Abstract
The aim of this analysis was to examine sex-based differences in renal segmental resistances in healthy controls (HCs) and patients with type 1 diabetes (T1D). We hypothesized that hyperfiltration—an early hemodynamic abnormality associated with diabetic nephropathy—would disproportionately affect women with T1D, thereby attenuating protection against the development of renal complications. Glomerular hemodynamic parameters were evaluated in HC (n = 30) and in normotensive, normoalbuminuric patients with T1D and either baseline normofiltration [n = 36, T1D-N, glomerular filtration rate (GFR) 90–134 ml·min−1·1.73 m2] or hyperfiltration (n = 32, T1D-H, GFR ≥ 135 ml·min−1·1.73 m2) during euglycemic conditions (4–6 mmol/l). Gomez’s equations were used to derive efferent (RE) and afferent (RA) arteriolar resistances, glomerular hydrostatic pressure (PGLO) from inulin (GFR) and paraaminohippurate [effective renal plasma flow (ERPF)] clearances, plasma protein and estimated ultrafiltration coefficients (KFG). Female patients with T1D with hyperfiltration (T1D-H) had higher RE (1,985 ± 487 vs. 1,381 ± 296 dyne·sec−1·cm−5, P < 0.001) and filtration fraction (FF, 0.20 ± 0.047 vs. 0.16 ± 0.03 P < 0.05) and lower ERPF (876 ± 245 vs. 1,111 ± 298 134 ml·min−1·1.73 m2 P < 0.05) compared with male T1D-H patients. Overall, T1D-H patients had higher PGLO and lower RA vs. HC subjects, although there were no sex-based differences. In conclusion, female T1D-H patients had higher RE and FF and lower ERPF than their male counterparts with no associated sex differences in RA. Prospective intervention studies should consider sex as a modifier of renal hemodynamic responses to renal protective therapies.
Keywords: glomerular hemodynamics, hyperfiltration, sex, type 1 diabetes
diabetes mellitus (DM) is the most common cause of chronic kidney disease (CKD) in North America, leading to great morbidity, mortality, and societal cost (1, 58a). In patients with nondiabetic CKD, women have a lower risk of progression to end-stage renal disease (ESRD). Conversely, in patients with diabetes, the association between sex and CKD risk is less clear, and the protective effect of female sex in patients with diabetes is generally attenuated (3, 19, 27).
To investigate the role of hemodynamic factors that may contribute to sex-related differences in the risk for CKD progression, previous studies have examined the relationship between sex and renal hemodynamic function, since renal hyperperfusion and hyperfiltration are risk factors for the initiation and progression of diabetic nephropathy (DN) (33, 44). Studies in the 1940s and 1950s examined renal hemodynamic function in healthy nondiabetic humans and demonstrated that women exhibited lower renal blood flow (RBF) than men (52). Similar findings have been documented in healthy patients with type 1 diabetes (T1D) (14). Despite these interesting observations, the mechanisms responsible for these sex differences in renal hemodynamic function—and possible relationships with risk factors for the development of renal disease—have not been established.
To better characterize sex differences in renal function, previous animal and human physiology experiments have characterized important modulators of renal hemodynamic function, such as activation of the renin angiotensin aldosterone system (RAAS). In animals, nondiabetic female rats have higher afferent and efferent arteriolar resistances and lower RBF compared with male rats (38). Despite the inability to directly measure segmental resistances in human kidney function, glomerular hemodynamic parameters such as afferent (RA) and efferent (RE) arteriolar resistances and glomerular hydrostatic pressure (PGLO) can be indirectly calculated by using equations that were derived by Gomez and colleagues in the 1950s (2, 22). Subsequently, these equations have been used in studies of nephrology, hypertension, and endocrinology (42, 50, 51, 57, 58). To our knowledge, these principles have not been applied to study sex differences in glomerular hemodynamic function in states of health or disease.
To better understand previously documented differences in renal hemodynamic function in men and women, our aim was to determine the sex differences in the glomerular hemodynamic profile estimated using Gomez’s equations (RA, RE, PGLO) of healthy control (HC) subjects compared with patients with T1D with hyperfiltration (T1D-H) and normofiltration (T1D-N). Differences in renal segmental resistances may offer insight into sex differences in responses to current and future renal protective therapies—such as RAAS inhibitors—that act by modulating renal arteriolar resistances. In this exploratory post hoc analysis, we hypothesized that women with T1D-H would exhibit a renal hemodynamic profile that could predispose them to renal injury such as lower RA and an increase in RE compared with men with T1D-H.
METHODS
A total of 30 HC patients, 36 T1D patients with normofiltration [TID-N, glomerular filtration rate (GFR) 90–134 ml·min−1·1.73 m2], and 32 T1D-H patients (GFR ≥ 135 ml·min−1·1.73 m2) were included in this retrospective post hoc analysis from previously completed studies (12, 16, 17, 36). Some previous studies included in the current analyses related to sex, as well as physiological effects of pharmacological agents in renal hemodynamic function. With this convenience sample, our aim was to gain further insight into sex-related differences by calculating renal segmental resistances by using the Gomez equations (discussed below). In this analysis, we included data from subjects who participated in four previously published studies, who had plasma protein data available to estimate oncotic pressure, and who met the inclusion criteria: T1D duration > 1 yr (T1D cohort), age > 18 yr, blood pressure < 140/90 mmHg, and normoalbuminuria. Patients were not taking medications that alter blood pressure or cardiovascular parameters. The study was approved by the Research Ethics Board at the University Health Network. All subjects provided informed consent before start of the study procedures.
Subjects adhered to a sodium replete (>140 mmol/day) and moderate protein (<1.5 g·kg−1·day−1) diet during the 7 days preceding the experiment as previously described (15). All studies were performed in the Renal Physiology Laboratory at the Toronto General Hospital. A modified glucose clamp technique was used to maintain euglycemic (4–6 mmol/l) conditions. Constant inulin and paraaminohippurate (PAH) infusions were used to determine GFR and effective renal plasma flow (ERPF). After a 10-min equilibration period, blood samples were collected for inulin, PAH, and hematocrit (Hct), with additional blood work drawn 30 min later. Two independent clearance periods were used for calculations, and the results averaged. All hemodynamic measurements were adjusted for body surface area.
Equations established by Gomez were used to estimate renal segmental resistances in the study cohorts (22). Gomez’s equations necessitate the following postulates: 1) intrarenal vascular resistances are divided into afferent, postglomerular, and efferent; 2) hydrostatic pressures within the renal tubules, venules, Bowman’s space, and interstitium are in equilibrium and given a value of 10 mmHg; 3) filtration disequilibrium in the glomerulus is assumed; 4) the gross filtration coefficient KFG is assumed to be 0.1733 ml/s per mmHg (25) in HC given a normal kidney physiology where GFR = 130 ml/min, PGLO = 60 mmHg, given Winton’s estimates in dog that glomerular pressure is roughly two-thirds of MAP (60) and normal glomerular oncotic pressure (πG) is 25 mmHg. Previous micropuncture studies in Munich-Wistar rats suggest different PGLO values in diabetic and control conditions. To replicate potential differences in HC vs. T1D patients, Gomez’s equations were modified in the T1D to calculate intraglomerular hemodynamic parameters assuming PGLO values of 56.4 mmHg (KFG = 0.1012 ml/s per mmHg) for T1D participants (30). To evaluate potential differences between male and female sex characteristics, KFG values were modified according to prior animal studies whereby male rats had measured KFG values higher by a correction factor of 1.84 compared with female rats (38, 43). We multiplied our previously determined KFG values by this correction factor to estimate male KFG values: HC male KFG = 0.318 ml/s per mmHg; HC female KFG = 0.1733 ml/s per mmHg; T1D male KFG = 0.186 ml/s per mmHg; T1D female KFG = 0.1012 ml/s per mmHg.
Clinical parameters including MAP (mmHg), ERPF (ml/s), GFR (ml/s), Hct (%), and TP (g/dl) were used to calculate RE (dyne·sec−1·cm−5) and RA (dyne·sec−1·cm−5) renal arteriolar resistances, PGLO (mmHg), and πG (mmHg): FF = GFR/RPF RBF = RPF/(1 – Hct). The filtration pressure across glomerular capillaries (ΔPF) is calculated by ΔPF = GFR/KFG. The πG is calculated from the plasma protein mean concentration (CM) within the capillaries: CM = TP/FF × ln(1/1−FF) and πG = 5 × (CM – 2). By substituting ΔPF, πG, and given that hydrostatic pressure in Bowman’s space (PBow) is assumed to be 10 mmHg, PGLO is calculated: PGLO = ΔPF + PBow + πG and PGLO = (GFR/KFG) + 10 mmHg + [5 × (TP/FF × ln(1/1−FF)−2)]. To calculate RA and RE using principles of Ohm’s law, where 1,328 is the conversion factor to dyne·sec−1·cm−5 (22): RA = [(MAP-PGLO)/RBF] × 1,328 and RE = [GFR/(KFG × (RBF−GFR)] × 1,328.
Statistical analysis.
Differences in continuous variables across the groups were examined by one-way ANOVA. Analyses with significant one-way ANOVA tests were examined for between-group differences using Tukey’s test. Differences were considered statistically significant at P < 0.05. Additional adjustments were not made for multiple comparisons as these data are considered hypothesis generating. Data are expressed as means ± SD. All statistical analyses were done with GraphPad Prism software (Version 5.0).
RESULTS
Baseline characteristics.
Clinical and demographic characteristics were similar among HC, T1D-N, and T1D-H patients (Table 1). In terms of sex, baseline characteristics were similar aside from the expected higher hematocrit and height values of female HC and T1D subjects in comparison to men. Within T1D-H subjects, women had lower systolic blood pressure (111 ± 8 vs. 121 ± 10 mmHg, P < 0.05), ERPF (876 ± 245 vs. 1,111 ± 298 ml·min−1·1.73 m2, P < 0.05), and RBF (1,326 ± 352 vs. 1,831 ± 465 ml·min−1·1.73m2, P < 0.001), with corresponding higher FF (0.20 ± 0.05 vs. 0.16 ± 0.03, P < 0.05) compared with men with T1D-H.
Table 1.
Clinical baseline characteristics of male and female normal healthy control subjects and patients with type 1 diabetes and either renal normofiltration or hyperfiltration
| HC Male | HC Female | T1D-N Male | T1D-N Female | T1D-H Male | T1D-H Female | |
|---|---|---|---|---|---|---|
| N | 12 | 18 | 23 | 13 | 16 | 16 |
| Age, yr | 27.0 ± 5.1 | 27.6 ± 7.8 | 24.8 ± 6.2 | 23.0 ± 2.7 | 23.0 ± 4.3 | 23.7 ± 4.0 |
| Diabetes duration, yr | — | — | 18.9 ± 6.2 | 18.8 ± 3.6 | 16.5 ± 6.7 | 14.9 ± 7.5 |
| Weight, kg | 75.6 ± 12 | 66.8 ± 9.2 | 75.0 ± 10.4 | 71.0 ± 16 | 73.0 ± 9.3 | 70.0 ± 11.5 |
| Height, m | 1.79 ± 0.06 | 1.69 ± 0.08* | 1.78 ± 0.06 | 1.65 ± 0.08* | 1.75 ± 0.06 | 1.66 ± 0.06* |
| Body mass index, kg/m2 | 23.5 ± 3.3 | 23.2 ± 2.7 | 23.4 ± 2.4 | 25.8 ± 4.5 | 23.4 ± 2.4 | 25.3 ± 3.0 |
| HbA1C - %, mmol/mol | — | — | 8.2 ± 1.6 | 8.5 ± 1.5 | 8.5 ± 1.3 | 7.8 ± 0.9 |
| 24 h urine sodium, mmol | 174 ± 65 | 177 ± 58 | 182 ± 108 | 162 ± 97 | 168 ± 84 | 166 ± 78 |
| 24 h urine urea, mmol | 349 ± 109 | 284 ± 84 | 366 ± 100 | 245 ± 66* | 369 ± 140 | 291 ± 113 |
| Hematocrit | 0.41 ± 0.03 | 0.37 ± 0.03* | 0.39 ± 0.02 | 0.35 ± 0.01* | 0.39 ± 0.03 | 0.34 ± 0.03§ |
| Systemic hemodynamic function | ||||||
| HR | 58 ± 9 | 59 ± 9 | 64 ± 11 | 75 ± 10 | 73 ± 13† | 77 ± 12 |
| SBP | 112 ± 8 | 109 ± 10 | 116 ± 8 | 107 ± 6 | 121 ± 10 | 111 ± 8* |
| DBP | 65 ± 6 | 66 ± 8 | 66 ± 7 | 64 ± 7 | 67 ± 7 | 65 ± 6 |
| MAP | 81 ± 6 | 81 ± 8 | 82 ± 6 | 78 ± 6 | 85 ± 6 | 80 ± 6 |
| Renal Hemodynamic function | ||||||
| ERPF | 645 ± 100 | 673 ± 205 | 652 ± 85 | 620 ± 103 | 1,111 ± 298†‡ | 87*6 ± 245*†‡ |
| GFR | 114 ± 11 | 119 ± 11 | 115 ± 11 | 116 ± 9 | 167 ± 25†‡ | 165 ± 22†‡ |
| FF | 0.18 ± 0.03 | 0.19 ± 0.05 | 0.18 ± 0.02 | 0.19 ± 0.03 | 0.16 ± 0.03 | 0.20 ± 0.05* |
| RBF | 1,086 ± 164 | 1,077 ± 349 | 1,066 ± 151 | 958 ± 159 | 1,831 ± 465†‡ | 1,326 ± 352§‡ |
| RVR | 76 ± 12 | 80 ± 23 | 79 ± 13 | 83 ± 14 | 49 ± 13†‡ | 65 ± 16†‡ |
Values are means ± SD. HC, healthy control; H, hyperfiltration; N, normofiltration; T1D, type 1 diabetes; HR, heart rate (beats per minute); SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); MAP, mean arterial pressure (mmHg); ERPF, effective renal plasma flow (ml·min−1·1.73m2); GFR, glomerular filtration rate (ml·min−1·1.73m2); FF, filtration fraction; RBF, renal blood flow (ml·min−1·1.73m2); RVR, renal vascular resistance (mmHg·l−1·min−1); PGLO, glomerular hydrostatic pressure (mmHg); πG, oncotic pressure (mmHg); RA, afferent arteriolar resistance (dyne·sec−1·cm−5); RE, efferent arteriolar resistance (dyne·sec−1·cm−5).
P < 0.05 vs. opposite sex within group.
P < 0.001 vs. opposite sex within group.
P < 0.05 vs. same sex healthy control group.
P < 0.05 vs. same sex type 1 diabetes and normofiltration group.
Glomerular hemodynamic parameters.
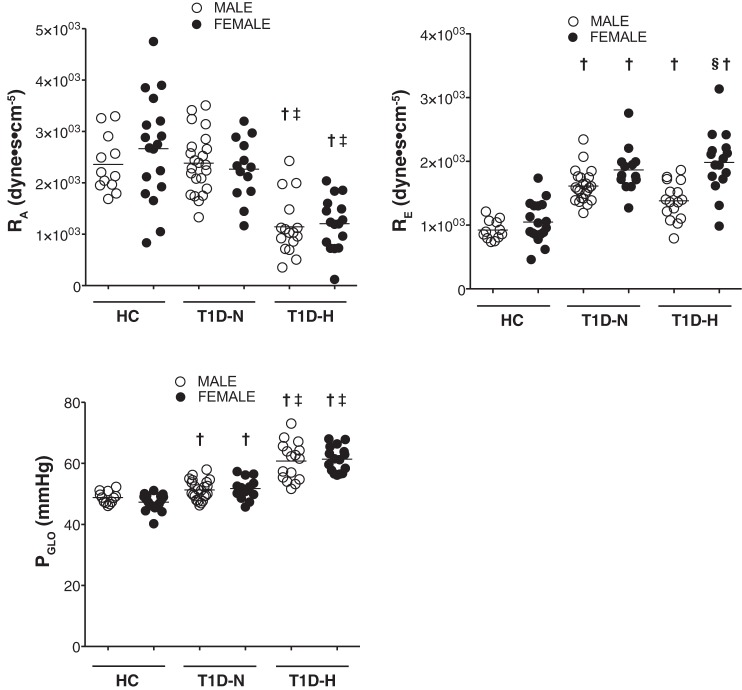
When using differential KFG values for normal and T1D subjects, but without making additional changes in KFG based on sex, female T1D-H subjects had significantly higher RE than men (1,985 ± 487 vs. 1,381 ± 296 dyne·sec−1·cm−5, P < 0.001, Fig. 1, Table 2), while no sex differences were observed for RA or PGLO. Similar to our previous studies, T1D-H subjects had significantly lower RA and higher PGLO compared with T1D-N and HC (Fig. 1).
Fig. 1.
Glomerular hemodynamics estimated by Gomez’s equations in normal healthy controls (HC), and patients with type 1 diabetes (T1D), renal normofiltration (T1D-N), and hyperfiltration (T1D-H) according to sex. PGLO, glomerular hydrostatic pressure; RA, afferent arteriolar resistance; RE, efferent arteriolar resistance. §P < 0.001 vs. opposite sex within group, †P < 0.05 vs. same sex healthy control group, ‡P < 0.05 vs. same sex Type 1 diabetes and normofiltration group using post hoc Tukey’s test after ANOVA analysis.
Table 2.
Glomerular hemodynamics calculated using differential KFG values for normal and T1D subjects
| HC Male | HC Female | T1D-N Male | T1D-N Female | T1D-H Male | T1D-H Female | |
|---|---|---|---|---|---|---|
| Glomerular hemodynamic parameters | ||||||
| N | 12 | 18 | 23 | 13 | 16 | 16 |
| PGLO | 48.8 ± 1.9 | 47.2 ± 2.6 | 51.3 ± 3.1 | 51.7 ± 3.5† | 60.8 ± 6.2†‡ | 61.4 ± 4.2†‡ |
| πG | 27.7 ± 2.4 | 25.7 ± 2.4 | 22.3 ± 2.3† | 22.6 ± 2.9† | 23.3 ± 3.0† | 24.1 ± 2.4 |
| RA | 2,359 ± 549 | 2,665 ± 1,019 | 2,383 ± 581 | 2,266 ± 599 | 1,144 ± 565†‡ | 1,206 ± 508†‡ |
| RE | 922 ± 153 | 1,049 ± 317 | 1,613 ± 256† | 1,867 ± 354† | 1,381 ± 296† | 1,985 ± 487§† |
Values are means ± SD. PGLO, glomerular hydrostatic pressure (mmHg); πG, oncotic pressure (mmHg); RA, afferent arteriolar resistance (dyne·sec−1·cm−5); RE, efferent arteriolar resistance (dyne·sec−1·cm−5).
P < 0.001 vs. opposite sex within group.
P < 0.05 vs. same sex healthy control group.
P < 0.05 vs. same sex Type 1 diabetes and normofiltration group.
When using estimated KFG values that differed based on sex and T1D status, we saw similar changes in RE: female T1D-H subjects had significantly higher RE than men (1,985 ± 487 vs. 751 ± 161 dyne·sec−1·cm−5, P < 0.001, Table 3). Under these assumptions, female T1D-N subjects had significantly higher RE than T1D-N males (1,867 ± 354 vs. 878 ± 139 dyne·sec−1·cm−5, P < 0.001, Table 3), and female HC subjects also had significantly higher RE than HC males (1,046 ± 317 vs. 501 ± 83 dyne·sec−1·cm−5, P < 0.001, Table 3). Sex differences in PGLO were seen where T1D-H females had significantly higher PGLO than T1D-H males (61.4 ± 4.2 vs. 48.3 ± 4.6 mmHg, P < 0.001, Table 3), and T1D-N females had significantly higher PGLO than T1D-N males (51.7 ± 3.5 vs. 42.7 ± 2.7 mmHg, P < 0.001, Table 3).
Table 3.
Glomerular hemodynamics using differential KFG values for sex within normal and T1D subjects
| HC Male | HC Female | T1D-N Male | T1D-N Female | T1D-H Male | T1D-H Female | |
|---|---|---|---|---|---|---|
| Glomerular hemodynamic parameters | ||||||
| N | 12 | 18 | 23 | 13 | 16 | 16 |
| PGLO | 43.8 ± 2.1 | 47.2 ± 2.7 | 42.7 ± 2.7 | 51.7 ± 3.5§† | 48.3 ± 4.6†‡ | 61.4 ± 4.2§†‡ |
| πG | 27.7 ± 2.4 | 25.7 ± 2.4 | 22.3 ± 2.3† | 22.6 ± 2.9† | 23.3 ± 3.0† | 24.1 ± 2.4 |
| RA | 2,734 ± 588 | 2,665 ± 1,019 | 3,036 ± 623 | 2,266 ± 599* | 1,711 ± 633†‡ | 1,206 ± 508†‡ |
| RE | 501 ± 83 | 1,049 ± 317§ | 878 ± 139 | 1,867 ± 354§† | 751 ± 161† | 1,985 ± 487§† |
Values are means ± SD. PGLO, glomerular hydrostatic pressure (mmHg); πG, oncotic pressure (mmHg); RA, afferent arteriolar resistance (dyne·sec−1·cm−5); RE, efferent arteriolar resistance (dyne·sec−1·cm−5).
P < 0.05 vs. opposite sex within group.
P < 0.001 vs. opposite sex within group.
P < 0.05 vs. same sex healthy control group.
P < 0.05 vs. same sex Type 1 diabetes and normofiltration group.
DISCUSSION
Measurements of afferent and efferent arteriolar resistances within the human glomerulus are not feasible. As a result, it is not clear how sex impacts renal segmental resistance and glomerular physiology in the presence or absence of diabetes. Previous studies have characterized human renal physiological responses to RAAS inhibition on the basis of sex (36), and shown that nondiabetic men may require higher doses of angiotensin receptor blockade therapy than nondiabetic women. To further clarify the preglomerular vs. postglomerular factors that are responsible for mediating sex-based pathophysiological differences in renal function early in the natural history of diabetes, we applied the equations derived by Gomez et al. (22) to calculate glomerular hemodynamic parameters in both male and female HC and T1D patients. It was important to separate T1D patients based on hyperfiltration status since patients with T1D-N vs. T1D-H respond differently to a variety of stimuli, including cyclooxygenase 2 inhibition, nitric oxide inhibition, angiotensin I-converting enzyme (ACE) inhibition, and sodium glucose cotransport-2 inhibition (7, 9–11, 53).
Long-term studies have shown that nondiabetic CKD progresses to ESRD more slowly in women than in men (23, 28, 40), especially before menopause (18, 49). Mass screening programs have similarly shown that ESRD incidence starts to rise 10 yr later in women compared with male age-matched controls (26). In addition to later onset of CKD, women with nondiabetic renal disease may also maintain better preservation of renal function over time compared with men (40). To further understand the influence of sex hormones on renal disease progression, in vitro studies have examined sex hormone effects on physiological factors that increase the risk of renal disease and have shown attenuated activation of profibrotic pathways under the influence of estrogen (29, 48). In the in vivo spontaneous hypertensive rat model, urine albumin excretion is greater in males than females—an effect that is attenuated by castration (55) or oophorectomy (24). In contrast with observations in nondiabetic CKD, the role of sex on renal outcomes in the context of diabetes is less clear.
As suggested from prior studies (14), T1D-H female subjects had lower RBF than their male counterparts. In the current analysis, we calculated that the lower RBF phenomenon may be due to a higher RE in T1D-H females. When reanalysis was done using altered KFG values for sex, T1D-N females also had higher RE, but there were no observed changes in RBF in this cohort. In contrast, in previous analyses, none of these hemodynamic parameters differed significantly in the HC or T1D-N groups based on sex (14). In terms of implications for the treatment of CKD, the higher baseline RE in T1D-H women is interesting because nondiabetic healthy women exhibit enhanced responsiveness to aldosterone receptor blockade therapy and greater responsiveness to ACE inhibition in the context of T1D (36). If RE was in fact elevated in women with T1D on the basis of RAAS activation in our previous work, then the greater responsiveness to RAAS inhibition may be on the basis of augmented blockade of the intrarenal RAAS leading to efferent arteriolar vasodilatation.
Although the mechanisms responsible for the increased RE and lower RBF in T1D-H women compared with their males with T1D-H are not known, intrarenal RAAS activation is a possible candidate mediator. Previous studies in rodents have demonstrated that males exhibit higher levels of angiotensinogen mRNA in kidney tissue compared with females, and that estrogen attenuates the renal hemodynamic responses to angiotensin II, as well as histological and molecular markers of renal injury—overall effects consistent with RAAS suppression (4, 6, 21, 34). Similarly, in humans, women exhibit lower systemic blood pressures compared with men—a pattern that is consistent with overall blood pressure levels seen in the current analysis, which have in the past been attributed to suppression of the RAAS (20, 47, 59). Similarly, estrogen decreases ACE activity (5), angiotensin I receptor density (41), and aldosterone (35), but activates angiotensin II receptor density (31) and natriuretic peptides (32)—a profile that would generally be consistent with vasodilatation and blood pressure lowering. Nevertheless, given the lack of direct measures of intrarenal RAAS activation in the current analysis, the role of the RAAS remains speculative.
With the overall suppression of the RAAS system in women presumably due to estrogen, renal efferent arteriolar vasodilatation (lower RE) would be expected. Instead, we observed an opposite interaction, with higher RE in women with T1D-H. While the factors responsible for increased RE levels in women with T1D-H are not yet known, several possible explanations exist. First, observations in women without diabetes demonstrating blunted ERPF and FF responses to an infusion of exogenous angiotensin II compared with men, suggesting maximal baseline upregulation of the RAAS resulting in attenuated angiotensin II responsiveness (35). Therefore, lower levels of RAAS mediators in the systemic circulating in animals and humans with diabetes may not accurately reflect functional intrarenal RAAS activation in women leading to increased RE, as suggested by others (37). Alternatively, other non-RAAS mediators may have important roles in altering renal hemodynamic based on sex. For example, endothelin infusion is associated with a primary efferent arteriolar vasoconstrictive effect, which is likely mediated by the endothelin-1 type A receptor (45, 54). At the afferent arteriole, nitric oxide and vasodilatory prostanoids have been implicated in the pathogenesis of hyperfiltration (8, 17), and may also mediate sex-based renal function differences in humans (13). Whether there are major functional contributions from other neurohormonal mediators such as endothelin, nitric oxide, or prostanoids to differences in RE based on sex requires further study.
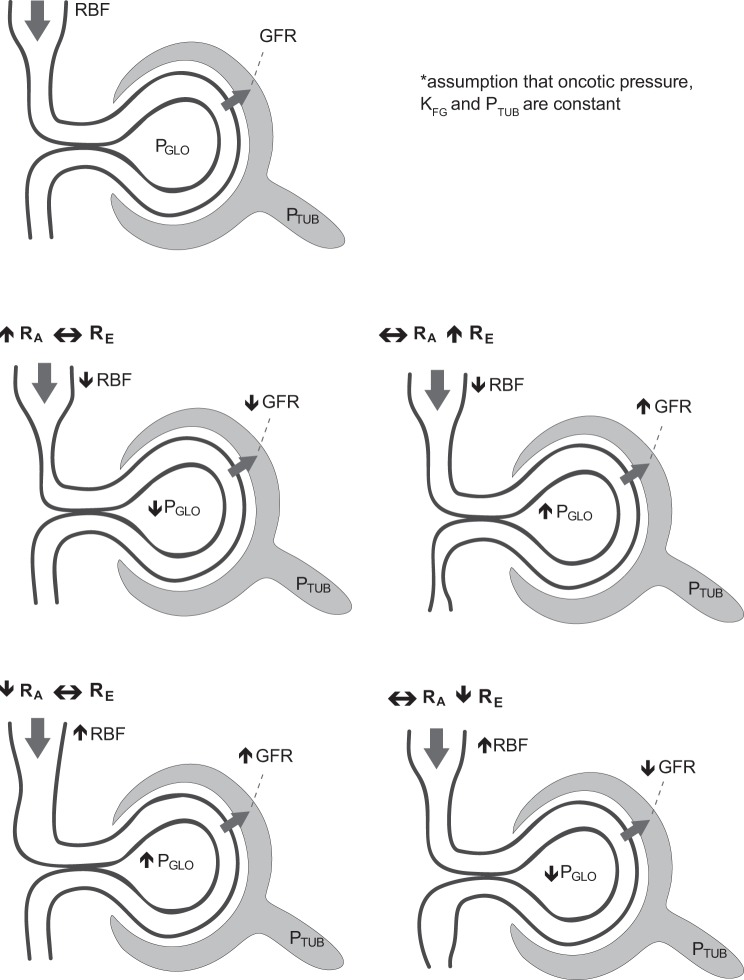
The difference in the renal hemodynamic profile observed in women vs. men was characterized by an increased FF and lower RBF in the context of similar GFR levels, which merits additional comment. These differences may have been on the basis of several mechanisms, including an increase in RE, or an increase in KFG with concomitant increases in RA and RE. If on the basis of efferent arteriolar vasoconstriction as estimated by the Gomez equations, it must be assumed that tubular hydraulic pressure and KFG remained constant in the presence of filtration disequilibrium (Fig. 2), which may not be the case in the setting of hyperfiltration (39, 46). For example, Scholey et al. (46) demonstrated that independent of insulin administration or ambient hyperglycemia, proximal tubular hydraulic pressure was ~3 mmHg lower in hyperfiltering animals with diabetes compared with nondiabetic control animals. In addition, in this model, the authors reported continued hyperfiltration despite a normalization of the glomerular transcapillary hydrostatic pressure difference in insulin-treated animals, which was due to an increase in calculated KFG. It is therefore important to recognize that in addition to the effect of sex on KFG, both tubular hydraulic pressure and KFG may change under dynamic physiological conditions. Therefore, when we repeated our glomerular hemodynamic calculations with alternate estimated KFG values for sex differences noted in animals, we observed consistent increased values for RE in both T1D-H and T1D-N females as well as HC subjects, highlighting that our main observation is maintained in the context of changing KFG values. Under this assumption, PGLO was also increased in both T1D-H and T1D-N females compared with men in the same groups(Table 3), although this was not seen in our primary analysis with constant KFG values (Table 2). Our main observation of higher RE in T1D-H females compared with men is consistent across analyses of constant and differential KFG values, highlighting the importance of understanding assumptions of filtration disequilibrium and the relevant physiological assumptions when doing calculations using Gomez formulae.
Fig. 2.
Intrarenal determinants of GFR. GFR, glomerular filtration rate; PGLO, glomerular hydrostatic pressure; RBF, renal blood flow; RA, afferent arteriolar resistance; RE, efferent arteriolar resistance; PTUB, tubular pressure.
The present study has limitations that are worth mentioning. First, the sample size in this analysis was small and may have limited our ability to detect between-group differences in HC and TID-N subjects. However, to minimize the effect of the sample size, we used careful physiological measurements, including RBF and GFR assessments by paraaminohippurate and inulin clearance, accepted as the gold standard methods. We also normalized glucose with a modified glucose clamp technique and standardized diet to eliminate the confounding impacts of acute glycemia and macronutrient content on renal measures. Another limitation is that the Gomez equations used in these calculations to determine glomerular hemodynamics only allow for indirect calculations of segmental resistance. The calculations of these parameters inferred ultrafiltration coefficients of normal and diabetic glomeruli determined from rat studies as described. With regard to mechanistic insights into the observed sex-based differences in renal hemodynamic function, mechanistic studies are now required to support our hypotheses. Finally, the observations described in this analysis are retrospective and cross-sectional and should therefore be considered to be exploratory and hypothesis generating. All experiments from the studies were performed in the one laboratory by the same clinical nurse and clinical supervisor with equivalent protocols to decrease potential confounders.
In conclusion, we have shown the first evidence in humans that women with T1D-H have higher RE compared with men. Our findings may help to explain some of the observed clinical and experimental differences in sex-based responses to RAAS blockade.
GRANTS
The studies that provided data for this pooled analysis were funded by CIHR, Heart and Stroke Foundation of Canada and Boehringer Ingelheim & Eli Lilly, and Company Diabetes Alliance. D. Cherney was also supported by a Kidney Foundation of Canada Scholarship and a Canadian Diabetes Association-KRESCENT Program Joint New Investigator Award.
DISCLOSURES
D. Z. I. Cherney has received speaker/consultant honoraria from Boehringer-Ingelheim, Eli Lilly, AstraZeneca, Sanofi, Merck, Mitsubishi-Tanabe and Janssen and has received operational funding for clinical trials from Boehringer-Ingelheim, Merck, Janssen and AstraZeneca.
AUTHOR CONTRIBUTIONS
D.Z.I.C., Y.L., and M.Š. performed analyses, interpreted data, and drafted the manuscript. P.B. contributed to data analysis, drafting the manuscript, and reviewed and edited the manuscript. H.N.R., J.W.S., P.Y., E.B.S., and B.P. contributed to the interpretation of data and reviewed and edited the manuscript. All authors approved the final version.
ACKNOWLEDGMENTS
The authors were fully responsible for all content and editorial decisions were involved at all stages of manuscript development and have approved the final version.
REFERENCES
- 1.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes : 279–286, 2014. doi: 10.1097/MED.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjornstad P, Škrtić M, Lytvyn Y, Maahs DM, Johnson RJ, Cherney DZ. The Gomez’ equations and renal hemodynamic function in kidney disease research. Am J Physiol Renal Physiol, in press. doi: 10.1152/ajprenal.00415.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breyer JA, Bain RP, Evans JK, Nahman NS Jr, Lewis EJ, Cooper M, McGill J, Berl T; The Collaborative Study Group . Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. Kidney Int : 1651–1658, 1996. doi: 10.1038/ki.1996.481. [DOI] [PubMed] [Google Scholar]
- 4.Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol Regul Integr Comp Physiol : R1908–R1915, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Brosnihan KB, Weddle D, Anthony MS, Heise C, Li P, Ferrario CM. Effects of chronic hormone replacement on the renin-angiotensin system in cynomolgus monkeys. J Hypertens : 719–726, 1997. doi: 10.1097/00004872-199715070-00003. [DOI] [PubMed] [Google Scholar]
- 6.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension : 456–463, 1992. doi: 10.1161/01.HYP.19.5.456. [DOI] [PubMed] [Google Scholar]
- 7.Cherney DZ, Konvalinka A, Zinman B, Diamandis EP, Soosaipillai A, Reich H, Lorraine J, Lai V, Scholey JW, Miller JA. Effect of protein kinase Cβ inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care : 91–93, 2009. doi: 10.2337/dc08-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with Type 1 diabetes. Diabetes : 688–695, 2008. doi: 10.2337/db07-1230. [DOI] [PubMed] [Google Scholar]
- 9.Cherney DZ, Miller JA, Scholey JW, Nasrallah R, Hébert RL, Dekker MG, Slorach C, Sochett EB, Bradley TJ. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in Type 1 diabetes. Diabetes Care : 1344–1346, 2010. doi: 10.2337/dc09-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with Type 1 diabetes mellitus. Circulation : 587–597, 2014. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 11.Cherney DZ, Reich HN, Jiang S, Har R, Nasrallah R, Hébert RL, Lai V, Scholey JW, Sochett EB. Hyperfiltration and effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated Type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol : R710–R718, 2012. doi: 10.1152/ajpregu.00286.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherney DZ, Scholey JW, Jiang S, Har R, Lai V, Sochett EB, Reich HN. The effect of direct renin inhibition alone and in combination with ACE inhibition on endothelial function, arterial stiffness, and renal function in Type 1 diabetes. Diabetes Care : 2324–2330, 2012. doi: 10.2337/dc12-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherney DZ, Scholey JW, Sochett EB. Sex differences in renal responses to hyperglycemia, L-arginine, and L-NMMA in humans with uncomplicated Type 1 diabetes. Diabetes Care : 1290–1296, 2013. doi: 10.2337/dc12-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherney DZ, Sochett EB, Miller JA. Gender differences in renal responses to hyperglycemia and angiotensin-converting enzyme inhibition in diabetes. Kidney Int : 1722–1728, 2005. doi: 10.1111/j.1523-1755.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 15.Cherney DZI, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with Type 1 diabetes. Diabetes : 688–695, 2008. doi: 10.2337/db07-1230. [DOI] [PubMed] [Google Scholar]
- 16.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with Type 1 diabetes mellitus. Circulation : 587–597, 2014. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 17.Cherney DZI, Reich HN, Jiang S, Har R, Nasrallah R, Hébert RL, Lai V, Scholey JW, Sochett EB. Hyperfiltration and effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated Type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol : R710–R718, 2012. doi: 10.1152/ajpregu.00286.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coggins CH, Breyer Lewis J, Caggiula AW, Castaldo LS, Klahr S, Wang SR. Differences between women and men with chronic renal disease. Nephrol Dial Transplant : 1430–1437, 1998. doi: 10.1093/ndt/13.6.1430. [DOI] [PubMed] [Google Scholar]
- 19.Coonrod BA, Ellis D, Becker DJ, Bunker CH, Kelsey SF, Lloyd CE, Drash AL, Kuller LH, Orchard TJ; Pittsburgh Epidemiology of Diabetes Complications Study . Predictors of microalbuminuria in individuals with IDDM. Diabetes Care : 1376–1383, 1993. doi: 10.2337/diacare.16.10.1376. [DOI] [PubMed] [Google Scholar]
- 20.Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens : 853–862, 1998. doi: 10.1097/00004872-199816060-00017. [DOI] [PubMed] [Google Scholar]
- 21.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest : 1941–1945, 1989. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest : 1143–1155, 1951. doi: 10.1172/JCI102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannedouche T, Chauveau P, Kalou F, Albouze G, Lacour B, Jungers P. Factors affecting progression in advanced chronic renal failure. Clin Nephrol : 312–320, 1993. [PubMed] [Google Scholar]
- 24.Hoshi-Fukushima R, Nakamoto H, Imai H, Kanno Y, Ishida Y, Yamanouchi Y, Suzuki H. Estrogen and angiotensin II interactions determine cardio-renal damage in Dahl salt-sensitive rats with heart failure. Am J Nephrol : 413–423, 2008. doi: 10.1159/000112806. [DOI] [PubMed] [Google Scholar]
- 25.Hostetter TH, Troy JL, and Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int : 410–415, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int : 800–805, 1996. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen P, Rossing K, Tarnow L, Rossing P, Mallet C, Poirier O, Cambien F, Parving HH. Progression of diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int Suppl : S101–S105, 1999. doi: 10.1046/j.1523-1755.1999.07125.x. [DOI] [PubMed] [Google Scholar]
- 28.Jungers P, Chauveau P, Descamps-Latscha B, Labrunie M, Giraud E, Man NK, Grünfeld JP, Jacobs C. Age and gender-related incidence of chronic renal failure in a French urban area: a prospective epidemiologic study. Nephrol Dial Transplant : 1542–1546, 1996. doi: 10.1093/oxfordjournals.ndt.a027610. [DOI] [PubMed] [Google Scholar]
- 29.Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, Silbiger S. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int : 1173–1179, 1996. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- 30.Lytvyn Y, Škrtić M, Yang GK, Lai V, Scholey JW, Yip PM, Perkins BA, Cherney DZ. Plasma uric acid effects on glomerular haemodynamic profile of patients with uncomplicated Type 1 diabetes mellitus. Diabet Med : 1102–1111, 2016. doi: 10.1111/dme.13051. [DOI] [PubMed] [Google Scholar]
- 31.Macova M, Armando I, Zhou J, Baiardi G, Tyurmin D, Larrayoz-Roldan IM, Saavedra JM. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology : 276–286, 2008. doi: 10.1159/000150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maffei S, Del Ry S, Prontera C, Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci (Lond) : 447–453, 2001. doi: 10.1042/cs1010447. [DOI] [PubMed] [Google Scholar]
- 33.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia : 691–697, 2009. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 34.Mankhey RW, Bhatti F, Maric C. 17β-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol : F399–F405, 2005. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 35.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int : 278–285, 1999. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 36.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol : 2554–2560, 2006. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 37.Moffett RB, McGowan RA, Gross KW. Modulation of kidney renin messenger RNA levels during experimentally induced hypertension. Hypertension : 874–882, 1986. doi: 10.1161/01.HYP.8.10.874. [DOI] [PubMed] [Google Scholar]
- 38.Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol Renal Physiol : F223–F231, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Nath KA, Kren SM, Hostetter TH. Dietary protein restriction in established renal injury in the rat. Selective role of glomerular capillary pressure in progressive glomerular dysfunction. J Clin Invest : 1199–1205, 1986. doi: 10.1172/JCI112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol : 319–329, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation : 2197–2201, 1998. doi: 10.1161/01.CIR.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 42.Ott C, Schneider MP, Raff U, Ritt M, Striepe K, Alberici M, Schmieder RE. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol : 129–135, 2013. doi: 10.1111/j.1365-2125.2012.04336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int : 481–486, 1988. doi: 10.1038/ki.1988.206. [DOI] [PubMed] [Google Scholar]
- 44.Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Dodesini AR, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators . Glomerular hyperfiltration and renal disease progression in Type 2 diabetes. Diabetes Care : 2061–2068, 2012. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schildroth J, Rettig-Zimmermann J, Kalk P, Steege A, Fähling M, Sendeski M, Paliege A, Lai EY, Bachmann S, Persson PB, Hocher B, Patzak A. Endothelin type A and B receptors in the control of afferent and efferent arterioles in mice. Nephrol Dial Transplant : 779–789, 2011. doi: 10.1093/ndt/gfq534. [DOI] [PubMed] [Google Scholar]
- 46.Scholey JW, Meyer TW. Control of glomerular hypertension by insulin administration in diabetic rats. J Clin Invest : 1384–1389, 1989. doi: 10.1172/JCI114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation : 39–45, 1997. doi: 10.1161/01.CIR.95.1.39. [DOI] [PubMed] [Google Scholar]
- 48.Silbiger S, Lei J, Ziyadeh FN, Neugarten J. Estradiol reverses TGF-β1-stimulated type IV collagen gene transcription in murine mesangial cells. Am J Physiol Renal Physiol : F1113–F1118, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Simon P, Ramée MP, Autuly V, Laruelle E, Charasse C, Cam G, Ang KS. Epidemiology of primary glomerular diseases in a French region. Variations according to period and age. Kidney Int : 1192–1198, 1994. doi: 10.1038/ki.1994.384. [DOI] [PubMed] [Google Scholar]
- 50.Škrtić M, Lytvyn Y, Yang GK, Yip P, Lai V, Silverman M, Cherney DZ. Glomerular haemodynamic profile of patients with Type 1 diabetes compared with healthy control subjects. Diabet Med : 972–979, 2015. doi: 10.1111/dme.12717. [DOI] [PubMed] [Google Scholar]
- 51.Škrtić M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, Woerle HJ, Johansen OE, Broedl UC, Hach T, Silverman M, Cherney DZ. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with Type 1 diabetes and renal hyperfiltration. Diabetologia : 2599–2602, 2014. doi: 10.1007/s00125-014-3396-4. [DOI] [PubMed] [Google Scholar]
- 52.Slack TK, Wilson DM. Normal renal function: CIN and CPAH in healthy donors before and after nephrectomy. Mayo Clin Proc : 296–300, 1976. [PubMed] [Google Scholar]
- 53.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol : 1703–1709, 2006. doi: 10.1681/ASN.2005080872. [DOI] [PubMed] [Google Scholar]
- 54.Sørensen SS, Madsen JK, Pedersen EB. Systemic and renal effect of intravenous infusion of endothelin-1 in healthy human volunteers. Am J Physiol Renal Physiol : F411–F418, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol : R1573–R1579, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 57.Tsuda A, Inaba M, Ichii M, Ochi A, Ohno Y, Nakatani S, Yamada S, Mori K, Tahara H, Ishimura E. Relationship between serum TSH levels and intrarenal hemodynamic parameters in euthyroid subjects. Eur J Endocrinol : 45–50, 2013. doi: 10.1530/EJE-13-0026. [DOI] [PubMed] [Google Scholar]
- 58.Tsuda A, Ishimura E, Ohno Y, Ichii M, Nakatani S, Mori K, Fukumoto S, Emoto M, Inaba M. Significant association of poor glycemic control with increased resistance in efferent arterioles—study of inulin and para-aminohippuric acid clearance in humans. Diabetes Res Clin Pract : 234–240, 2014. doi: 10.1016/j.diabres.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 58a.USRD System USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases, 2012. [Google Scholar]
- 59.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h Ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens : 978–986, 1995. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 60.Winton FR. Physical factors involved in the activities of the mammalian kidney. Physiol Rev : 408– 435, 1937. [Google Scholar]