Abstract
Delirium affects 18%–35% patients in the acute hospital setting, yet is often neither detected nor managed appropriately. It is associated with increased risk of falls, longer hospital stay and increased morbidity and mortality rates. It is a frightening and unpleasant experience for both patients and their families. We used quality improvement tools and a multicomponent intervention to promote detection and improve management of delirium on the acute medical unit (AMU). We reviewed whether a delirium screening tool (4AT) had been completed for all patients aged over 65 years admitted to the AMU over 1 week. If delirium was detected, we assessed whether investigation and management was adequate as per national guidance. After baseline data collection, we delivered focused sessions of delirium education for doctors and nursing staff, including training on use of the 4AT tool and the TIME (Triggers, Investigate, Manage, Engage) management bundle. We introduced TIME checklists, an online delirium order set and created a bedside orientation tool. We collected data following the interventions and identified areas for further improvement. Following our first PDSA (Plan, Do, Study, Act) cycle, use of the 4AT screening tool improved from 40% to 61%. Adequate assessment for the causes of and exacerbating factors for delirium increased from 73% to 94% of cases. Use of personal orientation tools improved from 0% to 38%. In summary, a targeted staff education programme and practical aids for the ward have improved the screening and management of delirium on the AMU. This may be improved further through more frequent training sessions to account for regular change-over of junior doctors and through implementing a nursing champion for delirium.
Keywords: quality improvement, PDSA, hospital medicine, medical education, mental health
Problem
We decided to undertake this quality improvement project as a review of services in our trust showed that there was significant room for improvement in the standard of care for older patients with cognitive impairment. In addition, we noted that rates of delirium in our trust were lower than expected, given that the prevalence of delirium in acute hospitals is reported at 18%–35%.1
The mental health liaison team at our trust noted a significant number of referrals from teams who were not aware of how to diagnose, investigate or manage delirium. The trust has an online delirium guideline compiled by geriatric and old-age psychiatry consultants, based on National Institute for Health and Care Excellence (NICE), British Geriatric Society (BGS) and Healthcare Improvement Scotland guidance.2–4 It became apparent through discussion with ward teams that this was not being accessed and implemented, due to a lack of awareness that a guideline existed. Through these observations, we identified a gap in clinical care that we wanted to close.
In this project, we sought to improve the detection and management of delirium in our trust. We carried out this project on the acute medical unit (AMU) as delirium should be promptly diagnosed on admission; delayed diagnosis is associated with worse outcomes.5
We used the PDSA (Plan, Do, Study, Act) framework to develop, test and implement our changes.6 Our overall aim was to improve the recognition and management of delirium as per NICE, BGS and Healthcare Improvement Scotland guidance. Our SMART aims were:
To improve the use of 4AT screening tool from 40% to 60% by July 2017.
To improve the proportion of patients with a diagnosed delirium having a thorough assessment and treatment of exacerbating causes (ie, following the TIME (Triggers, Investigate, Manage, Engage) bundle) from 73% to 100% by July 2017.
To improve the use of orientation signage for patients with delirium from 0% to 50% by July 2017.
Our trust is a large district general teaching hospital. It has two AMUs with a total of 68 beds.
Background
Delirium is a clinical syndrome involving disturbances in cognitive function, perception, attention and consciousness.7 8 It is acute in onset and has a fluctuating course.7 It is an unpleasant and frightening experience for the patient and is often associated with poor outcomes.1 9
Delirium is commonly seen in the acute hospital setting, occurring in 18%–35% of patients on general medical wards. Patients particularly at risk are those aged 65 years and above and those with a background of cognitive impairment.1 Delirium is associated with increased adverse incidents in hospital (eg, falls, pressure sores), increased length of hospital stay and associated cost, chance of institutional placement on discharge, likelihood of long-term cognitive impairment and higher mortality risk.1 10–13
The cause of delirium is usually multifactorial.14 Common causes include drugs (eg, steroids, opiates, benzodiazepines, anticholinergics), infections, postoperative or painful states, alcohol withdrawal, metabolic disturbances, myocardial infarction, constipation and urinary retention.15 In up to 20% of cases, the cause is never identified.16 Sensory deprivation can contribute to disorientation and delirium as, for example, rates of postoperative delirium have been noted to be higher in units without windows.17 Orientating patients to time and place as part of a multicomponent intervention has been shown to reduce the need for antipsychotic medications and reduce length of stay.18 The exact pathogenic mechanism has not yet been elicited; however, the common end pathway is thought to be a deficit in central cholinergic activity.19
As a result of its fluctuating course and multifactorial aetiology, delirium remains underdetected and misdiagnosed in healthcare settings.20 21 The awareness and detection of delirium has been shown to improve significantly with targeted education of healthcare professionals and focused care pathways.22 23 We therefore sought to implement and test these changes in our own trust, and to test whether education can also improve investigation and management.
The ‘4AT’ is a validated rapid initial assessment tool, testing for alertness, abbreviated mental test, attention and acute changes/fluctuating course24 (see online supplementary appendix 1). A score of 4 or above suggests delirium. It has a sensitivity of 89.7% and specificity of 84.1% for detecting delirium in the hospital setting, according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)criteria.25 26 It is particularly effective for testing attention, making it a good test for hypoactive as well as hyperactive delirium.27 28 It holds several advantages over traditionally used screening tools such as the Confusion Assessment Method (CAM), namely, it is quicker to use, does not require operator training, allows assessment of the drowsy patient and allows assessment of delirium superimposed on dementia.28 Its speed and ease of use in particular makes it highly suitable for acute medical practice. It is currently being rolled out across trusts in the UK.29 The 4AT has been validated in culturally diverse geriatric inpatients in an emergency or acute medical setting, making it a suitable screening tool for our patient population.26 30–32
bmjoq-2017-000200supp001.pdf (273.8KB, pdf)
The TIME bundle sets out critical actions to implement when there is a potential diagnosis of delirium; Think/Triggers, Investigate/Intervene, Manage, Engage/Explore (see online supplementary appendix 2). It is currently being rolled out in acute trusts in Scotland. It is based on Healthcare Improvement Scotland’s evidence-based delirium pathway.4 This quality improvement project particularly focused on education and implementation of the 4AT screening tool and TIME management bundle.
bmjoq-2017-000200supp002.pdf (37.1KB, pdf)
Measurement
We collected data from both of these units over a 1 week period (7 full days of admissions).
Inclusions
We reviewed the notes of all patients aged 65 years and above admitted to the AMUs (n=93) between 26 September 2016 and 2 October 2016. Patients with a background of cognitive impairment were included, as pre-existing cognitive impairment is a leading risk factor for the development of delirium.1
Exclusions
We intended to exclude any patients who had been admitted using the outdated trust clerking proforma which did not include a 4AT delirium screening tool (n=0), could not be assessed due to a language barrier (n=0) or were non-verbal with no collateral history available at the time of admission (n=0), as these factors make it difficult to perform the 4AT.
Data collection
We collected data on whether the 4AT screening tool (an integrated part of the clerking proforma) had been completed, and whether delirium had subsequently been diagnosed. If delirium was diagnosed, we assessed whether triggers and causes had been assessed for and treated according to the trust guidelines (based on NICE, BGS and Healthcare Improvement Scotland’s TIME guideline) via documentation in the clerking proforma. For patients with a diagnosed delirium, we assessed whether some form of orientation signage (eg, clock, calendar) had been placed within the patient’s view.
Our baseline data showed that 40% (n=37) of patients aged above 65 years had a 4AT tool completed on admission. Delirium was diagnosed in 12% of patients (n=11). Of these, 73% (n=8) had exacerbating factors adequately investigated and managed. None of the patients had orientation signage (eg, clock/calendar/whiteboard displaying date) placed by the bedside.
Design
Our team consisted of foundation doctors, consultant liaison psychiatrists and a consultant geriatrician. We worked with the speech and language therapy and occupational therapy teams to develop the interventions.
Following baseline data collection, we collected real-time informal verbal feedback from junior doctors and nurses on AMU to identify and prioritise the key drivers for bringing about improvement. This feedback strongly suggested that there was a lack of awareness of the importance of recognising and managing delirium promptly, as well as uncertainty surrounding the different clinical subtypes. It was also clear that staff were unaware of how to access the trust delirium guideline on the ward. The baseline findings were presented at the trust multidisciplinary dementia strategy board meeting, and drivers for interventions were discussed.
Based on our assessment of the key drivers that would facilitate our SMART aims, we implemented several interventions (figure 1). We felt that the root cause of the low use of 4AT screening tools and incomplete investigation and management was a lack of knowledge of the condition and its management. Therefore, we prioritised education of staff members and the provision of ward-based and online tools to complement the education programme.
Figure 1.
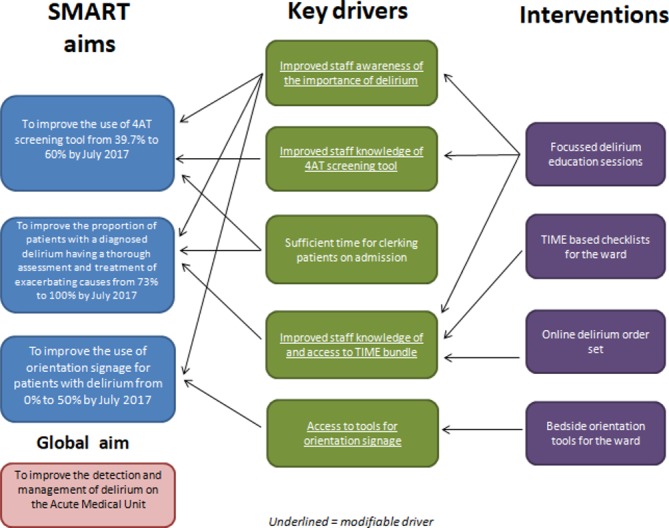
Driver diagram.
Strategy
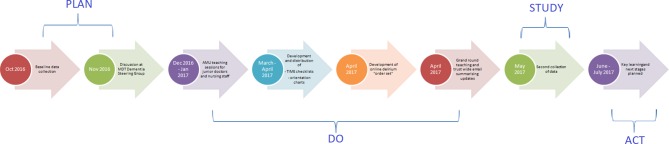
Given the multifactorial aetiology and complexity of delirium, we decided to implement a multicomponent intervention. We undertook a single multifactored PDSA cycle from October 2016 to July 2017 (figure 2). In the ‘plan’ phase, we set out our SMART aims (see the Problem section). In the ‘do’ phase, we carried out a multicomponent intervention to facilitate our SMART aims:
We aimed to improve the screening and detection rates of delirium. We carried out educational sessions led by consultant liaison psychiatrists for staff who were responsible for clerking patients and managing their care on AMU. This included a 1 hour session for doctors (junior to consultant) and a similar session for nursing staff. These sessions focused on the clinical subtypes, importance of recognition and outcomes of delirium and how to use the 4AT and TIME bundle.
We aimed to improve the investigation and management of delirium. We created a delirium ‘order set’ for our online ordering system to aid doctors in screening for treatable exacerbating factors. This was designed and approved by biochemistry and liaison psychiatry consultants in the trust, to be used when a patient presents with suspected delirium. The order set included full blood count, urea and electrolytes, C-reactive protein, bone profile, magnesium, phosphate, vitamin B12, folate, thyroid function and a midstream urine sample.
We created delirium ‘checklists’ for the ward. These were a single double-sided A4 sheet adapted from the existing trust delirium guidelines featuring the TIME bundle (see online supplementary appendix 2) and were designed to be placed in the front of a patient’s notes if they were diagnosed with delirium. These checklists give clear prompts for thorough investigation and management of the multiple factors that cause and contribute to delirium.
We aimed to improve the use of orientation signage for patients who had been diagnosed with delirium. We noticed that there were limited numbers of clocks and calendars available in the hospital. To this end, we worked with the speech and language therapy and occupational therapy teams to create reuseable laminated A3 bedside orientation tools for patients with delirium, to provide the name of hospital, ward, day of week, date and year.
Figure 2.
Timeline of events.
Results
In the ‘study’ phase, we recollected data over a 7-day period (18 May 2017 to 24 May 2017) for all patients aged above 65 (n=107) years admitted to the AMUs. We used the same inclusions and exclusions as for the baseline data collection. Patients were excluded if they were:admitted using an outdated clerking proforma which did not contain a delirium screen (n=13), could not be assessed due to a language barrier (n=2) or were non-verbal (n=0). When quoted, p values are calculated using χ2 test. Graphs were produced using GraphPad Prism (figure 3).
Figure 3.
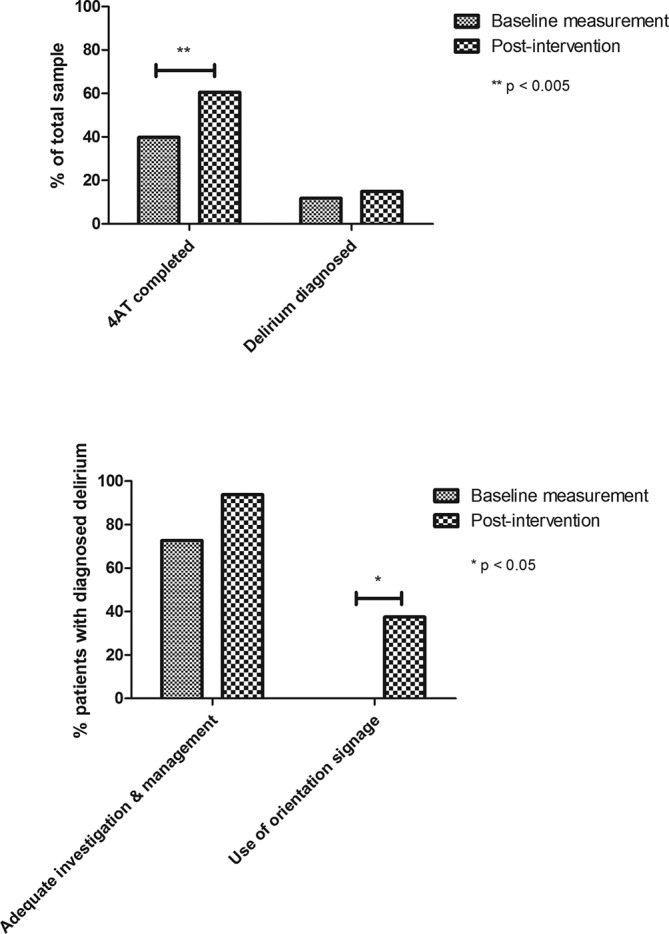
Comparison of results from baseline measurement and postintervention.
Sixty-one percentage of patients had a 4AT tool completed on admission (n=65), an improvement of 21% from baseline (p=0.003). Delirium was diagnosed in 15% patients (n=16), an increase of 3% from the baseline data collection (p=0.54).
Of these patients, 94% had exacerbating factors assessed for and treated according to the TIME bundle (n=15). This is an improvement of 21% from baseline (p=0.14).
Orientation tools such as a clock, calendar or whiteboard displaying the date was placed by the bedside in 38% of cases of patients with delirium (n=6), an improvement of 38% from baseline (p=0.02).
These results show an overall improvement in the screening, investigation and management of delirium, with statistically significant improvements in use of screening tool and use of orientation signage.
Lessons and limitations
This project was carried out over two AMUs with data collection over two periods of 7 days. Expanding this onto other wards and collecting data over a longer period of time will give us a stronger indication of whether our interventions have brought about improvements; the improvements in rates of delirium diagnosis and adequate investigation and management may be statistically non-significant due to small sample sizes. Due to time constraints, we implemented several interventions into one single PDSA cycle rather than carrying out multiple cycles. We felt that targeting several key areas at the same time would be more likely to improve the overall management of the condition. Nevertheless, as a result we cannot determine whether it was one single intervention in particular or the combination of interventions that brought about change.
Despite this, due to the complex nature of delirium and the multidisciplinary nature of the team involved, we believe that the decision to use a multicomponent intervention is warranted. As our project spanned over several months, a number of the junior doctors who took part in the education programme were no longer on AMU by the time the second set of data were collected, and we expect that for a sustained change to take place we would need to organise regular teaching to account for junior doctors rotating.
It is well recognised that in order to implement change successfully within a healthcare system, one needs to understand the problem, the target group, the setting and the obstacles to change.33 In our case, barriers at each of these levels contributed to for the shortcomings in fully reaching our SMART aims. The ‘problem’, that is, delirium itself can be confusing and frustrating for medical and nursing staff due to its multifactorial aetiology, varying presentation, fluctuating and often prolonged course. The ‘target group’ in this case were junior doctors on the acute medical take and nursing staff on AMU; two groups under significant time pressure who have a level of understandable reluctance to take on any extra measures that are time consuming. The ‘setting’, an AMU, is set up for short admissions and prompt transfers/discharges. The culture among staff on AMU very much reflects this. This makes implementing changes for a geriatric population of patients with multiple-morbidities challenging.
Nonetheless, this project contributes to the literature showing that educational interventions can improve delirium management in the acute medical setting.22 23 34 We note that other quality improvement projects in similar settings have not reported an increase in screening of delirium35 or report difficulties in accuracy of assessment by ward nursing staff.34 Factors that may have improved screening in this project may include (1) the use of 4AT as opposed to other screening tools and (2) the emphasis on the admitting junior doctor having the responsibility of recognition, rather than ward medical/nursing staff.
It has been frequently noted that it can be challenging to incorporate evidence and guidelines into practice when it involves a change in culture and routine. One way to tackle this is to use a more broad approach, targeting distinct members of the multidisciplinary team.36 This is why we felt it essential to include doctors, nurses, speech and language therapists and occupational therapists in this project.
Key themes we noted from staff feedback were particularly centred on attitudes towards delirium itself, in particular a belief that managing a multitude of small factors ‘won’t make a difference’ to a disease course, problems with time pressure for example, not having time to include a 4AT screening tool in a clerking on a busy medical take, and a reluctance to engage with ‘more paperwork’. The nursing staff felt that there was a lack of leadership among them regarding delirium. In retrospect, we may have benefited from recruiting a nurse onto the project team in order to increase nursing ownership and engagement with the problem. We are now planning to recruit a ‘delirium champion’ to each ward involved which we hope will help to increase and sustain change, as previous studies have shown that the appointment of ‘dementia champions’ has altered health professionals’ perception of dementia.37
There were often conflicting views from medical and nursing staff regarding an understanding of the use of the Mental Capacity Act38 in patients with delirium and acting in patients’ best interests, particularly regarding the need for patient consenting to the use of orientation tools by their bedside. This restricted its use in some cases. Further delirium education sessions for nursing staff could address this in order to provide clarity on this issue.
In the ‘act’ phase of this PDSA cycle, we will adapt our interventions and test again. Specifically, we will aim for multiple education sessions over several months in order to reach the maximum number of staff.
Conclusion
This project has improved the screening, detection, investigation and management of delirium on the AMU, with significant improvements in use of screening tools and orientation signage. However, there is still room for improvement.
This project adds to the literature on the ability of targeted education sessions to improve delirium management as a whole. We provide clear examples of ward-based and online tools to supplement education sessions that can be used by multidisciplinary staff on acute wards and enable trusts to develop clear and effective delirium care pathways.
The TIME bundle is a relatively new tool in clinical practice, currently being rolled out in acute trusts in the UK. This project gives further evidence for the clinical effectiveness of the TIME bundle, particularly in the context of an AMU.
Key learning points from this project include:
The effectiveness of staff education in tackling a culture reluctant for change but the need to maintain this on a regular basis.
The value of the 4AT tool, particularly its speed and lack of specific training, for an acute medical setting.
The involvement of the multidisciplinary team in managing delirium, and a need for corresponding wide representation on the quality improvement project team.
The need for appropriate delegation of responsibilities within the multidisciplinary team; that is, the admitting doctor responsible for recognition and appropriate investigation, and the nursing team responsible for bedside orientation intervention.
The applications of this project could be widened for future investigators to see whether these interventions can reduce the need for sedative/antipsychotic use on AMU or whether they reduce adverse consequences of delirium on the ward such as falls, prolonged admission or mortality.
Acknowledgments
We acknowledge the input of the North Middlesex University Hospital (NMUH) Mental Health Liaison Team in helping us to carry out this project. We also acknowledge the NMUH Speech and Language Therapy and Occupational Therapy teams for helping us to design the orientation tool, and the IT and biochemistry teams for helping us develop the online order set. We also acknowledge the junior doctors and nursing staff on NMUH AMU for their helpful feedback and patience throughout the project.
Footnotes
Contributors: All authors contributed to this project and to the manuscript. YB and MB collected the data and designed the project under the supervision of SR and ELS. YB and MB analysed the data. YB drafted the manuscript and all authors reviewed and provided feedback on drafts and approved the final manuscript.
Funding: NMUH procurement department funded the ward-based materials used in this project.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911–22. 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NICE. Delirium: prevention, diagnosis and management. 2010. https://www.nice.org.uk/guidance/cg103
- 3. British Geriatrics Society. Guidelines for the prevention, diagnosis and management of delirium in older people in hospital. London: British Geriatrics Society, 2006. [Google Scholar]
- 4. Healthcare Improvement Scotland. Delirium toolkit. Edinburgh: Healthcare Improvement Scotland, 2014. [Google Scholar]
- 5. Andrew MK, Freter SH, Rockwood K. Incomplete functional recovery after delirium in elderly people: a prospective cohort study. BMC Geriatr 2005;5:5 10.1186/1471-2318-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ACT Academy. Plan, Do, Study, Act: (PDSA) cycles and the model for improvement. London: NHS Improvement. [Google Scholar]
- 7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 8. World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization, 1993. [Google Scholar]
- 9. Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43:183–94. 10.1176/appi.psy.43.3.183 [DOI] [PubMed] [Google Scholar]
- 10. González M, Martínez G, Calderón J, et al. . Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009;50:234–8. 10.1176/appi.psy.50.3.234 [DOI] [PubMed] [Google Scholar]
- 11. Saczynski JS, Marcantonio ER, Quach L, et al. . Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30–9. 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fong TG, Jones RN, Marcantonio ER, et al. . Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med 2012;156:848–W296. 10.7326/0003-4819-156-12-201206190-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc 1997;45:174–8. 10.1111/j.1532-5415.1997.tb04503.x [DOI] [PubMed] [Google Scholar]
- 14. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852–7. [PubMed] [Google Scholar]
- 15. Rosen T, Connors S, Clark S, et al. . Assessment and management of delirium in older adults in the emergency department: literature review to inform development of a novel clinical protocol. Adv Emerg Nurs J 2015;37:183–96. 10.1097/TME.0000000000000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipowski ZJ. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am J Psychiatry 1983;140:1426–36. 10.1176/ajp.140.11.1426 [DOI] [PubMed] [Google Scholar]
- 17. Wilson LM. Intensive care delirium. The effect of outside deprivation in a windowless unit. Arch Intern Med 1972;130:225–6. [DOI] [PubMed] [Google Scholar]
- 18. Gorski S, Piotrowicz K, Rewiuk K, et al. . Nonpharmacological interventions targeted at delirium risk factors, delivered by trained volunteers (medical and psychology students), reduced need for antipsychotic medications and the length of hospital stay in aged patients admitted to an acute internal medicine ward: pilot study. Biomed Res Int 2017;2017:1–8. 10.1155/2017/1297164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hshieh TT, Fong TG, Marcantonio ER, et al. . Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci 2008;63:764–72. 10.1093/gerona/63.7.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong SC, Cozza KL, Watanabe KS. The misdiagnosis of delirium. Psychosomatics 1997;38:433–9. 10.1016/S0033-3182(97)71420-8 [DOI] [PubMed] [Google Scholar]
- 21. Faria RS, Moreno RP. Delirium in intensive care: an under-diagnosed reality. Rev Bras Ter Intensiva 2013;25:137–47. 10.5935/0103-507X.20130025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tabet N, Hudson S, Sweeney V, et al. . An educational intervention can prevent delirium on acute medical wards. Age Ageing 2005;34:152–6. 10.1093/ageing/afi031 [DOI] [PubMed] [Google Scholar]
- 23. Tauro R. Delirium awareness - Improving recognition and management through education and use of a care pathway. BMJ Qual Improv Rep 2014;2:u203195.w1451 10.1136/bmjquality.u203195.w1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacLullich A, Ryan T, Cash H. 4AT rapid clinical test for delirium. https://www.the4at.com/
- 25. American, Psychiatric, and Association. Diagnostic and statistical manual of mental disorders. 4th edn Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 26. Bellelli G, Morandi A, Davis DH, et al. . Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014;43:496–502. 10.1093/ageing/afu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosker C, Ward D. Hypoactive delirium. BMJ 2017;357:j2047 10.1136/bmj.j2047 [DOI] [PubMed] [Google Scholar]
- 28. De J, Wand AP. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist 2015;55:1079–99. 10.1093/geront/gnv100 [DOI] [PubMed] [Google Scholar]
- 29. Shenkin SD, Fox C, Godfrey M, et al. . Protocol for validation of the 4AT, a rapid screening tool for delirium: a multicentre prospective diagnostic test accuracy study. BMJ Open 2018;8:e015572 10.1136/bmjopen-2016-015572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Sullivan D, Brady N, Manning E, et al. . Validation of the 6-item cognitive impairment test and the 4AT test for combined delirium and dementia screening in older emergency department attendees. Age Ageing 2018;47:61–8. 10.1093/ageing/afx149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De J, Wand APF, Smerdely PI, et al. . Validating the 4A’s test in screening for delirium in a culturally diverse geriatric inpatient population. Int J Geriatr Psychiatry 2017;32:1322–9. 10.1002/gps.4615 [DOI] [PubMed] [Google Scholar]
- 32. Hendry K, Quinn TJ, Evans J, et al. . Evaluation of delirium screening tools in geriatric medical inpatients: a diagnostic test accuracy study. Age Ageing 2016;45:832–7. 10.1093/ageing/afw130 [DOI] [PubMed] [Google Scholar]
- 33. Grol R. Personal paper. Beliefs and evidence in changing clinical practice. BMJ 1997;315:418–21. 10.1136/bmj.315.7105.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solberg LM, Plummer CE, May KN, et al. . A quality improvement program to increase nurses’ detection of delirium on an acute medical unit. Geriatr Nurs 2013;34:75–9. 10.1016/j.gerinurse.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malik A, Harlan T, Cobb J. Stop. Think. Delirium! A quality improvement initiative to explore utilising a validated cognitive assessment tool in the acute inpatient medical setting to detect delirium and prompt early intervention. J Clin Nurs 2016;25:3400–8. 10.1111/jocn.13166 [DOI] [PubMed] [Google Scholar]
- 36. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet 2003;362:1225–30. 10.1016/S0140-6736(03)14546-1 [DOI] [PubMed] [Google Scholar]
- 37. Banks P, Waugh A, Henderson J, et al. . Enriching the care of patients with dementia in acute settings? The Dementia Champions Programme in Scotland. Dementia 2014;13:717–36. 10.1177/1471301213485084 [DOI] [PubMed] [Google Scholar]
- 38. Mental capacity act 2005. 2005. http://www.legislation.gov.uk/ukpga/2005/9/contents
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2017-000200supp001.pdf (273.8KB, pdf)
bmjoq-2017-000200supp002.pdf (37.1KB, pdf)