs 1, 3, or 4 having significantly higher (P < 0.05) zeaxanthin output from hours 2–6 and for the overall 6-h infusion period Fig. 6B) compared with rats given 20% lutein in SO. Zeaxanthin absorption from MDG 2 was significantly lower (P < 0.05) compared with MDGs 1, 3, and 4 and not different from rats given 20% lutein in SO (P > 0.05).
Fig. 6.
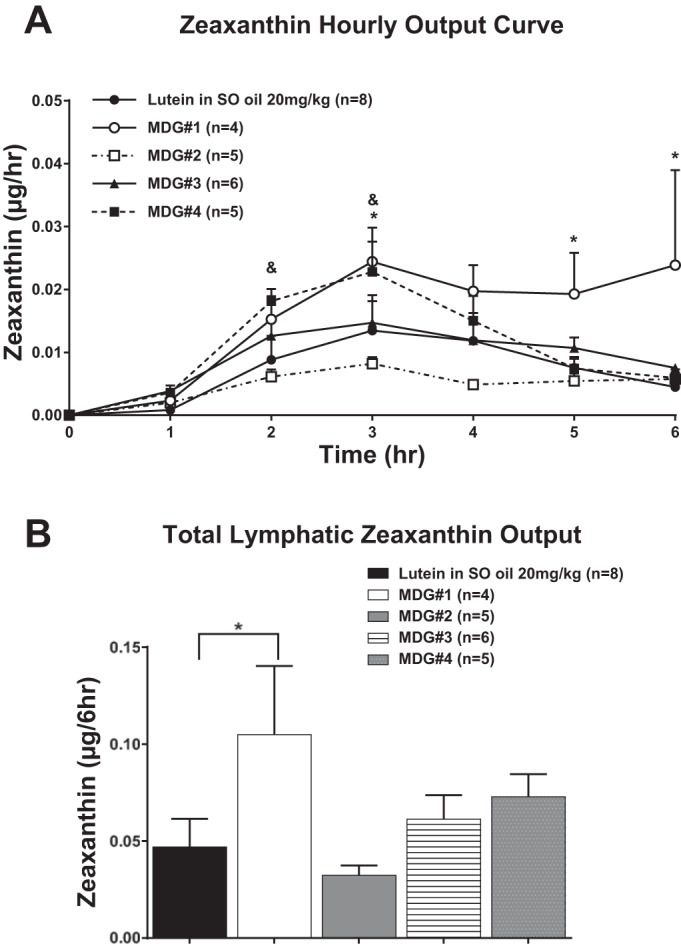
A: total lymphatic zeaxanthin output expressed as μg/h. Zeaxanthin concentrations were measured in lymph 1 h before (fasting) and hourly for 6 h after lutein infusion. The 5 groups of animals studied are the same as in Fig. 5. B: total lymphatic zeaxanthin output over the 6-h lutein infusion period (area under the curve) for the same 5 groups of animals shown in A. Values are means + SE. *MDG 1 vs. lutein in SO, +MDG 3 vs. lutein in SO, and &MDG 4 vs. lutein in SO.
DISCUSSION
With the growing awareness of the association between increased carotenoid intake and risk reduction in chronic diseases, the absorption of lutein from the diet becomes an important factor in its delivery and physiological action. One goal of this study was to gain a better understanding of how lutein is digested and absorbed from the GI tract. To assess this, a conscious lymph fistula rat model was used as an established method for studying the digestion and transport of lipids and lipophilic nutrients (4, 30). By infusing a physiological quantity of either lutein in SO or MDG into the stomachs of rats, the lymphatic and portal lutein and zeaxanthin outputs can be determined and quantitatively compared. Our results showed that gastric administration of lutein in SO is digested and transported to lymph with peak levels achieved in lymph occurring 3–4 h postinfusion. Interestingly, lutein is not transported to the liver through the portal circulation during the absorptive phase, as nondetectable levels of lutein in portal blood were found for all doses of lutein infused for all time points of portal blood collection. This suggests that the predominant transport of lutein from the GI tract is via chylomicrons generated after small-bowel digestion and absorption. Although we did not quantitate the percentage of lutein absorbed, it is quite low, given the lymphatic levels observed. An additional goal of this study was to identify a vehicle that would have properties to increase lutein absorption. To achieve this objective, we thought that it was necessary to first determine whether there was a saturable level at which lutein could be absorbed from the GI tract. The dose-response study showed that 20 mg/kg lutein in SO was the saturable level at which lutein could be absorbed, as evident by the similar lymphatic levels of lutein transported to lymph for doses of 20, 40, and 80 mg/kg. No lutein was detected in portal circulation at any dosage level.
Lutein is commercially available as a mixture of 90% lutein and 10% zeaxanthin. In addition to lutein, we had an opportunity for the first time to assess how zeaxanthin is digested and absorbed and whether any differences we observed for lutein absorption were also applicable to zeaxanthin to be transported from the GI tract. Similarly to lutein, zeaxanthin is absorbed through the lymph system with peak levels occurring after 3–4 h with no portal transport occurring from the GI tract. This absorption pattern makes sense given the fact that the polarities of lutein and zeaxanthin are similar. Interestingly the dose-response data suggest that, as the dose of lutein increased from 20 to 80 mg/kg, there was a reduction in the amount of lymphatic zeaxanthin output. This may have been due to the fact that the binding sites along the small-bowel mucosal cells were saturated with lutein with less room to accommodate for zeaxanthin (34).
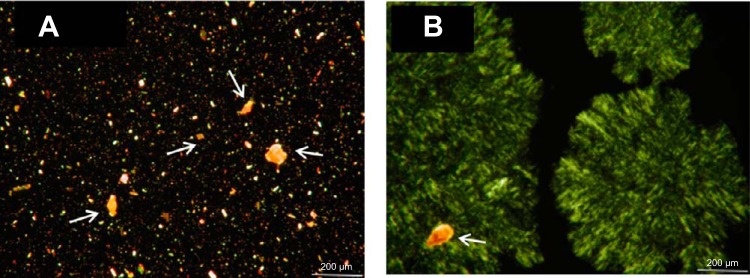
Currently, the most frequently used commercially available form of lutein is a crystalline lutein-zeaxanthin mixture suspended in a triglyceride-based oil. Our current study has agreed with other, previous studies showing that lutein in its crystalline form is poorly absorbed even in the presence of triglyceride oils containing long-chain fatty acids. Our next step was to identify a vehicle that would have properties to increase lutein absorption when equal amounts of lutein were delivered to the stomach. Preclinical testing to explore ways to increase lutein bioavailability led to the finding that if the solubility of lutein could be improved, its absorption might be increased as well. Combining lutein with MDG oil did improve its solubility, as evident by the microscopic images in Fig. 7, A and B, showing many lutein crystals present in Fig. 7A (lutein in SO) and using MDG 3 as an example, that many of the lutein crystals were absent, indicating that the MDG oil served as a better solubilization medium by reducing the amount of lutein in its crystalline structure.
Fig. 7.
Microscopic imaging (200 µM) comparing lutein’s crystal form when suspended in triglyceride oil (lutein in SO; A) vs. using MDG 3 (B) as an example. Many of the lutein crystals were absent, indicating that the MDG oil served as a solubilization medium by reducing the amount of lutein in its crystalline structure. Arrows shown in these images represent various lutein crystals of different sizes and shapes. Each oil suspension sample (~10 μl) was placed onto a microscope glass slide overlaid with a glass coverslip and examined using an Olympus BH-2 research microscope (Olympus Japan). This microscope was equipped with a halogen light source and a universal condenser. Microscope pictures were obtained using polarized light with an AmScope (MU1403) digital video camera system (AmScope), which was coupled with Adobe Photoshop software, v. 2015.0.1 (Adobe Systems).
Gastric infusion of lutein (20 mg/kg) suspended in MDG oil containing the higher amounts of MDGs and lower residual triglycerides significantly improved (70–211%, P < 0.05) lymphatic lutein output 2–6 h after lipid feeding vs. lutein in SO. This suggests that the level of residual triglyceride in the MDG mixture may directly affect lymphatic lutein absorption. Zeaxanthin absorption showed similar benefits within the MDG oil matrix. In addition to lutein solubility improvements, preliminary in vivo research has shown the ability of MDGs to improve micellar solubilization of lutein and zeaxanthin before being transferred to and absorbed by small-bowel enterocytes. The combination of lutein and MDG also aids in the formation and secretion of triglyceride- and carotenoid-rich chylomicrons into lymph. These combined benefits of MDG explain in part the absorption increases that we have observed. Further support of this approach was evident when healthy volunteers were randomized into a double-blind crossover study investigating the plasma kinetics of lutein and zeaxanthin provided as lutein in SO or MDG in capsule form. Subjects given the lutein in MDG capsules showed significantly higher plasma lutein and zeaxanthin throughout the early and late phases of the study (21).
The use of specific combinations of MDG is a different approach from the previous known ways to increase lutein absorption. Since lutein is a lipophilic carotenoid, the amount and type of dietary fat consumed with lutein can affect its absorption from the GI tract (14, 26). Baskaran et al. and others (12, 19, 28) have shown in mouse, rat, and human Caco-2 cells that the use of glycol and phospholipids may improve lutein bioavailability, thus suggesting that lutein absorption is dependent on the nature of the lipids (e.g., polarity, fatty acid profile, amount) when consumed together. Serum lutein levels are higher following egg consumption than with a comparable amount of green vegetables, further suggesting that lutein absorption is complex and dependent on the amount ingested, the matrix in which it is contained, and the type and amount of lipid present (7, 13, 29). However, all of these studies have used a change in plasma lutein as a measure of intestinal absorption. This has its limitations for interpretation, given the role of the liver in recirculating lutein after conjugation with lipoproteins. Our approach is based on a direct measurement of intestinal absorption and before the metabolism of lutein-containing chylomicrons by the peripheral organs.
Since lutein plays a significant role in maintaining and promoting overall health, its inclusion into commercially available multivitamin and mineral supplements is becoming commonplace by manufacturers. Many polar lipid nutrients that are typically included in nutritional products are generally less bioavailable upon consumption than desired, and as such, they are generally overfortified in the nutritional product to ensure that the plasma levels required by the consumer to achieve the desired nutritional benefits can be obtained. In some cases, the overfortification can be from about two times to about ten times the amount required in plasma to achieve the desired benefits. These high fortification rates lead to increased product costs without providing additional consumer benefits. Furthermore, and as demonstrated by our current study, more lutein in the diet does not translate to more lutein for the body. Therefore, there is a need for improved nutritional formulations containing lutein/MDG to have an improved bioavailability of lutein, so as to provide the same health benefits to consumers while allowing for lower fortification rates of the nutrient.
The present study has potential limitations. We performed this research using a blend of lutein and oils directly. Verification of these absorption benefits will have to be demonstrated when this combination is incorporated into complex nutritional supplement products. An additional limitation may be the choice of using a rat to assess the digestion and absorption of lutein/zeaxanthin. There exists a debate as to what is the most appropriate animal model that closely mimics human absorption of carotenoids (20). The uses of monkeys and gerbils have been considered superior models for carotenoid absorption research when one is focused on changes in plasma and tissue incorporation. Since our goals were limited to digestion and absorption from the GI tract and did not involve lutein metabolism or distribution in tissues, we chose to use the conscious lymph fistula rat model, which accurately measures and quantifies the absorption of physiological quantities of lipophilic compounds after a test meal. By infusing the lipid test meal together with lutein into the stomach, one can monitor directly the amount of lutein secreted by the small intestinal epithelial cells as triglyceride-rich chylomicrons into lymph as well as the transport into the portal blood.
In summary, our study data provide a better understanding of how lutein is digested and absorbed when given at different dosages in its crystalline form suspended in triglyceride oil. In addition, we were able to demonstrate that the use of specific mixtures of MDG helps solubilize lutein/zeaxanthin and facilitate GI micelle formation, thus improving lymphatic output compared with triglyceride oils. The next step is to assess whether the absorptive benefits of lutein/MDG can be preserved when incorporated into complex nutritional products. These results should also be useful for designing clinical studies to assess the effectiveness of lutein in MDG oil on some of lutein’s documented physiological benefits such as eye and brain health and antioxidant properties.
GRANTS
This research was supported by a grant from the Abbott Nutrition and also from National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-103557 and DK-59630.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.T., M.V., D.M.L., and SD conceived and designed research; P.T., M.V., D.M.L., and SD analyzed data; P.T., M.V., C.-W.K., and SD interpreted results of experiments; P.T., C.-W.K., and SD drafted manuscript; P.T., M.V., and SD edited and revised manuscript; P.T., M.V., C.-W.K., and SD approved final version of manuscript; D.M.L. and SD prepared figures.
ACKNOWLEDGMENTS
We gratefully acknowledge Gary Katz and Sarah Huesman for assistance in this study and Fei Wang and Hong Tang for performing statistical analyses.
REFERENCES
- 1.Baskaran V, Sugawara T, Nagao A. Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids 38: 705–711, 2003. doi: 10.1007/s11745-003-1118-5. [DOI] [PubMed] [Google Scholar]
- 2.Bettler J, Zimmer JP, Neuringer M, DeRusso PA. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur J Nutr 49: 45–51, 2010. doi: 10.1007/s00394-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33: 1349–1352, 1948. [PubMed] [Google Scholar]
- 5.Canfield LM, Clandinin MT, Davies DP, Fernandez MC, Jackson J, Hawkes J, Goldman WJ, Pramuk K, Reyes H, Sablan B, Sonobe T, Bo X. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr 42: 133–141, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Castenmiller JJ, West CE, Linssen JPH, van het Hof KH, Voragen AGJ. The food matrix of spinach is a limiting factor in determining the bioavailability of β-carotene and to a lesser extent of lutein in humans. J Nutr 129: 349–355, 1999. doi: 10.1093/jn/129.2.349. [DOI] [PubMed] [Google Scholar]
- 7.Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr 134: 1887–1893, 2004. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- 8.Craft NE. Chromatographic Techniques for Carotenoid Separation in Current Protocols in Food Analytical Chemistry. NewYork: Wiley, 2001. [Google Scholar]
- 9.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging 8: 156–162, 2004. [PubMed] [Google Scholar]
- 10.Ee LC, Zheng S, Yao L, Tso P. Lymphatic absorption of fatty acids and cholesterol in the neonatal rat. Am J Physiol Gastrointest Liver Physiol 279: G325–G331, 2000. 10.1152/ajpgi.2000.279.2.G325. PubMed [DOI] [PubMed] [Google Scholar]
- 11.Gitto E, Reiter RJ, Cordaro SP, La Rosa M, Chiurazzi P, Trimarchi G, Gitto P, Calabrò MP, Barberi I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am J Perinatol 21: 209–216, 2004. doi: 10.1055/s-2004-828610. [DOI] [PubMed] [Google Scholar]
- 12.Gorusupudi A, Vallikannan B. Glycolipids improve lutein bioavailability and accumulation in eyes in mice. Eur J Lipid Sci Technol 114: 710–717, 2012. doi: 10.1002/ejlt.201100183. [DOI] [Google Scholar]
- 13.Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, Blumberg JB. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am J Clin Nutr 70: 247–251, 1999. doi: 10.1093/ajcn.70.2.247. [DOI] [PubMed] [Google Scholar]
- 14.Hedrén E, Diaz V, Svanberg U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur J Clin Nutr 56: 425–430, 2002. doi: 10.1038/sj.ejcn.1601329. [DOI] [PubMed] [Google Scholar]
- 15.Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J Perinatol 21: 356–362, 2001. doi: 10.1038/sj.jp.7210548. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J, Nelson PT, Chung HY, Schalch W, Wittwer J, Poon LW. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res 13: 951786, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohan AB, Howles PN, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr Protoc Mouse Biol 2: 219–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 62: 604–610, 1995. doi: 10.1093/ajcn/62.3.604. [DOI] [PubMed] [Google Scholar]
- 19.Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V. Enhanced lutein bioavailability by lyso-phosphatidylcholine in rats. Mol Cell Biochem 281: 103–110, 2006. doi: 10.1007/s11010-006-1337-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW Jr. Review of animal models in carotenoid research. J Nutr 129: 2271–2277, 1999. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 21.Marriage BJ, Williams JA, Choe YS, Maki KC, Vurma M, DeMichele SJ. Mono- and diglycerides improve lutein absorption in healthy adults: a randomised, double-blind, cross-over, single-dose study. Br J Nutr 118: 813–821, 2017. doi: 10.1017/S0007114517002963. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa JJ, Ramirez-Tortosa MC, Quiles JL, Palomino N, Robles R, Mataix J, Huertas JR. Oxidative stress in erythrocytes from premature and full-term infants during their first 72 h of life. Free Radic Res 37: 317–322, 2003. doi: 10.1080/1071576021000050438. [DOI] [PubMed] [Google Scholar]
- 23.Ostrea EM Jr, Balun JE, Winkler R, Porter T. Influence of breast-feeding on the restoration of the low serum concentration of vitamin E and β-carotene in the newborn infant. Am J Obstet Gynecol 154: 1014–1017, 1986. doi: 10.1016/0002-9378(86)90740-4. [DOI] [PubMed] [Google Scholar]
- 24.Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr 129: 2170–2176, 1999. doi: 10.1093/jn/129.12.2170. [DOI] [PubMed] [Google Scholar]
- 25.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49, 2003. doi: 10.1016/S1084-2756(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 26.Schweigert FJ, Bok V. Vitamin A in blood plasma and urine of dogs is affected by the dietary level of vitamin A. Int J Vitam Nutr Res 70: 84–91, 2000. doi: 10.1024/0300-9831.70.3.84. [DOI] [PubMed] [Google Scholar]
- 27.Sommerburg O, Meissner K, Nelle M, Lenhartz H, Leichsenring M. Carotenoid supply in breast-fed and formula-fed neonates. Eur J Pediatr 159: 86–90, 2000. doi: 10.1007/PL00013811. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr 131: 2921–2927, 2001. doi: 10.1093/jn/131.11.2921. [DOI] [PubMed] [Google Scholar]
- 29.Surai PF, MacPherson A, Speake BK, Sparks NH. Designer egg evaluation in a controlled trial. Eur J Clin Nutr 54: 298–305, 2000. doi: 10.1038/sj.ejcn.1600939. [DOI] [PubMed] [Google Scholar]
- 30.Tso P, Lee T, DeMichele SJ. Lymphatic absorption of randomized structured triglycerides versus physical mix in a rat model of fat malabsorption. Am J Physiol Gastrointest Liver Physiol 277: G333–G340, 1999. [DOI] [PubMed] [Google Scholar]
- 31.van het Hof KH, Tijburg LB, Pietrzik K, Weststrate JA. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br J Nutr 82: 203–212, 1999. [PubMed] [Google Scholar]
- 32.Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci 16: 21–29, 2013. doi: 10.1179/1476830512Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 59: 659–665, 2014. doi: 10.1097/MPG.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 34.Vurma M, DeMichele S, Lee D, Wang F, Tso P. In vivo assessment of lipophilic nutrients absorption mechanisms using human milk and infant formula with novel mono- and diglycerides based emulsification technologies. J Pediatr Gastroenterol Nutr 62: 205, 2016. [Google Scholar]
- 35.Woollett LA, Wang Y, Buckley DD, Yao L, Chin S, Granholm N, Jones PJ, Setchell KD, Tso P, Heubi JE. Micellar solubilisation of cholesterol is essential for absorption in humans. Gut 55: 197–204, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr 64: 594–602, 1996. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- 37.Yeum KJ, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr 17: 442–447, 1998. doi: 10.1080/07315724.1998.10718791. [DOI] [PubMed] [Google Scholar]