Abstract
In the event of an injury, normal tissues exit quiescent homeostasis and rapidly engage a complex stromal and immune program. These tissue repair responses are hijacked and become dysregulated in carcinogenesis to form a growth-supportive tumor microenvironment. In pancreatic ductal adenocarcinoma (PDA), which remains one of the deadliest major cancers, the microenvironment is a key driver of tumor maintenance that impedes many avenues of therapy. In this review, we outline recent efforts made to uncover the microenvironmental cross-talk mechanisms that support pancreatic cancer cells, and we detail the strategies that have been undertaken to help overcome these barriers.
INTRODUCTION
In many organs, the epithelial, mesenchymal, and resident immune compartments exist in a relatively quiescent state. However, in response to damage, the individual compartments respond rapidly to activate the appropriate measures needed to restore tissue homeostasis. Each of these cellular compartments within the tissue is tasked with its own specialized role in this response. The immune cells first activate a proinflammatory response to clear foreign material, dead cells, and debris and then transition into an anti-inflammatory, growth-promoting state. The mesenchymal cells, such as resident fibroblasts, become activated to produce extracellular matrix proteins needed to provide structural support for the wound healing process. Finally, the normally quiescent epithelial cells enter a state of proliferation to replace the lost cell population. Importantly, all of these responses are transient, and quiescent homeostasis is restored once the injury subsides and the tissue has been repaired. In contrast, the failure of the immune, mesenchymal, and epithelial compartments to revert back to quiescence/homeostasis after activation can lead to irreparable tissue damage (19).
The pancreas is a dual-function organ that plays important roles in both blood glucose homeostasis and digestive enzyme production. The exocrine pancreas consists primarily of digestive enzyme-producing acinar cells and a network of ductal cells that transport these enzymes to the duodenum. Injury to the pancreas most commonly occurs in the exocrine pancreas in the form of pancreatitis. In pancreatitis, an acute injury such as gall stones or heavy alcohol abuse leads to tissue damage in the form of acinar cell necrosis (58, 60). The presence of this necrotic cell debris drives the recruitment of immune cells to first clear cellular debris and potential microbial infiltration, followed by the production of growth factors for repair. These immune cells also activate fibroblasts, which support tissue repair (37).
Stromal and immune responses also directly and indirectly promote the epithelial repair process. In addition to acinar cells, the exocrine pancreas also consists of ductal cells, which transport the enzymes to the digestive tract. As pancreatitis primarily results in the destruction of the acinar compartment, tissue repair takes place largely through the acinar-to-ductal metaplasia process (ADM), which functions to replace the lost epithelial cells (13). Again, in most instances, this wound healing process subsides after the tissue repair has occurred. However, in severe cases or after repeated bouts of acute injury, this process develops into chronic pancreatitis, where the cycle becomes a self-sustaining fibroinflammatory response (Fig. 1).
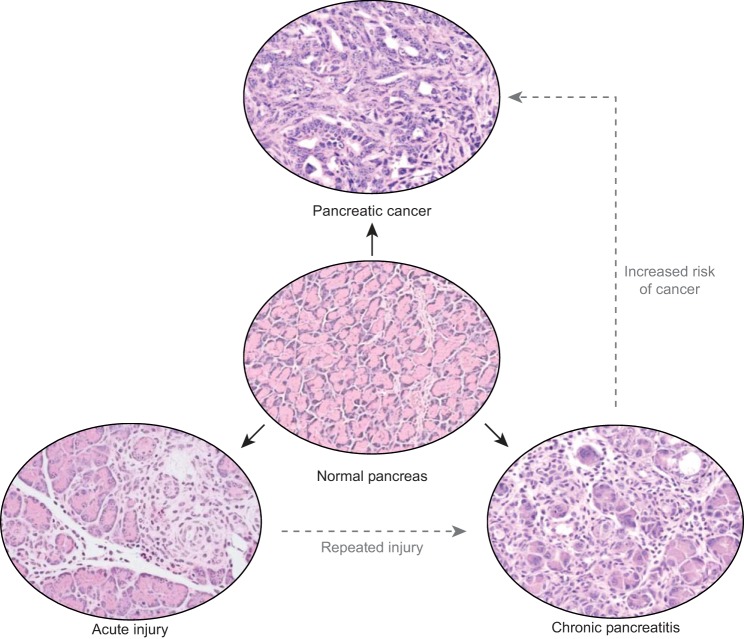
Fig. 1.
Pathophysiology of pancreatic disease. Normal exocrine pancreas tissue (H&E, middle) consists primarily of acinar cells with very few histologically apparent immune cells or fibroblasts. Acute injury leads to localized disruption of tissue architecture (H&E, bottom left), driving infiltration of immune cells and fibroblast activation. A more severe injury, or repeated bouts of acute injury, leads to tissue-wide disruption of organ homeostasis (H&E, bottom right), which drives a robust fibroinflammatory response. Oncogenic Kras mutations coupled with the loss of tumor suppressors, which can be promoted in the context of chronic inflammation, leads to the development of invasive carcinoma with an immune-rich desmoplastic response (H&E, top).
Importantly, recent studies have demonstrated that pancreatitis is driven in large part through heterocellular communication among the epithelial, stromal, and immune cell compartments. The recruited immune cells promote ADM and activate stromal fibroblasts (31, 59), driving proinflammatory cytokine production and propagating a feed-forward loop. Depletion of immune populations, such as macrophages, can prevent the initiation of ADM and establishment of pancreatitis, demonstrating the importance of immune-epithelial cross talk in this process (31). Furthermore, inhibition of signaling pathways in the epithelial cells can promote the restoration of tissue homeostasis, including a loss of inflammation and fibrosis, again demonstrating a central role for cross-compartmental communication in the maintenance of this disease (21).
Reminiscent of the microenvironment seen in chronic inflammatory diseases, tumors have long been described as wounds that do not heal (14). Given these similarities, it is not surprising that chronic pancreatitis is a leading risk factor for pancreatic ductal adenocarcinoma (PDA), although the precise link between pancreatitis and tumorigenesis has yet to be completely understood. PDA, the most common type of pancreatic cancer, is driven primarily through activating mutations in the Kras oncogene and is characterized by a unique tumor microenvironment. In this microenvironment, the stromal and immune compartments exist in a constant state of activation (1, 2) and communicate with each other and the proliferating cancer cells. These interactions are important mediators of the inflammatory and fibrotic tumor microenvironment, a hallmark of PDA (Fig. 1).
In this minireview, we provide a brief overview of several cross-talk relationships that support the growth of cancer cells, inhibit chemotherapy, and suppress responses to immunotherapy in PDA. The relationships described cover interactions between and among many different cellular compartments, with mechanisms that include both signaling and metabolic pathways.
STROMAL ACTIVATION REMODELS THE TUMOR MICROENVIRONMENT
Among the best described heterocellular cross-talk relationships in PDA is that between the epithelial cancer cells and stromal fibroblasts (Fig. 2). And this connection is especially prevalent, as the stromal fibroblasts in PDA typically outnumber the cancer cells in terms of the overall cellularity within the tumor. This heterogeneous population of fibroblasts is collectively referred to as cancer-associated fibroblast (40). Multiple mechanisms can stimulate the activation of fibroblasts in normal physiology, such as the hormone cholecystokinin (4) and signaling molecules including Hedgehog (HH) ligands. For example, the mammalian Hedgehog ligand Sonic Hedgehog (SHH), which is not expressed in the normal pancreas, is secreted by pancreatic cancer cells and prominently activates signaling in surrounding fibroblasts (63). This paracrine signaling loop occurs as an early event in tumorigenesis and promotes the progression of PDA (43, 56). The activated fibroblasts then deposit extensive extracellular matrix proteins into the tumor microenvironment, creating a densely fibrotic desmoplastic response. Among these highly enriched molecules is hyaluronic acid (HA), which has been identified as a potential therapeutic target (44). The deposition of HA leads to retention of fluid and results in an extremely high interstitial pressure within PDA tumors, often exceeding 10 times the pressure found in the normal pancreas (44).
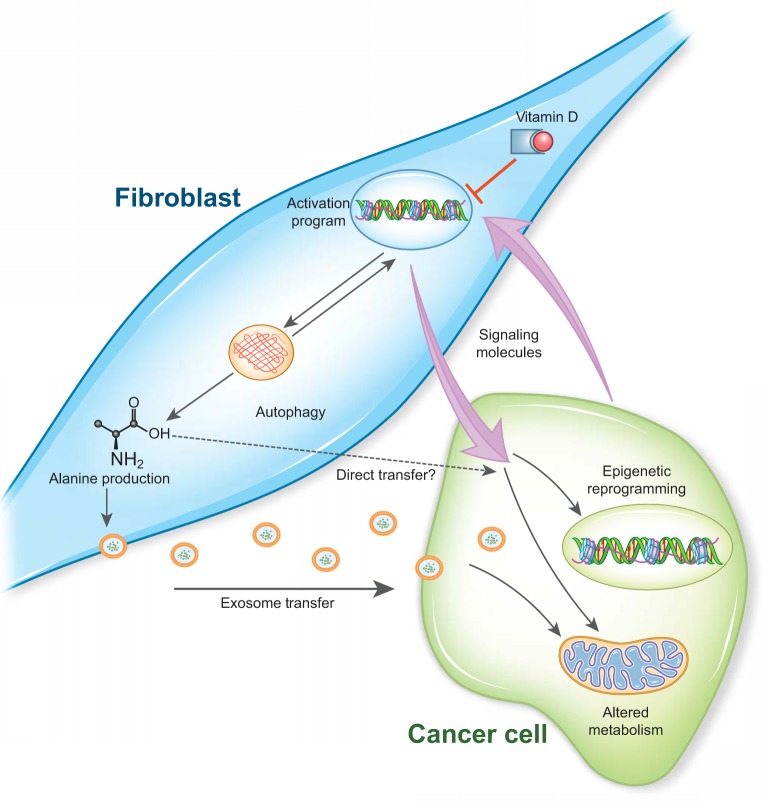
Fig. 2.
Reciprocal epithelial-mesenchymal cross talk in pancreatic ductal adenocarcinoma (PDA). Fibroblasts are activated by signaling molecules provided by PDA cells, and this is coupled to autophagy. This activation program can be suppressed by vitamin D receptor signaling. The fibroblasts in turn provide signaling molecules and metabolites that support the growth of the PDA cells. Fibroblast-derived support occurs through mechanisms that alter both epigenetic and metabolic programming of PDA cells.
Blood vessels collapse under the high pressure, which leads to hypoperfusion of the tumor with several important consequences. First, the lack of functional blood vessels limits nutrient availability to the cancer cells (27), forcing PDA cells to adopt metabolic programs to scavenge and recycle nutrients, such as macropinocytosis and autophagy (11, 26, 48, 61, 62). Second, the limited oxygen perfusion into the tumor imposes oxidative stress on the cancer cells that is compensated for by the upregulation of the Nrf2 reactive oxygen species detoxification program (9, 12). Finally, the impaired access to the bloodstream limits the delivery and efficacy of systemic chemotherapy agents, which constitute the standard of care treatments in PDA (41).
Direct targeting of pancreatic cancer fibroblasts has been challenging, in part due to our incomplete understanding of this cell population. As a key signaling pathway mediating the cross talk between tumor cells and fibroblasts, Hedgehog signaling has been targeted using clinically approved inhibitors of the key HH signaling component Smoothened (Smo). Initial preclinical studies showed a promising increase in survival in a genetically engineered mouse muse model of PDA treated with the HH inhibitor IPI-926 (41). Unfortunately, clinical trials that were started thereafter showed no benefit, but rather worsening of disease outcome in human patients. In parallel, clinical trials of a different Smo inhibitor, GDC-0449, showed no benefit (28). Interestingly, the latter trial included analysis of biopsies prior to and post-GDC-0449 treatment, and the posttreatment biopsies showed moderate to no changes in stromal quantity (28). The clinical studies were then re-created in experimental models, where ablation of SHH ligand in genetically engineered mouse models of PDA mice resulted in shorter survival and, depending on the model, a decrease in stroma (29, 47). Furthermore, a recent study confirmed that partial inhibition of HH signaling in pancreatic fibroblasts promotes tumor growth through the induction of angiogenesis (35). These results suggest that a treatment strategy combining stromal targeting (via HH inhibition) with approaches targeting angiogenesis (such as VEGF inhibition) may hold promise (47). In light of these collective studies, it is evident that a deeper understanding of the biological effect of HH signaling in fibroblasts is needed.
Like the HH studies above, genetic depletion of pancreatic fibroblasts also leads to a decrease in survival (42). In contrast, other approaches to targeting the stroma have been promising. For instance, stromal depletion via CAR T cells had an antitumor effect (32). The differences between these observations may lie in the efficiency of genetic stromal depletion vs. those that are immune mediated. Taken together, these studies may help explain in part the failure of combination gemcitabine- and HH-targeted therapies in clinical trials with PDA patients (7, 28). The results of these studies also started an active discussion in the pancreatic oncology community concerning the utility of targeting the stroma in PDA (6, 17).
Despite the early failures targeting stromal fibroblasts, additional new strategies are emerging, which may overcome some of the limitations discovered along the way. For example, fibroblasts in PDA express the vitamin D nuclear hormone receptor (VDR), which acts as a transcription factor upon activation (Fig. 2). Sherman et al., (49) demonstrated that VDR binding of calcitriol, the active form of vitamin D, initiates a transcriptional program that reprograms activated fibroblasts to a quiescent state. This inactivation of the fibroblasts within the tumor microenvironment is capable of sensitizing the cancer cells to chemotherapy in mouse models of pancreatic cancer, improving their overall survival. The results from this study demonstrate proof of principle that stromal reprogramming can increase treatment efficacy, a more nuanced approach than stromal ablation.
Finally, instead of targeting stromal signaling networks, new strategies have been developed that target the tissue remodeling and fibrosis that occur as a consequence of fibroblast activation. As an example, using preclinical autochthonous models of PDA, degradation of HA within the tumor extracellular matrix using a stabilized form of the HA degradative enzyme Hyaluronidase (HAase) leads to a decrease in the interstitial pressure, the restoration of tumor vasculature, and enhanced sensitivity to chemotherapy (25, 44). This concept has been translated to the clinic, with a recent phase 2 clinical trial reporting clinical benefit in patients treated with PEGylated recombinant HAase and gemcitabine (22, 23). Importantly, patient responses to HAase treatment can be correlated to the levels of HA present in the patient samples before treatment. HA “high” patients achieved a better progression-free and overall survival with the gemcitabine plus HAase treatment than HA “low” patients, who showed no additional benefit over gemcitabine alone. Overall, the results from this trial demonstrate that targeting of the extracellular matrix created by the activated stromal cells does indeed have the potential to improve our currently available strategies to treat PDA patients.
STROMAL CELLS SUPPORT CANCER CELL METABOLISM AND TUMOR GROWTH THROUGH METABOLIC CROSS TALK
In addition to the physical remodeling of the tumor microenvironment, there are also direct mechanisms by which the stromal cells support the proliferation of PDA cells (Fig. 2). This can occur though cell signaling cross-talk mechanisms. For example, the mesenchymal stem cell subpopulation within cancer-associated fibroblasts derived from patient tumors was reported to drive the growth and metastasis of PDA cells through a signaling axis involving the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) (57). It has further been demonstrated that stromal-derived signals can act to reprogram both the metabolism and epigenetic landscape of PDA cells (50, 55).
The stromal cells can also support cancer cells by directly fueling their metabolism. In this regard, we found that activated pancreatic stellate cells (PSCs), a type of fibroblast found in pancreatic tumors, enhance the oxidative metabolism of PDA cells through metabolite exchange (Fig. 2) (53). Using a metabolomics-based screening approach, we found that activated PSCs abundantly released the amino acid alanine and that this was a potent biosynthetic and bioenergetic substrate in nutrient-restricted PDA cells (53). Mechanistically, utilizing stable isotope tracing techniques, we demonstrated that exogenous alanine competes with glucose carbon within the cancer cells to fuel mitochondrial metabolism and thereby liberates the limited available glucose for other anabolic processes, including the serine biosynthetic pathway that makes amino acids, nucleotides, and glutathione.
We next sought to identify the source of alanine in the PSCs and hypothesized that this may be stored in protein and released through protein catabolism. In agreement with this, we found that PSCs in culture maintain a basal level of autophagy and that secreted factors from the cancer cells increased the levels of autophagy in the fibroblasts (53). And, most importantly, we found that the autophagy within the PSCs was required for the release of alanine. Genetic knockdown of the autophagy components Atg5 or Atg7 abrogated the ability of media from PSCs to enhance the oxidative metabolism of the PDA cells, which could be rescued with the addition of exogenous alanine (53). Functionally, whereas coinjection of PSCs with PDA cells into mice dramatically enhanced the rate of tumor growth, the ability of autophagy-deficient PSCs to promote tumor growth was significantly blunted. Collectively, our results defined a new mechanism of metabolic cross talk between stromal and malignant cells that facilitates the growth of PDA tumors (Fig. 2).
An open question from our study concerned how alanine was exchanged between the stromal fibroblasts and cancer cells. One such mechanism to exchange cellular contents is through membrane vesicle trafficking (51). Exosomes, defined as membrane vesicles between 30 and 100 nm, have been the subject of many recent studies in cell-cell communication. In pancreatic cancer, Zhao et al. (68) demonstrated that metabolite transfer can be mediated through the production and secretion of exosomes by cancer-associated fibroblasts. In the transferred exosomes, the authors found numerous metabolites that functionally reprogrammed cancer cells upon exposure, including alanine.
In line with our data demonstrating the relationship between autophagy in fibroblasts and their ability to support PDA (53), autophagy was recently reported to play an integral role in the biology of PSCs. Endo et al. (15) found that the inhibition of autophagy in PSCs results in the accumulation of markers of quiescence, whereas the induction of autophagy was sufficient to drive the activation of PSCs. The authors also noted that PSCs enhanced the growth of PDA xenografts, which was reversed following genetic or pharmacological inhibition of autophagy. Taken together, these data suggest that autophagy is required to maintain the activation state of PSCs.
In the nutrient-austere PDA tumor microenvironment, cancer cells need to scavenge nutrients to ensure their survival (20). Thus, future efforts to target these and other pathways of nutrient acquisition, using agents such as clinically available autophagy inhibitors, have the potential to starve the cancer cells to death through direct and indirect mechanisms.
INTRATUMORAL CROSS TALK DRIVES IMMUNOSUPPRESSION IN PDA
In addition to stromal fibroblasts, the tumor microenvironment in PDA is also richly infiltrated by immune cells. The immune compartment is increasingly being recognized as playing a key role in PDA. Several types of immune cells have been demonstrated to be critical in driving the initiation and progression of PDA (18, 30, 36, 45, 66, 69), and they have also been found to serve as a barrier to chemotherapy (38). Moreover, immunotherapy has proven largely ineffective in PDA (5).
Immunosuppressive programming is multifactorial in PDA tumors (Fig. 3). For example, PDA cells secrete the cytokine GM-CSF, which acts to drive the recruitment of immature myeloid-derived suppressor cells (MDSCs) to the tumor (3, 46, 54). These act to inhibit the recruitment and activation of cytotoxic T cells. Furthermore, oncogenic Kras signaling in cancer cells, the signature transforming pathway in this disease, has non-cell autonomous effects, which include altering the polarization of myeloid cell populations within the tumor microenvironment. This occurs through an EGFR/MAPK signaling axis that turns normal myeloid cells involved in tissue repair into drivers of carcinogenesis (67). Accordingly, inhibition of MDSC recruitment by GM-CSF blockade, depletion of myeloid cells through genetic means, or treatment with a pharmacological inhibitor of CSFR1 to block macrophage polarization/recruitment have all been demonstrated to relieve immune suppression. Furthermore, these methods enable the infiltration of cytotoxic T cells into the tumor (65, 70), which facilitate tumor regression.
Fig. 3.
Immune suppression in PDA is driven by intertumoral cross talk. The pancreatic tumor microenvironment is strongly immunosuppressive. Immune suppression is mediated through heterocellular signaling and metabolic cross-talk mechanisms that inhibit the infiltration and activation of cytotoxic T cells. Cancer-associated fibroblasts release CXCL12, which coats the surface of cancers cells and enables immune evasion. The fibroblasts also secrete IL-6, which drives the alternative polarization of macrophages to an immunosuppressive state. This can be mediated by the local depletion of arginine, which acts to inhibit activation of T cells, and conversion of tryptophan to kynurenine, which drives the production of regulatory T cells. Tumor-associated macrophages also secrete EGFR ligands, driving increased MAPK signaling in the PDA cells and promoting several mechanisms of immune suppression. Notably, MAPK signaling in PDA cells depletes the glucose required for cytotoxic T cell function and leads to lactate accumulation, which polarizes immunosuppressive macrophages. The increased MAPK signaling also enhances the expression of the checkpoint ligand PD-L1 by the cancer cells, which allows to them to directly inactivate cytotoxic T cells. Finally, MAPK activation by oncogenic Kras drives the secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF), leading to the accumulation of myeloid-derived suppressor cells (MDSCs) into the tumor microenvironment.
In addition to the signals from the epithelium, which attract immunosuppressive cells, it has also been demonstrated that the stromal compartment participates in the inhibition of the antitumor immune system. Cancer-associated fibroblasts marked by fibroblast activation protein (FAP) secrete chemokine (C-X-C motif) ligand 12 (CXCL12), which accumulates on the cancer cell surface and repels T cell infiltration (16). Tumor-associated mesenchymal stem cells also drive the alterative polarization of macrophages to an immunosuppressive phenotype through the secretion of cytokines including IL-6. This mesenchymal-to-immune cell cross talk also functions to inhibit the trafficking of T cells within the tumor (34).
This accumulation of alternatively polarized macrophages and other myeloid populations can also prime the cancer cells to directly resist elimination by cytotoxic T cells. We found that myeloid cells polarized by PDA cells reciprocally activate EGFR-MAPK signaling within the cancer cells, which induces the expression of checkpoint ligand PD-L1 (65). Inhibition of MAPK signaling by administration of a MEK inhibitor or ablation of the myeloid cells dramatically reduced PD-L1 expression on the cancer cells within the tumor microenvironment. Decreased PD-L1 expression was sufficient to allow influx of cytotoxic T cells into the tumor, leading to decreased tumor growth. However, inhibition of epithelial PD-L1 expression alone is unable to eliminate T cell exhaustion, as PD-L1 is also produced by other cells in the tumor. Accordingly, the treatment of PDA tumors with a MEK inhibitor to decrease epithelial PD-L1 expression, combined with coadministration of an anti-PD-1 immunotherapy regimen, resulted in a dramatic increase in T cell-mediated tumor regression. Interestingly, it has also been demonstrated that inhibition of CCK signaling through the use of small molecule antagonists led to a decreased accumulation of regulatory T cells in PDA tumors and enhanced the response of tumor-bearing mice the checkpoint inhibitor therapy (52).
In addition to immunosuppression driven through signaling pathways, metabolic cross-talk mechanisms within the tumor microenvironment have been reported to impair antitumor immunity in other cancers and may also be important in PDA (33). For example, glucose depletion blocks the antitumor T cell response in melanoma and sarcoma models (8, 24), and high levels of lactate are sufficient to drive the alternative polarization of macrophages (10). We (64) have previously demonstrated that oncogenic Kras signaling in PDA cells rewires the cancer cells to a maintain a highly glycolytic state. Thus, Kras signaling drives a glucose metabolic program that results in avid glucose consumption and lactate excretion into the tumor microenvironment, the buildup of which is exacerbated by a lack of vasculature to clear these metabolic byproducts. Based on these observations, it is reasonable to hypothesize that low intratumoral glucose levels and high lactate levels may block antitumor T cell responses while promoting the alternative polarization of macrophages, respectively. Furthermore, alternatively polarized macrophages are also known to deplete intratumoral arginine and tryptophan (39). Arginine depletion blocks the effector T cell response through metabolic competition, whereas tryptophan metabolism leads to the buildup of the tryptophan catabolite kynurenine, which promotes the formation of immune inhibitory regulatory T cells. Alternatively polarized macrophages are highly abundant in PDA tumors (65, 67), and the contribution of each of these mechanisms to the immune-inhibitory response remains to be determined.
Taken together, the current body of literature describes multiple avenues by which cell-cell signaling contributes to the overall immunosuppressive nature of the PDA tumor microenvironment and suggests additional nodes by which metabolic processes may be involved. Importantly, armed with a more detailed understanding of these pathways, many investigators are now exploring how immune suppression can be relieved to allow for successful use of immunotherapy in PDA patients in both preclinical and clinical studies.
CONCLUDING REMARKS
In contrast to many other major cancers, there have been few success stories in improving therapy for PDA patients. As a near-universally Kras-driven cancer, no durable targeted therapy options have emerged, and PDA has proved largely refractory to clinically available immunotherapeutic approaches. Accumulating evidence now suggests that much of the difficulty treating PDA is related to the unique challenges posed by the complex pancreatic tumor microenvironment. Accordingly, attempts have been made to deplete or eliminate several aspects of the tumor microenvironment, particularly the fibrotic stromal compartment or suppressive immune cells. However, alternative strategies are emerging that seek to reprogram, rather than eliminate, different components of the tumor microenvironment to enhance chemotherapy or to sensitize the tumor to immunotherapy. The continued evolution of these strategies could present a much-needed breakthrough for clinical treatment of pancreatic cancer patients.
GRANTS
C. J. Halbrook was supported by a grant from the Michigan Institute for Clinical and Health Research Postdoctoral Translational Scholars Program award (UL1TR000433) and a National Cancer Institute (NCI) Cancer Biology Training Grant (T32CA009676). M. Pasca di Magliano was supported by grants from the NCI (R01 CA-151588, R01 CA-198074, U01 CA-224145). C. A. Lyssiotis was supported by a Pancreatic Cancer Action Network/American Association for Cancer Research (AACR) Pathway to Leadership award (13-70-25-LYSS); Junior Scholar Award from The V Foundation for Cancer Research (V2016-009); Kimmel Scholar Award from the Sidney Kimmel Foundation for Cancer Research (SKF-16-005); and a 2017 AACR NextGen Grant for Transformative Cancer Research (17-20-01-LYSS). M. Pasca di Magliano and C. A. Lyssiotis were supported by the Cancer Center Core Grant P30 CA-46592.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.H., M.P.d.M., and C.A.L. conceived and designed research; C.J.H., M.P.d.M., and C.A.L. prepared figures; C.J.H., M.P.d.M., and C.A.L. drafted manuscript; C.J.H., M.P.d.M., and C.A.L. edited and revised manuscript; C.J.H., M.P.d.M., and C.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hanna Hong for feedback.
REFERENCES
- 1.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29: 179–187, 2004. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144: 1210–1219, 2013. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21: 822–835, 2012. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berna MJ, Seiz O, Nast JF, Benten D, Bläker M, Koch J, Lohse AW, Pace A. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem 285: 38905–38914, 2010. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465, 2012. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr RM, Fernandez-Zapico ME. Pancreatic cancer microenvironment, to target or not to target? EMBO Mol Med 8: 80–82, 2016. doi: 10.15252/emmm.201505948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catenacci DVT, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 33: 4284–4292, 2015. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162: 1229–1241, 2015. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chio IIC, Jafarnejad SM, Ponz-Sarvise M, Park Y, Rivera K, Palm W, Wilson J, Sangar V, Hao Y, Öhlund D, Wright K, Filippini D, Lee EJ, Da Silva B, Schoepfer C, Wilkinson JE, Buscaglia JM, DeNicola GM, Tiriac H, Hammell M, Crawford HC, Schmidt EE, Thompson CB, Pappin DJ, Sonenberg N, Tuveson DA. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell 166: 963–976, 2016. doi: 10.1016/j.cell.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513: 559–563, 2014. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497: 633–637, 2013. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475: 106–109, 2011. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117: 971–977, 2007. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1986. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 15.Endo S, Nakata K, Ohuchida K, Takesue S, Nakayama H, Abe T, Koikawa K, Okumura T, Sada M, Horioka K, Zheng B, Mizuuchi Y, Iwamoto C, Murata M, Moriyama T, Miyasaka Y, Ohtsuka T, Mizumoto K, Oda Y, Hashizume M, Nakamura M. Autophagy is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology 152: 1492–1506, 2017. doi: 10.1053/j.gastro.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 110: 20212–20217, 2013. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer Cell 25: 711–712, 2014. doi: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov 6: 270–285, 2016. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 453: 314–321, 2008. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 20.Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 31: 5–19, 2017. doi: 10.1016/j.ccell.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Halbrook CJ, Wen HJ, Ruggeri JM, Takeuchi KK, Zhang Y, Pasca di Magliano M, Crawford HC. Mitogen-activated protein kinase kinase activity maintains acinar-to-ductal metaplasia and is required for organ regeneration in pancreatitis. Cell Mol Gastroenterol Hepatol 3: 99–118, 2017. doi: 10.1016/j.jcmgh.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, Raghunand N, Dychter S, Jiang P, Shepard HM, Devoe CE. Phase Ib Study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 22: 2848–2854, 2016. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: randomized Phase II study of PEGPH20 plus Nab-paclitaxel/gemcitabine versus Nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol 36: 359–366, 2018. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 24.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, Kleinstein SH, Abel ED, Insogna KL, Feske S, Locasale JW, Bosenberg MW, Rathmell JC, Kaech SM. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor t cell responses. Cell 162: 1217–1228, 2015. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62: 112–120, 2013. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci USA 110: 8882–8887, 2013. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 75: 544–553, 2015. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, Zalupski MM, Simeone DM. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res 20: 5937–5945, 2014. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, Nagle JM, Deshpande V, Boucher Y, Kato T, Chen JK, Willmann JK, Bardeesy N, Beachy PA. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA 111: E3091–E3100, 2014. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC. Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov 6: 256–269, 2016. doi: 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liou GY, Döppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs. J Cell Biol 202: 563–577, 2013. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C, Durham AC, Buza EL, Vonderheide RH, June CH, Albelda SM, Puré E. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res 75: 2800–2810, 2015. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol 27: 863–875, 2017. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, Vinta A, Buckanovich RJ, di Magliano MP. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia 18: 142–151, 2016. doi: 10.1016/j.neo.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew E, Zhang Y, Holtz AM, Kane KT, Song JY, Allen BL, Pasca di Magliano M. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by hedgehog signaling. Cell Reports 9: 484–494, 2014. doi: 10.1016/j.celrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, Leach SD. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25: 621–637, 2014. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 50: 535–541, 2002. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73: 1128–1141, 2013. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray PJ. Amino acid auxotrophy as a system of immunological control nodes. Nat Immunol 17: 132–139, 2015. doi: 10.1038/ni.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214: 579–596, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324: 1457–1461, 2009. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25: 719–734, 2014. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 20: 3161–3173, 2006. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21: 418–429, 2012. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Discov 6: 247–255, 2016. doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21: 836–847, 2012. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25: 735–747, 2014. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM. p53 status determines the role of autophagy in pancreatic tumour development. Nature 504: 296–300, 2013. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 49.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O’Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159: 80–93, 2014. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu S, Truitt ML, He N, Ding N, Liddle C, Atkins AR, Leblanc M, Collisson EA, Asara JM, Kimmelman AC, Downes M, Evans RM. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci USA 114: 1129–1134, 2017. doi: 10.1073/pnas.1620164114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21: 575–581, 2009. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Smith JP, Wang S, Nadella S, Jablonski SA, Weiner LM. Cholecystokinin receptor antagonist alters pancreatic cancer microenvironment and increases efficacy of immune checkpoint antibody therapy in mice. Cancer Immunol Immunother 67: 195–207, 2018. doi: 10.1007/s00262-017-2077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536: 479–483, 2016. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S. Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res 75: 2629–2640, 2015. doi: 10.1158/0008-5472.CAN-14-2921. [DOI] [PubMed] [Google Scholar]
- 55.Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G, Lauffenburger DA, Jørgensen C. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165: 910–920, 2016. doi: 10.1016/j.cell.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425: 851–856, 2003. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waghray M, Yalamanchili M, Dziubinski M, Zeinali M, Erkkinen M, Yang H, Schradle KA, Urs S, Pasca Di Magliano M, Welling TH, Palmbos PL, Abel EV, Sahai V, Nagrath S, Wang L, Simeone DM. GM-CSF mediates mesenchymal-epithelial cross-talk in pancreatic cancer. Cancer Discov 6: 886–899, 2016. doi: 10.1158/2159-8290.CD-15-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 132: 1557–1573, 2007. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 6: 7158, 2015. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144: 1252–1261, 2013. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, Von Hoff DD, Maitra A, Kimmelman AC. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov 4: 905–913, 2014. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev 25: 717–729, 2011. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature 455: 406–410, 2008. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 64.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149: 656–670, 2012. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, Flannagan K, Rhim AD, Simeone DM, Beatty GL, Pasca di Magliano M. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66: 124–136, 2017. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Yan W, Mathew E, Bednar F, Wan S, Collins MA, Evans RA, Welling TH, Vonderheide RH, di Magliano MP. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol Res 2: 423–435, 2014. doi: 10.1158/2326-6066.CIR-14-0016-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Yan W, Mathew E, Kane KT, Brannon A III, Adoumie M, Vinta A, Crawford HC, Pasca di Magliano M. Epithelial-Myeloid cell cross-talk regulates acinar cell plasticity and pancreatic remodeling in mice. eLife 6: 6, 2017. doi: 10.7554/eLife.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5: e10250, 2016. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 144: 1230–1240, 2013. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74: 5057–5069, 2014. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]