Abstract
Blood culture-negative endocarditis (BCNE) is difficult to diagnose because one of the major criteria to raise suspicion for endocarditis, a positive blood culture, is absent. BCNE accounts for 2.5% to 31% of all cases of endocarditis. Our report describes a 69-year-old woman with end-stage renal disease who presented with altered mental status. Physical examination and testing, including complete blood count, comprehensive metabolic panel, chest X-ray and head CT were otherwise unremarkable. Brain MRI revealed multiple areas of decreased diffusion concerning for cardioembolic stroke. A transthoracic echocardiogram demonstrated an abnormality on the mitral valve. Operative evaluation revealed a purulent mitral valve with vegetative clumps. Cultures of the vegetation and the blood grew no organisms. BCNE is a rare entity; neurological abnormalities may be the only presenting signs/symptoms. Endocarditis should be considered among the causes of altered mental status, even in the absence of positive blood cultures.
Keywords: infectious diseases, valvar diseases
Background
The definition of blood culture-negative endocarditis (BCNE) is endocarditis in which three or more aerobic and anaerobic blood cultures that have been collected over 48 hours remain negative despite prolonged (ie, >1 week) incubation.1
While uncommon, BCNE accounts for 2.5% - 31% of all cases of endocarditis.2 There are three main categories of BCNE: (1) BCNE due to antibiotic administration before obtaining cultures, (2) BCNE due to fastidious organisms (ie, hard to culture organisms) and (3) BCNE due to bacteria that cannot be cultured.3
The most common organisms accounting for 45%–60% of cases of BCNE are Staphylococcus or Streptococcus due to antibiotic treatment preceding blood culture collection.4 The remainder of BCNE is likely due to fastidious organisms such as Bartonella, Coxiella and the HACEK group (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella and Kingella).1 While traditionally considered to be the most common agents responsible for BCNE, the HACEK organisms can be isolated with current blood culture systems when there is an incubation period of 5 days.5
This case is important because it highlights that a common presentation (altered mental status) may relate to a rare entity—true blood culture-negative endocarditis. Left-sided endocarditis and non-bacterial thrombotic endocarditis (NBTE) should be considered among the differential diagnoses of someone who presents with unexplained mental status or neurological changes and findings suggestive of emboli on brain MRI. NBTE involves vegetations on cardiac valves that are composed of fibrin and platelet aggregates and are devoid of inflammation or bacteria while BCNE implies an infected cardiac valve without identifiable bacteria on culture.6
Case presentation
The patient is a 69-year-old woman with a medical history of end-stage renal disease (ESRD) on peritoneal dialysis, hypertension, type 2 diabetes, hypothyroidism and combined systolic and diastolic heart failure who presented to the emergency department due to altered mental status. The day before admission, her husband noted she woke up feeling unwell and had one episode of vomiting and diarrhoea. Immediately afterwards, she walked unsteadily and passed out on the bed, no head trauma or injuries. She remained confused the rest of that day and the morning of presentation. The patient was not able to describe what had occurred or where she was. Her husband denied the patient had any fever, chills, cough, abdominal pain or headaches. Her peritoneal dialysis was completed uneventfully the day prior to this evaluation.
The patient was afebrile, and the remainder of her vital signs were within normal limits. Her neurological examination demonstrated intact strength, sensation and cranial nerves, but decreased alertness. She was oriented to person only. Her mental status waxed and waned; she had moments of clarity although she remained confused to her surroundings. Her cardiac examination demonstrated a regular rate and rhythm and no murmurs, rubs or gallops. Her lungs were clear to auscultation bilaterally. Her abdomen was soft, non-distended and non-tender. Her peritoneal dialysis catheter was in place without surrounding erythema or warmth. Her skin examination revealed no petechiae, Osler’s nodes, Janeway lesions or splinter haemorrhages of the nails.
Investigations
A complete blood count was unremarkable except for a mild chronic anaemia (haemoglobin 10 mg/dL). A comprehensive metabolic panel revealed elevated creatinine consistent with her ESRD and a potassium of 3.1. Thyroid-stimulating hormone and free T4 were normal. Urinalysis revealed 6–12 white cell count/high-powered field (HPF) and 16–22 red cell count/HPF, and urine culture demonstrated no organisms. Ammonia, thiamine and folate levels were normal. Chest X-ray revealed no acute abnormality. Initial blood cultures were negative after 5 days of incubation. During her hospitalisation, a total of five separate sets of blood cultures were obtained and were negative. Of note, the patient did not receive antibiotics prior to blood cultures being obtained. Peritoneal fluid demonstrated 6 white cell count (76% neutrophils). Peritoneal culture yielded no bacteria after 5 days of incubation. Non-contrast head CT revealed no acute abnormality.
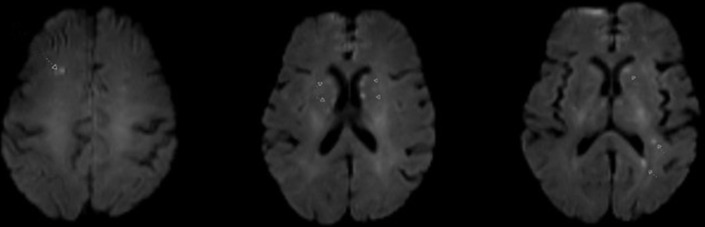
Neurological consultation was obtained and a non-contrast brain MRI was performed which revealed several small, scattered foci of restricted diffusion in the bilateral frontal lobes, left parietal lobe, bilateral basal ganglia and right pons, a pattern suspicious for a cardioembolic source (figure 1).
Figure 1.
Non-contrast brain MRI revealing multiple scattered foci of restricted diffusion, consistent with a cardioembolic aetiology.
A transthoracic echocardiogram (TTE) demonstrated mild mitral valve thickening, a 1.3×0.5 cm mobile echo density on the anterior leaflet of the mitral valve/chordae apparatus (figure 2). There was no significant mitral valvular regurgitation or stenosis. The TTE findings raised concerns about a vegetation or a papillary fibroelastoma with thrombus. A previous echocardiogram from 2 years earlier demonstrated normal mitral valve leaflets with trace regurgitation.
Figure 2.
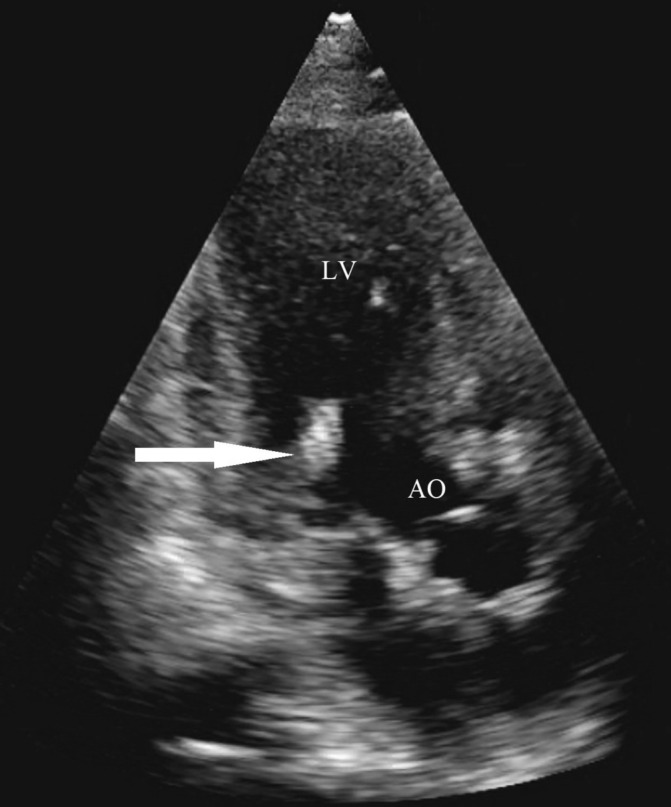
Mitral valve vegetation (arrow) seen on transthoracic echocardiogram. AO, aorta; LV, left ventricle.
Cardiothoracic surgery and cardiology consultants recommended mitral valve replacement to evaluate and remove the valvular density. During surgery, there was a large vegetation adherent to the ventricular aspect of the mitral valve’s posterior leaflet (ie, the P2–3 segments) which originated from the posterior mitral annulus. There were large abscesses at the P2–3 posterior mitral annulus. The annular abscesses were debrided into healthy tissue and a moderate amount of thick purulent material was obtained. The posterior annulus was repaired with a bovine pericardial patch. The operative findings of the mitral valve were consistent with endocarditis as the aetiology.
Surgical pathology demonstrated valvular tissue with myxoid degeneration, nodular calcifications and acute inflammation with necroinflammatory debris. Periodic acid-Schiff and Gomori methenamine silver stains performed on the valve did not identify any fungal forms; however, numerous bacterial clumps were seen. Culture of the valvular specimen never grew an organism. Fungal culture was followed for 21 days without any growth. Bacterial PCR was performed on the mitral valve without any bacteria detected. Bartonella PCR and antibodies and Coxiella antibodies were negative. Quantiferon gold testing for tuberculosis was negative as well.
Differential diagnosis
Based on the patient’s presenting symptom of altered mental status, the MRI findings consistent with a cardioembolic aetiology and the mitral valvular abnormality noted on TTE, endocarditis was high among the differential diagnostic possibilities. The patient’s negative blood cultures raised concerns for BCNE as well as non-bacterial aetiologies of valvular disease, including fungal endocarditis and papillary fibroelastoma. Additionally, NBTE could be included in the differential although it was effectively ruled out by the purulent appearance of the valve and by the bacterial clumps on pathology.
Treatment
The patient was initially started on an intravenous heparin drip to limit further cardiac emboli before the diagnosis of endocarditis was established.
The patient underwent cardiothoracic surgery to evaluate and replace the diseased mitral valve.
The patient was placed on a regimen of 6 weeks of intravenous vancomycin and ceftriaxone for BCNE.
Outcome and follow-up
The patient’s mental status had cleared and returned to her baseline by hospital day 3. The patient had a prolonged stay in the intensive care unit due to complications after surgery; however, she was eventually stabilised and discharged to a skilled nursing facility. Since discharge, she has slowly recovered and improved.
Discussion
The diagnosis of endocarditis is typically based on a combination of clinical findings, blood cultures or other microbiological/pathological data and the results of echocardiography. Using the modified Duke criteria,7 clinicians attempt to categorise the possibility of infective endocarditis into Definite, Possible or Rejected. Medical calculators, such as MD+Calc (https://www.mdcalc.com/duke-criteria-infective-endocarditis), help navigate the application of the modified Duke criteria during clinical care. Given our patient’s clinical presentation and lack of positive blood cultures, she fell into the Possible category per the modified Duke criteria.
BCNE can be suggested by echocardiogram findings of a vegetation or abscess despite negative blood cultures. Unfortunately, TTE is a relatively insensitive method to detect a vegetation or abscess. TTE has a sensitivity of 70% for native valve vegetation and only 50% for valve abscesses.7 Transoesophageal echocardiography (TEE) has a sensitivity of 96% for native valve vegetation. Both TTE and TEE have a specificity of roughly 90%. Of note, echocardiogram is less sensitive for vegetation of prosthetic valves (50% for TTE and 90% for TEE).7
There are case reports of endocarditis in patients with prosthetic valve replacements where blood cultures and initial echocardiogram are negative.8 As a result, TEE is the recommended test for those with prosthetic valves. If clinical suspicion remains high for infective endocarditis despite negative blood cultures and initial echocardiogram, repeat echocardiograms are recommended.8
If a vegetation is seen on echocardiogram in the setting of negative blood cultures, NBTE should be considered on the differential in addition to BCNE. NBTE is a rare entity of deposition of platelet thrombi on a heart valve that occurs in patients with underlying malignancy or hypercoagulable or inflammatory states, such as systemic lupus erythematosus.6 NBTE can present similarly to BCNE, with embolic stroke, negative blood cultures and vegetation on heart valves. Ultimately, the distinction between NBTE and BCNE relies on microbiological data and, in some cases, pathological data.6 In our case, our patient was initially started on heparin to prevent further emboli since BCNE was not confirmed until surgery.
The most common extracardiac sequela of infective endocarditis is a neurological complication, such as altered mental status or stroke.9 Cerebral embolism from left-sided heart valve vegetation is the currently accepted mechanism for neurological complications; however, it may not be the only mechanism. The body’s systemic inflammatory response to the infection may trigger cerebral vasculitis leading to neurological complications.10 Regardless of the mechanism of the neurological complications, appropriately chosen antibiotics are the treatment of choice. In the absence of culture data to guide treatment, one should consider risk factors and the patient’s presentation to determine best antibiotic selection.11 Antibiotic therapy can result in a decrease in the rate of stroke, with a 65% reduction in stroke being noted within 2 weeks of therapy.12 Anticoagulation is not generally recommended in those with native valve endocarditis due to scant evidence showing efficacy.11
Learning points.
Blood culture-negative endocarditis, while uncommon, accounts for 2.5% to 31% of all cases of endocarditis.
Patients with unexplained altered mental status and neuroimaging to suggest a cardioembolic aetiology should receive an echocardiogram to assess for the possibility of a valvular abnormality, such as a vegetation, despite negative blood cultures.
Neurological complications are the most common extracardiac manifestation of infective endocarditis.
Footnotes
Contributors: JRD conducted the literature review, created the manuscript, obtained the images and presented the manuscript to the related patient, obtaining consent from the patient. CRC coordinated the manuscript development and submission process and reviewed and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Katsouli A, Massad MG. Current issues in the diagnosis and management of blood culture-negative infective and non-infective endocarditis. Ann Thorac Surg 2013;95:1467–74. 10.1016/j.athoracsur.2012.10.044 [DOI] [PubMed] [Google Scholar]
- 2.Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 2001;14:177–207. 10.1128/CMR.14.1.177-207.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tattevin P, Watt G, Revest M, et al. Update on blood culture-negative endocarditis. Med Mal Infect 2015;45:1–8. 10.1016/j.medmal.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Madico GE, Rice PA. 16S-Ribosomal DNA to diagnose culture-negative endocarditis. Curr Infect Dis Rep 2008;10:280–6. 10.1007/s11908-008-0046-3 [DOI] [PubMed] [Google Scholar]
- 5.Petti CA, Bhally HS, Weinstein MP, et al. Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella organisms: a retrospective multicenter evaluation. J Clin Microbiol 2006;44:257–9. 10.1128/JCM.44.1.257-259.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007;32:696–701. 10.1016/j.ejcts.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 7.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the european society of cardiology (esc). endorsed by: european association for cardio-thoracic surgery (eacts), the european association of nuclear medicine (EANM). Eur Heart J 2015;36:3075–128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 8.Sumatani I, Kagiyama N, Saito C, et al. Infective endocarditis with negative blood culture and negative echocardiographic findings. J Echocardiogr 2015;13:66–8. 10.1007/s12574-015-0242-8 [DOI] [PubMed] [Google Scholar]
- 9.Pigretti S, Zurrú MC, Arias A, et al. [Neurologic complications of infective endocarditis: do they have an impact on prognosis?]. Medicina 2017;77:89–94. [PubMed] [Google Scholar]
- 10.Hart RG, Foster JW, Luther MF, et al. Stroke in infective endocarditis. Stroke 1990;21:695–700. 10.1161/01.STR.21.5.695 [DOI] [PubMed] [Google Scholar]
- 11.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation 2015;132:1435–86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 12.Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J 2007;154:1086–94. 10.1016/j.ahj.2007.07.023 [DOI] [PubMed] [Google Scholar]