Abstract
Protein ubiquitination serves many regulatory functions; in addition to degradation, ubiquitination has roles in intracellular trafficking, cell cycle, innate immunity, and more. Using mass spectrometry, it is possible to assess the ubiquitination state of every protein simultaneously. In this issue, Wade et al. have for the first time done just that in a hypoxic mouse model of pulmonary hypertension (PH). New techniques drive new discoveries; their work is important not just because they have found new ways to intervene in known PH-related pathways but have found regulation of proteins not previously associated with disease.
Keywords: animal models, pulmonary arterial hypertension, ubiquitination, and sumoylation
Ubiquitin is a 76 amino acid (8 kDa) protein. Its best-known role is in the targeted degradation of proteins (for which a Nobel prize was awarded in 2004). The first ubiquitin protein attaches to a substrate protein through binding between the carboxy terminus of the ubiquitin, usually to a lysine amino acid of the substrate protein (although cysteine and the initial methionine are also possible targets) (Fig. 1). Addition of this first ubiquitin to a substrate is monoubiquitination. For instance, ubiquitin binds to the Lys164 residue of proliferating cell nuclear antigen.1
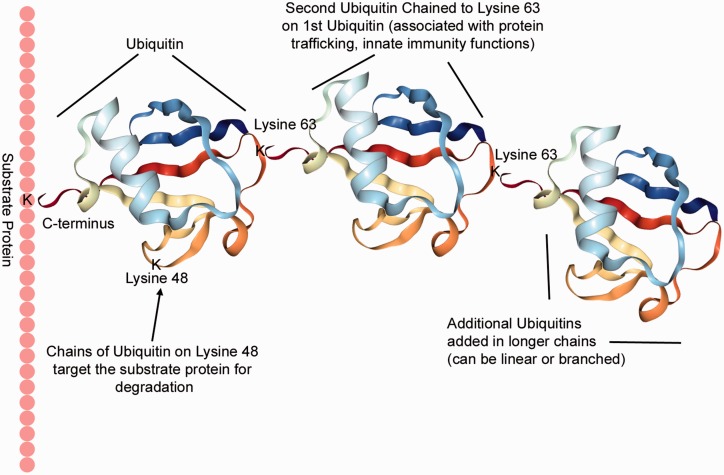
Fig. 1.
Ubiquitination is binding of the 76 amino acid protein ubiquitin, usually to a lysine residue of a target/substrate protein. Additional ubiquitin molecules attach to the original ubiquitin, with the C-terminus of the new ubiquitin binding to specific lysine residues of the earlier ubiquitins. Functional consequences of ubiquitination depend on the types of chains formed. A chain of at least four ubiquitins connected through Lysine 48 target the substrate for ubiquitination; linear and branched chains through M1, K6, K11, K27, K29, K33, K48, or K63 are all possible. Ubiquitin folding picture produced by NGL Viewer.9
A substrate can also undergo multimonoubiquitylation, when the binding of a single ubiquitin happens at multiple lysine residues on the substrate, as happens to epidermal growth factor receptor.2 Targeting of ubiquitin to substrates is regulated by E3 ubiquitin ligases and there are thousands of them.3
More interesting and powerful, however, is chain formation. Ubiquitin chains occur when ubiquitin proteins are linked by the carboxy terminus of one ubiquitin to one of eight sites on the previous ubiquitin: M1, K6, K11, K27, K29, K33, K48, or K63. K48 ubiquitin chains are best characterized – a minimum chain length of 4, each linked through the K48 lysine, attracts the 26 S proteasome complex, and results in degradation of the substrate protein.3 M1 chains have been associated with angiogenesis, K6 with DNA damage response, K11 with cell cycle control, K27 with nuclear translocation, K29 with Wnt signaling, K33 with AMPK-related kinase signaling, and K63 with protein trafficking and innate immunity. Each of these has other associations as well; in addition, branched and mixed chains and phosphorylation events all play regulatory roles.4
Addition of ubiquitin chains—and the types and locations of the ubiquitin chains—thus can tell us much more about what is happening within a cell than simply whether proteins are marked for degradation. What makes this powerful is that this is subject to a mass screening technique. When whole cellular proteins are fragmented with trypsin, the last two glycines in the ubiquitin protein remain attached to the protein to which the ubiquitin was attached. These residues can be precipitated with antibodies and then all protein fragments that had been ubiquitinated are identified through mass spectrometry. Types of ubiquitination are similarly identified through the locations on the ubiquitin proteins themselves that have the two glycine residues still attached.
In the current issue, Wade et al. have done exactly that to analyze changes in protein ubiquitination in hypoxic mice. Over the years, we have seen unbiased discovery of gene5 and protein6 expression from whole lungs, isolated vessels and cells, and circulating cells, from mice, rats, and patients, and we have seen large scale metabolomic data,7 but this is the first time we have seen wholesale data on ubiquitination from a pulmonary hypertension (PH) model. Because this is a level of regulation different than any that has been assessed before, it carries the potential of revealing entirely novel elements of disease etiology, in the same way that metabolomics did ten years ago.
First, and comfortingly, their analysis revealed that a little over half of the 131 proteins they found with altered ubiquitination pathways were already part of known pulmonary arterial hypertension (PAH)-related pathways. These include well-known vasoconstriction proteins such as RhoA, Rac1, and Calmodulin, as well as some genes associated with them, Cav1 (which had very complex patterns of ubiquitination), cell cycle genes such as CDC42, and others involving calcium signaling, cell migration, and nitric oxide.
However, they also found a large number of proteins with altered ubiquitination that had not previously been associated with PH. Of course, some of those may be spurious, but many of them may have never before been associated because they are not regulated at the level of expression. For instance, one of the proteins the authors follow up on is Profilin-1, a motility-related gene which had a decrease in ubiquitination at K54 (1.9 × fold change) and K126 (5.2 × fold change) in hypoxia, but no change in protein levels, implying that the ubiquitination was serving some other regulatory purpose. Lysine mutations in profilin-1 are known to enhance or inhibit profilin-1 interactions with actin,8 and so it seems likely that these ubiquitin modifications alter profilin-1 actin regulation. Others in the category of proteins never before directly associated with PAH include a tropomyosin, a tubulin polymerizing protein, and an F-actin capping protein, and so alterations in cytoskeletal mechanics are clearly implicated in these changes. No doubt other readers will find other interesting pathways in the list of novel proteins identified in Wade et al.’s manuscript as well.
This is an important paper, as the first report in the first model system of this technique. There are two reasons for its importance. First, many well-known PH-involved pathways are also apparently regulated through the ubiquitin system. Since this system has high specificity through the thousands of different E3 ligases, it may be possible to rescue some known PH targets through interventions in their specific E3 ligases. Second, it uncovers several new proteins differentially regulated in at least a mouse model of Group III PH. It will be important to see how these findings extend to other animal model systems and to patient samples: which of these new pathways are common to other models and to different categories of human patients, and which are specific to this model?
New techniques drive new discoveries; ubiquitinomics is a new tool in our fight against PH, and it finds its first application here.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Hoege C, Pfander B, Moldovan GL, et al. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002; 419: 135–141. [DOI] [PubMed] [Google Scholar]
- 2.Haglund K, Sigismund S, Polo S, et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol 2003; 5: 461–466. [DOI] [PubMed] [Google Scholar]
- 3.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem 2012; 81: 203–229. [DOI] [PubMed] [Google Scholar]
- 4.Akutsu M, Dikic I, Bremm A. Ubiquitin chain diversity at a glance. J Cell Sci 2016; 129: 875–880. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann J, Wilhelm J, Olschewski A, et al. Microarray analysis in pulmonary hypertension. Eur Respir J 2016; 48: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laudi S, Steudel W, Jonscher K, et al. Comparison of lung proteome profiles in two rodent models of pulmonary arterial hypertension. Proteomics 2007; 7: 2469–2478. [DOI] [PubMed] [Google Scholar]
- 7.Fessel JP, West JD. Redox biology in pulmonary arterial hypertension (2013 Grover Conference Series). Pulm Circ 2015; 5: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schluter K, Schleicher M, Jockusch BM. Effects of single amino acid substitutions in the actin-binding site on the biological activity of bovine profilin I. J Cell Sci 1998; 111 (Pt 22): 3261–3273. [DOI] [PubMed] [Google Scholar]
- 9.Rose AS, Hildebrand PW. NGL Viewer: a web application for molecular visualization. Nucleic Acids Res 2015; 43: W576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]