Abstract
This article is the result of a round-table discussion organised by ESMO Open in Vienna in December 2017. Its purpose is to discuss the background and advances in the evidence regarding cyclin-dependent kinase 4/6 inhibitors (palbociclib, ribociclib and abemaciclib) in the treatment of metastatic and early-stage breast cancer and to explore what the key open research questions are and next steps should be.
Keywords: CDK4/6 inhibitors, breast cancer, metastatic breast cancer, endocrine therapy, endocrine resistance, HR-positive breast cancer
Video 1.
Introduction
Hormone receptor (HR)-positive breast cancers (BCs), which represent up to 75% of invasive BCs, are characterised by the presence of receptors for oestrogen (ER) and/or progesterone (PgR). Endocrine therapy (ET) remains the therapeutic backbone for the treatment of HR-positive patients with BC.1 However, up to 50% of HR-positive patients with metastatic breast cancer (MBC) develop mechanisms of therapeutic resistance to the ET they receive.2 In order to modify the development of resistance to ET thereby enabling patients to receive effective HR-directed treatments, inhibitors of the cyclin-dependent kinases (CDK)4 and 6 have been developed and have recently shown a clinically meaningful efficacy and a good tolerability profile in patients with MBC.
Loss of cell cycle regulation leading to uncontrolled cellular proliferation is a hallmark of cancer.3 In the cell cycle, the progression from G1 (pre-DNA synthesis) to S (DNA synthesis) is a critical checkpoint in preventing the cell from abnormal proliferation. A key regulator of this process is the cyclin D-CDK4/6-inhibitor of CDK4 (INK4)-retinoblastoma (Rb) pathway which has emerged as a promising therapeutic approach in cancer.4
Initial clinical experience with first-generation pan-CDK inhibitors has shown poor efficacy and important toxicity.5 However, a new generation of highly selective CDK4/6 inhibitors including palbociclib, ribociclib and abemaciclib has overcome the challenges of non-specific pan-CDK inhibitors and targets tumour types in which the cyclin D-CDK4/6-INK4-Rb pathway has a key role, more effectively.6
Basics and rationale of CDK4/6 inhibition in general and in BC in particular
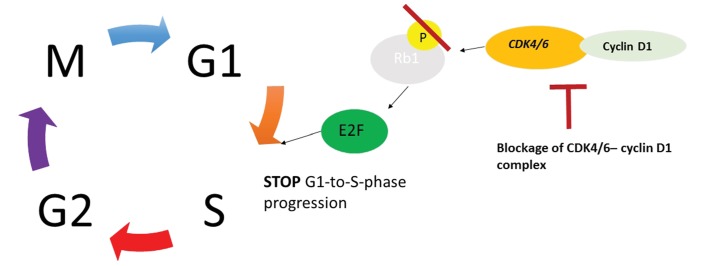
The CDK4/6-cyclin D1 complex phosphorylates Rb1 leading to a loss of repression of E2F transcription factors, resulting in cell cycle progression from G1 to S phase and then in cancer proliferation. CDK4/6 inhibitors, while blocking CDK4/6-cyclin D1 complex, prevent phosphorylation of Rb1, stop the cell cycle progression from G1 to S phase (figure 1).7
Figure 1.
Cell cycle progression in cancer and the role of cyclin-dependent kinases (CDK)4/6 inhibitors. Adapted with permission of John Wiley & Sons from: Murphy7. Permission conveyed through Copyright Clearance Center.
Several cancers such as melanoma, glioblastoma, head and neck cancer, non-small-cell lung cancer and BC have alterations that activate the cyclin D-CDK4/6-INK4-Rb pathway.8–11 In BC, alterations in the cyclin D-CDK4/6-INK4-Rb pathway are frequent. Amplification of the gene encoding cyclin D1 (CCND1) has been identified in about 15%–20% of human BC while overexpression of the protein has been demonstrated in a higher percentage.12–15 Loss of the tumour suppressor P16 has been found in up to 50% of BC.16
Preclinical studies have provided the rationale for the clinical development of CDK4/6 inhibitors in specific molecular subgroups of BC.17 18 Cell lines representing the luminal ER-positive BC subtype (including human epidermal growth factor receptor 2 (HER2)-amplified cell lines with luminal features) were the most sensitive to growth inhibition by palbociclib and ribociclib.18 19 By contrast, non-luminal or basal subtypes were shown to be the most resistant to palbociclib.18 19
In HR-positive BC, ER signalling upregulates cyclin D1 levels and potentiates multiple signalling pathways, resulting in upregulation of CDK4/6 activity.4 20
In addition, cyclin D1 facilitates ER transcriptional activity supporting the dependence of cyclin D1 to initiate cell cycle progression from G1 to S phase and to drive cancer cell proliferation, reinforcing the rationale for the combination of ET with CDK4/6 inhibitors.
Endocrine resistance in hormone-positive BC
Despite their name not all hormone responsive tumours are truly sensitive to ETs that inhibit the ER pathway. About 20% of HR-positive MBC are refractory to first-line ET (de novo or primary endocrine resistance).21 Although clinicians usually treat HR-positive MBC patients with further ET after initial response to first-line therapy, the clinical benefit rate declines from 70% at first-line treatment to about 30% for second and further lines representing acquired or secondary endocrine resistance.22
A possible definition of primary and secondary resistance is provided in table 1 1 with the definition based on clinical experience and not on biology.
Table 1.
Endocrine resistance in metastatic breast cancer (MBC)1
| Primary endocrine resistance | Relapse within 2 years of adjuvant ET PD within first six months of first-line ET for MBC |
| Secondary endocrine resistance | Relapse while on adjuvant ET but after the first two years or relapse within 12 months after completing adjuvant ET PD≥6 months after initiating ET for MBC |
ET, endocrine therapy; PD, progressive disease.
Acquired resistance to ET occurs due to the loss of ER functionality or loss of previously present ER dependence. Loss of ER is infrequent and observed in only 10% of MBCs23 24 and in <10% of BCs during neoadjuvant chemotherapy.25 An increased genetic instability further complicates tumour heterogeneity, even though not all newly acquired mutations may be important for drug resistance. Besides tumour heterogeneity, dormancy with the risk of experiencing a late recurrence, and mutations in the gene ESR1, which encodes ERα, are further mechanisms of endocrine resistance. Moreover, overexpression and/or amplifications of growth factor receptors are associated with the appearance of endocrine resistance23 and cell cycle checkpoint alterations can also contribute to loss of endocrine responsiveness.7
Mutations in ESR1 are rare in untreated patients and were not identified by the Cancer Genome Atlas analysis as untreated primary BC specimen were analysed in this work.26 Activating mutations of ESR1 27 28 are likely a mechanism of resistance to ET and were found in patients that had been treated with ETs.
However, in metastatic and pretreated BC, ESR1 mutations occur more frequently. ESR1 mutation status in cell-free tumour DNA (ctDNA) showed high concordance with contemporaneous tumour biopsies29 and liquid biopsy has been used to detect ESR1 mutations which could identify patients who have become resistant to particular ETs.30 Clalot and coauthors found ESR1 mutations in ctDNA in 75% of blood samples 3 and 6 months before progression on first-line therapy with aromatase inhibitors (AIs).31 In recently published clinical data from two large randomised phase II trials, the PALOMA-3 and the SoFEA study, ESR1 mutations were found in 29% and 39% in specimens from patients who received prior AI therapy, respectively. In comparison to treatment-naive patients with a very low rate of ESR1 mutations, these data are in line with the existing evidence that ESR1 mutations emerge more commonly with an acquired resistance after AI treatment.32
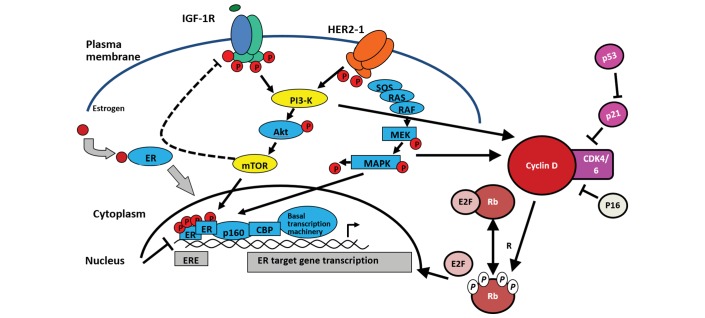
Components of the growth factor receptor pathways including fibroblast growth factor receptor 1, HER2, HER3, epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor (IGF-1R) converge on the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) and Raf/mitogen-activated protein kinase/ERK kinase/extracellular-signal-regulated kinase (RAF/MEK/ERK) pathways and are frequently mutated in BC. These pathways regulate cell survival and proliferation and aberrations in the PI3K signalling pathways and lead to a pathway hyper-activation that promotes ER-independent ER transcriptional activation (figure 2).
Figure 2.
Cross-talk between oestrogen (ER) and epidermal growth factor receptor (EGFR)/insulin-like growth factor 1 receptor (IGF-1R) signalling pathway and cyclin-dependent kinase (CDK)4/6 function. Adapted with permission from Springer Nature: Di Cosimo S and Baselga J.65 Copyright 2010. HER2-1, human epidermal growth factor receptor 2; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase.
The aberrations include both mutations of PIK3 catalytic alpha polypeptide (PIK3CA), AKT1, AKT2 and PDK1 and loss of inhibitory signals PTEN and INPP4B that occur in about 70% of BCs.33 Blockage of PI3K pathway alteration results in a disturbed cross-talk and consecutively in an increased EER dependence that provides the rationale for an ET.34
The recently presented results of the phase II LORELEI trial (NCT02273973) showed a promising piece of evidence for the combination of PI3K inhibitor and non-steroidal aromatase inhibitor (NSAI) in the neoadjuvant setting as the combination of PI3K inhibitor taselisib with letrozole led to an improved objective response rate (ORR) (OR 1.55; 95% CI 1.00 to 2.38, p=0.049 for all and OR 2.03; 95% CI 1.06 to 3.88, p=0033 for PIK3CA mutations compared with letrozole alone).35
mTOR plays a key role in the regulation of protein translation, cell growth and metabolism. It exists in two distinct protein kinase complexes, that is, mammalian target of rapamycin complex (mTORC) 1 and 2.36 Phosphorylation of AKT leads to an increased mTORC1 kinase activity that promotes protein synthesis. It is another target that can be blocked to reverse emerging endocrine resistance. The concept of a mTOR blockade was successfully proven in the BOLERO-2 study, a phase III trial that compared the mTOR inhibitor everolimus in combination with exemestane versus exemestane with placebo in postmenopausal women with ER-positive, HER2-negative advanced breast cancer whose disease recurred during or within 12 months after the end of adjuvant treatment or progressed during or within 1 month after the end of treatment for advanced disease. The combination of everolimus and exemestane led to an improvement of progression-free survival (PFS) by 4.1 months (6.9 vs 2.8 months).37
In the recently presented results of the MANTA trial, the dual mTOR inhibitor vistusertib was inferior to everolimus.38
The cyclin D-CDK4/6-INK4-Rb pathway plays a key role in cell cycle regulation as it is downstream of multiple mitogenic cascades, making it a further important target for overcoming endocrine resistance.39 Cyclin D associates with and activates the protein kinases CDK4 and CDK6 that have been associated with poor response and resistance to ET. Cyclin D1 amplification is a common event in ER-positive BC, identified in 58% of luminal B cancers and 29% of luminal A cancers.26 Therefore, CDK4/6 inhibition is currently one of the most promising approaches to overcome endocrine resistance. In big trial programmes, CDK4/6 inhibition such as the PALOMA trials for palbociclib, the MONALEESA trials for ribociclib and the MONARCH trials for abemaciclib the efficacy of a CDK4/6 inhibition was evaluated. The trials included patients with endocrine sensitive and in the PALOMA-3 and MONARCH2 trial also endocrine-resistant disease.
State of the art of CDK4/6 inhibitor efficacy in advanced HR-positive BC
Palbociclib
The first-in-class, oral CDK4/6 inhibitor palbociclib has been evaluated in several randomised clinical trials including patients with metastatic HR-positive, HER2-negative BC: the PALOMA-1/TRIO-18 trial was an open-label, phase II study that randomised 165 postmenopausal women with advanced HR-positive/HER2-negative BC in first-line therapy to receive either letrozole or letrozole plus palbociclib.40 The study had two sequentially accrued cohorts: cohort 1, including patients with HR-positive/HER2-negative BC, and cohort 2, including patients bearing either cyclin D1 gene (CCND1) amplification or loss of p16, or both. The addition of palbociclib to letrozole significantly improved PFS compared with letrozole alone (20.2 vs 10.2 months, respectively HR, 0.488; p=0.0004).40 The most frequently observed adverse event (AE) was grade 3–4 neutropenia (54% in the experimental arm vs 1% in the control arm).40
The PALOMA-2 trial confirmed the superiority of palbociclib and letrozole over letrozole for treatment-naive patients with HR-positive/HER2-negative MBC within the frame of a prospective randomised phase III clinical trial.41 In total, 666 postmenopausal women were randomly assigned in a 2:1 ratio to receive letrozole with palbociclib or placebo, respectively. The median PFS was 24.8 months in the palbociclib arm versus 14.5 months in the placebo arm (HR 0.58; p<0.001).42 Again, grade 3 or 4 neutropenia was the most common AE (66.4% in the palbociclib arm vs 1.4% in the placebo arm), with 1.8% rate of febrile neutropenia in the experimental arm and none in the control arm.41
The phase III PALOMA-3 trial randomised 521 women with pretreated HR-positive/HER2-negative MBC—regardless of menopausal status—to receive either fulvestrant plus palbociclib or fulvestrant plus placebo.42 The combination of palbociclib and fulvestrant significantly improved PFS (9.2 vs 3.8 months, HR 0.42; p<0.001). The most common AE was again grade 3/4 AE neutropenia (62.0% in the palbociclib arm vs 0.6% in the placebo arm).42 However, febrile neutropenia occurred in only 0.6% of patients who received palbociclib and in 0.6% of patients who received placebo. At the final analysis of the PALOMA-3 trial, the median PFS was 9.5 months in the palbociclib arm and 4.6 months in the placebo arm (HR 0.46, p<0.0001). The addition of palbociclib was superior in all subgroups.43
Based on these results, palbociclib has been approved by the US Food and Drug Administration (FDA) and European Medicines Agency for clinical use in the metastatic setting in combination with letrozole in patients with newly diagnosed BC or with fulvestrant in patients pretreated with ET.
Very recently, the first overall survival (OS) data from a randomised trial of palbociclib plus ET were reported. Namely, in the PALOMA-1 trial, median OS was 37.5 months for palbociclib/letrozole versus 34.5 months for letrozole (HR 0.897, p=0.281).44 Median OS was 37.5 versus 33.3 months (HR 0.837, p=0.280) in cohort 1 and 35.1 versus 35.7 months (HR 0.935, p=0.388) in cohort 2, respectively. OS data from the larger PALOMA-2 trial are awaited.
Ribociclib
Ribociclib is another selective CDK4/6 inhibitor in the therapeutic armamentarium for BC.
The phase III MONALEESA-2 trial randomised 668 postmenopausal women with newly diagnosed HR-positive/HER2-negative MBC to receive either letrozole plus ribociclib or placebo. The experimental treatment resulted in a significantly improved PFS: at a median follow-up of 18 months, PFS was 63.0% in the ribociclib arm and 42.2% in the placebo arm.45 The median duration of PFS was 14.7 months (95% CI 13.0 to 16.5) in the placebo group (HR 0.56; 95% CI 0.43 to 0.72, p=3.29×10–6 for superiority) versus not reached in the ribociclib group (95% CI 19.3 to not reached). Moreover, ribociclib led to a higher ORR compared with the letrozole/placebo (52.7% vs 37.1%, p<0.001). The most common AE was grade 3/4 neutropenia (59.3% in the ribociclib arm vs 0.9% in the placebo arm).45
The MONALEESA-7 is the first phase III trial dedicated to the evaluation of a CDK4/6 inhibitor as first-line therapy for premenopausal and perimenopausal patients with HR-positive/HER2-negative MBC.44 In this study, the addition of ribociclib to either tamoxifen or a NSAI plus goserelin significantly improved PFS over placebo plus tamoxifen/NSAI plus goserelin (23.8 vs 13.0 months, respectively, HR 0.553, p=0.0000000983).46 Treatment benefit was consistent across patient subgroups and regardless of endocrine agent.46
Abemaciclib
Abemaciclib is another potent selective CDK4/6 inhibitor with more pronounced inhibition of CDK4.47 Equally to the other CDK4/6 inhibitors, the greatest benefit was observed among patients with HR-positive BC, with a disease control rate of 81% versus 33% for patients with HR-negative BC. Only patients with HR-positive BC experienced a treatment response. Heavily pretreated metastatic patients with HR-positive/HER2-positive and HR-positive/HER2-negative BC had a clinical benefit rate (CBR) of 54.5% and 64%, respectively.48 In the HR-positive subgroup, median PFS was 8.8 months (95% CI 4.2 to 16.0).48 Based on these results, abemaciclib has been granted FDA breakthrough therapy designation for patients with pretreated HR-positive MBC.
Abemaciclib monotherapy was assessed in the phase II single-arm MONARCH 1 study, which enrolled 132 women with HR-positive/HER2-negative MBC, whose disease progressed on or after ET and chemotherapy.49 Abemaciclib led to an ORR of 19.7%, CBR of 42.4% and median PFS of 6.0 months. The most common AEs all grades were diarrhoea (90.2%), fatigue (65.2%), nausea (64.4%), decreased appetite (45.5%) and abdominal pain (38.6%).
MONARCH 2 assessed abemaciclib plus fulvestrant in 669 women with HR-positive/HER2-negative MBC who had been pretreated with ET. These patients were randomised in a 2:1 ratio to receive either fulvestrant plus abemaciclib or fulvestrant plus placebo.50 The combination of fulvestrant plus abemaciclib resulted in a significantly prolonged PFS over fulvestrant/placebo (16.4 vs 9.3 months, HR 0.553, p<0.001). Higher rates of ORR were achieved in the abemaciclib plus fulvestrant arm (48.1%) compared with the placebo arm (21.3%).50 The double-blind phase III MONARCH 3 study randomised 493 postmenopausal women with HR-positive/HER2-negative advanced BC previously untreated in the advanced setting to receive either abemaciclib or placebo plus a NSAI. Median PFS was significantly longer in the abemaciclib arm (HR 0.54; 95% CI 0.41 to 0.72, p=0.000021; median: not reached in the abemaciclib arm, 14.7 months in the placebo arm). In patients with measurable disease, the ORR was 59% in the abemaciclib arm versus 44% in the placebo arm (p=0.004). In the abemaciclib arm, diarrhoea was the most frequent AE (81.3%) but was mainly grade 1 (44.6%). Comparing abemaciclib and placebo, the most frequent grade 3 or 4 AEs were neutropenia (21.1% vs 1.2%), diarrhoea (9.5% vs 1.2%) and leucopenia (7.6% vs 0.6%).51
Tables 2–4 give an overview on the phase III trials in patients with HR+/HER2 negative MBC.41–43 45 51
Table 2.
Phase III trials with cyclin-dependent kinase4/6 inhibitors in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer: median progression-free survival (PFS) and objective response rate
| Trial | Line in the metastatic setting | Patients (n) | Treatment | HR for PFS | Median PFS, months | Objective response rate (ITT population) (%) |
| PALOMA-2 | 1L | 666 | Palbociclib + letrozole vs placebo + letrozole | 0.58 | 24.8 vs 14.5 | 42.1 vs 34.7 |
| PALOMA-3 | >1L | 521 | Palbociclib + fulvestrant vs placebo + fulvestrant | 0.46 | 9.5 vs 4.6 | 19 vs 8 |
| MONALEESA-2 | 1L | 668 | Ribociclib + letrozole vs placebo + letrozole | 0.56 | NR vs 14.7 | 40.7 vs 27.5 |
| MONARCH 3 | 1L | 493 | Abemaciclib + NSAI vs placebo + NSAI |
0.54 | NR vs 14.7 | 59 vs 44 |
| MONARCH 2 | 1L or 2L | 669 | Abemaciclib + fulvestrant vs placebo + fulvestrant | 0.55 | 16.4 vs 9.3 | 48.1 vs 21.3 |
ITT, intentiontion to treat; NR, not reached; NSAI, non-steroidal aromatase inhibitor.
Table 3.
Phase III trials with cyclin-dependent kinase4/6 in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer: safety data
| Adverse event (AE) | PALOMA-2 | PALOMA-3 | MONALEESA-2 | MONARCH 3 | MONARCH 2 | ||||||||||
| Grade % | Grade % | Grade % | Grade % | Grade % | |||||||||||
| All | 3 | 4 | All | 3 | 4 | All | 3 | 4 | All | 3 | 4 | All | 3 | 4 | |
| Rash | 18 | 1 | – | NR | NR | NR | 17.1 | 0.6 | – | NR | NR | NR | 11.1 | 1.1 | 0 |
| Fatigue | 37 | 2 | – | 38 | 2 | – | 36.5 | 2.1 | 0.3 | 40.1 | 1.8 | – | 39.9 | 2.7 | – |
| Diarrhea | 26 | 1 | – | 19 | – | – | 35 | 1.2 | – | 81.3 | 9.5 | – | 86.4 | 13.4 | 0 |
| Nausea | 35 | <1 | – | 29 | – | – | 51.4 | 2.4 | – | 38.5 | 0.9 | – | 45.1 | 2.7 | – |
| Decreased appetite | 15 | 1 | – | 12.8 | 0.9 | – | 18.6 | 1.5 | – | 24.5 | 1.2 | – | 26.5 | 1.1 | 0 |
| Neutropenia | 80 | 56 | 10 | 78.8 | 53.3 | 8.7 | 74.3 | 49.7 | 9.6 | 41.3 | 19.6 | 1.5 | 46.0 | 23.6 | 2.9 |
| Anaemia | 24 | 5 | <1 | 26.1 | 2.6 | 0 | 18.6 | 0.9 | 0.3 | 28.4 | 5.8 | – | 29.0 | 7.0 | 0.2 |
| Thrombocytopenia | 16 | 1 | <1 | 19.4 | 1.7 | 0.6 | 9 | 0.6 | – | NR | NR | NR | 15.6 | 2.0 | 1.4 |
| Alopecia | 33 | – | – | 14.8 | – | – | 33.2 | – | – | 26.6 | – | – | 15.6 | – | – |
| QTcF Prolongation | – | – | 3.3 | – | – | ||||||||||
| Increased creatinine | – | – | – | 19 | 2.1 | – | 11.8 | 0.9 | 0 | ||||||
Table 4.
Phase III trials with cyclin-dependent kinase4/6 in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer: inclusion criteria
| PALOMA-2 (%) | PALOMA-3 (%) | MONALEESA-2 (%) | MONARCH 3 (%) | MONARCH 2 (%) | ||||||
| Palbociclib + Letrozol |
Placebo + Letrozol |
Palbociclib + Fulvestrant |
Placebo + Fulvestrant |
Ribociclib + Letrozol |
Placebo + Letrozol |
Abemaciclib + NSAI |
Placebo + NSAI |
Abemaciclib + Fulvestrant |
Placebo + Fulvestrant |
|
| Menopausal status | ||||||||||
| Pre/perimenopausal | 0 | 0 | 21 | 21 | 0 | 0 | 0 | 0 | 16 | 19 |
| Postmenopausal | 100 | 100 | 79 | 79 | 100 | 100 | 100 | 100 | 83 | 81 |
| Gender | ||||||||||
| Female | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Male | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Disease-free interval | ||||||||||
| Newly metastatic | 38 | 36 | 0 | 0 | 34 | 34 | 41 | 37 | na | na |
| <12 months | 22 | 22 | 5 | 2 | 1 | 3 | na | na | ||
| >12 months | 40 | 42 | 95 | 98 | 65 | 63 | na | na | ||
| Treatment-free interval | ||||||||||
| <36 months | na | na | na | na | na | na | 28 | 40 | na | na |
| ≥36 months | 63 | 50 | ||||||||
| unknown | 9 | 10 | ||||||||
| Prior neo-/adjuvant chemotherapy | 48 | 49 | 40 | 43 | 44 | 43 | 38 | 40 | 60 | 60 |
| Prior therapies for advanced BC | ||||||||||
| 1 | 0 | 0 | 38 | 40 | 0 | 0 | 0 | 0 | 38 | 38 |
| >2 (%) | 0 | 0 | 38 | 34 | 0 | 0 | 0 | 0 | 0 | 0 |
na, not available; NSAI, non-steroidal aromatase inhibitor.
CDK4/6 inhibitors in early-stage BC
Several studies were recently initiated evaluating the potential role of CDK4/6 inhibitors in the neoadjuvant and adjuvant settings aiming at reducing the risk of BC recurrence:
Neoadjuvant setting
While preoperative ET is not commonly applied in patients with early stage BC, the combination of ET with CDK4/6 inhibitors offers the chance of applying neoadjuvant ET in a wider population, thereby offering the chance to forgo chemotherapy. In two studies, a major decrease in proliferation rate was demonstrated with the combination of palbociclib or ribociclib plus AIs.52 53
The French phase II NEOPAL trial directly compared standard neoadjuvant chemotherapy to ET plus palbociclib in 106 early-stage HER2-negative/luminal patients with BC. The percentage of patients with residual cancer burden (RCB) score 0/1 at surgery was defined as the primary study end point. While the rate of patients with RCB 0/1 was higher in the chemotherapy arm (15.5% vs 7.7%), response rates and breast conservation rates were comparable.54 Finally, the phase II NeoMonarch trial evaluated abemaciclib as neoadjuvant treatment for early-stage HR-positive/HER2-negative BC. Overall, 223 postmenopausal women were included; patients received anastrozole, abemaciclib or the combination of both drugs for 2 weeks followed by abemaciclib plus anastrozole for an additional 14 weeks until surgery. The change in Ki-67 protein levels from baseline to week 2 was defined as primary study end point and this was significantly greater in patients receiving abemaciclib.55
Overall, these results suggest that further evaluation of CDK4/6 inhibitors as part of preoperative treatment concepts is warranted. Currently the NeoRHEA trial (NCT03065621), a single-arm phase II trial of neoadjuvant palbociclib plus ET, is recruiting premenopausal and postmenopausal patients. This study aims to identify novel biomarkers of palbociclib sensitivity or resistance.
Adjuvant setting
In addition to the neoadjuvant setting, two prospective randomised phase III trials evaluating the role of the three different CDK4/6 inhibitors as adjuvant therapy when added to standard ET are currently ongoing (palbociclib: PALLAS, NCT02513394; abemaciclib: MonarchE, NCT03155997). In these trials focusing on intermediate and high-risk luminal patients, CDK4/6-inhibtor therapy is administered for a total duration of 2 years; of note, premenopausal patients may also be included when treated with additional gonadotropin-releasing hormone analogues.
Patients with poor response to neoadjuvant chemotherapy are at increased recurrence risk; therefore, the phase III PENELOPE-B trial (NCT01864746) investigates the addition of palbociclib for 1 year to standard ET as post-neoadjuvant therapy in patients with significant residual invasive disease after neoadjuvant chemotherapy. This study has recently finished accrual and results are eagerly awaited.
CDK 4/6 inhibition and rationale for their combination with other compounds beyond ET
CDK4/6 inhibitors with standard ET have changed the treatment of BC patients with metastatic HR-positive/HER2-negative disease. The antitumour activity with a favourable toxicity profile has been demonstrated in several phase III trials and is now the standard of care.41 42 45 51 These results also led to the investigation of this class of with different combinations including:
Combination with anti-HER2 agents
CDK4/6 inhibitors overcome anti-HER2 resistance by increasing tumour cell dependence on EGFR family kinase signalling; therefore, CDK4/6 inhibitors may resensitise tumour cells to HER2-targeted therapies. Concomitant HER2 and CDK4/6 inhibition synergistically inhibits cell proliferation, controls tumour growth in vivo and delays tumour recurrence in transgenic mouse models.56 There are several ongoing trials testing CDK4/6 inhibitors with different anti-HER2 drugs including trastuzumab, pertuzumab, neratinib, tucatinib and TDM1 (eg, NCT02530424; NCT02947685; NCT02448420; NCT02675231; NCT02657343; NCT03054363; NCT03054363).
Combination with PI3K/AKT/mTOR pathway inhibitors
Relapse of ER-positive BC is often associated with constitutive PI3K pathway activation. Targeting PI3K/mTOR and CDK4/6 pathways could be effective in ER-positive BCs and could overcome resistance to ER-targeted therapies. A combinatorial drug screen on multiple PIK3CA mutant cancers with decreased sensitivity to PI3K inhibitors revealed that combined CDK4/6-PI3K inhibition synergistically reduces cell viability. Importantly, the combination of PI3K and CDK4/6 inhibitors overcomes intrinsic and adaptive resistance to PI3K inhibitors leading to tumour regression in PIK3CA mutant xenografts.57 There are several ongoing trials testing CDK4/6 inhibitors with different PI3K/mTOR inhibitors including copanlisib, taselisib, everolimus, vistursertib, pictilisib, gedatolisib and GDC-0077 (NCT03128619, NCT03006172, NCT02684032, NCT02389842, NCT02732119, NCT02871791, NCT02599714).
Combination with immunotherapy
In preclinical models, CDK4/6 inhibitors promote antitumour immunity by (a) increasing tumour antigen presentation plus suppressing the proliferation of regulatory T cells, and (b) promoting cytotoxic T-cell-mediated clearance of tumour cells. First, CDK4/6 inhibitors activate tumour cell expression of endogenous retroviral elements, thus increasing intracellular levels of double-stranded RNA and stimulating production of type III interferon, resulting in tumour antigen presentation. Second, CDK4/6 inhibitors markedly suppress the proliferation of regulatory T cells.58 There are several ongoing trials testing CDK4/6 inhibitors with different PI3K/mTOR inhibitors including pembrolizumab and avelumab (eg, NCT02778685; NCT02779751; NCT03147287).
Combination with androgen blockade
Luminal androgen receptor (AR) represents a subgroup of triple-negative BC that may benefit from CDK4/6 inhibitors. However, the sensitivity to these inhibitors is correlated with the AR expression, and the absence/or low levels of cyclin E1 in cell lines.59 Ongoing trials combining bicalutamide and CDK4/6 inhibitors are being conducted (eg, NCT02605486, NCT03090165).
CDK4/6 inhibitors in patients with brain metastases
Given the high efficacy and favourable tolerability of CDK4/6 inhibitors in MBC, this class of drugs may also constitute a novel treatment option for patients with brain metastases of BC. Differences in brain penetration between CDK4/6 inhibitors have been documented. P-glycoprotein and BC resistance protein have been shown to restrict the brain penetration of palbociclib, while abemaciclib crosses the blood–brain barrier more efficiently.60 61 Ongoing clinical trials are evaluating the activity of palbociclib (NCT02774681) and abemaciclib (NCT02308020) in patients with brain metastases of BC.
Open questions regarding the best use of CDK4/6 inhibitors
CDK4/6 inhibitors with standard ET showed impressive improvement in PFS in first-line and second-line settings compared with ET alone. However, most of the patients with HR-positive tumours receive many lines of treatment over several years over the course of their disease. So far, no OS benefit with the addition of CDK4/6 inhibitors could be shown. However, the median follow-up is still short and further analysis needs to be awaited. Therefore, the real value of this new class of drugs cannot conclusively be judged yet. Several open questions accompany the discussion of their best use.
A key challenge is the search of patient populations that benefit most from the CDK4/6ET combination. The selection could be made clinically and/or biologically.
Several potential biomarkers such as TP53 mutation status, complexity of tumour genomics and PI3K/AKT/mTOR pathway alterations have been investigated and did not show any predictive value for treatment with CDK4/6 inhibitors.62
In all trials (first and second line), the different clinical subgroups including proliferation rate, number of organs involved, HR expression, age and race showed the same relative benefit by the addition of CDK4/6 inhibitors.
All these factors were prognostic, but not predictive. In the MONARCH 2 and 3 trials, an exploratory analysis of combined data of the first-line and second-line studies was performed showing the largest absolute difference in the populations with tumours with higher grading, with liver metastases, with PgR-negative disease and with a shorter treatment-free interval.63
Although these are signals, sequential trials are needed for better patient selection with the primary end point after two lines of treatment, particularly regarding the missing data about cross over after progression in the mono-endocrine arms.
Additionally, it is unclear what to do after progression on CDK4/6-ET combination therapy. One option—without much data so far—might be to change the backbone of ET only, for example, from an AI to fulvestrant while continuing CDK4/6 inhibition beyond progression. Another option could be drug holidays according to some preclinical data.64
Other important research areas regarding the use of CDK4/6 inhibitors include
Should they best be implemented in first-line or second-line treatment? A Dutch group has launched a phase lll sequential trial with the primary end point after two lines of treatment to solve this question (NCT03425838).
Does each patient need a combination ET from the beginning?
How does the existing combination ET with everolimus in second-line compare with CDK4/6 inhibitors?
Also, the value of everolimus after failure of a CDK4/6 inhibitor is unclear and needs to be investigated.
Another research field will be the comparison of the CDK4/6 inhibitor-ET combination with upfront chemotherapy regarding the speed of response in patients with relevant visceral ER-positive disease.
Also, are CDK4/6 inhibitors able to replace chemotherapy in patients without a life-threatening disease (visceral crisis)?
If the efficacy of CDK4/6 inhibitors can be demonstrated in patients with brain metastases, further studies should focus on investigating the optimal sequence and combination of these drugs with other treatments used for brain metastases, such as stereotactic radiotherapy.
Conclusion
The use of CDK4/6 inhibitors with ET has changed the treatment landscape of BC patients with metastatic HR-positive/HER2-negative disease. Relevant antitumour activity with a favourable toxicity profile has been demonstrated in several phase III trials. Consistently, all three drugs (palbociclib, ribociclib, abemaciclib) have shown a significant and clinically meaningful prolongation of PFS over ET alone, thereby establishing a novel treatment standard not only in pretreated patients but also in the first-line setting of HR-positive MBC. Initial results of studies evaluating the potential role of CDK4/6 inhibitors in the neoadjuvant setting suggest that further research is warranted in these patients.
With regards to CDK4/6 inhibitors in other combinations than with ET (ie, anti-HER2 agents, PIK3/AKT/mTOR inhibitors, immunotherapy and androgen blockers), trials are ongoing and results are awaited.
There are still many open questions in trial design, selection of patients and time points for the best use of CDK4/6 inhibitors that need to be addressed. One of the key challenges is the identification of predictive biomarkers for better patient selection. Sequential trials are needed.
Acknowledgments
The authors acknowledge Dr Christiane Rehwagen for her work organising the round-table discussion and her medical writing support on the manuscript.
Footnotes
Funding: This initiative was sponsored by Lilly through the provision of an unrestricted educational grant. Lilly has had no influence over the content other than a review of the paper for medical accuracy. The participants/authors received an honorarium for their participation in the round-table from BMJ.
Competing interests: MP: honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Merck Sharp & Dome. MT: advisory role at: Amgen, AstraZeneca, Celgene, Eisai, Genomic Health, Lilly, Neodynamics, Novartis, Pfizer, pfm Medical, Roche, RTI Surgical, SurgicEye, Tesaro, Teva. Speaker engagements and travel support: Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Eisai, Genomic Health, Medtronic, Myriad, Novartis, Pfizer, pfm Medical, Roche, RTI Surgical, Teva. Manuscript fees: Roche, Celgene. Trial funding: Genomic Health. RB: advisory role: Eli-Lilly, Novartis, Pfizer; Lecture honoraria: Eli-Lilly, Novartis, Pfizer; research support: Novartis; Travel support: Pfizer. TR: advisory role for: Lilly, Roche, Novartis, Pfizer, AstraZeneca; Travel support: Roche, Amgen. EdA: honoraria and advisory board: Roche/GNE; travel grants: Roche/GNE and GSK/Novartis; research grant: Roche/GNE. CZ: honoraria from Roche, Novartis, BMS, MSD, Imugene, Ariad, Pfizer, Merrimack, Merck KGaA, Fibrogen, AstraZeneca, Tesaro, Gilead, Servier, Shire.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:3111 10.1093/annonc/mdx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233–47. 10.1146/annurev-med-070909-182917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016;18:17 10.1186/s13058-015-0661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 2015;14:130–46. 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417–30. 10.1038/nrclinonc.2016.26 [DOI] [PubMed] [Google Scholar]
- 7. Murphy CG, Dickler MN. The Role of CDK4/6 Inhibition in Breast Cancer. Oncologist 2015;20:483–90. 10.1634/theoncologist.2014-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gautschi O, Ratschiller D, Gugger M, et al. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer 2007;55:1–14. 10.1016/j.lungcan.2006.09.024 [DOI] [PubMed] [Google Scholar]
- 9. Kenny FS, Hui R, Musgrove EA, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res 1999;5:2069–76. [PubMed] [Google Scholar]
- 10. Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47. 10.1056/NEJMoa050092 [DOI] [PubMed] [Google Scholar]
- 11. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462–77. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buckley MF, Sweeney KJ, Hamilton JA, et al. Expression and amplification of cyclin genes in human breast cancer. Oncogene 1993;8:2127–33. [PubMed] [Google Scholar]
- 13. Dickson C, Fantl V, Gillett C, et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett 1995;90:43–50. 10.1016/0304-3835(94)03676-A [DOI] [PubMed] [Google Scholar]
- 14. Bartkova J, Lukas J, Müller H, et al. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 1994;57:353–61. 10.1002/ijc.2910570311 [DOI] [PubMed] [Google Scholar]
- 15. Gillett C, Fantl V, Smith R, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 1994;54:1812–7. [PubMed] [Google Scholar]
- 16. Geradts J, Wilson PA. High frequency of aberrant p16(INK4A) expression in human breast cancer. Am J Pathol 1996;149:15–20. [PMC free article] [PubMed] [Google Scholar]
- 17. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427–38. [PubMed] [Google Scholar]
- 18. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77 10.1186/bcr2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Brien NA, Tomaso ED, Ayala R, et al. Abstract 4756: In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Cancer Res 2014:74. [Google Scholar]
- 20. Lundgren K, Brown M, Pineda S, et al. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res 2012;14:R57 10.1186/bcr3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGuire WL, Horwitz KB, Pearson OH, et al. Current status of estrogen and progesterone receptors in breast cancer. Cancer 1977;39(6 Suppl):2934–47. [DOI] [PubMed] [Google Scholar]
- 22. Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 2008;26:1664–70. 10.1200/JCO.2007.13.5822 [DOI] [PubMed] [Google Scholar]
- 23. Clarke R, Tyson JJ, Dixon JM. Endocrine resistance in breast cancer--An overview and update. Mol Cell Endocrinol 2015;418(Pt 3):220–34. 10.1016/j.mce.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sighoko D, Liu J, Hou N, et al. Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: biological difference or misclassification? Oncologist 2014;19:592–601. 10.1634/theoncologist.2013-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8. 10.1093/jnci/djn309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 2013;45:1439–45. 10.1038/ng.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013;45:1446–51. 10.1038/ng.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015;7:313ra182 10.1126/scitranslmed.aac7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beddowes E, Sammut SJ, Gao M, et al. Predicting treatment resistance and relapse through circulating DNA. Breast 2017;34(Suppl 1):S31–S35. 10.1016/j.breast.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 31. Clatot F, Perdrix A, Augusto L, et al. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget 2016;7:74448–59. 10.18632/oncotarget.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol 2016;34:2961–8. 10.1200/JCO.2016.67.3061 [DOI] [PubMed] [Google Scholar]
- 33. Fu X, Osborne CK, Schiff R. Biology and therapeutic potential of PI3K signaling in ER+/HER2-negative breast cancer. Breast 2013;22:S12–S18. 10.1016/j.breast.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller TW, Rexer BN, Garrett JT, et al. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011;13:224 10.1186/bcr3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saura C. Primary results of LORELEI: a phase II randomized, double-blind study of neoadjuvant letrozole (LET) plus taselisib versus LET plus placebo (PLA) in postmenopausal patients (pts) with ER+/HER2-negative early breast cancer (EBC). 2017. Abstract presented at ESMO#LBA10.
- 36. Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw 2013;11:670–8. 10.6004/jnccn.2013.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520–9. 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmid P, Zaiss M, Harper-Wynne C, et al. MANTA - A randomized phase II study of fulvestrant in combination with the dual mTOR inhibitor AZD2014 or everolimus or fulvestrant alone in estrogen receptor-positive advanced or metastatic breast cancer. 2017. SABCS. #GS2-07.
- 39. Murphy CG, Dickler MN. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer 2016;23:R337–52. 10.1530/ERC-16-0121 [DOI] [PubMed] [Google Scholar]
- 40. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 41. Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925–36. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 42. Turner NC, Ro J, André F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 2015;373:209–19. 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 43. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 44. Finn RS, Crown J, Lang I, et al. Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) versus letrozole alone for frontline treatment of ER-positive/HER2-advanced breast cancer (PALOMA-1; TRIO-18). J Clin Oncol 2017;35(15_suppl):1001. [Google Scholar]
- 45. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738–48. 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 46. Tripathy D, Bardia A, Hurvitz SA, et al. Phase III, randomized, double-blind, placebo-controlled study of ribociclib (LEE011) in combination with either tamoxifen and goserelin or a non-steroidal aromatase inhibitor (NSAI) and goserelin for the treatment of premenopausal women with HR+, HER2-advanced breast cancer (aBC): MONALEESA-7. J Clin Oncol 2015;33(15_suppl). [Google Scholar]
- 47. Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–37. 10.1007/s10637-014-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov 2016;6:740–53. 10.1158/2159-8290.CD-16-0095 [DOI] [PubMed] [Google Scholar]
- 49. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2-breast cancer, after chemotherapy for advanced disease. J Clin Oncol 2016;34(15_suppl):510. [Google Scholar]
- 50. Sledge GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875–84. 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 51. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35:3638–46. 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 52. Curigliano G, Gómez Pardo P, Meric-Bernstam F, et al. Ribociclib plus letrozole in early breast cancer: A presurgical, window-of-opportunity study. Breast 2016;28:191–8. 10.1016/j.breast.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 53. Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin Cancer Res 2017;23:4055–65. 10.1158/1078-0432.CCR-16-3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cottu P, D’Hondt V, Dureau S, et al. LBA9Letrozole and palbociclib versus 3rd generation chemotherapy as neoadjuvant treatment of minal breast cancer. Results of the UNICANCER-eoPAL study. Annals of Oncology 2017;28(suppl_5):AbstractLBA9 10.1093/annonc/mdx440 [DOI] [Google Scholar]
- 55. Martin M, Hurvitz SA, Chan D, et al. Abstract PD5-01: Final results of NeoMONARCH: A phase 2 neoadjuvant study of abemaciclib in postmenopausal women with hormone receptor positive (HR+), HER2 negative breast cancer (BC). Cancer Res 2018;78:PD5-01 10.1158/1538-7445.SABCS17-PD5-01 [DOI] [Google Scholar]
- 56. Goel S, Wang Q, Watt AC, et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016;29:255–69. 10.1016/j.ccell.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vora SR, Juric D, Kim N, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014;26:136–49. 10.1016/j.ccr.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471–5. 10.1038/nature23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Asghar US, Barr AR, Cutts R, et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin Cancer Res 2017;23:5561–72. 10.1158/1078-0432.CCR-17-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Gooijer MC, Zhang P, Thota N, et al. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs 2015;33:1012–9. 10.1007/s10637-015-0266-y [DOI] [PubMed] [Google Scholar]
- 61. Raub TJ, Wishart GN, Kulanthaivel P, et al. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab Dispos 2015;43:1360–71. 10.1124/dmd.114.062745 [DOI] [PubMed] [Google Scholar]
- 62. Spring L, Niemierko A, Juric D, et al. Tumor genomics and response to CDK 4/6 inhibitors for patients with hormone receptor-positive (HR+) metastatic breast cancer (MBC). J Clin Oncol 2017;35(15_suppl):1046. [Google Scholar]
- 63. Goetz MP, O’Shaughnessy J, Sledge GW, et al. Abstract GS6-02: The benefit of abemaciclib in prognostic subgroups: An exploratory analysis of combined data from the MONARCH 2 and 3 studies. Cancer Res 2018;78:GS6-02 10.1158/1538-7445.SABCS17-GS6-02 [DOI] [Google Scholar]
- 64. Martin L-A, Pancholi S, Ribas R, et al. Abstract P3-03-09: Resistance to palbociclib depends on multiple targetable mechanisms highlighting the potential of drug holidays and drug switching to improve therapeutic outcome. Cancer Res 2017;77:P3-03-09 10.1158/1538-7445.SABCS16-P3-03-09 [DOI] [Google Scholar]
- 65. Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: importance of heterogeneity. [corrected]. Nat Rev Clin Oncol 2010;7:139–47. 10.1038/nrclinonc.2009.234 [DOI] [PubMed] [Google Scholar]