Abstract
Peripheral factors likely play a role in at least a subset of irritable bowel syndrome (IBS) patients. Few studies have investigated mucosal gene expression using an unbiased approach. Here, we performed mucosal gene profiling in a sex-balanced sample to identify relevant signaling pathways and gene networks and compare with publicly available profiling data from additional cohorts. Twenty Rome III+ IBS patients [10 IBS with constipation (IBS-C), 10 IBS with diarrhea (IBS-D), 5 men/women each), and 10 age-/sex-matched healthy controls (HCs)] underwent sigmoidoscopy with biopsy for gene microarray analysis, including differential expression, weighted gene coexpression network analysis (WGCNA), gene set enrichment analysis, and comparison with publicly available data. Expression levels of 67 genes were validated in an expanded cohort, including the above samples and 18 additional participants (6 each of IBS-C, IBS-D, HCs) using NanoString nCounter technology. There were 1,270 differentially expressed genes (FDR < 0.05) in IBS-C vs. HCs but none in IBS or IBS-D vs. HCs. WGNCA analysis identified activation of the cAMP/protein kinase A signaling pathway. Nine of 67 genes were validated by the NanoString nCounter technology (FDR < 0.05) in the expanded sample. Comparison with publicly available microarray data from the Mayo Clinic and University of Nottingham supports the reproducibility of 17 genes from the microarray analysis and three of nine genes validated by nCounter in IBS-C vs. HCs. This study supports the involvement of peripheral mechanisms in IBS-C, particularly pathways mediating neuronal signaling.
NEW & NOTEWORTHY Peripheral factors play a role in the pathophysiology of irritable bowel syndrome (IBS), which, to date, has been mostly evident in IBS with diarrhea. Here, we show that sigmoid colon mucosal gene expression profiles differentiate IBS with constipation from healthy controls. These profiling data and analysis of additional cohorts also support the concept that peripheral neuronal pathways contribute to IBS pathophysiology.
Keywords: colon, constipation, gene expression, irritable bowel syndrome
INTRODUCTION
Irritable bowel syndrome (IBS) is a prevalent and costly condition with limited therapies and poorly understood pathophysiology. Affecting around 11% of the general population in the US (84), IBS is associated with a significant healthcare and economic burden, and a lack of treatment satisfaction (25, 49). Improved understanding of the molecular basis for the dysregulated gastrointestinal function and sensation in IBS may lead to development of targeted therapies and improved quality of life.
There is clear and growing evidence that peripheral mechanisms play a role in IBS pathophysiology for at least a subset of IBS patients (24). Peripheral mechanisms manifest in a range of phenotypes, including, but not limited to, alterations in intestinal permeability (41, 132), serotonin signaling (7, 36, 38, 45, 54, 63, 122), immune milieu (2, 4, 12, 13, 20, 30, 39, 94), bile acids (21), microbiota, and neuronal sensitivity (9, 16–18, 27, 57, 95, 118, 124). It is not known whether these phenotypes result from universally deregulated molecular pathways or whether IBS represents a syndrome caused by heterogeneous pathophysiological mechanisms. In order to identify molecular pathways associated with IBS, we conducted a microarray analysis of sigmoid mucosal gene expression in a pilot study of Rome III+ IBS patients and age- and sex-matched healthy controls (HCs) who were free of active psychiatric disease, and had limited use of medications.
The National Institutes of Health recently launched an initiative to improve experimental reproducibility (37). This has notoriously been a challenge in IBS. This likely results from the combination of heterogeneity in both population and study methodology, and pathophysiology resulting from subtle changes; a large sample is needed to detect small differences when standard deviations are large. Alternatively, IBS may not be caused by a universal pathophysiological mechanism. This difficulty with reproducibility may actually be underrepresented in the literature due to publication bias for positive results, since very few studies have used unbiased approaches (e.g., microarray or RNA sequencing evaluation of mucosal gene expression). In order to identify reproducible results within our own cohort, we compared our findings with publicly available data. Our comparison data come from two microarray studies comparing well-phenotyped IBS patients with HCs. The first study, referred to as “Mayo”, evaluated gene expression in sigmoid biopsies from 36 Rome II+ IBS patients (15 IBS with constipation, IBS-C) and 25 HCs (2). The second study, “Nottingham,” evaluated rectal biopsies in 56 Rome II+ (115) IBS patients (19 IBS-C) and 25 HCs in addition to a group of postinfection IBS patients (112). The only currently published RNA sequencing study in IBS (22), as well as the comprehensive follow-up studies (19, 23) have focused on IBS-D.
Here, we report on the 1) differential expression of transcripts found by microarray, 2) comparison with results from two prior microarray studies (Mayo and Nottingham), 3) weighted gene coexpression network analysis (WGCNA) and gene set enrichment analysis (GSEA) to identify pathways and networks relevant to IBS, and 4) validation of selected genes in a larger cohort. We have also created a website (http://uclacns.org/ibs-microarray-comparison/), which displays tabular and graphic results of gene expression data from this study, Mayo, and Nottingham for any gene present on the arrays.
MATERIALS AND METHODS
Participants and Protocol
Rome III+ (80) IBS patients and HCs (men and women, aged 18–55 yr) were recruited primarily by community advertisement. Bowel habit subtypes were based on Rome III criteria. The diagnosis was confirmed by a clinician with expertise in IBS. HCs had no personal or family history of IBS or other chronic pain conditions. Additional exclusion criteria for all subjects included infectious or inflammatory disorders, active psychiatric illness over the past 6 mo assessed by structured clinical interview for the DSM-IV (MINI) (105), use of corticosteroids in the past 6 mo, use of narcotics, antidepressants, or other medications that could affect neuroendocrine function in the past 2 mo, or current tobacco or alcohol abuse. Participants were compensated. The study was approved by the UCLA Institutional Review Board, and all subjects signed a written informed consent form prior to starting the study. Overall IBS symptom, abdominal pain, and bloating severity over the prior week were each assessed with a numeric rating scale (0–20) (90). Current anxiety and depression symptoms were measured with the Hospital Anxiety and Depression (HAD) scale (133). Sigmoid biopsies (30 cm) were obtained by flexible sigmoidoscopy following tap water enemas. Specimens were flash-frozen in liquid nitrogen, and RNA was extracted with TRIzol reagent (ThermoFisher Scientific, Waltham, MA). Participants for the microarray study as well as the additional participants included in the nCounter validation were selected from the same biorepository at UCLA. The only criteria used to select participants for inclusion in both the microarray and validation cohorts was optimal matching of sex and age within each bowel habit subtype.
Microarray
Gene expression was measured using the Arraystar Human LncRNA Microarray v. 3.0 (Arraystar, Rockville, MD), which profiles over 30,500 LncRNAs and 26,100 mRNAs. RNA quantity and quality were first measured using the NanoDrop ND-1000 (ThermoFisher Scientific), and RNA integrity was assessed by standard denaturing agarose gel electrophoresis. Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technologies, Santa Clara, CA), with minor modifications. Briefly, mRNA was purified from total RNA after removal of rRNA (mRNA-ONLY Eukaryotic mRNA Isolation Kit; Epicentre, Madison, WI). Then, each sample was amplified and transcribed into fluorescent cRNA along the entire length of the transcripts without 3′ bias, utilizing a mixture of oligo(dT) and random priming method (Flash RNA Labeling Kit, Arraystar). The labeled cRNAs were purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). The concentration and specific activity of the labeled cRNAs (pmol Cy3/μg cRNA) were measured with the NanoDrop ND-1000. One microgram of each labeled cRNA was fragmented by adding 5 μl of 10× blocking agent and 1 μl of 25× fragmentation buffer, heated to 60°C for 30 min, and 25 μl of 2× GE hybridization buffer was added to dilute the labeled cRNA. Fifty microliters of hybridization solution was dispensed into the gasket slide and assembled to the LncRNA expression microarray slide. The slides were incubated for 17 h at 65°C in an Agilent hybridization oven. The hybridized arrays were washed, fixed, and scanned using the Agilent DNA Microarray Scanner (part no. G2505C). Agilent Feature Extraction software (v. 11.0.1.1) was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v. 12.0 software package (Agilent Technologies). After quantile normalization of the raw data, LncRNAs and mRNAs that had flags in present or marginal (“all targets value”) in at least 10 of 30 samples were chosen for further data analysis. Targets with mean raw signal intensity of less than 200 in both IBS or HCs were excluded. Data have been deposited in the ArrayExpress database at EMBL-EBI under accession no. E-MTAB-5811 (https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5811.
Validation of Selected Targets
RNA (100 ng) from the 30 samples analyzed by microarray and 18 additional samples (identical recruitment and protocol) were analyzed using a custom nCounter panel including 67 targets and three reference genes (GAPDH, BACT, PPIA; NanoString Technologies, Seattle, WA).
Statistical Analysis
Code used for analyses in R is provided in the online Supplement.
Sample size.
Based on the proportion of differentially expressed transcripts (DETs) in the Mayo study (2), (23/47,000) and an approximate standard deviation of 0.5, we estimated that eight per group would provide 80% power to detect DETs with a fold change (FC) of ≥2 (78).
Microarray differential expression.
DETs were determined using the Linear Models for Microarray Analysis (limma) (107) package in R v. 3.3.1 (99) following log2 transformation. An adjusted P value threshold (Benjamini-Hochberg) of 0.05 determined statistical significance.
Acquisition and analysis of external data.
External data [Mayo:E-TABM-176 (2), Nottingham:E-GEOD-36701 (112)] were retrieved using the ArrayExpress package (61), and Affymetrix probe annotations were updated [annotate package (47)]. Data were normalized using the robust multiarray average function [affy package (46)]. The limma package was used to account for technical replicates (duplicateCorrelation function) and to determine DETs (107).
Determination of microarray probe transcript specificity.
The “ReAnnotator” pipeline (5) was used to obtain accurate alignments of Arraystar probe sequences from the current study to the exome. Updated annotations of Affymetrix probes (2015) were downloaded from ThermoFisher. Among nonspecific probes in the current study, the probe with the maximum variance across samples was retained. Common results were defined as probes targeting the same transcript or gene and differentially expressed in the same direction (i.e., up- or downregulated in both) with a threshold of P < 0.05.
WGCNA.
WGCNA (67) was performed in R on targets with mean raw signal intensity over 200 using a soft-thresholding power 14 and minimum module size 30. Highly correlated modules (r > 0.75) were merged.
Functional annotation.
Gene set enrichment analysis (GSEA) (111) was performed using the Java GSEA Desktop Application (v. 2.2.4) on all annotated mRNAs (log fold change and P value were entered) with mean signal intensity > 200 in either group (n = 14,775), using default parameters against the following gene sets obtained from Molecular Signatures Database (v. 6.0) (89): Reactome (43), KEGG (60), and Gene Ontology (GO) Biological Process (BP) (6). Overrepresented GO terms for Mayo/Nottingham comparison and WGCNA were determined using the hypergeometric function in the GOstats (44) package in R. These overrepresented terms as well as those yielded from analysis in DAVID (55, 56) were used to select descriptive module names. Pathway analysis was performed using Ingenuity Pathway Analysis (Qiagen, Valencia, CA) using experimentally observed direct relationships in the Ingenuity Knowledge Base.
NanoString nCounter expression and association with symptoms.
nCounter expression data was analyzed with the NanoStringNorm and limma packages in R (107, 120). Counts were normalized to the geometric mean of all samples to adjust for technical variation between the four cartridges. Due to high variation of the housekeeping genes included in the code set (GAPDH, ACTB, PPIA), RNA quantity was normalized to the geometric mean of genes with the lowest coefficients of variation (GABBR1, RBM5, MUC4, IL17RE, RNF19A, ELMOD3, USP34, CDC42EP5, OPLAH, SGSM2), which was determined using NanoStringNorm (results were unchanged when MUC4, which was differentially expressed, was removed from the normalization set). Three outliers (mean count z-scores > 3) were excluded from analysis (an additional with z-score 3.03 was retained) yielding 45 samples with nCounter data. Using the voom function (69) in the limma package, count data were prepared for analysis with linear models, and DETs were determined with limma. An adjusted-P value threshold of 0.05 was used to determine statistical significance. Association with symptoms was determined using linear models. If sex, age, or BMI was a predictor of the outcome variable, it was included in the model (sex for overall symptoms and all 3 for abdominal pain).
RESULTS
Study Population
Characteristics of the study participants are shown in Table 1. The total cohort with available NanoString nCounter gene expression data (excluding outliers, n = 3) included 31 IBS patients and 14 HCs (51.6 and 42.9% women, respectively). These included a total of 15 IBS-C patients (10 women, 5 men), 16 IBS-D patients (6 women, 10 men), and 14 HCs (6 women, 8 men).
Table 1.
Participant characteristics
| Variable (range) | Microarray |
nCounter |
||
|---|---|---|---|---|
| Group | IBS | HC | IBS | HC |
| n | 20 | 10 | 31 | 14 |
| %Women | 50 | 50 | 51.6 | 42.9 |
| Age | 33.8 (10.8) | 31.7 (8.5) | 34 (12) | 35.4 (8.4) |
| BMI | 24.4 (6.4) | 24.7 (2.5) | 24.5 (5.5) | 25.8 (3) |
| Ethnicity | ||||
| Hispanic | 1 (5%) | 2 (20%) | 4 (12.9%) | 1 (7.1%) |
| Race | ||||
| Asian | 5 (25%) | 3 (30%) | 8 (25.8%) | 3 (21.4%) |
| Black | 5 (10%) | 3 (10%) | 8 (9.7%) | 3 (7.1%) |
| White | 5 (50%) | 3 (40%) | 8 (48.4%) | 3 (57.1%) |
| Other/mixed | 3 (15%) | 2 (20%) | 5 (16.1%) | 2 (14.3%) |
| Bowel habit subtype | ||||
| IBS-C | 10 (50%) | 15 (48.4%) | ||
| IBS-D | 10 (50%) | 16 (51.6%) | ||
| HAD anxiety score (0-21) | 5.6 (6) | 3.3 (2.8) | 6.4 (6)* | 2.9 (2.4) |
| HAD depression score (0-21) | 3 (3.6) | 1.9 (2.1) | 3 (3.1) | 1.4 (1.8) |
| Overall IBS severity (0-20) | 10.4 (4.4) | 10.1 (4) | ||
| Abdominal pain (0-20) | 9.8 (4.6) | 9.7 (4.2) | ||
Values are means (SD) or frequency (%). nCounter, counter cohort is inclusive of microarray cohort. HC, healthy control; IBS, irritable bowel syndrome; BMI, body mass index; HAD, Hospital Anxiety and Depression; IBS-C, IBS with constipation; IBS-D, IBS with diarrhea.
P < 0.05 between IBS and HCs.
Only 24 participants (all IBS) reported any medication use, and most used laxatives/antidiarrheals and ibuprofen only on an as-needed basis. All participants were instructed to hold aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) for 72 h prior to the procedure. No participants in the microarray cohort reported use of antidepressants/neuromodulators. In the expanded cohort, one participant took sertraline (100 mg/day) and bupropion (150 mg/day), and another took trazodone (50 mg/day). Participants were instructed not to take unnecessary medications 24–48 h prior to the procedure.
Identification of IBS Gene Signature by Microarray Profiling Analysis
Differentially expressed transcripts.
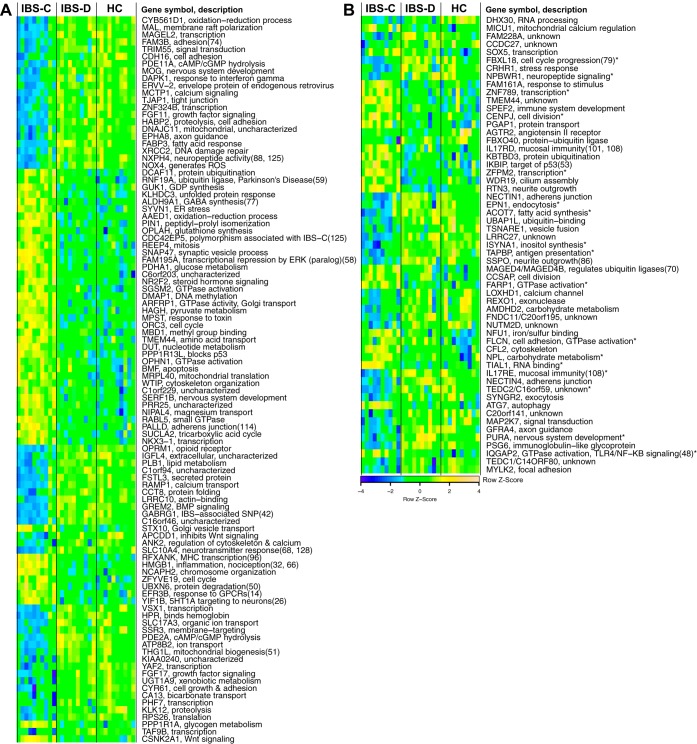
For IBS vs. HCs and IBS-D vs. HCs, there were no DETs meeting the threshold of FDR < 0.05. However, there were 120 and 66 DETs using an unadjusted P value threshold of 0.001. For IBS-C, there were 1,270 DETs with FDR < 0.05 (790 upregulated and 480 downregulated), mapping to 1,246 specific transcripts (retaining the probe with highest variance across samples among nonspecific probes; Supplement 1). A heat map of the top 100 genes is shown in Fig. 1A. Since our sample was age and sex matched, our primary analysis did not include sex. There were six genes for which there was a significant effect of sex (FDR < 0.05), and the effect of IBS-C vs. HCs remained significant (FDR < 0.05) in all six after controlling for sex.
Fig. 1.
A: top 100 differentially expressed genes in irritable bowel syndrome with constipation (IBS-C) vs. healthy controls (HCs). B: differentially expressed genes in IBS-C vs. HC that are replicated in the Mayo Clinic cohort. Expression values are scaled. Descriptions are from gene ontology or KEGG pathways, except where referenced. Genes and samples (within bowel habit subtypes) are clustered by Euclidean distance. Sample clustering is based on top 500 probes after ordering by variance across all 30 sample. IBS-D, IBS with diarrhea; cAMP/cGMP, cyclic adenosine/guansosine monophosphate; GTP, guanosine triphosphate; GTPase, enzymatic function catalyzing formation of GDP from GTP; BMP, bone-morphogenic protein; SNP, single nucleotide polymorphism; MHC, major histocompatibility complex; ATP, adenosine triphosphate; GPCR, G protein-coupled receptor; PKA, protein kinase A.
Comparison with external gene expression data.
Demographics of the samples and study protocols from Mayo Clinic and the University of Nottingham are shown in Table 2. Probes from the Arraystar (Agilent) array used in our study and the Affymetrix array used in Mayo and Nottingham were matched using the following algorithm. First, we identified transcripts or genes matched by only a single probe in each array. There were 452 probes uniquely matched to a specific transcript, and one probe uniquely matched to a specific gene. There were 90 Affymetrix probes that were nonspecific and overlapped with more than one Arraystar probe (e.g., two Arraystar probes were transcript specific with multiple Affymetrix probes matching). In these cases, the affected Arraystar probes were reduced to retain the probe with the highest variance across samples (48 Arraystar probes excluded). This yielded a set of Affymetrix probes wherein each only matched a single Arraystar probe, although in many cases there were more than one Affymetrix probes matched with each Arraystar probe. The final matches were determined separately for Mayo and Nottingham by reducing the multiple matching Affymetrix probes to the one with the highest variance across samples. Among the 1,246 DETs (IBS-C vs. HCs) in the current study, 859 were matched with probes from the Mayo and Nottingham data. Among these, the number of DETs with the same direction of FC (P < 0.05) in IBS-C and HC samples from Mayo and Nottingham were 57 and 185, respectively, with 17 matched in all three data sets. To address a possible limitation of comparing rectal (Nottingham) expression with sigmoid, we focused further investigation on the 57 common genes between our study and the Mayo study (Fig. 1B and Table 3). Upregulated genes included reticulon 3, which is important in neurite outgrowth (29). Downregulated genes included corticotropin-releasing factor receptor 1 (CRHR1), SCO-spondin (SSPO), which we previously found to be hypermethylated in peripheral blood mononuclear cells from IBS vs. HCs (82), and the glial cell line-derived neurotrophic factor receptor GRFA4.
Table 2.
External sample and study characteristics
| Mayo |
Nottingham |
|||||
|---|---|---|---|---|---|---|
| Sample | IBS-D | IBS-C | HC | IBS-D | IBS-C | HC |
| n | 21 | 15 | 25 | 27 | 18 | 26 |
| %Women | 86% | 100% | 92% | 67% | 89% | 62% |
| Recruitment | By mail from database, Rome II | Outpatient GI clinic, Rome II | ||||
| Protocol (prep) | Sigmoidoscopy (Fleet’s enema) | Sigmoidoscopy (none) | ||||
| Biopsy site | Sigmoid colon | Rectum | ||||
| Microarray | Human Genome U133-Plus2.0 (Affymetrix) | |||||
Table 3.
Genes differentially expressed in IBS-C vs. HC concordant in Mayo
| Name (Symbol) | AveExpr | FC (FDR) |
|---|---|---|
| Upregulated | ||
| Autophagy related 7 (ATG7) | 10.99 | 1.2 (0.031) |
| Centriole, cilia and spindle associated protein (CCSAP) | 8.90 | 1.4 (0.04) |
| Centromere protein J (CENPJ)* | 7.63 | 1.4 (0.032) |
| Cofilin 2 (CFL2) | 10.05 | 1.5 (0.015) |
| Family with sequence similarity 161 member A (FAM161A) | 7.47 | 1.4 (0.022) |
| FERM, ARH/RhoGEF and pleckstrin domain protein 1 (FARP1)* | 8.97 | 1.3 (0.026) |
| Folliculin (FLCN)* | 9.81 | 1.3 (0.041) |
| IKBKB interacting protein (IKBIP) | 8.38 | 1.5 (0.035) |
| Interleukin 17 receptor D (IL17RD) | 8.26 | 1.4 (0.029) |
| IQ motif containing GTPase activating protein 2 (IQGAP2)* | 12.94 | 1.3 (0.048) |
| Kelch repeat and BTB domain containing 3 (KBTBD3) | 8.24 | 1.3 (0.025) |
| MAGE family member D4B (MAGED4/MAGED4B) | 9.07 | 1.4 (0.024) |
| Mitochondrial calcium uptake 1 (MICU1) | 8.00 | 1.3 (0.046) |
| NFU1 iron-sulfur cluster scaffold (NFU1) | 9.91 | 1.4 (0.032) |
| N-acetylneuraminate pyruvate lyase (NPL)* | 9.61 | 1.7 (0.022) |
| PostGPI attachment to proteins 1 (PGAP1) | 7.54 | 1.6 (0.031) |
| Reticulon 3 (RTN3) | 8.57 | 1.3 (0.049) |
| SRY-box 5 (SOX5) | 8.02 | 1.4 (0.03) |
| Sperm flagellar 2 (SPEF2) | 7.31 | 1.6 (0.033) |
| TIA1 cytotoxic granule associated RNA binding protein like 1 (TIAL1)* | 9.36 | 1.3 (0.027) |
| Transmembrane protein 44 (TMEM44) | 7.44 | 1.5 (0.003) |
| WD repeat domain 19 (WDR19) | 8.66 | 1.3 (0.047) |
| Zinc finger protein, FOG family member 2 (ZFPM2)* | 8.43 | 1.7 (0.01) |
| Zinc finger protein 789 (ZNF789)* | 7.44 | 1.3 (0.032) |
| Downregulated | ||
| Acyl-CoA thioesterase 7 (ACOT7)* | 8.58 | 0.6 (0.019) |
| Angiotensin II receptor type 2 (AGTR2) | 7.35 | 0.6 (0.019) |
| Amidohydrolase domain containing 2 (AMDHD2) | 9.54 | 0.8 (0.028) |
| Chromosome 20 open reading frame 141 (c20orf141) | 11.70 | 0.8 (0.029) |
| Coiled-coil domain containing 27 (CCDC27) | 7.81 | 0.8 (0.046) |
| Corticotropin releasing hormone receptor 1 (CRHR1) | 7.53 | 0.7 (0.018) |
| DExH-box helicase 30 (DHX30) | 8.16 | 0.8 (0.012) |
| Epsin 1 (EPN1)* | 8.23 | 0.7 (0.041) |
| Family with sequence similarity 228 member A (FAM228A) | 7.90 | 0.8 (0.038) |
| F-box and leucine rich repeat protein 18 (FBXL18)* | 7.55 | 0.7 (0.013) |
| F-box protein 40 (FBXO40) | 7.81 | 0.4 (0.032) |
| Fibronectin type III domain containing 11 (FNDC11/C20orf195) | 9.56 | 0.8 (0.03) |
| GDNF family receptor alpha 4 (GFRA4) | 11.93 | 0.7 (0.045) |
| Interleukin 17 receptor E (IL17RE)* | 10.46 | 0.7 (0.025) |
| Inositol-3-phosphate synthase 1 (ISYNA1)* | 8.84 | 0.8 (0.01) |
| Lipoxygenase homology domains 1 (LOXHD1) | 9.48 | 0.7 (0.021) |
| Leucine rich repeat containing 27 (LRRC27) | 8.87 | 0.8 (0.029) |
| Mitogen-activated protein kinase kinase 7 (MAP2K7) | 11.66 | 0.7 (0.036) |
| Myosin light chain kinase 2 (MYLK2) | 14.59 | 0.5 (0.008) |
| Nectin cell adhesion molecule 1 (NECTIN1) | 8.27 | 0.6 (0.01) |
| Nectin cell adhesion molecule 4 (NECTIN4) | 10.16 | 0.7 (0.029) |
| Neuropeptides B and W receptor 1 (NPBWR1)* | 7.73 | 0.6 (0.017) |
| NUT family member 2D (NUTM2D) | 9.59 | 0.8 (0.046) |
| Pregnancy specific beta-1-glycoprotein 6 (PSG6) | 12.64 | 0.6 (0.01) |
| Purine rich element binding protein A (PURA)* | 12.90 | 0.7 (0.026) |
| RNA exonuclease 1 homolog (REXO1) | 9.43 | 0.7 (0.039) |
| SCO-spondin (SSPO) | 9.15 | 0.8 (0.029) |
| Synaptogyrin 2 (SYNGR2) | 11.12 | 0.8 (0.034) |
| TAP binding protein (TAPBP)* | 9.20 | 0.7 (0.013) |
| Chromosome 14 open reading frame 80 (TEDC1) | 13.37 | 0.5 (0.014) |
| Chromosome 16 open reading frame 59 (TEDC2)* | 10.55 | 0.5 (0.008) |
| T-SNARE domain containing 1 (TSNARE1) | 8.89 | 0.7 (0.008) |
| Ubiquitin associated protein 1 like (UBAP1L) | 8.95 | 0.8 (0.041) |
AveExpr, average expression (log2 transformed); FC, fold change, FDR, Benjamini-Hochberg adjusted P value.
Also differentially expressed in this direction in Nottingham.
Identification of IBS gene networks by performing WGCNA analysis.
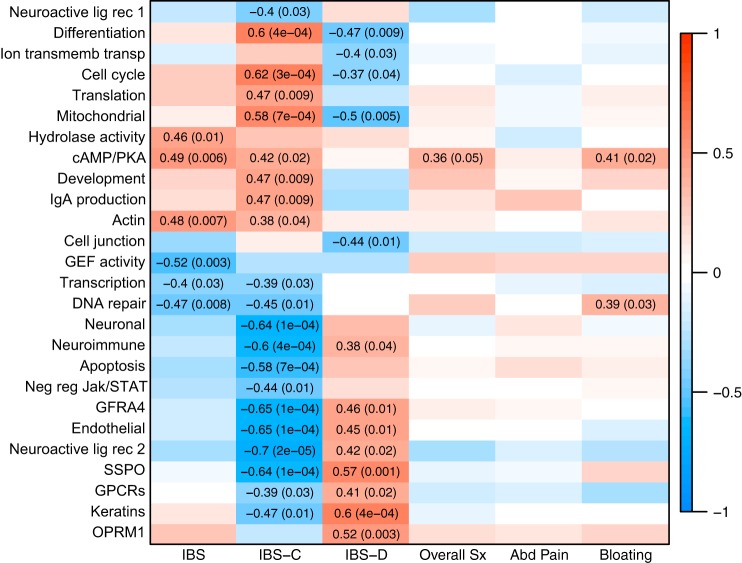
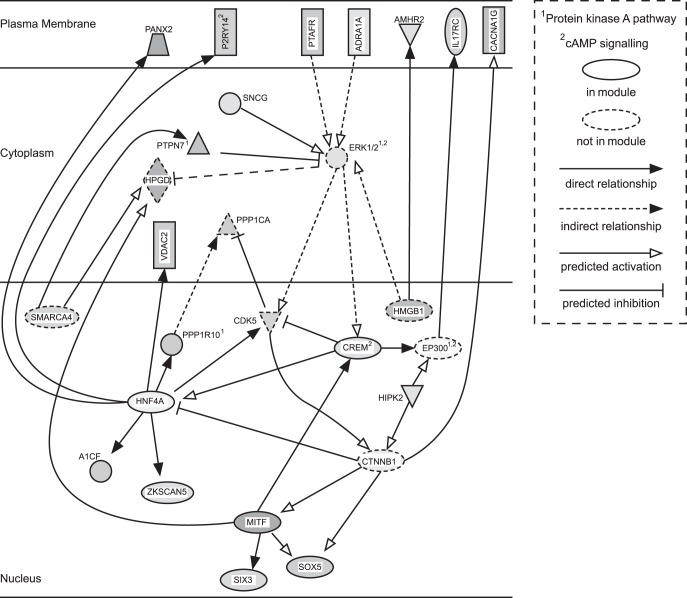
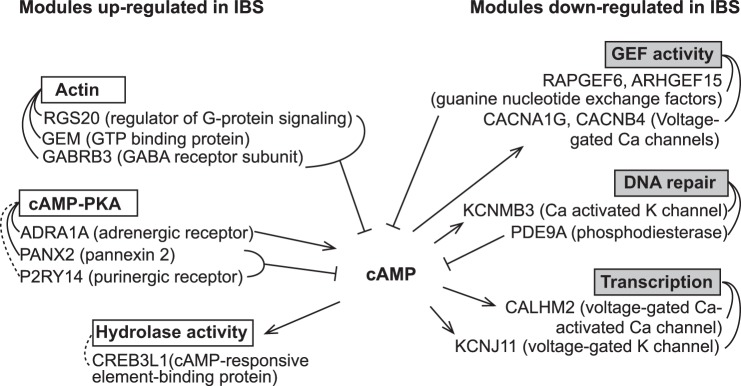
WGCNA yielded 45 gene modules, of which 26 significantly correlated with IBS status (P < 0.05; Fig. 2). None of the modules was significantly associated with sex. One module, subsequently named “cAMP-PKA”, was associated with IBS, IBS-C, overall symptom, and bloating severity. Top GO terms associated with this module are shown in Table 4, and the other IBS-associated modules are described in Table 5. Results of the pathway analysis for the “cAMP-PKA” module are shown in Fig. 3, which is displayed as a subset of two networks. The IPA analysis highlights upregulation of cAMP/PKA signaling. Interestingly, following review of the “hub” genes, those with the highest “module membership” (correlations with module “eigengene”), revealed a role for cAMP signaling in other modules that were associated with IBS, and the function of the gene products was consistent with increased cAMP (Fig. 4 and Table 6). For example, phosphodiesterase (PDE)9A, which hydrolyzes cAMP, is in a module that is downregulated in IBS-C. IPA pathway analysis settings, main results, and references for the relationships in the network are provided as a supplementary file.
Fig. 2.
Weighted gene coexpression network analysis (WGCNA) modules associated with IBS. Values are correlations between module eigengene and IBS status or overall symptom severity (0–20) with associated P value. Red and blue indicate positive and negative correlations, respectively.
Table 4.
Gene ontology terms overrepresented among genes in the cAMP/PKA module
| GO ID | P Value | Term | Genes |
|---|---|---|---|
| 0035589 | 0.001 | G-protein coupled purinergic nucleotide receptor signaling pathway | PTAFR, P2RY14 |
| 0051058 | 0.002 | Negative regulation of small GTPase mediated signal transduction | ADRA1A, MYOC, RASAL2 |
| 1902692 | 0.004 | Regulation of neuroblast proliferation | CX3CR1, SIX3 |
| 0045907 | 0.006 | Positive regulation of vasoconstriction | ADRA1A, PTAFR |
| 0048246 | 0.008 | Macrophage chemotaxis | AZU1, CX3CR1 |
| 0071560 | 0.010 | Cellular response to transforming growth factor beta stimulus | AMHR2, LDLRAD4, CDK9, CX3CR1, SOX5, HIPK2, CHST11 |
| 0042063 | 0.012 | Gliogenesis | AZU1, ERCC2, ETV5, MBD1, MYOC, SOX5 |
| 0046578 | 0.013 | Regulation of Ras protein signal transduction | ADRA1A, MYOC, RASAL2 |
| 0050808 | 0.016 | Synapse organization | ACHE, ETV5, SNCG, PDLIM5 |
| 0061351 | 0.018 | Neural precursor cell proliferation | CX3CR1, SIX3, SOX5, TACC2 |
| 0030182 | 0.020 | Neuron differentiation | CNGA3, ERCC2, ETV5, FES, MBD1, MYOC, ROBO1, SIX3, SOX5, SPTAN1, LAMTOR3, CACNA1G, VAPA, RASAL2, PDLIM5, NSMF, HIPK2, MDGA2 |
| 1903307 | 0.020 | Positive regulation of regulated secretory pathway | PTAFR, CACNA1G |
| 0051591 | 0.022 | Response to cAMP | CNGA3, CREM, HNF4A, PTAFR, PYGM, SLC26A6 |
| 0007155 | 0.024 | Cell adhesion | ACHE, AZU1, DDR1, CX3CR1, FES, MYOC, PTAFR, ROBO1, ADAM12, EFS, FBLIM1, PCDHA11, ANTXR1 |
| 0007600 | 0.030 | Sensory perception | AOC2, CNGA3, PTAFR, SCNN1D, SIX3, OR10H2, CNGB3 |
| 0019048 | 0.030 | Modulation by virus of host morphology or physiology | VAPA, HIPK2 |
| 0034220 | 0.030 | Ion transmembrane transport | AQP5, CNGA3, PTAFR, S100A1, SCNN1D, VDAC2, CACNA1G, ATP5J2, CNGB3, SLC26A6, SLC26A9 |
| 0099536 | 0.031 | Synaptic signaling | ACHE, ADRA1A, CX3CR1, ETV5, SNCG, CACNA1G, NSMF, PANX2, SHISA7 |
| 0060395 | 0.042 | SMAD protein signal transduction | HNF4A, HIPK2 |
Overrepresented terms were determined using the hypergeometric function in the GOstats package for R (44).
Table 5.
Description of IBS-associated modules
| Module (size) | Description |
|---|---|
| Neuroactive ligand receptors 1 (758) | Hub genes enriched (0.01) for KEGG pathway “Neuroactive ligand-receptor interaction” and include 5-hydroxytryptamine receptor 4 (HTR4), cholinergic receptor muscarinic 1 (CHRM1), dopamine receptor D4 (DRD4), and glutamate metabotropic receptor 2 (GRM2). Full module also enriched for GO-BP “negative regulation of cytokine production” (0.002), and “negative regulation of tumor necrosis factor production” (0.0005) |
| Differentiation (435) | Hub genes enriched (FDR <0.05) for cell differentiation and nervous system development. Representative genes for the latter included glial cell derived neurotrophic factor (GDNF) and beta tubulin (TUBB2B). The module overall was enriched for RNA transcription (0.005). |
| Ion transmembrane transport (248) | Enriched for GO:BP “regulation of transmembrane ion transport” (0.005) |
| Cell cycle (273) | Very heavily enriched for cell-cycle genes (GO:BP, FDR = 1.5e-49) |
| Translation (2685) | Highly enriched for terms related to protein translation. Top two are GO:BP “RNA splicing via transesterification reactions” and “ribonucleoprotein complex biogenesis” (1.8e-07 for each) |
| Mitochondrial (1915) | Module and hub genes heavily enriched for terms and pathways related to cellular respiration and oxidative phosphorylation (FDR <1e-15 for several) |
| Hydrolase activity (165) | No terms or pathways enriched P < 0.005 or FDR <0.05. Nine of 45 hub genes map to GO:BP “regulation of hydrolase activity” (0.02) |
| cAMP/PKA (184) | Based on Ingenuity Pathway Analysis. |
| Development (234) | Hub genes marginally (0.006) enriched for “anatomical structure development” and “single organism developmental process.” Relevant genes include the transcriptional regulators GATA binding protein 2 (GATA2), Sp3 transcription factor (SP3) and homeobox B3 (HOXB3). |
| IgA production (127) | Heavily enriched for GO terms related to adaptive immune response (<0.005) as well as KEGG pathway “Intestinal immune network for IgA production” (FDR <0.0005). Hub genes include three HLA class molecules (DMA, DRA, DMB) integrin subunit alpha and beta (ITGA4, ITGB7). |
| Actin (77) | Enriched (<0.005) for several terms related to regulation of actin polymerization. |
| Cell junction (889) | Enriched (FDR <0.05) for GO:MF “cadherin-binding in cell adhesion” and CC “adherens junction.” Relevant genes include vinculin (VCL), tight junction protein 1 (TJP1) and catenin delta 1 (CTNND1) |
| GEF activity (224) | Hub genes enriched for GO:BP “guanyl-nucleotide exchange factor activity” (<0.005). |
| Transcription (143) | GO:BP “DNA-templated transcription” is most representative (19 genes, 0.04) |
| DNA repair (168) | Enriched for several terms related to DNA repair (i.e., GO:BP “double-strand break repair via nonhomologous end joining”, 0.001) |
| Neuronal (578) | GO:BP: “protein localization to synapse” is most enriched (0.0001) and there are 25 genes mapping to GO:BP “nervous system development” (0.03). Relevant genes include neuronal cell adhesion molecule (NRCAM) and neuropeptide Y (NPY). |
| Neuroimmune (1074) | Hub genes are enriched for sensory perception (FDR <0.05) and include 6 olfactory receptors. Hub genes also contain multiple genes related to the inflammatory response and innate immune system. These include tumor necrosis factor (TNF), CD70, CD80, macrophage expressed 1/Perforin-2 (MPEG1), toll like receptor 9 (TLR9), mucin 22 (MUC22), defensin beta 113 (DEFB113), cysteine rich secretory protein 3 (CRISP3), and the serine protease inhibitors serpin family E members 1&2 (SERPINE1/2). |
| Apoptosis (275) | Enriched for GO:BP “positive regulation of apoptotic process” (<0.005). Relevant genes include caspase 2 (CASP2) and slit guidance ligand 2 (SLIT2). |
| Negative regulation of JAK/STAT (139) | The top GO:BP term is “negative regulation of JAK-STAT cascade (0.003) based on caveolin 1 (CAV1) and inositol polyphosphate-5-phosphatase F (INPP5F). |
| GFRA4 (161) | No significantly enriched terms or pathways. GDNF family receptor alpha is one of top genes (MM = 0.93). Nonsignificantly enriched terms include GO:BP “regulation of GTPase activity” (0.05), “cellular response to BMP stimulus” (0.02), “glial cell-derived neurotrophic factor receptor signaling pathway” (0.04), and “catechol-containing compound metabolic process” (0.01). |
| Endothelial (107) | Enriched (0.001) for GO:BP “regulation of tube size” based on expression of endothelial nitric oxide synthase (eNOS, NOS3) and adrenoceptor alpha 1A (ADRA1A). |
| Neuroactive ligand receptors 2 (892) | Hub genes enriched for membrane proteins (GO:CC, FDR <0.05). Top 100 genes include receptors for NPY (NPY1R) and neuropeptides B and W (NPBWR1), melanocortin (MC5R) and GABA (GABRG1). |
| SSPO (186) | SCO-Spondin (SSPO) is among top two genes in module (MM = 0.95). GO:BP “ion transport” is also enriched (<0.005) |
| GPCRs (328) | Enriched (<0.005) for GO:BP “G-protein coupled receptor signaling pathway”. Relevant genes include 5-hydroxytryptamine receptors 6 and 3D(HTR6, HTR3D), urocortin 2 (UCN2), and four olfactory receptors. |
| Keratins (827) | Hub genes enriched for GO:CC “Intermediate filament cytoskeleton” (FDR = 0.05). The majority of these are keratins or keratin associated proteins. The module overall (but not hub genes exclusively) is enriched for GO:BP “response to steroid hormone” (0.009). Relevant genes include hepatocyte nuclear factor 4 alpha (HNF4A), KRAS proto-oncogene, GTPase (KRAS) and estrogen related receptor alpha (ESRRA). |
| OPRM1 (198) | Not significantly enriched for pathways or terms. Opioid receptor mu 1 (OPRM1) is a hub gene (MM = 0.84). Marginally enriched for GO:BP “sensory response to pain” (P = 0.02) due to OPRM1 and purinergic receptor P2X 4 (P2RX4). |
Module membership (MM) is the correlation with module eigengene. Hub genes are those with MM > 0.8. MM P values < 1e-7 for all hub genes. Overrepresented terms were determined using the hypergeometric function in the GOstats package for R(44) or DAVID. (55, 56). Numbers in parentheses are unadjusted P values except where noted. GO, gene ontology; BP, biological process; MF, molecular function; CC, cellular component; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; GEF, guanine nucleotide exchange factor; GPCRs, G-protein coupled receptors.
Fig. 3.
IPA pathway analysis network for the cAMP/PKA module. Genes not in the cAMP/PKA module have shapes outlined in dashed lines. Fill is proportional to fold change from the microarray data (darker = greater fold change), and all are upregulated except for P2RY14. Figure generated using IPA (Qiagen, Valencia, CA).
Fig. 4.
cAMP signaling roles for hub genes in IBS-associated modules. Directionality of arrows indicates upstream/downstream of cAMP. Dotted lines indicate a negative correlation with the module eigengene. Note that CACNA1G in the guanine nucleotide exchange factor (GEF) activity module is a different isoform than in the cAMP/PKA (Fig. 3). Module memberships and P values are shown in Table 6.
Table 6.
Correlation of cAMP-related genes with module eigengenes
| Gene | MM* | P | Module |
|---|---|---|---|
| Upregulated modules | |||
| ADRA1A | 0.711 | 1.1E-05 | cAMP/PKA |
| CREB3L1 | −0.682 | 3.3E-05 | Hydrolase activity |
| GABRB3 | 0.714 | 9.6E-06 | Actin |
| GEM | 0.772 | 5.9E-07 | Actin |
| PANX2 | 0.830 | 1.4E-08 | cAMP/PKA |
| P2RY14 | −0.660 | 7.3E-05 | cAMP/PKA |
| RGS20 | 0.500 | 4.9E-03 | Actin |
| Downregulated modules | |||
| ARHGEF15 | 0.564 | 1.2E-03 | GEF activity |
| CACNA1G** | 0.934 | 4.6E-14 | GEF activity |
| CACNB4 | 0.776 | 4.6E-07 | GEF activity |
| CALHM2 | 0.818 | 3.3E-08 | Transcription |
| KCNJ11 | 0.928 | 1.6E-13 | Transcription |
| KCNMB3 | 0.896 | 2.3E-11 | DNA repair |
| PDE9A | 0.669 | 5.2E-05 | DNA repair |
| RAPGEF6 | 0.918 | 8.9E-13 | GEF activity |
MM (module membership) is the correlation between the gene and the module eigengene;
a separate isoform from that in the orangered4 module.
Identification of IBS signaling pathways based on the gene expression data.
GSEA results are provided in a supplementary file. There were no gene sets enriched in IBS-C or HCs meeting a threshold of FDR < 0.25 (recommended GSEA threshold). Most gene sets that were enriched among HCs with nominal (unadjusted) P < 0.05 were thematically related to synaptic transmission or G protein-coupled receptor (GPCR) signaling. These included Reactome pathways “Activation of kainite receptors upon glutamate binding” (P = 0.024), “Gα(s) signaling” (P = 0.026), “GPCR downstream signaling” (P = 0.036), KEGG pathway “neuroactive ligand receptor interaction” (P = 0.014), and GO:BP gene-sets “neuronal synaptic plasticity” (P = 0.004), and “regulation of synaptic transmission glutaminergic” (P = 0.021). Despite the absence of DETs in IBS vs. HCs, the Reactome pathway “signaling by FGFR1 mutants” was enriched in HCs vs. IBS (p/FDR 0.012/0.19). The 20 genes in this gene set include growth factors and downstream effectors (JAK/STAT pathway). The top enriched Reactome gene set was smooth muscle contraction (p/FDR 0.004/0.29); however, these genes (actins, myosin kinases) likely represent expression at cell junctions rather than muscle. No gene sets were enriched at FDR < 0.25 in IBS-D vs. HC, IBS-C vs. IBS-D or in men vs. women. Within IBS, the following KEGG gene sets were enriched in men vs. women (p/FDR): “allograft rejection” (0.002/0.12), “graft vs. host disease” (0.006/.086), and “autoimmune thyroid disease” (0.002/0.139). These gene sets overlap and include human leukocyte antigen (HLA) complex proteins and tumor necrosis factor (TNF).
Validation of Selected Targets
Selection of genes to validate (n = 67) was based on the following: 1) visualization of dot plots to ensure consistent group difference, 2) programmatic search (annotate package in R) of abstracts linked to each Entrez ID for the words “pain” or “gastrointestinal” and manual search of retrieved abstracts, 3) comparison with external datasets, 4) comparison with results of IBS GWA studies [HTR3A, CDC42EP5 (125)], and 5) results of other studies.
Differential expression.
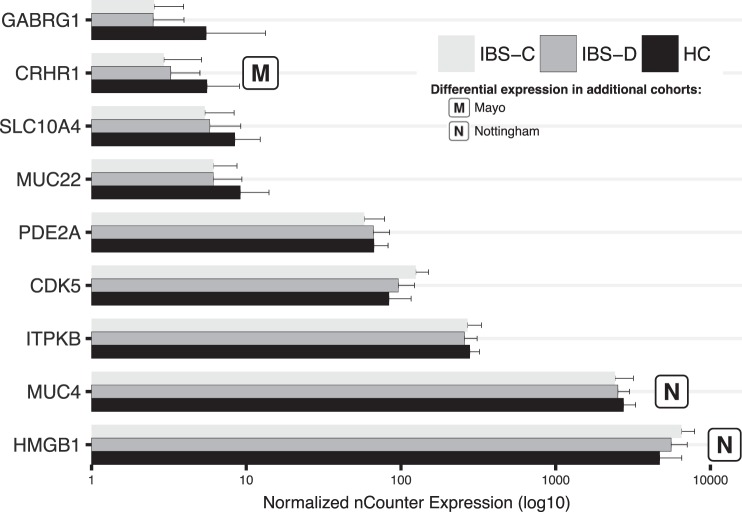
In the expanded cohort (15 IBS-C vs. 14 HCs), differential expression of 9/67 DETs was validated with nCounter (FDR < 0.05, same direction of FC as in microarray, Fig. 5 and Table 7). There were an additional six (ALDH3A1, ELMOD3, GH1, RAMP1, SSPO, TMEM80) with unadjusted P < 0.05 and five (ALDAH9A1, CLDN15, EZH2, RNF19A, YIF1B) with significant positive correlations (P < 0.05) between microarray and nCounter values.
Fig. 5:
nCounter expression of 9 validated genes in IBS-C vs. HC. Plotted results are mean (SD) for nCounter expression (n = 45). Short description of genes not described in paper: ITPKB is expressed in cells with a brush border (104) but is also important in immune cells (92) and neurons (110). Its molecular function is related to intracellular calcium regulation (113). SLC10A4 is a bile acid transporter, about which relatively little is known, including whether it is able to transport bile acids or neurotransmitters (1, 103, 109). Slc10a4 knockout mice show altered dopamine homeostasis (87, 128). MUC4 is major component of intestinal mucus. Roles include barrier function and host-microbe interactions. Expression of MUC22, a susceptibility locus for panbronchiolitis and childhood asthma, is upregulated in bronchial epithelial cells after exposure to a virus-like molecule or lipopolysaccharide (31, 52).
Table 7.
Results of nCounter validation
| IBS-C vs. HC | IBS-D vs. HC | IBS vs. HC | ||
|---|---|---|---|---|
| Gene | AveExpr (all n = 45) | FC (FDR) | FC (FDR) | FC (FDR) |
| AK9 | 11.7 | 1.1 (0.74) | 1.1 (0.63) | 1.1 (0.61) |
| ALDH3A1 | 11.1 | 0.7 (0.07)* | 0.8 (0.3) | 0.7 (0.06)* |
| ALDH9A1 | 14.8 | 1.1 (0.5) | 1.0 (0.79) | 1.0 (0.86) |
| AQP9 | 8.5 | 0.6 (0.17) | 0.5 (0.21) | 0.6 (0.06)* |
| CCDC134 | 11.9 | 1.0 (0.76) | 0.9 (0.54) | 0.9 (0.48) |
| CDC42EP5 | 16.7 | 1.1 (0.3) | 1.0 (0.67) | 1.1 (0.44) |
| CDK5 | 11.2 | 1.3 (0.05) | 1.1 (0.57) | 1.2 (0.09)* |
| CLDN15 | 12.5 | 1.0 (0.91) | 0.9 (0.61) | 1 (0.85) |
| CRHR1 | 6.4 | 0.5 (0.05) | 0.6 (0.41) | 0.5 (0.06)* |
| DBI | 16.2 | 1.1 (0.33) | 1.0 (1.0) | 1.0 (0.59) |
| DHRS12 | 12.4 | 0.9 (0.48) | 0.9 (0.61) | 0.9 (0.48) |
| EFR3B | 9.6 | 1.0 (0.87) | 0.9 (0.79) | 1.0 (0.93) |
| ELMOD3 | 12.3 | 0.8 (0.11)* | 0.9 (0.61) | 0.9 (0.18) |
| EZH2 | 13.0 | 1.0 (0.74) | 1.0 (0.83) | 1.0 (0.94) |
| FABP3 | 11.5 | 1.0 (0.94) | 1.0 (0.96) | 1.0 (0.97) |
| GABBR1 | 12.8 | 0.6 (0.01) | 0.8 (0.54) | 0.7 (0.04) |
| GABRA1 | 8.6 | 0.9 (0.76) | 1.0 (1.0) | 1.0 (0.88) |
| GABRG1 | 6.2 | 0.5 (0.05) | 0.6 (0.21) | 0.6 (0.04) |
| GH1 | 8.0 | 0.7 (0.12)* | 0.6 (0.21)* | 0.7 (0.06)* |
| HMGA1 | 14.3 | 1.0 (0.87) | 1.1 (0.57) | 1.1 (0.59) |
| HMGB1 | 17.0 | 1.2 (0.05) | 1.1 (0.3) | 1.2 (0.06)* |
| HTR3A | 7.9 | 0.8 (0.33) | 0.7 (0.21) | 0.7 (0.14)* |
| IL17RD | 9.9 | 0.8 (0.12) | 0.9 (0.54) | 0.8 (0.16) |
| IL17RE | 14.9 | 0.9 (0.29) | 1.0 (0.82) | 0.9 (0.48) |
| ITPKB | 12.6 | 0.8 (0.05) | 0.8 (0.21)* | 0.8 (0.04) |
| JRK | 11.4 | 0.9 (0.63) | 0.9 (0.61) | 0.9 (0.48) |
| KAT5 | 14.2 | 1.1 (0.59) | 1.1 (0.61) | 1.1 (0.48) |
| MLN | 6.5 | 1.1 (0.87) | 0.7 (0.45) | 0.9 (0.61) |
| MUC22 | 7.3 | 0.6 (0.05) | 0.7 (0.21) | 0.6 (0.04) |
| MUC4 | 15.9 | 0.7 (0.02) | 0.8 (0.41) | 0.8 (0.04) |
| MUCL1 | 6.4 | 0.8 (0.62) | 0.8 (0.61) | 0.8 (0.48) |
| NECTIN1 | 13.3 | 1.0 (0.76) | 1.0 (0.83) | 1.0 (0.97) |
| NKX2-1 | 7.1 | 0.6 (0.08) | 0.6 (0.21) | 0.6 (0.04) |
| NKX3-1 | 8.1 | 0.9 (0.48) | 0.9 (0.61) | 0.9 (0.48) |
| NOTCH4 | 10.8 | 0.9 (0.64) | 1.0 (0.99) | 1.0 (0.85) |
| NPBWR1 | 7.5 | 1.1 (0.74) | 0.9 (0.82) | 1.0 (0.97) |
| NRG3 | 8.3 | 0.9 (0.74) | 1.0 (0.82) | 0.9 (0.77) |
| OPHN1 | 12.5 | 1.1 (0.51) | 1.1 (0.61) | 1.1 (0.48) |
| OPLAH | 12.2 | 0.7 (0.11) | 0.8 (0.3) | 0.8 (0.09) |
| OPRM1 | 6.7 | 0.8 (0.62) | 0.7 (0.59) | 0.8 (0.48) |
| PALLD | 15.3 | 1.1 (0.62) | 1.1 (0.61) | 1.1 (0.51) |
| PCDHA3 | 8.5 | 0.6 (0.02) | 0.8 (0.29) | 0.7 (0.04) |
| PDE11A | 9.5 | 0.8 (0.32) | 0.9 (0.61) | 0.9 (0.36) |
| PDE2A | 10.5 | 0.7 (0.02) | 0.9 (0.61) | 0.8 (0.06)* |
| PPP1CA | 17.0 | 1.1 (0.18) | 1.2 (0.3) | 1.2 (0.13)* |
| PPP1R1A | 8.9 | 0.7 (0.16) | 0.8 (0.54) | 0.8 (0.18) |
| RAMP1 | 9.4 | 0.7 (0.09)* | 0.7 (0.21)* | 0.7 (0.04) |
| RBM5 | 14.9 | 0.9 (0.33) | 1.0 (0.61) | 0.9 (0.44) |
| RNF19A | 14.9 | 1.0 (0.76) | 1.0 (0.99) | 1.0 (0.86) |
| S100A1 | 7.7 | 0.8 (0.37) | 1.2 (0.61) | 1.0 (0.86) |
| SGSM2 | 13.0 | 0.8 (0.05) | 0.9 (0.62) | 0.8 (0.15) |
| SIGMAR1 | 13.5 | 1.0 (0.76) | 1.0 (0.82) | 1.0 (0.78) |
| SLC10A4 | 7.2 | 0.5 (0.02) | 0.6 (0.21) | 0.6 (0.03) |
| SLC17A3 | 8.4 | 1.0 (0.91) | 0.9 (0.61) | 1.0 (0.85) |
| SMARCA4 | 14.7 | 1.1 (0.24) | 1.1 (0.54) | 1.1 (0.22) |
| SOX5 | 9.9 | 0.7 (0.01) | 0.8 (0.21) | 0.7 (0.03) |
| SPATS2 | 12.6 | 1.2 (0.16) | 1.1 (0.57) | 1.1 (0.21) |
| SSPO | 6.2 | 0.5 (0.08)* | 0.5 (0.21) | 0.5 (0.04) |
| ST3GAL3 | 12.2 | 0.9 (0.32) | 0.9 (0.56) | 0.9 (0.29) |
| THBD | 11.7 | 0.7 (0.12) | 0.8 (0.54) | 0.8 (0.15) |
| TMEM44 | 13.7 | 0.9 (0.76) | 0.9 (0.72) | 0.9 (0.73) |
| TMEM80 | 13.6 | 1.2 (0.12)* | 1.0 (0.98) | 1.1 (0.44) |
| TRIM37 | 13.4 | 1.1 (0.51) | 1.1 (0.61) | 1.1 (0.48) |
| TXLNG | 13.3 | 0.9 (0.65) | 0.9 (0.61) | 0.9 (0.53) |
| UGT1A9 | 15.2 | 1.0 (0.78) | 1.0 (0.98) | 1.0 (0.87) |
| USP34 | 15.1 | 0.9 (0.09) | 1.0 (0.61) | 0.9 (0.2) |
| YIF1B | 12.9 | 1.1 (0.33) | 0.9 (0.61) | 1.0 (0.87) |
Boldface indicates FDR < 0.05. Unmarked values < 0.05 indicate direction did not match microarray. AveExpr, average expression (log2 transformed); FC, fold change, FDR, Benjamini-Hochberg adjusted P value.
Unadjusted P value < 0.05.
Associations between gene expression and IBS symptoms were found, although these did not retain significance after adjusting for multiple comparisons (n = 67 genes).
Discussion
The most important findings from this study are that 1) sigmoid mucosal gene expression profiles differentiate IBS-C from HCs and 2) results of multiple analyses and in several cohorts support the relevance of neurally mediated mechanisms in IBS overall and specifically in IBS-C.
Defining a Molecular Signature for IBS-C
Very few studies have reported DETs in IBS-C vs. HCs. A survey of the literature revealed ~90 DETs in IBS (those included in our microarray are shown in Table 8). Recently, Peters et al. (97) compared expression of tight junction proteins in sigmoid colonic mucosal biopsies from women with IBS-C vs. HC women, and none were reported as differentially expressed. As a demonstration of the heterogeneity in findings with different samples and methodology, some of the genes in this study were DETs in our study (FDR < 0.05; Table 9). An important strength of our study is that our microarray sample included no individuals using SSRIs, and there were only two with antidepressant use in the validation sample. Medication use was not reported in the Nottingham comparison study, but in the Mayo study, 42% of IBS participants took medications (SSRI, SNRI, TCA, or dopaminergic agent) vs. 1% of HCs. It is possible that this difference in medication use contributed to our findings. Divergent results by bowel habit subtype is supported by other studies (28, 57), but notably, there is evidence for the absence of subtype-specific effects as well (38, 93). We caution the reader against overinterpretation of these findings. Although gene networks may differ in IBS-C and IBS-D, one cannot conclude that these differences are causative and therefore represent different underlying pathophysiology. These differences are also not evidence of bowel habit differences at the phenotype level (i.e., nerve sensitivity). Furthermore, in accord with our sample size calculation, one can only conclude that there are unlikely to be genes with greater than a twofold difference between IBS-D and HC. We are underpowered to make a negative conclusion for smaller differences that could still be biologically relevant.
Table 8.
Comparison of findings from current study for genes found to be differentially expressed in the literature
| Fold Change, P Value |
|||||
|---|---|---|---|---|---|
| Symbol | Published Change & Subgroup (Ref.) | IBS | IBS-C | IBS-D | Product |
| ALDOB*** | ↑D (22) | 0.9 (0.3) | 0.8 (0.06) | 1.0 (0.88) | Fructose-bisphosphate aldolase B |
| ALDOC*** | ↑D (22) | 1.3 (0.05) | 1.6 (0.01) | 1.1 (0.45) | Fructose-bisphosphate aldolase C |
| AQP8 | ↓D (121) | 1.3 (0.25) | 1.7 (0.06) | 1.0 (0.94) | Aquaporin-8 |
| BIRC5 | ↑D (22) | 1.1 (0.53) | 1.5 (0.02) | 0.8 (0.16) | Baculoviral IAP repeat-containing protein 5 isof. 3 |
| C4BPA*** | ↑D (21) | 0.9 (0.29) | 0.7 (0.002) | 1.2 (0.13) | Complement component 4 binding protein, alpha |
| CACNA1H*** | ↑ (102) | 1.1 (0.57) | 1.2 (0.07) | 0.9 (0.36) | Voltage-dep. T-type calcium channel sub. Alpha-1h isof. A |
| CARD16*** | ↑ (2) | 1.1 (0.44) | 1.1 (0.25) | 1.0 (0.86) | Caspase recruitment domain-containing protein 16 isof. 1 |
| CASP1*** | ↓ (2) | 0.9 (0.55) | 1.0 (0.7) | 0.8 (0.16) | Caspase-1 isof. Gamma |
| CCL20*** | ↑D (21, 22) | 0.8 (0.12) | 0.6 (0.004) | 1.1 (0.7) | C-C motif chemokine 20 isof.1 precursor |
| CD163L1*** | ↑ (2) | 1.2 (0.25) | 1.6 (0.03) | 1.0 (0.84) | Scavenger recep. Cysteine-rich type 1 protein m160 precursor |
| CKB | ↑ (2) | 0.9 (0.33) | 0.9 (0.41) | 0.9 (0.38) | Creatine kinase B-type |
| DTL | ↑ (2) | 0.8 (0.03) | 0.9 (0.12) | 0.8 (0.03) | Denticleless protein homolog |
| DUOX2** | ↑ (2) | 1.3 (0.06) | 1.3 (0.05) | 1.2 (0.18) | Dual oxidase 2 precursor |
| DUSP27 | ↓D (22) | 1.0 (0.95) | 0.9 (0.81) | 1.0 (0.9) | Dual specificity phosphatase 27 (putative) |
| ERAP2 | ↑ (2) | 0.9 (0.8) | 1.4 (0.5) | 0.6 (0.27) | Endoplasmic reticulum aminopeptidase 2 |
| F2RL1 | ↑ (75) | 0.9 (0.63) | 0.9 (0.29) | 1.0 (0.82) | Proteinase-activated receptor 2 precursor |
| F2RL3*** | ↓ (130) | 1.0 (0.58) | 1.0 (0.99) | 0.9 (0.34) | PAR4 coagulation factor II (thrombin) receptor-like 3 |
| FAAH | ↓C (31) | 1.1 (0.09) | 1.2 (0.05) | 1.1 (0.31) | Fatty-acid amide hydrolase 1 |
| FCGR2A | ↑ (2) | 0.5 (0.09) | 0.7 (0.3) | 0.5 (0.05) | Low affinity Ig gamma Fc region recep, II-a isof.1 precursor |
| FGFR4* | ↓D (21) | 0.9 (0.12) | 1.0 (0.86) | 0.8 (0.01) | Fibroblast growth factor receptor 4 isof. 2 precursor |
| FN1 | ↓D (21, 22) | 0.8 (0.07) | 0.8 (0.12) | 0.8 (0.12) | Fibronectin isof. 5 preproprotein |
| FOXP3 | ↓ (13) | 0.6 (0.001) | 0.5 (0.001) | 0.7 (0.02) | Forkhead box P3 |
| GPBAR1 | ↑C (21) | 0.9 (0.2) | 1.0 (0.46) | 0.9 (0.14) | G protein-coupled bile acid receptor 1 |
| GPER1 | ↑D (98) | 1.3 (0.01) | 1.5 (0.01) | 1.2 (0.11) | G protein-coupled estrogen receptor 1 |
| GUCA2B*** | ↑D (22) | 1.2 (0.4) | 1.4 (0.25) | 1.1 (0.75) | Guanylate cyclase activator 2B (uroguanylin) |
| HELLS*** | ↑ (2) | 1.2 (0.4) | 1.6 (0.01) | 1.1 (0.75) | Helicase, lymphoid-specific |
| HLA-DRB5 | ↓D (22) | 1.2 (0.55) | 1.0 (0.73) | 1.3 (0.49) | MHC, class II, DR beta 5 precursor |
| HSPA1A*** | ↓D (22) | 0.9 (0.48) | 0.9 (0.57) | 0.9 (0.51) | Heat shock 70 kda protein 1A/1B |
| HSPA1B | ↓D (22) | 1.0 (0.92) | 1.0 (0.77) | 1.0 (0.9) | Heat shock 70 kda protein 1B |
| IFIT3** | ↓D (21, 22) | 0.9 (0.07) | 0.9 (0.19) | 0.9 (0.07) | Interferon-induced protein with tetratricopeptide repeats 3 |
| IGFBP2*** | ↑D (22) | 1.6 (0.07) | 2.1 (0.01) | 1.2 (0.61) | Insulin-like growth factor-binding protein 2 precursor |
| IL10*** | ↓ (13, 30) | 0.8 (0.28) | 0.9 (0.62) | 0.7 (0.18) | Interleukin-10 precursor |
| IL13 | ↑ (125) | 0.9 (0.14) | 0.8 (0.02) | 1.0 (0.85) | Interleukin-13 precursor |
| IL1B | ↑ (4) | 0.8 (0.31) | 0.8 (0.31) | 0.8 (0.45) | Interleukin-1 beta proprotein |
| KCNS3 | ↑ (2) | 1.0 (0.81) | 0.8 (0.26) | 1.1 (0.48) | Potassium voltage-gated channel subfamily S member 3 |
| KDELR2 | ↑ (42) | 1.1 (0.45) | 1.2 (0.1) | 1.0 (0.73) | ER lumen protein retaining receptor 2 isof. 1 |
| LCN15*** | ↑D (22) | 1.0 (0.9) | 0.8 (0.16) | 1.2 (0.23) | Lipocalin 15 |
| LYZ | ↓ (2) | 1.0 (0.96) | 1.3 (0.32) | 0.8 (0.36) | Lysozyme C precursor |
| MCM5*** | ↑ (2) | 1.0 (0.7) | 1.2 (0.1) | 0.9 (0.32) | DNA replication licensing factor MCM5 |
| MS4A4A** | ↑ (2) | 1.4 (0.07) | 1.8 (0.01) | 1.1 (0.71) | Membrane-spanning 4-domains subfam. A member 4a isof. 1 |
| MUC20* | ↑ (2) | 1.2 (0.04) | 1.3 (0.01) | 1.1 (0.49) | Mucin-20 isof. S |
| MX1*** | ↓D (22) | 1.0 (0.94) | 1.1 (0.76) | 0.9 (0.67) | Interferon-induced GTP-binding protein Mx1 |
| NCF4* | ↑ (2) | 1.3 (0.04) | 1.6 (0.001) | 1.0 (0.99) | Neutrophil cytosol factor 4 isof. 2 |
| NOS2 | ↑ (4) | 0.9 (0.07) | 1.0 (0.48) | 0.8 (0.02) | Nitric oxide synthase, inducible |
| NR1H4 | ↑D (21) | 1.1 (0.88) | 1.9 (0.11) | 0.6 (0.18) | Bile acid receptor isof. 2 |
| OCLN** | ↑C (21) | 1.2 (0.06) | 1.2 (0.06) | 1.1 (0.17) | Occludin isof. B precursor |
| P2RY1*** | ↑ (81) | 1.1 (0.51) | 1.1 (0.37) | 1.0 (0.79) | P2Y purinoceptor 1 |
| P2RY2 | ↑ (81) | 1.0 (0.83) | 1.0 (0.31) | 0.9 (0.17) | P2Y purinoceptor 2 |
| P2RY4*** | ↑D (21, 22) | 1.0 (0.89) | 0.9 (0.13) | 1.1 (0.2) | Pyrimidinergic receptor P2Y, G-protein coupled, 4 |
| PDZD3*** | ↑D (21, 22) | 1.1 (0.25) | 1.2 (0.25) | 1.1 (0.4) | Na(+)/H(+) exchange regulatory cofactor NHE-RF4 isof. 2 |
| PSME2*** | ↑ (2) | 1.0 (0.62) | 1.2 (0.01) | 0.9 (0.09) | Proteasome activator complex subunit 2 |
| RBP2 | ↑D (22) | 1.2 (0.33) | 1.4 (0.08) | 1.0 (0.89) | Retinol-binding protein 2 |
| RFC4*** | ↑ (2) | 1.1 (0.27) | 1.3 (0.04) | 1.0 (0.81) | Replication factor C subunit 4 |
| S100A10 | ↑ (20, 106) | 0.9 (0.11) | 0.9 (0.34) | 0.8 (0.07) | Protein S100-A10 |
| SLC10A2 | ↑D (21) | 1.0 (0.86) | 1.1 (0.33) | 0.9 (0.51) | Ileal sodium/bile acid cotransporter |
| SLC6A4 | ↓ (36, 45, 63, 76, 122) | 1.9 (0.01) | 1.3 (0.38) | 2.9 (0.001) | Sodium-dependent serotonin transporter |
| TAP2*** | ↑ (2) | 1.1 (0.79) | 1.9 (0.05) | 0.6 (0.12) | Antigen peptide transporter 2 isof. 2 |
| TFF1 | ↑D (22) | 1.0 (0.92) | 1.1 (0.62) | 0.9 (0.76) | Trefoil factor 1 precursor |
| TH | ↑D (129) | 0.9 (0.25) | 0.9 (0.37) | 0.9 (0.26) | Tyrosine 3-monooxygenase isof. B |
| TJP1*** | ↓ (21, 119) | 0.9 (0.21) | 0.9 (0.5) | 0.9 (0.13) | Tight junction protein ZO-1 isof. A |
| TLR2*** | ↑ (12) | 1.0 (0.86) | 1.1 (0.64) | 1.0 (0.86) | Toll-like receptor 2 precursor |
| TLR4*** | ↑ (12) | 1.0 (0.63) | 1.0 (0.92) | 1.1 (0.36) | Toll-like receptor 4 isoform A precursor |
| TNFSF15 | ↓ (21, 112) | 1.0 (0.88) | 1.0 (0.68) | 1.0 (0.88) | TNF ligand superfam. member 15 isof. VEGI-251 precursor |
| TPH1 | ↓ (63) | 1.3 (0.17) | 1.5 (0.12) | 1.2 (0.4) | Tryptophan 5-hydroxylase 1 |
| TPSAB1 | ↑ (4, 75) | 0.8 (0.03) | 0.9 (0.17) | 0.8 (0.02) | Tryptase alpha/beta-1 precursor |
| TRPV1 | ↑ (64, 81, 131) | 0.8 (0.05) | 0.7 (0.08) | 0.8 (0.1) | TRP cation channel subfamily V member 1 |
| VIP* | ↑D (21, 22) | 0.7 (0.06) | 0.6 (0.03) | 0.8 (0.25) | VIP peptides isoform 2 preproprotein |
| VSIG2 | ↑ (2) | 0.8 (0.07) | 0.8 (0.16) | 0.8 (0.08) | V-set and Ig domain-containing protein 2 precursor |
| VSIG4* | ↑ (2) | 1.4 (0.03) | 1.7 (0) | 1.1 (0.5) | V-set and Ig domain-containing protein 4 isof. 1 precursor |
| WDR72*** | ↓D (14) | 0.8 (0.02) | 0.8 (0.01) | 0.9 (0.15) | WD repeat-containing protein 72 |
↑, increased vs. healthy controls (HCs); ↓ decreased vs. HCs; D, IBS with diarrhea; C, IBS with constipation; isof., isoform; dep., dependent; sub., subunit; Ig, immunoglobulin; recep., receptor; subfam., subfamily; MHC, major histocompatibility complex; TNF, tumor necrosis factor; TRP, transient receptor potential.
Our results agree with reported findings at P < 0.05,
P < 0.1 and
not significant but same direction, respectively.
Table 9.
Differentially expressed tight junction genes
| Peters | UCLA | Mayo/Aerssens | |
|---|---|---|---|
| Symbol | FC (P) | FC (FDR) | FC (P, FDR) |
| MARK2 | 1.2 (0.02) | 1.4 (0.03) | 0.9 (0.49, 0.78) |
| LLGL2 | 1.2 (0.03) | 1.3 (0.02) | 1.0 (0.34, 0.69) |
| TJAP1 | 1.2 (0.10) | 0.6 (0.01) | 1.0 (0.35, 0.69) |
| PRKCZ | 1.2 (0.11) | 1.8 (0.01) | 1.0 (1.00, 1.00) |
| CLDN15 | 1.2 (0.22) | 1.4 (0.02) | 0.9 (0.01, 0.32) |
| MLLT4 | 1.2 (0.36) | 1.5 (0.01) | 1.1 (0.02, 0.34) |
| PRKCI | 1.1 (0.46) | 1.3 (0.04) | 1.1 (0.10, 0.47) |
| CSNK2A1 | 2.0 (0.50) | 1.7 (0.00) | 0.9 (0.01, 0.32) |
| CLDN6 | 1.1 (0.56) | 0.7 (0.01) | 0.9 (0.08, 0.45) |
| SPTA1 | 0.9 (0.69) | 1.4 (0.03) | 1.0 (0.20, 0.58) |
| CSDA | 1.0 (0.75) | 0.7 (0.02) | 0.8 (0.00, 0.30) |
| MPDZ | 1.1 (0.77) | 0.7 (0.02) | 1.1 (0.15, 0.52) |
| B2M | 3.1 (0.83) | 1.4 (0.03) | 1.0 (0.04, 0.39) |
Tight junction genes measured in IBS-C vs. HC by Peters et al. (97); 13 of 88 were differentially expressed (FDR < 0.05). FC, fold change, FDR, Benjamini-Hochberg adjusted P value.
Evidence for Neurally Mediated Mechanisms in IBS
cAMP-PKA signaling.
WGCNA and IPA analysis identified upregulation of cAMP-PKA signaling in IBS and IBS-C biopsies compared with HCs. The downregulation of PDE2A (validated by nCounter), which hydrolyzes cAMP, is also supportive. PKA signaling activates T-type calcium channels, and in our dataset, expression of the T-type calcium channel Cav3.1 (CACNA1G) was increased in IBS (both IBS-C and IBS-D). The potential importance of this channel in IBS is supported by a study in rats, in which knockdown of Cav3.1 attenuated butyrate-induced visceral hypersensitivity (83). In addition, Scanzi et al. (127) found increased expression of an alternate isoform with similar properties (Cav3.2, CACNA1H) in biopsies from IBS patients vs. HCs and in a mouse model of visceral pain.
While cAMP-PKA signaling is important in nociceptive pain signaling (11), this pathway regulates many aspects of cellular physiology including chloride secretion (15) and serotonin release by enterochromaffin cells (34). Furthermore, while evidence exists for altered neuronal sensitivity in IBS (9, 16–18, 27, 38, 95, 100, 118, 124), both desensitization (95) and sensitization (124) may occur. For these reasons, as well as the increased relative abundance of mucosal projections of enteric neurons compared with extrinsic afferents in the colonic mucosa, our findings are more likely to represent signaling mechanisms unrelated to pain. It is also possible that the differences in cAMP-PKA signaling may involve other cell types. Interestingly one of the hub genes in the cAMP-PKA module is an epithelial cell apical sodium channel (SCNN1D, correlated with module eigengene: 0.91), which may influence fluid transport and contribute to the constipation phenotype (62).
Neurotransmitter and neuropeptide signaling.
CRHR1 was downregulated (Mayo study, current study microarray, and nCounter), perhaps involving a response to increased stress-induced release of CRH. In a previous study (30), CRHR1 mRNA was not detectable in 81% of samples by quantitative real-time PCR, but microarray and nCounter are both more sensitive detection methods.
The γ-aminobutyric acid type A (GABA A) receptor γ1 subunit (GABRG1) was also downregulated in IBS. In a genomewide association study, a variant in this gene was associated with IBS within the discovery (but not replication) sample (42). In the enteric nervous system, activation of GABA A receptors exerts an excitatory effect (33). These receptors are also located on epithelial cells, and agonists stimulate luminal chloride secretion (73). Thus, the potential relevance of GABA receptor downregulation in IBS-C could be decreased peristalsis and/or secretion. An additional mechanism may be related to GABA receptor subunit stoichiometry. There are 19 GABA receptor subunit genes in the human genome, and several dozen subunit combinations for the pentameric receptor have been described (35). Differential composition of GABA receptors can affect membrane targeting and ligand sensitivity, which may be relevant in IBS (35, 65). It is also possible that the relevance to IBS is related to neuroimmune mechanisms, as GABA receptors are present on immune cells (8).
Neurite outgrowth.
Several genes related to neurite outgrowth were dysregulated in IBS-C vs. HCs both in our cohort and in Mayo (reticulon 3, SSPO, GRFA4). SSPO, which encodes the secreted glycoprotein SCO-spondin, which may participate in neurite outgrowth and neuron/glial interaction (85), was previously found to be hypermethylated in peripheral blood mononuclear cells in IBS (82). Interestingly, changes in neuron density have been reported in full-thickness samples from animal models of IBS (72, 116) and in patients with slow-transit constipation (123).
Additional Notable Findings
We also found that high-mobility group box 1 (HMGB1) was upregulated in IBS-C. HMGB1 is a chromatin-binding protein as well as a proinflammatory cytokine capable of increasing permeability of intestinal epithelial cell monolayers and ileal permeability and bacterial translocation in mice. While HMGB1 has well-defined roles in inflammation, it has recently emerged as a potential mediator of nociceptive pain signaling (3, 40, 66). For example, in a mouse model of cyclophosphamide-induced cystitis, it mediated mechanical bladder sensitivity induced by the protease-activated receptor PAR4 (66). HMGB1 is a target of cyclin-dependent kinase-5 (CDK5) (117), which was also upregulated and validated in our cohort. In a colon cancer-derived cell line, CDK5 phosphorylated HMGB1 at Ser181, a posttranslational modification that has been associated with increased secretion of HMGB1 (71). CDK5 activation is also pronociceptive through interactions with TRPV and purinergic receptors (91, 126).
We would also like to note the enrichment of a few gene sets that met the threshold of FDR < 0.25. Specifically, enrichment of “signaling by fibroblast growth factor receptor 1 (FGFR1) mutants” in HCs vs. IBS overall may warrant further investigation into this pathway. In addition, enrichment of the gene sets “allograft rejection”, “graft vs. host disease”, and “autoimmune thyroid disease” in men vs. women with IBS suggests that immune regulation may play a larger role in IBS among men.
Limitations
The most important limitation to consider in the interpretation of these results is that the results are not cell type specific; furthermore, there may be group differences in cell type composition of the biopsies with no reliable methods to measure or account for these differences. The upregulated gene sets in HCs vs. IBS-C suggest that there may be more neurons and/or increased transcriptional activity in neurons. However, decreased enteric neurons in the submucosal plexus has previously been associated with constipation (10). We attempted to address this question by looking at expression of traditional neuronal and astrocyte/glial markers (Table 10), which did not reveal significant differences. Additionally, there are limitations to drawing conclusions on neuronal physiology based on findings from mucosal biopsies. Although the mucosa is innervated by sensory nerve fibers, and biopsies frequently include some submucosa, an alternative explanation is that the expression changes may reflect alterations in transcriptional activity of glial cells or enteroendocrine cells.
Table 10.
Expression of neuronal and glial/astrocytic markers
| IBS-C vs. HC | IBS vs. HC | IBS-D vs. HC | ||
|---|---|---|---|---|
| Gene Name, Symbol (alias, Entrez ID) | Mean | FC (p) | FC (p) | FC (p) |
| Neuronal | ||||
| Enolase2, ENO2 (neuron speific enolase, 2026) | 6.8 | 1.0 (0.62) | 1.0 (0.74) | 1.1 (0.29) |
| Ubiquitin C-terminal hydrolase L1, UCHL1 (PGP9.5, 7345) | 9.0 | 0.8 (0.2) | 1.0 (0.9) | 1.3 (0.1) |
| Microtubule associated protein 2, MAP2 (4133) | 5.0 | 1.2 (0.12) | 1.1 (0.42) | 1.2 (0.31) |
| 8.2 | 0.9 (0.35) | 1.0 (0.96) | 1.0 (0.84) | |
| Synaptophysin, SYP (6855) | 6.5 | 1.1 (0.15) | 1.1 (0.43) | 1.0 (0.93) |
| Neurofilament, heavy, NEFH (4744) | 15.2 | 1.0 (0.74) | 1.0 (0.39) | 0.9 (0.24) |
| Calbindin 2, CALB2 (calretinin, 794) | 9.2 | 0.8 (0.14) | 0.9 (0.3) | 1.0 (0.77) |
| 7.5 | 0.6 (0.04) | 0.7 (0.04) | 0.7 (0.13) | |
| Astrocyte/glial | ||||
| Proteolipid protein 1, PLP1 (5354) | 7.3 | 0.8 (0.08) | 0.9 (0.49) | 1.1 (0.54) |
| 6.3 | 0.9 (0.46) | 1.0 (0.72) | 1.0 (0.9) | |
| S100 calcium binding protein B, S100B (6285) | 6.1 | 0.8 (0.14) | 1.0 (0.8) | 0.9 (0.28) |
| Glial fibrillary acidic protein, GFAP (2670) | 7.9 | 0.9 (0.56) | 0.9 (0.4) | 0.9 (0.38) |
Mean is mean log2 normalized signal; FC, fold change.
Another significant limitation is the small sample size of the microarray study. However, the composition (equal number of men and women, medication use) and rigor of phenotyping increases the probability that significant findings are not due to sampling bias. Despite the larger sample size included in the IBS vs. HC comparison (n = 20 vs. n = 10 for IBS-C), we did not find DETs meeting a FDR < 0.05 threshold. This may be have been due to bowel habit-specific differences in peripheral mechanisms, as has been suggested by others (57). Importantly, our study was powered to detect only larger changes (FC ≥ 2) at an FDR ≤0.05. Therefore, caution should be used in the interpretation of negative results (absence of changes).
Reproducibility between IBS studies remains a challenge. There were 85 DETs in the literature that were present on our microarray, and we found the same direction of change (up or down in IBS vs. HCs) for 44 of these (Table 8). We did not find differences in SERT, which has been reported in several (36, 63, 122) but not all (20, 22) studies. Although we have attempted to highlight findings that are reproducible though comparison with publicly available data, this approach has computational limitations as well as limitations due to differences in protocol (e.g., different bowel preparation) and patient populations. In particular, the current study used Rome III (80) criteria, whereas Mayo and Nottingham used Rome II (115), which are less specific and may identify a less severe population. Given that inclusion of IBS patients in all three cohorts was supervised by experts in the field, the differences in diagnostic criteria are less likely to have resulted in sample differences than for survey-based studies. We recommend pooling resources and increasing collaboration with the goal of larger samples that are uniformly phenotyped and processed in order to yield robust findings.
Conclusions
In conclusion, using a small but well-phenotyped and age- and sex-balanced sample, we have found additional support for peripheral mechanisms in IBS and demonstrate that these findings were more significant in IBS-C patients within our cohort, suggesting that pathophysiological processes differ between IBS-C and IBS-D. Based on some of the observed changes, future investigations should focus on alterations in neuronal physiology.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants P50 DK-64539 (E. A. Mayer, L. Chang) and T32 DK-07180 (E. J. Videlock) and Barbara and Joel Marcus GI Fellowship Seed Grant (E. J. Videlock). Study personnel: P30 DK-41301 (PI, J. E. Rozengurt). Core services: NCATS UL1TR000124.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.J.V. and L.C. conceived and designed research; E.J.V., S.M.-J., and L.C. performed experiments; E.J.V. and S.M.-J. analyzed data; E.J.V., S.M.-J., J.M.H., D.I., C.P., E.A.M., and L.C. interpreted results of experiments; E.J.V. prepared figures; E.J.V. drafted manuscript; E.J.V., S.M.-J., J.M.H., D.I., C.P., E.A.M., and L.C. edited and revised manuscript; L.C. approved final version of manuscript.
Supplemental Data
Differentially expressed transcripts in IBS-C vs healthy controls - .xlsx (246 KB)
ACKNOWLEDGMENTS
We thank the study participants and staff of the Oppenheimer Center for Neurobiology of Stress and Resilience (CNSR), UCLA Clinical and Translational Research Center, E. Faure-Kumar in the UCLA Center for Systems Biomedicine, and Cathy Liu in the CNSR for assistance with WebApp publication.
REFERENCES
- 1.Abe T, Kanemitu Y, Nakasone M, Kawahata I, Yamakuni T, Nakajima A, Suzuki N, Nishikawa M, Hishinuma T, Tomioka Y. SLC10A4 is a protease-activated transporter that transports bile acids. J Biochem 154: 93–101, 2013. doi: 10.1093/jb/mvt031. [DOI] [PubMed] [Google Scholar]
- 2.Aerssens J, Camilleri M, Talloen W, Thielemans L, Göhlmann HW, Van Den Wyngaert I, Thielemans T, De Hoogt R, Andrews CN, Bharucha AE, Carlson PJ, Busciglio I, Burton DD, Smyrk T, Urrutia R, Coulie B. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 194–205, 2008. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agalave NM, Svensson CI. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol Med 20: 569–578, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An S, Zong G, Wang Z, Shi J, Du H, Hu J. Expression of inducible nitric oxide synthase in mast cells contributes to the regulation of inflammatory cytokines in irritable bowel syndrome with diarrhea. Neurogastroenterol Motil 28: 1083–1093, 2016. doi: 10.1111/nmo.12811. [DOI] [PubMed] [Google Scholar]
- 5.Arloth J, Bader DM, Röh S, Altmann A. Re-annotator: annotation pipeline for microarray probe sequences. PLoS One 10: e0139516, 2015. doi: 10.1371/journal.pone.0139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 130: 34–43, 2006. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Auteri M, Zizzo MG, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93: 11–21, 2015. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Bassotti G, Villanacci V, Nascimbeni R, Asteria CR, Fisogni S, Nesi G, Legrenzi L, Mariano M, Tonelli F, Morelli A, Salerni B. Colonic neuropathological aspects in patients with intractable constipation due to obstructed defecation. Mod Pathol 20: 367–374, 2007. doi: 10.1038/modpathol.3800748. [DOI] [PubMed] [Google Scholar]
- 11.Bavencoffe A, Li Y, Wu Z, Yang Q, Herrera J, Kennedy EJ, Walters ET, Dessauer CW. Persistent electrical activity in primary nociceptors after spinal cord injury is maintained by scaffolded adenylyl cyclase and protein kinase A and is associated with altered adenylyl cyclase regulation. J Neurosci 36: 1660–1668, 2016. doi: 10.1523/JNEUROSCI.0895-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belmonte L, Beutheu Youmba S, Bertiaux-Vandaële N, Antonietti M, Lecleire S, Zalar A, Gourcerol G, Leroi AM, Déchelotte P, Coëffier M, Ducrotté P. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS One 7: e42777, 2012. doi: 10.1371/journal.pone.0042777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennet SM, Polster A, Törnblom H, Isaksson S, Capronnier S, Tessier A, Le Nevé B, Simrén M, Öhman L. Global cytokine profiles and association with clinical characteristics in patients with irritable bowel syndrome. Am J Gastroenterol 111: 1165–1176, 2016. doi: 10.1038/ajg.2016.223. [DOI] [PubMed] [Google Scholar]
- 14.Bojjireddy N, Guzman-Hernandez ML, Reinhard NR, Jovic M, Balla T. EFR3s are palmitoylated plasma membrane proteins that control responsiveness to G-protein-coupled receptors. J Cell Sci 128: 118–128, 2015. doi: 10.1242/jcs.157495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borthwick LA, McGaw J, Conner G, Taylor CJ, Gerke V, Mehta A, Robson L, Muimo R. The formation of the cAMP/protein kinase A-dependent annexin 2-S100A10 complex with cystic fibrosis conductance regulator protein (CFTR) regulates CFTR channel function. Mol Biol Cell 18: 3388–3397, 2007. doi: 10.1091/mbc.E07-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buhner S, Braak B, Li Q, Kugler EM, Klooker T, Wouters M, Donovan J, Vignali S, Mazzuoli-Weber G, Grundy D, Boeckxstaens G, Schemann M. Neuronal activation by mucosal biopsy supernatants from irritable bowel syndrome patients is linked to visceral sensitivity. Exp Physiol 99: 1299–1311, 2014. doi: 10.1113/expphysiol.2014.080036. [DOI] [PubMed] [Google Scholar]
- 17.Buhner S, Li Q, Berger T, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Schemann M. Submucous rather than myenteric neurons are activated by mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol Motil 24: 1134, e572, 2012. doi: 10.1111/nmo.12011. [DOI] [PubMed] [Google Scholar]
- 18.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434, 2009. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol 592: 2967–2980, 2014. doi: 10.1113/jphysiol.2014.270892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilleri M, Andrews CN, Bharucha AE, Carlson PJ, Ferber I, Stephens D, Smyrk TC, Urrutia R, Aerssens J, Thielemans L, Göhlmann H, van den Wyngaert I, Coulie B. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology 132: 17–25, 2007. doi: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, Carlson P, Acosta A, Busciglio I. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol 309: G10–G20, 2015. doi: 10.1152/ajpgi.00080.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M, Carlson P, Acosta A, Busciglio I, Nair AA, Gibbons SJ, Farrugia G, Klee EW. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol Gastrointest Liver Physiol 306: G1089–G1098, 2014. doi: 10.1152/ajpgi.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camilleri M, Carlson P, Valentin N, Acosta A, O’Neill J, Eckert D, Dyer R, Na J, Klee EW, Murray JA. Pilot study of small bowel mucosal gene expression in patients with irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol 311: G365–G376, 2016. doi: 10.1152/ajpgi.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M, Oduyebo I, Halawi H. Chemical and molecular factors in irritable bowel syndrome: current knowledge, challenges, and unanswered questions. Am J Physiol Gastrointest Liver Physiol 311: G777–G784, 2016. doi: 10.1152/ajpgi.00242.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 40: 1023–1034, 2014. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 26.Carrel D, Masson J, Al Awabdh S, Capra CB, Lenkei Z, Hamon M, Emerit MB, Darmon M. Targeting of the 5-HT1A serotonin receptor to neuronal dendrites is mediated by Yif1B. J Neurosci 28: 8063–8073, 2008. doi: 10.1523/JNEUROSCI.4487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 117: 636–647, 2007. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, Bertrand J, Liedtke W, Dubourdeau M, Bertrand-Michel J, Zecchi L, Stanghellini V, Bunnett NW, Barbara G, Vergnolle N. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 149: 433–444, 2015. doi: 10.1053/j.gastro.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Chang K, Seabold GK, Wang CY, Wenthold RJ. Reticulon 3 is an interacting partner of the SALM family of adhesion molecules. J Neurosci Res 88: 266–274, 2010. doi: 10.1002/jnr.22209. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, Presson AP, Yuan PQ, Cortina G, Gong H, Singh S, Licudine A, Mayer M, Tache Y, Pothoulakis C, Mayer EA. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol 107: 262–272, 2012. doi: 10.1038/ajg.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JB, Zhang J, Hu HZ, Xue M, Jin YJ. Polymorphisms of TGFB1, TLE4 and MUC22 are associated with childhood asthma in Chinese population. Allergol Immunopathol (Madr) 45: 432–438, 2017. doi: 10.1016/j.aller.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Li L, Khan MN, Shi L, Wang Z, Zheng F, Gong F, Fang M. HMGB1 exacerbates experimental mouse colitis by enhancing innate lymphoid cells 3 inflammatory responses via promoted IL-23 production. Innate Immun 22: 696–705, 2016. doi: 10.1177/1753425916669862. [DOI] [PubMed] [Google Scholar]
- 33.Cherubini E, North RA. Actions of gamma-aminobutyric acid on neurones of guinea-pig myenteric plexus. Br J Pharmacol 82: 93–100, 1984. doi: 10.1111/j.1476-5381.1984.tb16445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, Timberlake A, Sumpio B, Pfragner R, Modlin IM, Kidd M. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol 302: G397–G405, 2012. doi: 10.1152/ajpgi.00087.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua HC, Chebib M. GABAA Receptors and the Diversity in their Structure and Pharmacology. Adv Pharmacol 79: 1–34, 2017. doi: 10.1016/bs.apha.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664, 2004. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature 505: 612–613, 2014. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De Ponti F, Corinaldesi R, Barbara G. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 106: 1290–1298, 2011. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 39.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol 104: 392–400, 2009. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 40.Das N, Dewan V, Grace PM, Gunn RJ, Tamura R, Tzarum N, Watkins LR, Wilson IA, Yin H. HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Reports 17: 1128–1140, 2016. doi: 10.1016/j.celrep.2016.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101: 1288–1294, 2006. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 42.Ek WE, Reznichenko A, Ripke S, Niesler B, Zucchelli M, Rivera NV, Schmidt PT, Pedersen NL, Magnusson P, Talley NJ, Holliday EG, Houghton L, Gazouli M, Karamanolis G, Rappold G, Burwinkel B, Surowy H, Rafter J, Assadi G, Li L, Papadaki E, Gambaccini D, Marchi S, Colucci R, Blandizzi C, Barbaro R, Karling P, Walter S, Ohlsson B, Tornblom H, Bresso F, Andreasson A, Dlugosz A, Simren M, Agreus L, Lindberg G, Boeckxstaens G, Bellini M, Stanghellini V, Barbara G, Daly MJ, Camilleri M, Wouters MM, D’Amato M. Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut 64: 1774–1782, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, Matthews L, May B, Milacic M, Rothfels K, Shamovsky V, Webber M, Weiser J, Williams M, Wu G, Stein L, Hermjakob H, D’Eustachio P. The Reactome pathway Knowledgebase. Nucleic Acids Res 44, D1: D481–D487, 2016. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics 23: 257–258, 2007. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- 45.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139: 249–258, 2010. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315, 2004. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 47.Gentleman R. annotate: Annotation for microarrays. In: R Package Version 1550. 2017. [Google Scholar]
- 48.Ghaleb AM, Bialkowska AB, Snider AJ, Gnatenko DV, Hannun YA, Yang VW, Schmidt VAIQ. IQ motif-containing GTPase-activating protein 2 (IQGAP2) is a novel regulator of colonic inflammation in mice. PLoS One 10: e0129314, 2015. doi: 10.1371/journal.pone.0129314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gralnek IM, Hays RD, Kilbourne A, Mayer EA. Impact of irritable bowel syndrome on health-related quality of life (HRQOL). Gastroenterology 116: A1038, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Guo X, Qi X. VCP cooperates with UBXD1 to degrade mitochondrial outer membrane protein MCL1 in model of Huntington’s disease. Biochim Biophys Acta 1863: 552–559, 2017. doi: 10.1016/j.bbadis.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hickey FB, Corcoran JB, Docherty NG, Griffin B, Bhreathnach U, Furlong F, Martin F, Godson C, Murphy M. IHG-1 promotes mitochondrial biogenesis by stabilizing PGC-1α. J Am Soc Nephrol 22: 1475–1485, 2011. doi: 10.1681/ASN.2010111154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hijikata M, Matsushita I, Tanaka G, Tsuchiya T, Ito H, Tokunaga K, Ohashi J, Homma S, Kobashi Y, Taguchi Y, Azuma A, Kudoh S, Keicho N. Molecular cloning of two novel mucin-like genes in the disease-susceptibility locus for diffuse panbronchiolitis. Hum Genet 129: 117–128, 2011. doi: 10.1007/s00439-010-0906-4. [DOI] [PubMed] [Google Scholar]
- 53.Hofer-Warbinek R, Schmid JA, Mayer H, Winsauer G, Orel L, Mueller B, Wiesner C, Binder BR, de Martin R. A highly conserved proapoptotic gene, IKIP, located next to the APAF1 gene locus, is regulated by p53. Cell Death Differ 11: 1317–1325, 2004. doi: 10.1038/sj.cdd.4401502. [DOI] [PubMed] [Google Scholar]
- 54.Houghton LA, Atkinson W, Lockhart C, Whorwell PJ, Keevil B. Sigmoid-colonic motility in health and irritable bowel syndrome: a role for 5-hydroxytryptamine. Neurogastroenterol Motil 19: 724–731, 2007. doi: 10.1111/j.1365-2982.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 55.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 57.Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, Isaacs NJ, Maldeniya L, Martin CM, Persson J, Andrews JM, Holtmann G, Blackshaw LA, Brierley SM. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62: 1456–1465, 2013. doi: 10.1136/gutjnl-2011-301856. [DOI] [PubMed] [Google Scholar]
- 58.Ichikawa K, Kubota Y, Nakamura T, Weng JS, Tomida T, Saito H, Takekawa M. MCRIP1, an ERK substrate, mediates ERK-induced gene silencing during epithelial-mesenchymal transition by regulating the co-repressor CtBP. Mol Cell 58: 35–46, 2015. doi: 10.1016/j.molcel.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 59.Ito T, Niwa J, Hishikawa N, Ishigaki S, Doyu M, Sobue G. Dorfin localizes to Lewy bodies and ubiquitylates synphilin-1. J Biol Chem 278: 29106–29114, 2003. doi: 10.1074/jbc.M302763200. [DOI] [PubMed] [Google Scholar]
- 60.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30, 2000. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kauffmann A, Rayner TF, Parkinson H, Kapushesky M, Lukk M, Brazma A, Huber W. Importing ArrayExpress datasets into R/Bioconductor. Bioinformatics 25: 2092–2094, 2009. doi: 10.1093/bioinformatics/btp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 63.Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol 302: G1053–G1060, 2012. doi: 10.1152/ajpgi.00153.2011. [DOI] [PubMed] [Google Scholar]
- 64.Keszthelyi D, Troost FJ, Jonkers DM, Helyes Z, Hamer HM, Ludidi S, Vanhoutvin S, Venema K, Dekker J, Szolcsányi J, Masclee AA. Alterations in mucosal neuropeptides in patients with irritable bowel syndrome and ulcerative colitis in remission: a role in pain symptom generation? Eur J Pain 17: 1299–1306, 2013. doi: 10.1002/j.1532-2149.2013.00309.x. [DOI] [PubMed] [Google Scholar]
- 65.Khom S, Baburin I, Timin EN, Hohaus A, Sieghart W, Hering S. Pharmacological properties of GABAA receptors containing gamma1 subunits. Mol Pharmacol 69: 640–649, 2005. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- 66.Kouzoukas DE, Ma F, Meyer-Siegler KL, Westlund KN, Hunt DE, Vera PL. Protease-activated receptor 4 induces bladder pain through high mobility group box-1. PLoS One 11: e0152055, 2016. doi: 10.1371/journal.pone.0152055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larhammar M, Patra K, Blunder M, Emilsson L, Peuckert C, Arvidsson E, Rönnlund D, Preobraschenski J, Birgner C, Limbach C, Widengren J, Blom H, Jahn R, Wallén-Mackenzie Å, Kullander K. SLC10A4 is a vesicular amine-associated transporter modulating dopamine homeostasis. Biol Psychiatry 77: 526–536, 2015. doi: 10.1016/j.biopsych.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 69.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15: R29, 2014. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee AK, Potts PR. A comprehensive guide to the MAGE family of ubiquitin ligases. J Mol Biol 429: 1114–1142, 2017. doi: 10.1016/j.jmb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee H, Park M, Shin N, Kim G, Kim YG, Shin JS, Kim H. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun 424: 321–326, 2012. doi: 10.1016/j.bbrc.2012.06.116. [DOI] [PubMed] [Google Scholar]
- 72.Li S, Fei G, Fang X, Yang X, Sun X, Qian J, Wood JD, Ke M. Changes in enteric neurons of small intestine in a rat model of irritable bowel syndrome with diarrhea. J Neurogastroenterol Motil 22: 310–320, 2016. doi: 10.5056/jnm15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Xiang YY, Lu WY, Liu C, Li J. A novel role of intestine epithelial GABAergic signaling in regulating intestinal fluid secretion. Am J Physiol Gastrointest Liver Physiol 303: G453–G460, 2012. doi: 10.1152/ajpgi.00497.2011. [DOI] [PubMed] [Google Scholar]
- 74.Li Z, Mou H, Wang T, Xue J, Deng B, Qian L, Zhou Y, Gong W, Wang JM, Wu G, Zhou CF, Fang J, Le Y. A non-secretory form of FAM3B promotes invasion and metastasis of human colon cancer cells by upregulating Slug expression. Cancer Lett 328: 278–284, 2013. doi: 10.1016/j.canlet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang WJ, Zhang G, Luo HS, Liang LX, Huang D, Zhang FC. Tryptase and protease-activated receptor 2 expression levels in irritable bowel syndrome. Gut Liver 10: 382–390, 2016. doi: 10.5009/gnl14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun 469: 288–293, 2016. doi: 10.1016/j.bbrc.2015.11.102. [DOI] [PubMed] [Google Scholar]
- 77.Lin SW, Chen JC, Hsu LC, Hsieh CL, Yoshida A. Human gamma-aminobutyraldehyde dehydrogenase (ALDH9): cDNA sequence, genomic organization, polymorphism, chromosomal localization, and tissue expression. Genomics 34: 376–380, 1996. doi: 10.1006/geno.1996.0300. [DOI] [PubMed] [Google Scholar]
- 78.Liu P, Hwang JT. Quick calculation for sample size while controlling false discovery rate with application to microarray analysis. Bioinformatics 23: 739–746, 2007. doi: 10.1093/bioinformatics/btl664. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Lear T, Zhao Y, Zhao J, Zou C, Chen BB, Mallampalli RK. F-box protein Fbxl18 mediates polyubiquitylation and proteasomal degradation of the pro-apoptotic SCF subunit Fbxl7. Cell Death Dis 6: e1630, 2015. doi: 10.1038/cddis.2014.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 130: 1480–1491, 2006. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 81.Luo Y, Feng C, Wu J, Wu Y, Liu D, Wu J, Dai F, Zhang J. P2Y1, P2Y2, and TRPV1 receptors are increased in diarrhea-predominant irritable bowel syndrome and P2Y2 correlates with abdominal pain. Dig Dis Sci 61: 2878–2886, 2016. doi: 10.1007/s10620-016-4211-5. [DOI] [PubMed] [Google Scholar]
- 82.Mahurkar S, Polytarchou C, Iliopoulos D, Pothoulakis C, Mayer EA, Chang L. Genome-wide DNA methylation profiling of peripheral blood mononuclear cells in irritable bowel syndrome. Neurogastroenterol Motil 28: 410–422, 2016. doi: 10.1111/nmo.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrère C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, Eschalier A, Bourinet E, Ardid D. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci USA 108: 11268–11273, 2011. doi: 10.1073/pnas.1100869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel disorders. Gastroenterology pii; S0016-5085(16)00222-5, 2016. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27144627&dopt=Abstract 2016 Feb 18 [Epub ahead of print]. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 85.Meiniel A, Meiniel R, Gonçalves-Mendes N, Creveaux I, Didier R, Dastugue B. The thrombospondin type 1 repeat (TSR) and neuronal differentiation: roles of SCO-spondin oligopeptides on neuronal cell types and cell lines. Int Rev Cytol 230: 1–39, 2003. doi: 10.1016/S0074-7696(03)30001-4. [DOI] [PubMed] [Google Scholar]
- 86.Meiniel O, Meiniel A. The complex multidomain organization of SCO-spondin protein is highly conserved in mammals. Brain Res Brain Res Rev 53: 321–327, 2007. doi: 10.1016/j.brainresrev.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Melief EJ, Gibbs JT, Li X, Morgan RG, Keene CD, Montine TJ, Palmiter RD, Darvas M. Characterization of cognitive impairments and neurotransmitter changes in a novel transgenic mouse lacking Slc10a4. Neuroscience 324: 399–406, 2016. doi: 10.1016/j.neuroscience.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Missler M, Südhof TC. Neurexophilins form a conserved family of neuropeptide-like glycoproteins. J Neurosci 18: 3630–3638, 1998. doi: 10.1523/JNEUROSCI.18-10-03630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 90.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology 112: 55–63, 1997. doi: 10.1016/S0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 91.Nair A, Simonetti M, Fabbretti E, Nistri A. The Cdk5 kinase downregulates ATP-gated ionotropic P2X3 receptor function via serine phosphorylation. Cell Mol Neurobiol 30: 505–509, 2010. doi: 10.1007/s10571-009-9483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nalaskowski MM, Fliegert R, Ernst O, Brehm MA, Fanick W, Windhorst S, Lin H, Giehler S, Hein J, Lin YN, Mayr GW. Human inositol 1,4,5-trisphosphate 3-kinase isoform B (IP3KB) is a nucleocytoplasmic shuttling protein specifically enriched at cortical actin filaments and at invaginations of the nuclear envelope. J Biol Chem 286: 4500–4510, 2011. doi: 10.1074/jbc.M110.173062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Malley D, Buckley MM, McKernan DP, Quigley EM, Cryan JF, Dinan TG. Soluble mediators in plasma from irritable bowel syndrome patients excite rat submucosal neurons. Brain Behav Immun 44: 57–67, 2015. doi: 10.1016/j.bbi.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Ohman L, Lindmark AC, Isaksson S, Posserud I, Strid H, Sjövall H, Simrén M. Increased TLR2 expression on blood monocytes in irritable bowel syndrome patients. Eur J Gastroenterol Hepatol 24: 398–405, 2012. [DOI] [PubMed] [Google Scholar]
- 95.Ostertag D, Buhner S, Michel K, Pehl C, Kurjak M, Götzberger M, Schulte-Frohlinde E, Frieling T, Enck P, Phillip J, Schemann M. Reduced responses of submucous neurons from irritable bowel syndrome patients to a cocktail containing histamine, serotonin, TNFalpha, and tryptase (IBS-cocktail). Front Neurosci 9: 465, 2015. doi: 10.3389/fnins.2015.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ouederni M, Vincent QB, Frange P, Touzot F, Scerra S, Bejaoui M, Bousfiha A, Levy Y, Lisowska-Grospierre B, Canioni D, Bruneau J, Debré M, Blanche S, Abel L, Casanova JL, Fischer A, Picard C. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood 118: 5108–5118, 2011. doi: 10.1182/blood-2011-05-352716. [DOI] [PubMed] [Google Scholar]
- 97.Peters SA, Edogawa S, Sundt WJ, Dyer RB, Dalenberg DA, Mazzone A, Singh RJ, Moses N, Smyrk TC, Weber C, Linden DR, MacNaughton WK, Turner JR, Camilleri M, Katzka DA, Farrugia G, Grover M. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am J Gastroenterol 112: 913–923, 2017. doi: 10.1038/ajg.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin B, Dong L, Guo X, Jiang J, He Y, Wang X, Li L, Zhao J. Expression of G protein-coupled estrogen receptor in irritable bowel syndrome and its clinical significance. Int J Clin Exp Pathol 7: 2238–2246, 2014. [PMC free article] [PubMed] [Google Scholar]
- 99.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 100.Rolland-Fourcade C, Denadai-Souza A, Cirillo C, Lopez C, Jaramillo JO, Desormeaux C, Cenac N, Motta JP, Larauche M, Taché Y, Berghe PV, Neunlist M, Coron E, Kirzin S, Portier G, Bonnet D, Alric L, Vanner S, Deraison C, Vergnolle N. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut 66: 1767–1778, 2017. doi: 10.1136/gutjnl-2016-312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rong Z, Wang A, Li Z, Ren Y, Cheng L, Li Y, Wang Y, Ren F, Zhang X, Hu J, Chang Z. IL-17RD (Sef or IL-17RLM) interacts with IL-17 receptor and mediates IL-17 signaling. Cell Res 19: 208–215, 2009. doi: 10.1038/cr.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scanzi J, Accarie A, Muller E, Pereira B, Aissouni Y, Goutte M, Joubert-Zakeyh J, Picard E, Boudieu L, Mallet C, Gelot A, Ardid D, Carvalho FA, Dapoigny M. Colonic overexpression of the T-type calcium channel Cav 3.2 in a mouse model of visceral hypersensitivity and in irritable bowel syndrome patients. Neurogastroenterol Motil 28: 1632–1640, 2016. doi: 10.1111/nmo.12860. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt S, Moncada M, Burger S, Geyer J. Expression, sorting and transport studies for the orphan carrier SLC10A4 in neuronal and non-neuronal cell lines and in Xenopus laevis oocytes. BMC Neurosci 16: 35, 2015. doi: 10.1186/s12868-015-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scoumanne A, Molina-Ortiz P, Monteyne D, Perez-Morga D, Erneux C, Schurmans S. Specific expression and function of inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) in wild type and knock-out mice. Adv Biol Regul 62: 1–10, 2016. doi: 10.1016/j.jbior.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, Suppl 20: 22–33, 1998. [PubMed] [Google Scholar]
- 106.Shiotani A, Kusunoki H, Kimura Y, Ishii M, Imamura H, Tarumi K, Manabe N, Kamada T, Hata J, Haruma K. S100A expression and interleukin-10 polymorphisms are associated with ulcerative colitis and diarrhea predominant irritable bowel syndrome. Dig Dis Sci 58: 2314–2323, 2013. doi: 10.1007/s10620-013-2677-y. [DOI] [PubMed] [Google Scholar]
- 107.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 108.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 12: 1151–1158, 2011. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 109.Splinter PL, Lazaridis KN, Dawson PA, LaRusso NF. Cloning and expression of SLC10A4, a putative organic anion transport protein. World J Gastroenterol 12: 6797–6805, 2006. doi: 10.3748/wjg.v12.i42.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stygelbout V, Leroy K, Pouillon V, Ando K, D’Amico E, Jia Y, Luo HR, Duyckaerts C, Erneux C, Schurmans S, Brion JP. Inositol trisphosphate 3-kinase B is increased in human Alzheimer brain and exacerbates mouse Alzheimer pathology. Brain 137: 537–552, 2014. doi: 10.1093/brain/awt344. [DOI] [PubMed] [Google Scholar]
- 111.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swan C, Duroudier NP, Campbell E, Zaitoun A, Hastings M, Dukes GE, Cox J, Kelly FM, Wilde J, Lennon MG, Neal KR, Whorwell PJ, Hall IP, Spiller RC. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut 62: 985–994, 2013. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 113.Szlufcik K, Missiaen L, Parys JB, Callewaert G, Smedt H. Uncoupled IP3 receptor can function as a Ca2+-leak channel: cell biological and pathological consequences. Biol Cell 98: 1–14, 2006. doi: 10.1042/BC20050031. [DOI] [PubMed] [Google Scholar]
- 114.Tay PN, Tan P, Lan Y, Leung CH, Laban M, Tan TC, Ni H, Manikandan J, Rashid SB, Yan B, Yap CT, Lim LH, Lim YC, Hooi SC. Palladin, an actin-associated protein, is required for adherens junction formation and intercellular adhesion in HCT116 colorectal cancer cells. Int J Oncol 37: 909–926, 2010. [DOI] [PubMed] [Google Scholar]
- 115.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut 45, Suppl 2: ii43–ii47, 1999. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Traini C, Evangelista S, Girod V, Faussone-Pellegrini MS, Vannucchi MG. Changes of excitatory and inhibitory neurotransmitters in the colon of rats underwent to the wrap partial restraint stress. Neurogastroenterol Motil 28: 1172–1185, 2016. doi: 10.1111/nmo.12816. [DOI] [PubMed] [Google Scholar]
- 117.Ugrinova I, Pashev IG, Pasheva EA. Cyclin-dependent kinase 5 phosphorylates mammalian HMGB1 protein only if acetylated. J Biochem 149: 563–568, 2011. doi: 10.1093/jb/mvr005. [DOI] [PubMed] [Google Scholar]
- 118.Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol 108: 1634–1643, 2013. doi: 10.1038/ajg.2013.241. [DOI] [PubMed] [Google Scholar]
- 119.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, O’Neill J, Carlson P, Lamsam J, Eckert D, Janzow D, Burton D, Ryks M, Rhoten D, Zinsmeister AR. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol 303: G1262–G1269, 2012. doi: 10.1152/ajpgi.00294.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waggott D, Chu K, Yin S, Wouters BG, Liu FF, Boutros PC. NanoStringNorm: an extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics 28: 1546–1548, 2012. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang JP, Hou XH. Expression of aquaporin 8 in colonic epithelium with diarrhoea-predominant irritable bowel syndrome. Chin Med J (Engl) 120: 313–316, 2007. [PubMed] [Google Scholar]
- 122.Wang YM, Chang Y, Chang YY, Cheng J, Li J, Wang T, Zhang QY, Liang DC, Sun B, Wang BM. Serotonin transporter gene promoter region polymorphisms and serotonin transporter expression in the colonic mucosa of irritable bowel syndrome patients. Neurogastroenterol Motil 24: 560–565, e254–e565, 2012. doi: 10.1111/j.1365-2982.2012.01902.x. PubMed [DOI] [PubMed] [Google Scholar]
- 123.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology 123: 1459–1467, 2002. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 124.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, Van der Merwe S, Mols R, Ghesquiere B, Cirillo C, Kortekaas I, Carmeliet P, Peetermans WE, Vermeire S, Rutgeerts P, Augustijns P, Hellings PW, Belmans A, Vanner S, Bulmer DC, Talavera K, Vanden Berghe P, Liston A, Boeckxstaens GE. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150: 875–887, e879, 2016. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 125.Wouters MM, Lambrechts D, Knapp M, Cleynen I, Whorwell P, Agréus L, Dlugosz A, Schmidt PT, Halfvarson J, Simrén M, Ohlsson B, Karling P, Van Wanrooy S, Mondelaers S, Vermeire S, Lindberg G, Spiller R, Dukes G, D’Amato M, Boeckxstaens G. Genetic variants in CDC42 and NXPH1 as susceptibility factors for constipation and diarrhoea predominant irritable bowel syndrome. Gut 63: 1103–1111, 2014. doi: 10.1136/gutjnl-2013-304570. [DOI] [PubMed] [Google Scholar]
- 126.Yang YR, He Y, Zhang Y, Li Y, Li Y, Han Y, Zhu H, Wang Y. Activation of cyclin-dependent kinase 5 (Cdk5) in primary sensory and dorsal horn neurons by peripheral inflammation contributes to heat hyperalgesia. Pain 127: 109–120, 2007. doi: 10.1016/j.pain.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 127.Yunker AM. Modulation and pharmacology of low voltage-activated (“T-Type”) calcium channels. J Bioenerg Biomembr 35: 577–598, 2003. doi: 10.1023/B:JOBB.0000008025.65675.37. [DOI] [PubMed] [Google Scholar]
- 128.Zelano J, Mikulovic S, Patra K, Kühnemund M, Larhammar M, Emilsson L, Leão RN, Kullander K. The synaptic protein encoded by the gene Slc10A4 suppresses epileptiform activity and regulates sensitivity to cholinergic chemoconvulsants. Exp Neurol 239: 73–81, 2013. doi: 10.1016/j.expneurol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 129.Zhang R, Zou N, Li J, Lv H, Wei J, Fang XC, Qian JM. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): a new target for IBS treatment. Int J Colorectal Dis 26: 1035–1044, 2011. doi: 10.1007/s00384-011-1153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao JH, Dong L, Shi HT, Wang ZY, Shi HY, Ding H. The expression of protease-activated receptor 2 and 4 in the colon of irritable bowel syndrome patients. Dig Dis Sci 57: 58–64, 2012. doi: 10.1007/s10620-011-1827-3. [DOI] [PubMed] [Google Scholar]
- 131.Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 65: 797–805, 2016. doi: 10.1136/gutjnl-2013-306464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146: 41–46, 2009. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differentially expressed transcripts in IBS-C vs healthy controls - .xlsx (246 KB)