Abstract
Objective: Prescription drug use is on the rise, and the use of dietary supplementation remains common. In the United States, more than half of all adults take a dietary supplement in any given month. As a result, drug-nutrient interactions are becoming an important consideration when pharmacists counsel patients about their drug regimens. We reviewed the literature to identify common and/or clinically relevant drug-nutrient interactions that pharmacists may encounter in practice. Data Sources: A MEDLINE search for English-language publications from 1970 through March 2017 was performed using search terms (and variations) related to drugs, medications, micronutrients, and interactions. Study Selection and Data Extraction: Relevant studies, case reports, and reviews describing drug-nutrient interactions were selected for inclusion. Data Synthesis: Some drug-nutrient interactions may result in micronutrient insufficiencies or even frank deficiencies, thereby necessitating augmentation with multivitamin/minerals or individual vitamin/mineral dietary supplements. This most often occurs with long-term therapy for chronic conditions, such as treatment with proton-pump inhibitors and histamine-2 receptor antagonists. In addition, some chronic diseases themselves, such as diabetes, may predispose patients to micronutrient insufficiencies, and dietary supplementation may be advisable. Conclusions: Drug-nutrient interactions can often be resolved through specific dosing strategies to ensure that the full effect of the medication or the dietary supplement is not compromised by the other. In rare cases, the dietary supplement may need to be discontinued or monitored during treatment. Pharmacists are in a key position to identify and discuss these drug-nutrient interactions with patients and the health care team.
Keywords: nutrition, dietary supplements, diet, vitamins, trace elements/minerals, drug interactions
Introduction
The majority of US adults take prescription drugs, with their use increasing in recent years from 51% in 1999-2000 to 59% in 2011-2012 based on National Health and Nutrition Examination Survey (NHANES) data.1 In a given 30-day period, it is estimated that more than half of Americans use at least 1 prescription drug, and this pattern of use tends to increase with age. Polypharmacy (defined as use of ⩾5 drugs) has also been rising and is also more common among older adults,1 making the elderly population particularly susceptible to potential drug interactions.
Dietary supplementation with multivitamins/minerals (MVMs) or individual vitamins and minerals is widespread. According to NHANES data collected from 2011 to 2012,2 52% of the US adult population reported use of any dietary supplement product (including MVMs, individual vitamin/mineral supplements, and non-vitamin, non-mineral specialty supplements) in the prior 30 days; 48% took a supplement containing ⩾1 vitamin; and 39% took a supplement containing ⩾1 mineral. Less than 10% of individuals report using 4 or more supplement products.2 Although their use has decreased somewhat in recent years, MVMs remain the most common type of dietary supplement used, being reported by almost one third of US adults.2 As expected, use of a daily MVM supplement decreases the risk of nutritional inadequacies and increases the prevalence of micronutrient intake exceeding the upper intake level that is considered safe and tolerable (although this remains relatively uncommon in large population-based studies [⩽4% for any single micronutrient]).3 Use of supplement products increased by age, with 72% of individuals ⩾65 years of age taking at least 1 dietary supplement a month.2 Supplementation was also more common among women, non-Hispanic whites, and those who had attained higher levels of education.2 Individuals take dietary supplements for numerous reasons, with improving and maintaining overall health being the primary drivers (Table 1).4
Table 1.
Prevalence of Adults (⩾20 Years of Age) in the United States Taking Various Types of Supplements and Their Most Commonly Reported Motivation for Use, 2007 to 2010.
| Type of Supplement | Users, n | Overall, % (SE) (N = 11 956) | Most Common Reported Motivation | Users Reporting Motivation, % (SE) |
|---|---|---|---|---|
| Multivitamin/mineral | 3404 | 31.9 (0.8) | To improve overall health | 48 (1) |
| Calcium | 1342 | 11.6 (0.6) | For bone health | 74 (2) |
| Vitamin C | 764 | 7.1 (0.5) | To boost immune system, prevent colds | 45 (3) |
| Mutlivitamin | 632 | 5.7 (0.4) | To improve overall health | 31 (2) |
| Vitamin D | 542 | 4.9 (0.4) | For bone health | 38 (2) |
| Vitamin E | 439 | 3.7 (0.2) | To improve overall health | 40 (3) |
| Vitamin B12 | 408 | 3.3 (0.2) | To improve overall health | 31 (3) |
| Iron | 245 | 1.8 (0.1) | For anemia, low iron | 67 (4) |
| Folic acid | 194 | 1.5 (0.2) | Other reason | 15 (4) |
| Potassium | 119 | 0.9 (0.1) | For muscle-related issues | 24 (5) |
| Magnesium | 125 | 1.1 (0.1) | To improve overall health | 18 (4) |
| Vitamin B6 | 106 | 0.9 (0.1) | To improve overall health | 24 (5) |
| Vitamin A | 103 | 0.8 (0.1) | For eye health | 44 (6) |
| Vitamin B3 (niacin) | 70 | 0.7 (0.1) | For heart health, lower cholesterol | 77 (6) |
Abbreviation: SE, standard error.
Source: Adapted with permission from JAMA Intern Med. 2013;173(5): 355-361. DOI 10.1001/jamainternmed.2013.2299. Copyright ©2013 American Medical Association. All rights reserved.4
The pharmacokinetics of some drugs can be affected when administered with food or dietary supplements containing certain micronutrients5 (ie, substances such as vitamins, essential minerals, and other trace elements that are required in small amounts to support normal physiologic functions). Drug-nutrient interactions refer to biochemical, physicochemical, physiological, or pathophysiological relationships between medications and the specific micronutrients involved in the interactions.6 Many of these drug-nutrient interactions, with the exception of major nutritional issues associated with prescription medications, are not top of mind and may be underrecognized in the pharmacy setting since dispensing software only flags interactions with drugs that are captured in the system. As a result, over-the-counter (OTC) products, including nutritional supplements, may not be included. In addition, there are a number of chronic conditions for which there are special nutritional concerns, including diabetes, inflammatory bowel disease, cardiovascular disease, and alcoholism. As many as 48% of patients taking dietary supplements concomitantly with prescription drugs have been found to be at risk for a drug-micronutrient interaction.5,7-10 In one study, approximately 29% of supplement-drug interactions were classified as interactions that were potentially clinically significant and required either monitoring or a change in drug therapy.10
Because pharmacists have regular access to patients’ medication histories, as well as insight on their use of dietary supplements and other OTC products, they are well positioned to monitor for drug-micronutrient interactions and recommend therapeutic strategies to the prescribing health care provider to address or avoid potential interactions for at-risk patients. If a drug is known to significantly affect the pharmacokinetics of specific micronutrients, any potential deficiencies that may occur can be reversed through supplementation with multivitamins or specific micronutrients.5 The purpose of this review is to highlight the most common and/or clinically relevant drug-nutrient interactions that pharmacists may encounter in practice, with an emphasis on medications for chronic conditions that can predispose individuals to micronutrient gaps.
Data Sources
We conducted MEDLINE searches for English-language publications from 1970 through March 2017 using search terms related to drugs, medications, micronutrients (including related terms such as vitamin, mineral, antioxidant, etc), and interactions. Additional relevant papers were identified via cross-referencing of the articles identified via these literature searches. Additional studies and reviews were evaluated to provide background and context.
Bidirectional Relationship Between Micronutrients and Medications
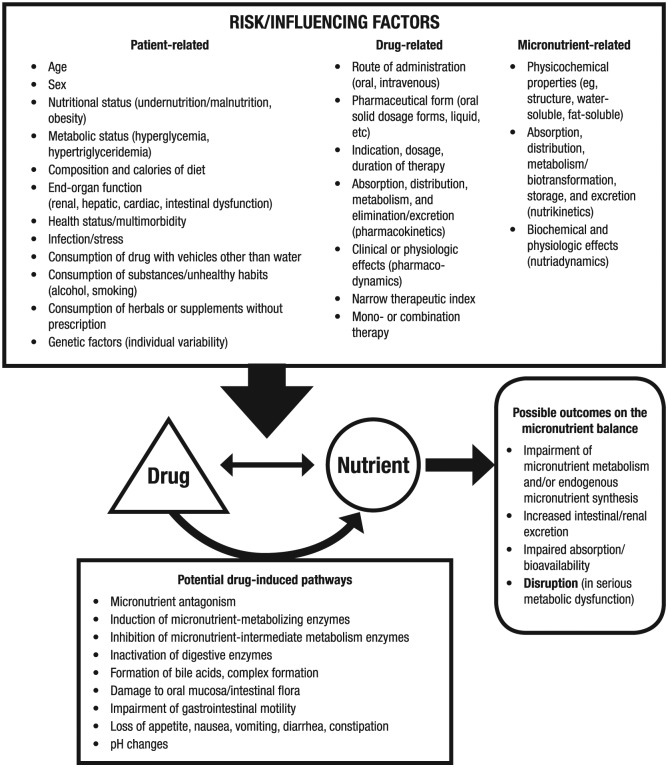
Medications can affect a patient’s micronutrient status either directly or indirectly (Figure 1).11,12 Some medications may directly affect the pharmacokinetic properties (absorption, distribution, metabolism, or excretion) of micronutrients because both may use the same metabolic and transport pathways in the body. In addition, physiologic changes resulting from the drug’s mechanism of action may directly affect the micronutrient. The medication itself may indirectly affect the patient’s health due to its effect on overall nutritional (eg, weight gain, weight loss), metabolic (eg, hyperglycemia, hypertriglyceridemia), or specific micronutrient or mineral (eg, hypokalemia, zinc deficiency) status.5,13 Many medications have gastrointestinal (GI) adverse effects, such as nausea, vomiting, and diarrhea.5,13 Other adverse effects of medications, including cognitive, visual, and gait disturbances, may alter a patient’s ability to obtain, prepare, and consume food. The elderly are particularly at risk for these adverse effects given their more prevalent use of medications and lower tolerance for adverse effects.5,13,14 As an example, antacids can affect the absorption of concomitantly administered oral preparations via alterations in GI transit time or by binding to or chelating the substances.15 Thus, iron or folic acid supplements should be separated by 2 hours, and citrus fruit/juice or calcium citrate supplements should be separated by 3 hours from antacid use.16 Additionally, prolonged use of gastric acid suppressants (eg, proton-pump inhibitors [PPIs] and histamine-2 receptor antagonists [H2RAs]) has been associated with vitamin B12 malabsorption and deficiency, although the clinical significance of this vitamin-drug interaction is not clear.17,18
Figure 1.
Bidirectional relationship of drug-micronutrient interactions. Reproduced from Karadima et al.12
Conversely, micronutrients can influence the pharmacokinetics and pharmacodynamics of medications.6 Dietary supplements may interact with medications through various mechanistic pathways, such as alterations in transport proteins or enzymes; complexation, chelation, or deactivation processes occurring in the gut; and in some cases, pharmacodynamic interactions occurring at the site of action.6 The micronutrient status of the patient can indirectly affect the efficacy of medications because nutritional deficiencies may affect drug absorption and metabolism.19 When a patient experiences severe energy or protein deficiencies, enzyme concentrations in tissues can be decreased, reducing drug absorption or protein binding and possibly causing liver dysfunction. Alterations in the GI tract can also reduce drug absorption and modify response. A specific example is the association of hypomagnesemia and digoxin toxicity.20 Digoxin inhibits the magnesium-dependent enzyme Na+/K+-ATPase, and therefore, in patients who are magnesium-deficient, plasma potassium is also reduced, thereby enhancing the effects of digoxin.20,21 A deficiency of vitamin C may also reduce the activity of drug-metabolizing enzymes, as suggested in scorbutic guinea pigs who demonstrated significantly decreased levels of various components of the P450 cytochrome enzyme complex.19,22-24
Supplementation with micronutrients may also be recommended to improve the efficacy of a medication. For example, raloxifene and teriparatide should be administered with adequate dietary intake of calcium and vitamin D; otherwise, dietary supplementation may be necessary to ensure the efficacy of these medications.16 Likewise, epoetin-alfa may need to be administered with supplemental iron, vitamin B12, and folic acid to provide effective therapy.16
Drug-Nutrient Interactions of Concern in Clinical Practice
Table 2 summarizes several drug-nutrient interactions that commonly occur in clinical practice.11,16,25-34 In many cases, the effect of short-term use of these medications in healthy individuals is negligible and does not require intervention. In addition, some interactions may be clinically significant only when micronutrient intake is very low; therefore, average daily intake of the micronutrient may need to be considered. However, long-term medication use in chronic conditions may require an increased or decreased intake of specific micronutrients or monitoring of the intake of these micronutrients to prevent adverse outcomes.
Table 2.
Common Drug-Micronutrient Interactions, Their MOAs, Consequences of the Interaction(s), and Potential Action(s) Required.11,16,25-34
| Drug | Micronutrient | MOA | Consequence(s) | Potential Action(s) Required |
|---|---|---|---|---|
| Antacids and acid reducers | ||||
| Antacids containing aluminum/magnesium hydroxide | Folate, iron, phosphorus | Decreased absorption of folate, iron, and phosphorus | Potential decreased effect of iron or folic acid supplementation if administered concurrently; hypophosphatemia | Take iron or folic acid separately by 2 hours; take calcium citrate separately by 3 hours |
| Proton-pump inhibitors | Vitamin B12 | Increased gastric pH, decreased release of vitamin B12 from R-protein, decreased absorption of dietary, but not supplemental, vitamin B12 | Vitamin B12 deficiency (megaloblastic anemia), hyperhomocysteinemia | Monitor vitamin B12 status, supplementation may be needed |
| Calcium | Decreased solubility of calcium due to higher gastric pH, potential decreased absorption of insoluble calcium | Reduced bioavailability of insoluble calcium salts | Ensure recommended daily intake of calcium (via diet and/or supplementation) | |
| Take calcium carbonate supplements with meals to improve bioavailability | ||||
| Consider use of soluble calcium salts (calcium citrate) | ||||
| Iron | Decreased absorption of carbonyl iron | Reduced bioavailability of carbonyl iron-containing supplements | Use an alternative iron supplement | |
| Histamine-2 receptor antagonists | Vitamin B12 | Bacterial colonization, decreased absorption of dietary, but not supplemental, vitamin B12 | Vitamin B12 deficiency | Monitor vitamin B12 status with long-term use |
| Iron | Iron absorption decreased | Iron status decreased | Take ⩾2 hours before or after iron | |
| Antibiotics | ||||
| Penicillins | Biotin, vitamin K | Inhibition of intestinal biotin and vitamin K synthesis | Adverse effects on biotin and vitamin K status | Caution with vitamin K supplementation |
| Zinc | Decreased zinc absorption | Zinc status decreased | May need to increase zinc intake | |
| Cephalosporins | Vitamin K | Inhibition of endogenous vitamin K synthesis | Decreased vitamin K status, potentially leading to bleeding abnormalities | May need to increase vitamin K intake |
| Fluoroquinolones | Calcium, magnesium, zinc, iron | Decreased absorption and bioavailability of drug | Decreased antibiotic efficacy | Take calcium, magnesium, zinc, iron, or MVM supplement at least 2 hours before or 6 hours after |
| Tetracyclines | Calcium, iron, magnesium, zinc | Formation of complex, decreased absorption of antibiotic | Decreased antibiotic efficacy | Take calcium, iron, magnesium, zinc, or MVM supplement separately by 3 hours before or 1 hour after drug |
| Vitamin C | Increased renal vitamin C excretion | Decreased WBC vitamin C status | Take vitamin C supplement | |
| Trimethoprim-sulfamethoxazole | Folate | Inhibitory effect on dihydrofolate reductase | Folate deficiency | May need folic acid supplementation, particularly pregnant women |
| Antidiabetics | ||||
| Metformin | Vitamin B12 | Inhibition of calcium-dependent receptor-mediated endocytosis of the IF-B12 complex (impaired absorption) | Vitamin B12 deficiency (megaloblastic anemia), hyperhomocysteinemia | Monitor vitamin B12 status |
| Anti-epileptics | ||||
| Phenytoin, phenobarbital, primidone, carbamazepine | Calcium, vitamin D | Cytochrome P450 induction, which increases vitamin D metabolism; calcium absorption is decreased and calcium utilization decreased | 25-OH-D3 and 1,25-(OH)2-D3 decreased, PTH increased, pyridinoline increased, all leading to bone abnormalities | May need vitamin D, calcium supplementation with long-term use |
| Phenytoin | Folate | Increased folate metabolism, decreased folate absorption, increased oxidative phenytoin metabolism | Folate deficiency (increased homocysteine, megaloblastic anemia, gingival hyperplasia), decreased antiepileptic efficacy | ⩾1 mg folic acid daily, often started with phenytoin |
| Vitamin D | Reduces activity of vitamin D 25-hydroxylase in liver | 25-OH-D decreased, potentially leading to bone abnormalities | May need vitamin D, calcium supplementation with long-term use | |
| Valproic acid | L-Carnitine | Decreased carnitine-acyl-carnitine translocase, increased valproyl-carnitine excretion, decreased carnitine concentration | Carnitine deficiency, cardiac dysfunction, fatigue, liver pathologies | Those with inadequate dietary intake may need carnitine supplementation |
| Vitamin D | Increased vitamin D metabolism | Increased risk of osteoporosis with long-term use | Increase calcium and vitamin D intake or supplement | |
| Antihypertensives | ||||
| ACEIs and ARBs | Potassium | Renal potassium excretion decreased | Hyperkalemia | Monitor/limit potassium intake |
| Zinc | Renal zinc excretion increased | Zinc depletion (eg, hypogeusia) | None | |
| Hydralazine | Vitamin B6 | Covalent bonding between hydralazine (hydrazine) and vitamin B6 (pyridoxal) | Vitamin B6 deficiency, increased peripheral neuropathy | Vitamin B6 100-200 mg supplementation |
| Anticoagulants | ||||
| Vitamin K antagonists (warfarin) | Coenzyme Q10 | Structural similarity between coenzyme Q10 and vitamin K | Higher doses of coenzyme Q10 can reduce efficacy of warfarin (monitor INR) | Avoid coenzyme Q10 supplements |
| Vitamin K | Antagonism | Anticoagulant antagonism | Consistent intake of vitamin K essential | |
| Anti-inflammatories | ||||
| Aspirin | Vitamin C | Decreased absorption, increased renal excretion, decreased intragastric vitamin C concentration | Increased gastric mucosa damage | Increase foods high in vitamin C with long-term high dose |
| Iron | Intensification of irritant action on mucous membranes | Increased GI intolerability, risk of ulcers | No specific action; monitor for clinical signs of AEs | |
| Vitamin E | Interaction with vitamin K at vitamin E dosage ⩾800 IU/day | Prolongation of bleeding time | No specific action; monitor for clinical signs of AEs | |
| Diclofenac, ibuprofen, indomethacin | Vitamin E | Synergy; COX-2 inhibition potentiated by vitamin E | May enhance anti-inflammatory efficacy and reduce likelihood of gastric mucosal injury associated with NSAIDs | No action generally required |
| Methotrexate | Folate | Folate antagonist (THF reductase inhibitor) | Folate deficiency, leukopenia, thrombocytopenia, stomatitis, gingivitis, hyperhomocysteinemia | Leucovorin (folinic acid) rescue prescribed with methotrexate to decrease oral and GI effects |
| Sulfasalazine | Folate | Folate absorption decreased | Folate deficiency, hyperhomocysteinemia | Folic acid supplementation when used with methotrexate in rheumatoid arthritis |
| Antituberculotics | ||||
| Cycloserine | Vitamin B6 | Inactivation of pyridoxal phosphate due to complex formation | Pyridoxal phosphate deficiency, increased neurotoxicity, paresthesias | Supplementation with large doses of vitamin B6 may be needed (>50 mg/day) |
| Ethambutol | Zinc | Complex formation, renal zinc excretion increased | Zinc deficiency, possible disturbances of visual function and optic nerve damage | Zinc supplementation may be required |
| Isoniazid | Vitamin B6 | Inactivation of pyridoxal phosphate due to complex formation (Schiff base) | Vitamin B6 deficiency, increased neurotoxicity (eg, seizures, peripheral neuritis, optic neuritis) | Vitamin B6 supplementation (25-50 mg daily) to prevent peripheral neuropathy |
| Rifampin | Vitamin D | Vitamin D breakdown due to induction of microsomal liver enzymes | 25-OH-D3 concentration decreases, risk of hypocalcemia and hypophosphatemia | May need vitamin D supplementation |
| Bisphosphonates, oral | ||||
| Alendronate, risedronate | Calcium, iron, magnesium | Formation of poorly absorbable complexes | Efficacy of bisphosphonates decreased | Adequate calcium/vitamin D intake essential; supplementation may be needed if dietary intake is lacking; ensure interval of several hours between administration of calcium, magnesium, or iron supplements |
| Zinc | Decreased absorption of both zinc and bisphosphonate | Efficacy of bisphosphonates decreased; zinc status decreased | Separate supplements including zinc from bisphosphonate dosing | |
| Corticosteroids | ||||
| Dexamethasone, methylprednisolone, prednisolone | Calcium, vitamin D | Anti–vitamin D effect: calcium absorption decreased, renal excretion increased, serum osteocalcin concentration decreased | Corticoid-induced osteoporosis | Calcium/vitamin D supplementation recommended with long-term use |
| Vitamin A, vitamin C, potassium | Increased urinary excretion of vitamin C and potassium | Decreased blood concentrations of vitamin A, vitamin C, potassium | May need increased intake (via diet or supplementation) of potassium, vitamins A and C | |
| Diuretics | ||||
| Thiazides, furosemide | Magnesium, potassium | Magnesium and potassium excretion increased, myocardial potassium and magnesium decreased | Vasoconstriction, blood pressure increased, LVEF decreased, (hyperlipidemia, glucose tolerance decreased) | Increase magnesium and potassium (or supplement) |
| Hydrochlorothiazide/triamterene | Calcium, vitamin D | Renal calcium excretion decreased | Blood calcium concentration increased | Monitor blood calcium concentrations |
| Spironolactone | Potassium | Potassium excretion decreased | Hyperkalemia | Avoid excessive potassium intake and potassium supplementation |
| Anti-gout drugs | ||||
| Colchicine | Vitamin B12 | GI tract: mucosal damage, vitamin B12 absorption from food decreased | Vitamin B12 deficiency, megaloblastic anemia | Higher doses of vitamin B12 supplements are recommended for chronic use |
| Allopurinol | Iron | Increased iron storage in liver | Increased risk of hepatocellular toxicity | Avoid combination |
| Oral contraceptives | ||||
| Ethinyl estradiol and progestins | Calcium, copper, folate, iron, magnesium, vitamins A, C, B5, and B6, zinc, possibly vitamin B12 | Various mechanisms, including malabsorption, increased excretion, decreased protein binding, and altered metabolism | Anemia, leukopenia, thrombocytopenia, increased risk of thromboembolism, low serotonin levels, headache, muscle spasms, osteoporosis, increased blood pressure, neural tube defects in babies born shortly after discontinuation of oral contraceptives, pregnancy complications | Multivitamin/mineral supplementation containing vitamin B complex, vitamins C and E, magnesium, selenium, zinc |
| Miscellaneous | ||||
| Cholestyramine | Folate | Reduction of red cell folate | Folate deficiency | Folic acid supplementation recommended with long-term use |
| Vitamins A, D, E, K | Binds bile acids, thereby preventing absorption of fat-soluble vitamins | Decreased vitamin A, D, E, K status; bleeding due to vitamin K deficiency, osteomalacia/osteoporosis with long-term use | Supplementation with fat-soluble vitamins in water-miscible or parenteral form recommended with long-term use | |
| Other vitamins/minerals | Resin may bind to other substances and interfere with absorption | Administer supplements 1 hour prior or 4-6 hours after to avoid interference with absorption | ||
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AE, adverse event; ARB, angiotensin II receptor antagonists; COX-2, cyclooxygenase-2; GI, gastrointestinal; IF, intrinsic factor; INR, international normalized ratio; LVEF, left ventricular ejection fraction; MVM, multivitamin/mineral; MOA, mechanism of action; NSAIDs, nonsteroidal anti-inflammatory drugs; PTH, parathyroid hormone; THF, tetrahydrofolate; WBC, white blood cell.
Gastric Acid Suppressants
OTC, as well as prescribed, PPIs and H2RA acid suppressants are often used for the treatment of heartburn and gastroesophageal reflux disease.18,35 Due to the reduction in the secretion of gastric acid and pepsin that they produce, prolonged use of PPIs and H2RAs has been associated with vitamin B12 malabsorption and deficiency.17,18,35 Aside from the obvious consequences of vitamin B12 deficiency (ie, megaloblastic anemia and neurologic symptoms), there is also the risk that slight vitamin B12 deficiency will increase serum homocysteine, which has been associated with cardiac and vascular conditions, impaired cognition, and other adverse effects, particularly in the elderly and in those who have had gastric surgery.17,30 Therefore, a supplement containing vitamin B12 may be advisable in patients receiving long-term PPI and H2RA therapy.16,18,30
Because an acidic environment in the GI tract is needed for absorption of insoluble calcium, long-term acid suppression could theoretically decrease the solubility and absorption of calcium from the gut, leading to a reduction in bone mineral density and increased risk of osteoporosis and fracture.36 A modest increased risk of fracture has been observed in patients taking PPIs.37 Because of this potential association between PPIs and fractures, the US Food and Drug Administration issued a warning regarding the possible increased risk of fractures among patients using high-dose PPIs for extended periods.38 However, it is important to note that these findings are limited by the potential confounding factors and biases inherent to observational data, and evidence supporting whether the association is mediated through impaired calcium absorption from the gut has been mixed.37 Nevertheless, several practical strategies can be recommended by the pharmacist for individuals taking PPIs long-term to address the issue.36 When calcium intake is adequate, the impact of gastric acidity on the solubility of calcium salts relative to overall absorption would be minimal, so patients chronically using PPIs should be instructed to ensure they are obtaining the recommended amount of daily calcium for their age/sex (via either dietary intake or supplementation).36 The calcium content of the typical vegan diet is of concern,39 so vegans in particular may consider taking calcium supplements with a PPI. Soluble calcium salts, such as calcium citrate, would be less subject to changes in bioavailability related to gastric pH.36 Absorption of insoluble salts, such as calcium carbonate, can be improved when administered with meals, which is routinely recommended but may be particularly important in the context of acid suppression.36 Likewise, gastric acid is needed for iron absorption, and therefore, the use of acid-suppressive therapy could decrease iron absorption. Use of H2RAs, for example, has been shown to decrease the absorption of iron by as much as 65%.35,40 This interaction can be avoided by taking H2RAs at least 2 hours before or after iron intake.16
Anticoagulants and Vitamin K Stability
Drug-nutrient interactions with anticoagulants such as warfarin, and vitamin K are well known and can lead to life-threatening hemorrhagic events.41 Studies have demonstrated that clinicians have deficiencies in their knowledge of warfarin-vitamin K interactions, which may result in inaccurate patient counseling and adverse outcomes.42,43 Patients should be counseled that they should continue to consume the same amount of vitamin K in their diet, including that contained in dietary supplements, while taking concomitant anticoagulant therapy.42 Diets low in vitamin K can result in unstable international normalized ratios (INRs); therefore, supplementation with vitamin K may be needed in these patients.44 Both phylloquinone (vitamin K1) and menaquinone (vitamin K2) in doses from 25 to 100 µg are contained in MVM supplements that are available in the United States.33 For patients using vitamin K–containing dietary supplements with anticoagulants, pharmacists should be aware of differences in potency between the different forms of vitamin K. Specifically, vitamin K2 is 3 to 4 times more potent than vitamin K1 in counteracting coumarin-derived anticoagulants. As such, the recommended upper limit for safe intake of vitamin K2 in patients taking oral anticoagulants is 50 µg/day compared with 100 µg/day with vitamin K1.45 However, when administered in combination with properly adjusted anticoagulant doses, the longer half-life of vitamin K2 may provide more stable levels of anticoagulation effect.45 Moreover, anticoagulants that directly inhibit factor Xa or thrombin are now available and have little or no interaction with food or dietary supplements and other medications.46
Chemotherapy
Patients being treated for cancer may require individualized micronutrient supplementation management rather than following generalized recommendations due to the complex nature of these therapies. This may be further complicated by the fact that many cancer patients, without the knowledge of their physician, take micronutrients to help alleviate the disease itself or the adverse effects of therapy, or to increase the efficacy of treatment.47
Chemotherapy is an area where there is a particular need for individualized assessments of nutritional intake during treatment. As reviewed by Ozben,48 evidence for the benefits or risks associated with antioxidant supplementation in patients undergoing cancer treatment is conflicting, and results vary according to the form and intake level. Some studies have suggested a potential benefit for vitamin E in terms of enhanced effectiveness or reduced toxicity when taken concurrently with chemotherapy.48 Mechanistic studies using preclinical disease models have demonstrated that vitamin C, as an inhibitor of hypoxia-induced factor-1 (HIF-1)–dependent angiogenesis, has the ability to counteract cancer-promoting processes.49 However, studies are needed to determine how oxidative homeostasis differs in normal and malignant cells in order to find how antioxidants may be incorporated into cancer treatment.47
Niacin supplementation at a dose of 14 to 16 mg/day in adults has been recommended to treat the symptoms of pellagra (dermatitis, diarrhea, and dementia) that are often associated with long-term chemotherapy in patients who may be niacin deficient.50 In addition, niacin deficiency in cancer patients has been shown to sensitize bone marrow and increase the risk of chemically induced leukemia as a result of the suppressive effects of chemotherapy.50
Thus, not only may chemotherapy itself negatively affect the nutrient status of the body, but the adverse effects of chemotherapy (ie, anorexia, stomatitis, and diarrhea) can also cause nutrient deficiencies.51,52 However, because supplementation with certain micronutrients may decrease the efficacy of treatment in some circumstances, patients should be advised of appropriate supplementation as part of their overall treatment plan.47,48
Folic Acid Supplementation in Methotrexate Therapy for Autoimmune Disorders
Folic acid supplementation in the context of anti-folate therapy with methotrexate for treatment of psoriasis or rheumatoid arthritis is widely used despite the absence of generally accepted evidence-based guidelines.53 The goal of supplementation with folic acid or folinic acid (5-formyl tetrahydrofolate, a cofactor of methotrexate’s target dihydrofolate reductase) is to reduce adverse reactions to methotrexate treatment.54 Baran et al reviewed literature reports published between 1960 and March 2014 pertaining to the vitamin-drug interaction in psoriasis patients and concluded that folic acid supplementation may be effective in reducing the severity of methotrexate-related adverse effects.53 In a meta-analysis of 6 trials with 624 rheumatoid arthritis patients, folic/folinic acid was found to reduce the incidence of GI tract adverse effects in patients on methotrexate therapy.55 However, a meaningful effect of supplementation on the hematological adverse effects of methotrexate cannot be established. Importantly, folic/folinic acid supplementation does not appear to affect the efficacy of methotrexate treatment of rheumatoid arthritis.55 A study comparing the effects of 2 dose regimes of folic acid supplementation (5 mg/week vs 30 mg/week) on the tolerability and efficacy of methotrexate in treatment of rheumatoid arthritis patients found no differences with regard to adverse effects or methotrexate discontinuation.56 However, the lower-dose group showed a lower rheumatoid arthritis disease index, indicating that the 5 mg/week dose had a smaller negative effect on methotrexate efficacy. Rheumatoid arthritis patients who receive counseling from a rheumatologist are more likely to be prescribed folic acid-methotrexate combination therapy than patients counseled by other health care providers, highlighting the need for guidelines on folic acid supplementation in this clinical setting.57
Chronic Disease Contribution to Vitamin/Mineral Insufficiencies
Some chronic diseases can predispose patients to vitamin/mineral insufficiencies that may require dietary supplementation. Diabetes, malabsorptive disorders (including Crohn’s disease and ulcerative colitis), cardiovascular disease, and alcoholism are some of the most common conditions seen by pharmacists in clinical practice that may necessitate patient counseling on the need for micronutrient supplementation.
Diabetes
Type 2 diabetes mellitus (T2DM) has been associated with poor nutrition and deficiencies in micronutrients involved in glucose metabolism, pancreatic β-cell function, and insulin signaling.51 Therefore, deficiencies in these micronutrients could also contribute to the development of T2DM.58
It has been demonstrated that 14% to 48% of patients with T2DM have hypomagnesemia, while patients without diabetes have an incidence of only 3% to 15%.59-65 Low serum magnesium concentrations are associated with complications of diabetes, including an increase in the risk of cardiovascular disease and diabetic retinopathy.59 A meta-analysis of studies of oral magnesium use found evidence that supplementation for 4 to 16 weeks may be effective in reducing fasting plasma glucose concentrations and raising high-density lipoprotein cholesterol in patients with T2DM; however, the authors noted that the long-term efficacy and safety remained to be determined.66
Thiamin (vitamin B1) deficiencies have been observed in 17% to 79% of patients with diabetes,58,67,68 and studies suggest a beneficial role for supplementation in reducing risk and severity of T2DM or its associated complications.58,67,69-71 It has also been observed that compared with healthy controls, patients with diabetes have lower plasma concentrations of vitamin C72 and lower circulating concentrations of biotin, with an inverse correlation between biotin status and fasting plasma glucose.73,74 Low levels of serum vitamin D have been found to significantly increase cardiometabolic risk (including insulin resistance, metabolic syndrome, and cardiovascular disease risk),75 and preliminary evidence suggests that vitamin D with or without calcium supplementation may improve glucose metabolism and insulin signaling.76-79
Antidiabetic medications can also affect micronutrient status. For example, it has been demonstrated that metformin hydrochloride lowers blood concentrations of vitamin B12 and folate by decreasing their absorption in the GI tract, thereby causing neuropathies of the hands and feet.80-83 Therefore, supplementation with folic acid (the synthetic form of folate) in this patient population should be considered. The precise role of vitamin B12 supplementation in diabetic patients taking metformin has not been established, but it would be wise to monitor vitamin B12 status in this group.84
Malabsorptive Disorders (eg, Crohn’s Disease, Ulcerative Colitis)
Inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis, involve chronic inflammation that ultimately damages the GI tract, and thereby impairs absorption of nutrients.85 Patients suffering from Crohn’s disease or ulcerative colitis are thus at increased risk of micronutrient insufficiency.85 Vitamin B12 malabsorption is of particular concern in IBD, and dietary supplementation in the form of intramuscular injection is recommended in those with clinical deficiency or severe B12 malabsorptive disease (eg, patients with disease in the ileum or those who have undergone small bowel surgery).86 Vitamin D deficiency is associated with morbidity and the course of inflammatory bowel disease,87 and therefore, screening and supplementation as appropriate are recommended.87,88 Crohn’s disease has been associated with reduced bone density and with osteoporosis due to reduced intake of vitamin D–fortified dairy products, malabsorption of vitamin D, or bacterial overgrowth.89 As a result, patients should have an intake of at least 1500 mg of calcium daily, either via diet or supplementation.89 In addition, iron deficiencies frequently occur in individuals with ulcerative colitis and Crohn’s disease due to intestinal bleeding,87,90,91 and intravenous or oral iron supplementation should be considered when iron-deficiency anemia is present.91
Certain medications used in IBD may also cause nutrient deficiencies. For example, folate deficiency may occur in patients who are receiving sulfasalazine, and supplementation with folic or folinic acid is recommended to replenish folate stores.92 Long-term use of prednisone and other anti-inflammatory steroids can both inhibit the absorption of calcium and increase renal calcium loss, thus having an unfavorable effect on bone health.89 Patients initiating corticosteroid medication should therefore also start supplementation with calcium and vitamin D.89 The bile acid sequestrant cholestyramine (used to treat diarrhea following ileac resection) interferes with the absorption of fat and may increase the risk for deficiency of vitamin D and other fat-soluble vitamins in patients with Crohn’s disease.34,89
Individuals with obesity who undergo bariatric surgery are at increased risk of nutrient deficiencies due to malabsorption.93,94 At a minimum, a multivitamin supplement is recommended for all patients after bariatric surgery; additional supplementation may be necessary depending on the type of surgery that was performed and the associated risk of malabsorptive disease.93
Cardiovascular Disease
Vitamin and mineral deficiencies can cause significant clinical problems that should be monitored in patients with cardiovascular disease. Some research has suggested that diets high in potassium, magnesium, and possibly calcium may have beneficial effects on blood pressure, as well as reducing the risk of stroke, and supplementation may be beneficial if these needs are not met through diet alone.95,96 The Atherosclerosis Risk in Communities Study found that the lower the serum magnesium level, the greater the risk of coronary heart disease.97 In addition, a meta-analysis of hypertensive and normotensive individuals found that supplemental magnesium produced a small but clinically significant reduction in blood pressure, particularly for those who received a higher dosage (>370 mg/day).98 Increased dietary potassium intake is associated with a reduction in blood pressure,99 which, in turn, has been shown to result in corresponding decreases in vascular disease. A meta-analysis of prospective studies further demonstrated that higher daily potassium intakes were associated with a 21% lower risk of stroke.100
A number of medications used to treat cardiovascular disease can affect vitamin status. Cholesterol-lowering drugs, such as cholestyramine, can limit the absorption of dietary fats in the intestine, as well as the fat-soluble vitamins A, D, E, and K.101 Diuretics, such as hydrochlorothiazide, increase levels of homocysteine, which may in turn counter the cardioprotection resulting from lower blood pressure.102 Supplementation with folic acid, vitamin B6, and vitamin B12 can help reduce homocysteine levels.102
Alcoholism
Individuals who suffer from alcoholism usually have insufficient intake of certain micronutrients because most of their calories come from alcohol.51 Most notably, deficiencies in vitamin B6, vitamin B12, folate, and thiamin may contribute to the development of alcoholic liver disease.51,103,104 Higher intake of thiamin is needed during alcohol withdrawal as a result of increased metabolism.104 Therefore, vitamin supplements are recommended for patients who suffer from alcoholism to help prevent thiamin deficiency, as well as deficiencies in other micronutrients.103,104 In addition, during alcohol withdrawal, it is recommended that thiamin be administered either orally or parenterally to offset the greater thiamin requirement.104
Drug-Micronutrient Interactions and Unmet Nutritional Needs: Opportunities for pharmacist intervention
Recognition of At-Risk Individuals
One of the main factors that affect drug-nutrient interactions is the patient’s nutritional status prior to the beginning of treatment.11 Underlying nutrient deficiencies, as well as multiple comorbidities and polypharmacy, increase the risk for drug-nutrient interactions. However, the most susceptible patients are those who are critically ill, as well as the elderly, obese, frail, severely malnourished, and those with underlying intestinal dysfunction.6 Additional factors that can affect micronutrient status include gender, self-medication with supplements, and liver and kidney function.11,13 Length of treatment with a medication should also be considered, as short-term use usually does not require treatment with dietary supplements.
Opportunities for the Pharmacist
The majority of health care professionals believe that pharmacists are in the best position to discuss drug-nutrient interactions with patients.105 Most pharmacists have access to full medication histories and regularly provide counseling and disease state education; therefore, they can identify areas of direct and indirect drug-nutrient interactions and assess dietary supplement needs. Additionally, the availability of MVMs and other dietary supplements in most pharmacies gives pharmacists a direct opportunity to identify and educate patients on appropriate use.
Pharmacists are in a position to evaluate drug-micronutrient interactions for patients in whom the intake of a micronutrient is already inadequate, particularly in patients with chronic disease who use long-term maintenance medications. In addition, the elderly may be at particular risk due to comorbidities and polypharmacy.11,106 Pharmacists can also ask their patients about dietary habits, MVM and other dietary supplement use, including dose and duration of use, and concurrent drug therapies during counseling sessions to get a comprehensive overview of their patients’ medication histories.12
Collaboration between pharmacists and other providers, including physicians, nutritionists/dietitians, and nurses, can facilitate identification of recommendations that will decrease drug-nutrient interactions and prevent adverse events.12 Some drug-nutrient interactions may require specific dosing strategies to ensure that the desired effect of the medication is not compromised by dietary supplementation. The timing of administration may be important to ensure that the medication is taken apart from these supplements. For example, calcium, magnesium, iron, and zinc can form a complex with tetracycline that may decrease its absorption, and therefore, these supplements should be administered 3 hours before or 1 hour after tetracycline.16 Another example is that antacids can increase or decrease the rate and/or extent of absorption of concomitantly administered dietary supplements, and as a result, their administration should be separated by 2 to 3 hours.15,16
Conclusions
Dietary supplement use with vitamins and minerals is widespread, as is prescription drug use in the United States; therefore, drug-nutrient interactions are an important consideration. Some drug-nutrient interactions, as well as some chronic diseases themselves, may result in micronutrient deficiencies, thereby requiring augmentation with MVMs or other dietary supplements. Pharmacists should be knowledgeable of potential drug-nutrient interactions, monitor for their occurrence, and recommend strategies to prevent adverse outcomes when necessary.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JDP declares that there is no conflict of interest. VJD is principal investigator of research sponsored by Pfizer and has received an honorarium from Pfizer in connection with development of a review article. JFS declares that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing support was provided by Virginia Schad, PharmD, and Diane Sloan, PharmD, of Peloton Advantage, LLC, and was funded by Pfizer.
References
- 1. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;316:1464-1474. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blumberg JB, Frei BB, Fulgoni VL, Weaver CM, Zeisel SH. Impact of frequency of multi-vitamin/multi-mineral supplement intake on nutritional adequacy and nutrient deficiencies in US adults. Nutrients. 2017;9:E849. doi: 10.3390/nu9080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173:355-361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 5. Boullata JI. Drug and nutrition interactions: not just food for thought. J Clin Pharm Ther. 2013;38:269-271. doi: 10.1111/jcpt.12075. [DOI] [PubMed] [Google Scholar]
- 6. Chan LN. Drug-nutrient interactions. JPEN J Parenter Enteral Nutr. 2013;37:450-459. doi: 10.1177/0148607113488799. [DOI] [PubMed] [Google Scholar]
- 7. Lee AH, Ingraham SE, Kopp M, Foraida MI, Jazieh AR. The incidence of potential interactions between dietary supplements and prescription medications in cancer patients at a Veterans Administration Hospital. Am J Clin Oncol. 2006;29:178-182. doi: 10.1097/01.coc.0000209369.44100.25. [DOI] [PubMed] [Google Scholar]
- 8. Loya AM, Gonzalez-Stuart A, Rivera JO. Prevalence of polypharmacy, polyherbacy, nutritional supplement use and potential product interactions among older adults living on the United States-Mexico border: a descriptive, questionnaire-based study. Drugs Aging. 2009;26:423-436. doi: 10.2165/00002512-200926050-00006. [DOI] [PubMed] [Google Scholar]
- 9. Peng CC, Glassman PA, Trilli LE, Hayes-Hunter J, Good CB. Incidence and severity of potential drug-dietary supplement interactions in primary care patients: an exploratory study of 2 outpatient practices. Arch Intern Med. 2004;164:630-636. doi: 10.1001/archinte.164.6.630. [DOI] [PubMed] [Google Scholar]
- 10. Sood A, Sood R, Brinker FJ, Mann R, Loehrer LL, Wahner-Roedler DL. Potential for interactions between dietary supplements and prescription medications. Am J Med. 2008;121:207-211. doi: 10.1016/j.amjmed.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11. Lechner P. Important drug-micronutrient interactions to know. Pharmacy Times. http://www.pharmacytimes.com/contributor/petra-lechner-pharmd/2015/12/important-drug-micronutrient-interactions-to-know. Accessed August 14, 2017.
- 12. Karadima V, Kraniotou C, Bellos G, Tsangaris GT. Drug-micronutrient interactions: food for thought and thought for action. EPMA J. 2016;7:10. doi: 10.1186/s13167-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White R. Drugs and nutrition: how side effects can influence nutritional intake. Proc Nutr Soc. 2010;69:558-564. doi: 10.1017/s0029665110001989. [DOI] [PubMed] [Google Scholar]
- 14. Zadak Z, Hyspler R, Ticha A, Vlcek J. Polypharmacy and malnutrition. Curr Opin Clin Nutr Metab Care. 2013;16:50-55. doi: 10.1097/MCO.0b013e32835b612e. [DOI] [PubMed] [Google Scholar]
- 15. Antacids. In: McEvoy GK, Miller J, Snow EK, eds. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2003:2732. [Google Scholar]
- 16. Pronsky ZM, Elbe D, Ayoob K. Food Medication Interactions. 18th ed. Birchrunville, PA: Food Medication Interactions; 2015. [Google Scholar]
- 17. Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2008;27:1110-1121. doi: 10.1111/j.1365-2036.2008.03658.x. [DOI] [PubMed] [Google Scholar]
- 18. Linder L, Tamboue C, Clements JN. Drug-induced vitamin B12 deficiency: a focus on proton pump inhibitors and histamine-2 antagonists. J Pharm Pract. 2017;30:639-642. doi: 10.1177/0897190016663092. [DOI] [PubMed] [Google Scholar]
- 19. Youdim A. Nutrient-drug interactions. http://www.merckmanuals.com/professional/nutritional-disorders/nutrition-general-considerations/nutrient-drug-interactions. Accessed August 14, 2017.
- 20. Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24:47-66. [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan MP. Interrelationships of magnesium and potassium homeostasis. Miner Electrolyte Metab. 1993;19:290-295. [PubMed] [Google Scholar]
- 22. Zannoni VG, Lynch MM. The role of ascorbic acid in drug metabolism. Drug Metab Rev. 1973;2:57-69. [DOI] [PubMed] [Google Scholar]
- 23. Zannoni VG, Flynn EJ, Lynch M. Ascorbic acid and drug metabolism. Biochem Pharmacol. 1972;21:1377-1392. [DOI] [PubMed] [Google Scholar]
- 24. Wilson CW. Vitamins and drug metabolism with particular reference to vitamin C. Proc Nutr Soc. 1974;33:231-238. [PubMed] [Google Scholar]
- 25. Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183:1851-1858. doi: 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palmery M, Saraceno A, Vaiarelli A, Carlomagno G. Oral contraceptives and changes in nutritional requirements. Eur Rev Med Pharmacol Sci. 2013;17:1804-1813. [PubMed] [Google Scholar]
- 27. Jansen G, van der Heijden J, Oerlemans R, et al. Sulfasalazine is a potent inhibitor of the reduced folate carrier: implications for combination therapies with methotrexate in rheumatoid arthritis. Arthritis Rheum. 2004;50:2130-2139. doi: 10.1002/art.20375. [DOI] [PubMed] [Google Scholar]
- 28. King AB, Schwartz R. Effects of the antituberculous drug ethambutol on zinc absorption, turnover and distribution in rats fed diet marginal and adequate in zinc. J Nutr. 1987;117:704-708. [DOI] [PubMed] [Google Scholar]
- 29. Bhagavan HN, Brin M. Drug-vitamin B6 interaction. Curr Concepts Nutr. 1983;12:1-12. [PubMed] [Google Scholar]
- 30. Delage B, Drake VJ, Higdon J. Vitamin B12. http://lpi.oregonstate.edu/mic/vitamins/vitamin-B12. Accessed June 26, 2017.
- 31. Drake VJ, Higdon J. Zinc. http://lpi.oregonstate.edu/mic/minerals/zinc. Accessed June 26, 2017.
- 32. O’Neill LW, Culpepper BL, Galdo JA. Long-term consequences of chronic proton pump inhibitor use. US Pharmacist. 2013;38:38042. [Google Scholar]
- 33. Hendler SS, Rorvik D. PDR for Nutritional Supplements. 2nd ed. Monvale, NJ: Thomson Reuters; 2008. [Google Scholar]
- 34. Cholestyramine [package insert]. Maple Grove, MN: Upsher-Smith Laboratories; 2015. [Google Scholar]
- 35. Aymard JP, Aymard B, Netter P, Bannwarth B, Trechot P, Streiff F. Haematological adverse effects of histamine H2-receptor antagonists. Med Toxicol Adverse Drug Exp. 1988;3:430-448. [DOI] [PubMed] [Google Scholar]
- 36. Yang YX. Chronic proton pump inhibitor therapy and calcium metabolism. Curr Gastroenterol Rep. 2012;14:473-479. doi: 10.1007/s11894-012-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou B, Huang Y, Li H, Sun W, Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;23:339-347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 38. US Food and Drug Administration. FDA drug safety communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed June 13, 2017.
- 39. Craig WJ. Nutrition concerns and health effects of vegetarian diets. Nutr Clin Pract. 2010;25:613-620. doi: 10.1177/0884533610385707. [DOI] [PubMed] [Google Scholar]
- 40. Skikne BS, Lynch SR, Cook JD. Role of gastric acid in food iron absorption. Gastroenterology. 1981;81:1068-1071. [PubMed] [Google Scholar]
- 41. Wells PS, Holbrook AM, Crowther NR, Hirsh J. Interactions of warfarin with drugs and food. Ann Intern Med. 1994;121:676-683. [DOI] [PubMed] [Google Scholar]
- 42. Couris RR, Tataronis GR, Dallal GE, Blumberg JB, Dwyer JT. Assessment of healthcare professionals’ knowledge about warfarin-vitamin K drug-nutrient interactions. J Am Coll Nutr. 2000;19:439-445. [DOI] [PubMed] [Google Scholar]
- 43. Benni JM, Jayanthi MK, Tubaki BR, Renuka M. Knowledge and awareness of food and drug interactions (FDI): a survey among health care professionals. Int J Pharmacol Clin Sci. 2012;1:97-105. [Google Scholar]
- 44. Holmes MV, Hunt BJ, Shearer MJ. The role of dietary vitamin K in the management of oral vitamin K antagonists. Blood Rev. 2012;26:1-14. doi: 10.1016/j.blre.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 45. Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279-3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 46. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52:69-82. doi: 10.1007/s40262-012-0030-9. [DOI] [PubMed] [Google Scholar]
- 47. Grober U, Holzhauer P, Kisters K, Holick MF, Adamietz IA. Micronutrients in oncological intervention. Nutrients. 2016;8:163. doi: 10.3390/nu8030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ozben T. Antioxidant supplementation on cancer risk and during cancer therapy: an update. Curr Top Med Chem. 2015;15:170-178. [PubMed] [Google Scholar]
- 49. Miles SL, Fischer AP, Joshi SJ, Niles RM. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer. 2015;15:867. doi: 10.1186/s12885-015-1878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delage B, Drake VJ, Higdon J. Vitamins: niacin. http://lpi.oregonstate.edu/mic/vitamins/niacin. Accessed June 26, 2017.
- 51. Tremblay A, Kushner PR. Vitamin and mineral shortfalls: frequently asked questions about closing dietary gaps. Consultant. 2016;56:312-318. [Google Scholar]
- 52. Dreizen S, McCredie KB, Keating MJ, Andersson BS. Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad Med. 1990;87:163-167. [DOI] [PubMed] [Google Scholar]
- 53. Baran W, Batycka-Baran A, Zychowska M, Bieniek A, Szepietowski JC. Folate supplementation reduces the side effects of methotrexate therapy for psoriasis. Expert Opin Drug Saf. 2014;13:1015-1021. doi: 10.1517/14740338.2014.933805. [DOI] [PubMed] [Google Scholar]
- 54. Visentin M, Zhao R, Goldman ID. The antifolates. Hematol Oncol Clin North Am. 2012;26:629-648. doi: 10.1016/j.hoc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shea B, Swinden MV, Tanjong Ghogomu E, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;(5):CD000951. doi: 10.1002/14651858.CD000951.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koh KT, Teh CL, Cheah CK, et al. Real-world experiences of folic acid supplementation (5 versus 30 mg/week) with methotrexate in rheumatoid arthritis patients: a comparison study. Reumatismo. 2016;68:90-96. doi: 10.4081/reumatismo.2016.872. [DOI] [PubMed] [Google Scholar]
- 57. Schmajuk G, Tonner C, Miao Y, et al. Folic acid supplementation is suboptimal in a national cohort of older veterans receiving low dose oral methotrexate. PLoS One. 2016;11:e0168369. doi: 10.1371/journal.pone.0168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Via M. The malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012;2012:103472. doi: 10.5402/2012/103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366-373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 60. Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol. 2005;63:429-436. [DOI] [PubMed] [Google Scholar]
- 61. McNair P, Christensen MS, Christiansen C, Madsbad S, Transbol I. Renal hypomagnesaemia in human diabetes mellitus: its relation to glucose homeostasis. Eur J Clin Invest. 1982;12:81-85. [DOI] [PubMed] [Google Scholar]
- 62. Mather HM, Nisbet JA, Burton GH, et al. Hypomagnesaemia in diabetes. Clin Chim Acta. 1979;95:235-242. [DOI] [PubMed] [Google Scholar]
- 63. de Lordes Lima M, Cruz T, Pousada JC, Rodrigues LE, Barbosa K, Cangucu V. The effect of magnesium supplementation in increasing doses on the control of type 2 diabetes. Diabetes Care. 1998;21:682-686. [DOI] [PubMed] [Google Scholar]
- 64. Walti MK, Zimmermann MB, Spinas GA, Hurrell RF. Low plasma magnesium in type 2 diabetes. Swiss Med Wkly. 2003;133:289-292. [DOI] [PubMed] [Google Scholar]
- 65. Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48:927-940. [DOI] [PubMed] [Google Scholar]
- 66. Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050-1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 67. Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J Nutr Sci Vitaminol (Tokyo). 1987;33:421-430. [DOI] [PubMed] [Google Scholar]
- 68. Thornalley PJ, Babaei-Jadidi R, Al Ali H, et al. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164-2170. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ascher E, Gade PV, Hingorani A, et al. Thiamine reverses hyperglycemia-induced dysfunction in cultured endothelial cells. Surgery. 2001;130:851-858. doi: 10.1067/msy.2001.117194. [DOI] [PubMed] [Google Scholar]
- 70. Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29:2064-2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 71. Wong CY, Qiuwaxi J, Chen H, et al. Daily intake of thiamine correlates with the circulating level of endothelial progenitor cells and the endothelial function in patients with type II diabetes. Mol Nutr Food Res. 2008;52:1421-1427. doi: 10.1002/mnfr.200800056. [DOI] [PubMed] [Google Scholar]
- 72. Shim JE, Paik HY, Shin CS, Park KS, Lee HK. Vitamin C nutriture in newly diagnosed diabetes. J Nutr Sci Vitaminol (Tokyo). 2010;56:217-221. [DOI] [PubMed] [Google Scholar]
- 73. Coggeshall JC, Heggers JP, Robson MC, Baker H. Biotin status and plasma glucose levels in diabetes. Ann N Y Acad Sci. 1985;447:389-392. [Google Scholar]
- 74. Maebashi M, Makino Y, Furukawa Y, Ohinata K, Kimura S, Sato T. Therapeutic evaluation of the effect of biotin on hyperglycemia in patients with non-insulin dependent diabetes mellitus. J Clin Biochem Nutr. 1993;14:211-218. [Google Scholar]
- 75. Al-Khalidi B, Kimball SM, Rotondi MA, Ardern CI. Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in US adults: a cross-sectional analysis of NHANES, 2001-2010. Nutr J. 2017;16:16. doi: 10.1186/s12937-017-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980-986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 77. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017-2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29:e142-e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 79. Talaei A, Mohamadi M, Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aghamohammadi V, Gargari BP, Aliasgharzadeh A. Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr. 2011;30:210-215. [DOI] [PubMed] [Google Scholar]
- 81. Palomba S, Falbo A, Giallauria F, et al. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care. 2010;33:246-251. doi: 10.2337/dc09-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications. 2007;21:118-123. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 83. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care. 2000;23:1227-1231. [DOI] [PubMed] [Google Scholar]
- 84. Langan RC, Zawistoski KJ. Update on vitamin B12 deficiency. Am Fam Physician. 2011;83:1425-1430. [PubMed] [Google Scholar]
- 85. Geerling BJ, Badart-Smook A, Stockbrugger RW, Brummer RJ. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514-521. [DOI] [PubMed] [Google Scholar]
- 86. Battat R, Kopylov U, Szilagyi A, et al. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis. 2014;20:1120-1128. doi: 10.1097/MIB.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 87. Owczarek D, Rodacki T, Domagala-Rodacka R, Cibor D, Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol. 2016;22:895-905. doi: 10.3748/wjg.v22.i3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 89. Valentine JF, Sninsky CA. Prevention and treatment of osteoporosis in patients with inflammatory bowel disease. Am J Gastroenterol. 1999;94:878-883. doi: 10.1111/j.1572-0241.1999.981_d.x. [DOI] [PubMed] [Google Scholar]
- 90. Weisshof R, Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2015;18:576-581. doi: 10.1097/mco.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 91. Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211-222. doi: 10.1093/ecco-jcc/jju009. [DOI] [PubMed] [Google Scholar]
- 92. Pironi L, Cornia GL, Ursitti MA, et al. Evaluation of oral administration of folic and folinic acid to prevent folate deficiency in patients with inflammatory bowel disease treated with salicylazosulfapyridine. Int J Clin Pharmacol Res. 1988;8:143-148. [PubMed] [Google Scholar]
- 93. Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009;56:1105-1121. doi: 10.1016/j.pcl.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26:1031-1037. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 95. Houston M. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens (Greenwich). 2011;13:843-847. doi: 10.1111/j.1751-7176.2011.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ascherio A, Rimm EB, Hernan MA, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198-1204. [DOI] [PubMed] [Google Scholar]
- 97. Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998;136:480-490. [DOI] [PubMed] [Google Scholar]
- 98. Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66:411-418. doi: 10.1038/ejcn.2012.4. [DOI] [PubMed] [Google Scholar]
- 99. Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13:309-317. doi: 10.1007/s11906-011-0197-8. [DOI] [PubMed] [Google Scholar]
- 100. D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210-1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 101. Thurnham DI. An overview of interactions between micronutrients and of micronutrients with drugs, genes and immune mechanisms. Nutr Res Rev. 2004;17:211-240. doi: 10.1079/nrr200486. [DOI] [PubMed] [Google Scholar]
- 102. Westphal S, Rading A, Luley C, Dierkes J. Antihypertensive treatment and homocysteine concentrations. Metabolism. 2003;52:261-263. doi: 10.1053/meta.2003.50060. [DOI] [PubMed] [Google Scholar]
- 103. Halsted CH, Medici V. Vitamin-dependent methionine metabolism and alcoholic liver disease. Adv Nutr. 2011;2:421-427. doi: 10.3945/an.111.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rees E, Gowing LR. Supplementary thiamine is still important in alcohol dependence. Alcohol Alcohol. 2013;48:88-92. doi: 10.1093/alcalc/ags120. [DOI] [PubMed] [Google Scholar]
- 105. Teresi ME, Morgan DE. Attitudes of healthcare professionals toward patient counseling on drug-nutrient interactions. Ann Pharmacother. 1994;28:576-580. [DOI] [PubMed] [Google Scholar]
- 106. van Orten-Luiten AC, Janse A, Dhonukshe-Rutten RA, Witkamp RF. Vitamin D deficiency as adverse drug reaction? A cross-sectional study in Dutch geriatric outpatients. Eur J Clin Pharmacol. 2016;72:605-614. doi: 10.1007/s00228-016-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]