Abstract
Retromer is a phylogenetically conserved, multisubunit coat complex that controls endosomal protein trafficking and sorting. Mutations in the retromer gene VPS35 cause late-onset Parkinson disease, suggesting that trafficking defects cause neurodegeneration. Sorting nexins assist retromer to guide cell surface proteins to their assigned destinations, and our interest here is sorting nexin 3 (Snx3). Snx3 binds to membranes via a phox homolog (PX) domain that binds phosphatidylinositol 3-phosphate (PI3P), and in human cells its cargo proteins are the transferrin and Wnt receptors and the divalent metal ion transporter, whereas in yeast the best characterized cargo is the iron permease Ftr1. We recently discovered that α-synuclein inhibits Snx3-retromer recycling of Ftr1 in an unexpected way: α-synuclein, which avidly binds to negatively charged lipids, blocks the association of Snx3 to early endosomes. Here, we discuss mechanisms by which α-synuclein can disrupt Snx3-retromer–mediated recycling.
Keywords: α-synuclein, endosome, Parkinson disease, phox homolog domain, retromer
Comment on: Patel D, Xu C, Nagarajan S, et al. Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum Mol Genet. 2018;27:1514–1532. PubMed PMID: 29452354. https://www.ncbi.nlm.nih.gov/pubmed/29452354
Parkinson disease (PD) is the most common neurodegenerative movement disease.1 The disease affects approximately 1% of the people over the age of 65, and, currently, more than 4 million people are afflicted worldwide. Age is the primary risk factor. In PD, the progressive degeneration of dopaminergic neurons occurs in the mid-brain region called the substantia nigra par compacta (SNpc), and the loss of these neurons causes both a deficit of dopamine and the classic symptoms of resting tremor, slowness of movement, and disturbances in gait and balance. Affected neurons often contain inclusion bodies called Lewy bodies, and the principal component of Lewy bodies is the protein α-synuclein.2 α-Synuclein has been implicated in neurodegeneration in sporadic PD, where the term “sporadic” indicates no known genetic causes. Missense mutations or multiplications of the α-synuclein gene (SNCA) cause familial or early-onset PD. The current therapy for the disease, l-DOPA, which replaces dopamine in the brain, has been used since the early 1950s, but this drug ultimately fails because it does not stop neurodegeneration. New treatments are needed, and to discover new treatments, scientists need to elucidate the molecular pathways involved in PD.
α-Synuclein is highly expressed in dopaminergic neurons. The protein consists of 3 domains: the N-terminal helical domain (residues 1-65), a hydrophobic domain (residues 65-95) (referred to as the NAC domain for “non-Aβ component” of amyloid plaques), and the unstructured, acidic C-terminal domain (residues 100-140). Intrinsically unfolded in solution,3 α-synuclein adopts a bent α-helix on binding to membranes, and at elevated concentrations the protein self-associates because of the hydrophobic middle domain into a myriad array of soluble protofibrils, some of which may be cytotoxic, and insoluble amyloid fibers. Elevated levels of α-synuclein due to the multiplication of the SNCA locus or from diminished lysosomal function and/or posttranslation modification such as phosphorylation can promote α-synuclein aggregation/self-association. Functionally, α-synuclein is thought to regulate synaptic vesicle fusion with the presynaptic membrane. Emerging evidence indicates that α-synuclein is also intimately involved in endocytosis, which is the focus of this Commentary.
α-Synuclein Blocks Endoplasmic Reticulum-to-Golgi Traffic
A pioneering genetic screen revealed that α-synuclein blocks endoplasmic reticulum (ER)-to-Golgi protein traffic in yeast and that overexpression of Rab1 GTPase in yeast, flies, worms, and human neurons rescues the trafficking defect.4 The specific trafficking defect in yeast was that α-synuclein inhibits the trafficking of alkaline phosphatase (ALP) and carboxypeptidase (CPY) from the ER to the Golgi. These 2 proteins normally transit with their receptor, Vps10, en route to their final destination, the vacuole. Given that α-synuclein blocks the trafficking of ALP and CPY, we infer that α-synuclein probably also blocks the ER-to-Golgi trafficking of Vps10. The human ortholog of Vps10 is the cation-independent mannose-6-phosphate receptor (CI-MPR), and, curiously, CI-MPR mislocalizes in cells that overexpress α-synuclein.
Mutations in Retromer Cause Late-Onset PD
Most (>90%) of the PD cases are sporadic in that there are no known genetic causes (other than the aggregation of α-synuclein), whereas a small percentage (<10%) of cases are due to mutations in a variety of genes. Missense mutations in DJ-1, PINK1, LRRK2, PRKN, or SNCA cause early-onset PD, whereas mutations in VPS35 cause late-onset PD.5 That mutations in VPS35 cause late-onset PD strongly suggests that defects in retromer-mediated protein trafficking are a cause of PD. Moreover, further illustrating the importance of endocytosis vis-à-vis α-synuclein, overexpression of endocytosis-related genes6 rescues α-synuclein aggregation and toxicity in cell models of PD.
In the retrograde endosomal protein trafficking pathway, upon being endocytosed, cell surface receptors and transporters have 2 fates: they are either degraded in the lysosome or recycled back to the plasma membrane. The retromer complex,7 which localizes to early endosomes, retrieves cell surface proteins from the degradative pathway and recycles them either to the trans-Golgi for redelivery to the plasma membrane or the plasma membrane directly. Retromer, which is composed of a trimer and dimer, coats the cytosolic face of early endosomes. The trimer is composed of Vps26-Vps29-Vps35 (Vps26, Vps29, and Vps35 in yeast) and functions in cargo selection. The dimer is composed Snx1/Snx2 (Vps5 in yeast) and Snx5/Snx6 (Vps17 in yeast), and this subcomplex senses and induces curvature and membrane tubulation. The sorting nexins Snx1 and Snx2 (and Snx5/Snx6) each contain 2 membrane-binding domains: a PX domain, which binds PI3P, and a BIN/Amphiphysin/Rvs (BAR) domain that senses membrane curvature and induces vesicle tubulation. Other sorting nexins, such as Snx3, can also bind cargo proteins and physically interact with the retromer machinery. Snx3 is an adapter that binds the endocytic recycling sequence of the yeast permease Ftr1 and the human iron importer divalent metal ion transporter. For cargo to be retrieved from the degradative pathway, the multimeric retromer complex must bind to the endocytic recycling sequence on the cargo in early endosomes. This is followed by the formation of endosomal tubules from which retromer-cargo vesicles bud off and then transit to the trans-Golgi for recycling to the plasma membrane.
α-Synuclein Inhibits Snx3-Retromer–Mediated Endosomal Trafficking of Fet3-Ftr1
We recently reported that α-synuclein inhibits Snx3-retromer–mediated recycling of Fet3-Ftr1 in yeast by blocking the association of the 2 PX-domain proteins, ie, Snx3 and Vps17, to the surface of the early endosomes.8 Specifically, α-synuclein, which binds to negatively charged lipids, appears to inhibit the binding of the Snx3 PX domain to PI3P, which is negatively charged. This was the first paper to report such a novel activity for α-synuclein. Although this finding was made in yeast, the ability of α-synuclein to inhibit the association of PX proteins with PI3P will likely translate to human cells. Yeast Snx3 binds to PI3P-containing artificial membranes with a Kd of 2 µM. If human Snx3 (and other PX proteins) bind to PI3P with a similar affinity, then α-synuclein should also block the association of these human PX domain proteins to the surface of vesicles. Factors other than PI3P content that will determine whether α-synuclein binds to vesicles are PS content and the presence of lipid rafts, which are composed of cholesterol and sphingolipids. Collectively, we propose that neuronal pathology in PD is instigated by some species of α-synuclein—be it monomer, dimer, or oligomer—blocking ER-to-Golgi traffic, by α-synuclein disrupting retromer components, or by mutations in retromer components.
Chlamydia trachomatis Protein IncE Binds to and Subverts the Function of the Human PX-BAR Sorting Nexin and Retromer Component Snx5
Chlamydia trachomatis is a sexually transmitted bacterial pathogen that colonizes host cells. On entry into the host cells, the bacteria become enclosed in a membrane that is referred to as an inclusion. The membrane-enclosed pathogen expresses proteins that interact with host proteins, with a goal of blocking retrograde transport of the membrane-enclosed pathogens to the lysosome for degradation. In essence, this bacterium subverts or hijacks the host’s retrograde transport system to avoid detection, gain nutrients, and reproduce. Until recently, the mechanisms by which C trachomatis subvert the host’s retrograde transport pathway were unknown.
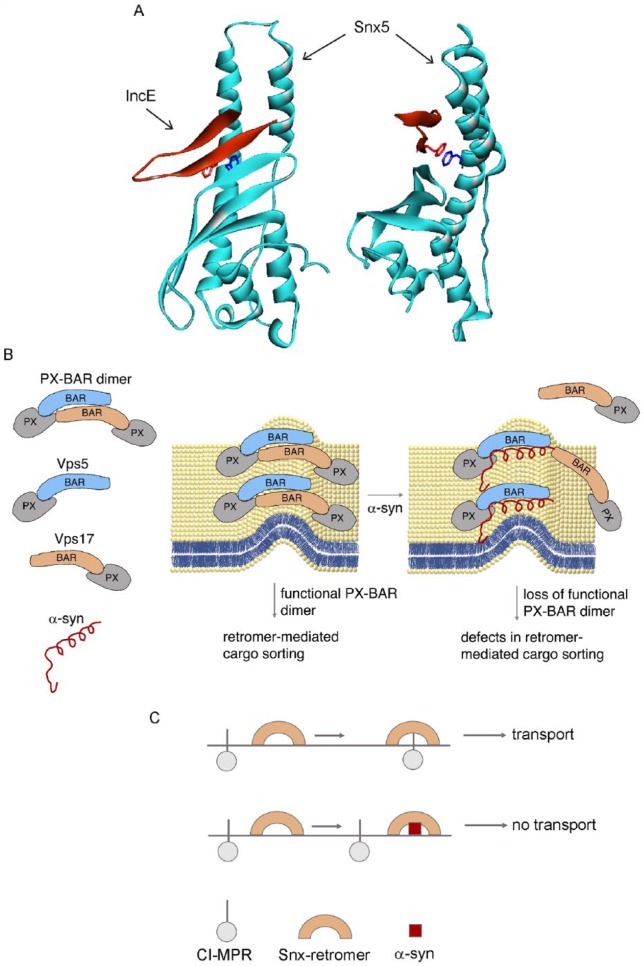
A breakthrough report revealed that the C trachomatis effector protein IncE suppresses retromer-mediated recycling of CI-MPR by specifically binding to a conserved patch on Snx5 (Figure 1A).10 Snx5, which is a retromer component, binds to an endosome-to-TGN (trans-Golgi network) sorting motif on CI-MPR. IncE is a membrane-bound protein that loops through the membrane enclosure that surrounds the pathogen such that the N- and C-termini of IncE are on the cytoplasmic face of the inclusion membrane. The C-terminus of IncE also specifically binds to Snx5, Snx6, and Snx32 but not to Snx1. In essence, IncE recruits these 3 PX-BAR nexins to the surface of the C trachomatis inclusions, where they probably reshape the membrane to promote tubule formation. The question is how does the recruitment of Snx5, Snx6, and Snx32 to the pathogen inclusion membranes affect retromer? The retromer dimer is composed of combinations of Snx1 or Snx2 and Snx5 or Snx6. For example, the functional retromer dimer may be Snx1-Snx5, Snx1-Snx6, or Snx2-Snx5, and so on. By binding to Snx5 on the pathogen membranes, IncE depletes host cells of this molecule, consequently fewer molecules of Snx5 are available to bind to CI-MPR; thus, IncE disrupts retromer-mediated trafficking of CI-MPR. In addition, the IncE peptide directly inhibits the interaction of CI-MPR with Snx5. This example of a bacterial pathogen subverting a human host cell’s vesicular trafficking machinery is germane to this discussion of vesicular trafficking and PD, as discussed below.
Figure 1.
α-Synuclein disrupts retromer function. (A) Crystal structure of the C-terminal fragment of C trachomatis IncE bound to the PX domain of mouse Snx5 (5TP1 PDB file.). The binding of IncE to Snx5 inhibits retromer-mediated trafficking of CI-MPR. (B) Proposed model showing α-synuclein bound to the retromer protein Snx1. (C) How this interaction inhibits dimer formation and hence retromer-mediated recycling of CI-MPR.
Source: Adapted from Sun et al.9
α-Synuclein interacts with a network of proteins involved in 2 cellular pathways: the endocytic pathway and RNA metabolism.11 This conclusion was based on a recent study that used a cross-linking technique in conjunction with mass spectroscopy to uncover proteins in the vicinity of α-synuclein. The identified α-synuclein-protein interaction network components were further evaluated by co-immunoprecipitation and a membrane-2-hybrid assay to test for physical interactions. Focusing on the endocytic pathway, curiously, α-synuclein specifically binds to the retromer trimer proteins Vps26 and Vps29 and to the retromer dimer protein Snx1.11 The authors concluded that “the retromer emerges as a multi-protein complex that interacts physically with α-synuclein and plays a key role in its toxicity.”
We speculate that the binding of α-synuclein to Snx1 inhibits the formation of the retromer dimer and the inability to form the dimer inhibits retromer-mediated recycling of receptors such as CI-MPR (Figure 1B and C). This proposed effect exactly parallels the case of IncE/Snx5. The failure to properly traffic/recycle CI-MPR would cause the mislocalization of CI-MPR in cells and, perhaps more ominously, CI-MPR ligands such as the lysosomal cathepsin D would fail to transit to and enter the lysosome. The accumulation of cathepsin D in the cytosol could promote the cleavage of α-synuclein to yield short highly aggregation prone, cytotoxic fragments.
Collectively, we propose that α-synuclein can disrupt or alter retromer by (1) blocking the recruitment of adapter proteins like Snx3 and SNX-BAR dimers like Snx1/Snx2 and Snx5/Snx6 to vesicular membranes or (2) by blocking formation of the retromer dimer. The role of α-synuclein in modulating retromer function is just emerging, and we expect that in the near future that these 2 models will be tested in human cells.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: DP and SNW designed/conceived the review. SNW wrote and edited the review. DP edited the review and made the figure.
ORCID iD: Stephan N Witt  https://orcid.org/0000-0002-6462-2840
https://orcid.org/0000-0002-6462-2840
References
- 1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 2. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. [DOI] [PubMed] [Google Scholar]
- 3. Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. [DOI] [PubMed] [Google Scholar]
- 4. Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vilariño-Güell C, Wider C, Ross OA, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonçalves SA, Macedo D, Raquel H, et al. shRNA-based screen identifies endocytic recycling pathway components that act as genetic modifiers of alpha-synuclein aggregation, secretion and toxicity. PLoS Genet. 2016;12:e1005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucas M, Hierro A. Retromer. Curr Biol. 2017;27:R687–R689. [DOI] [PubMed] [Google Scholar]
- 8. Patel D, Xu C, Nagarajan S, et al. Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum Mol Genet. 2018;27:1514–1532. [DOI] [PubMed] [Google Scholar]
- 9. Sun Q, Yong X, Sun X, et al. Structural and functional insights into sorting nexin 5/6 interaction with bacterial effector IncE. Signal Transduct Target Ther. 2017;2:e17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paul B, Kim HS, Kerr MC, Huston WM, Teasdale RD, Collins BM. Structural basis for the hijacking of endosomal sorting nexin proteins by Chlamydia trachomatis. Elife. 2017;6:e22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung CY, Khurana V, Yi S, et al. In situ peroxidase labeling and mass-spectrometry connects alpha-synuclein directly to endocytic trafficking and mRNA metabolism in neurons. Cell Syst. 2017;4:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]