Abstract
Ductus arteriosus aneurysm (DAA) is rare in adults, but often involves life-threatening complications. Open repair is common, but is invasive and relatively dangerous. With the continued development of endovascular devices, we can now choose endovascular repair for DAA. However, endovascular repair for infected lesion is controversial. We report a successful case of thoracic endovascular aortic repair with perioperative antibiotic therapy for infected DAA in a 59-year-old man.
INTRODUCTION
Ductus arteriosus aneurysm (DAA) in adulthood is rare, and the exact incidence is unclear [1]. This pathology sometimes involves dangerous complications, including rupture, infection, pulmonary hypertension and fistula formation [2]. Open repair has been the standard treatment for DAA, but is invasive and carries a relatively high risk of complications. Endovascular repair is both less invasive and less risky. However, endovascular repair for mycotic aortic aneurysm (MAA) is controversial [3]. We report a case of successful thoracic endovascular aortic repair (TEVAR) with perioperative antibiotic therapy for infected DAA in an adult.
CASE PRESENTATION
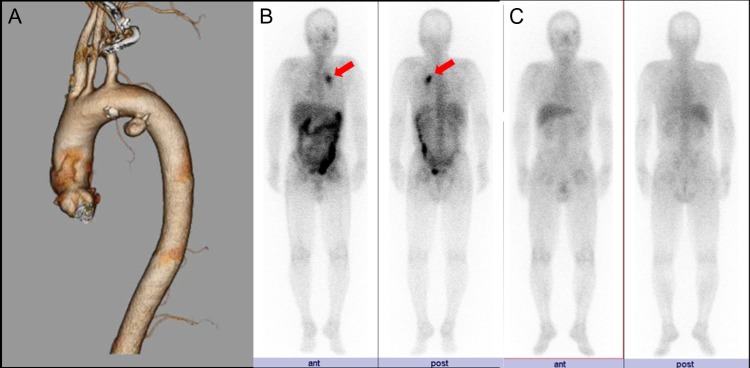
A 59-year-old man with chest pain and prolonged fever was referred to a local clinic. The only past medical history was polyps of the colon. Computed tomography (CT) revealed thoracic aortic aneurysm, and he was transported to our hospital for further investigations. CT angiography (CTA) demonstrated a 2 × 1.5 cm aneurysm on the lesser curvature of the aortic arch (Fig. 1A). We suspected DAA because of the location of the aneurysm and the lack of atherosclerosis in the aorta. Echocardiography showed DAA, but there was no shunt flow. Gallium 67 scintigraphy revealed inflammation at the exact location of the DAA (Fig. 1B). Laboratory tests also revealed inflammation, with an elevated white blood cell count (12.5 × 109/L) and a high C-reactive protein (CRP) level (19 g/L), but blood cultures were negative. Based on the prolonged fever, chest pain and the presence of inflammation of the exact location of the DAA, we made a diagnosis of infected DAA. The treatment strategy was to address the infection with antibiotic therapy as long as the size of the DAA did not change, with treatment of DAA planned for later. Antibiotics were administered intravenously for 3 weeks until CRP reached <1 g/L. He was discharged home after the end of intravenous antibiotic therapy. He continued to take oral antibiotics for 5 months until CRP reached <0.30 g/L. Follow-up CTA showed no change in the size of the DAA. Gallium 67 scintigraphy showed no inflammation at the DAA (Fig. 1C), and laboratory tests showed negative results for inflammation. We chose TEVAR to treat the DAA, as this option was minimally invasive and no infection was apparent at the DAA. TEVAR was performed under general anesthesia. A Valiant stent graft (Medtronic, Minneapolis, MN) was deployed from zone 3, but migrated slightly distally, so we deployed another stent graft from just beneath the left subclavian artery. Postoperative CTA revealed complete closure of the DAA (Fig. 2). The postoperative course was uneventful and the patient was discharged on postoperative Day 7. He continued to take oral antibiotics for 6 months after TEVAR. Laboratory tests and CT showed no recurrent infection during the two years’ follow-up.
FIGURE 1:
(A) CTA demonstrates DAA on the lesser curvature of the aortic arch. (B) Gallium 67 scintigraphy on first admission demonstrates uptake into the DAA (arrows). (C) Preoperative gallium 67 scintigraphy shows no uptake into the DAA.
FIGURE 2:
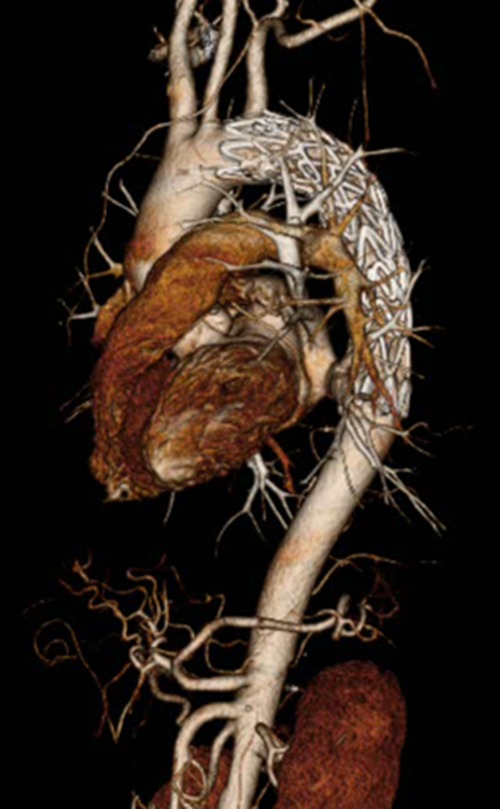
Postoperative CTA shows complete closure of the DAA.
DISCUSSION
DAA is rarely seen in adults. Incomplete closure of the ductus arteriosus at the aortic site, hypertension and atherosclerosis in aging have been reported as factors associated with the formation of DAA [4]. Lund et al. [1] reported that 47% of adult DAA cases occur with life-threatening complications such as rupture (28%), thromboembolism (10%), erosion into the bronchus or esophagus (6%) and infection (3%). Mitchell et al. [5] recommended treatment for DAA with a diameter >3 cm. In our case, we decided on treatment because of the risk of recurrent infection at the DAA, although the diameter was only 2 cm. Our patient was young and healthy enough to undergo open repair, but that approach requires thoracotomy or median sternotomy and cardiopulmonary bypass. TEVAR is much less invasive than open repair. However, TEVAR for an actively infected lesion is controversial [3]; therefore, we first administered antibiotic therapy, and after confirming the absence of infection at the DAA from gallium 67 scintigraphy and laboratory tests, we performed TEVAR. Sörelius et al. [6] reported good short-term outcome (91% survival at 30 days) and relatively good long-term outcome (55% survival at 5 years) of endovascular treatment for MAA, whereas reported short-term mortality (20–40%) and long-term outcome (35% survival at 5 years) of open repair were worse. Aoki et al. [7] also described that TEVAR for MAA in descending aorta is appropriate if infection is controlled by antibiotic therapy. Postoperative antibiotic therapy is important for good outcome, but there is no consensus on its duration [7]. Sörelius et al. [6] recommend at least 6–12 months postoperative antibiotic therapy after endovascular repair for MAA because most recurrent infections occur first year, especially within the first 6 months. In our case, we continued postoperative antibiotic therapy for 6 months, and no recurrent infection occurs during the 2 years’ follow-up.
In conclusion, this case suggests that TEVAR with enough perioperative antibiotic therapy for infected DAA is feasible and less invasive than the open approach, but further careful follow-up is needed for long-term outcome.
CONFLICT OF INTEREST STATEMENT
All authors declared they have no conflict of interests.
FUNDING
None of authors received any funding.
REFERENCES
- 1. Lund JT, Jensen MB, Hjelms E. Aneurysm of the ductus arteriosus. A review of literature and the surgical implications. Eur J Cardiothorac Surg 1991;5:566–70. [DOI] [PubMed] [Google Scholar]
- 2. De Freitas S, Connolly C, Neary C, Sultan S. Ductus arteriosus aneurysm presenting as hoarseness: successful repair with an endovascular approach. J Surg Case Rep 2016;4:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clough RE, Black SA, Lyons OT, Zayed HA, Bell RE, Carrell T, et al. Is endovascular repair of mycotic aortic aneurysm a durable treatment option? Eur J Vasc Endovasc Surg 2009;37:407–12. [DOI] [PubMed] [Google Scholar]
- 4. Pastuszko P, Eisenberg JA, Diehl JT. Ductus arteriosus aneurysm in an adult patient presenting with hoarseness. J Card Surg 2005;20:386–8. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell RS, Seifert FC, Miller DC, Jamieson SW, Shumway NE. Aneurysm of the diverticulum of the ductus arteriosus in the adult. Successful surgical treatment in five patients and review of the literature. J Thorac Cardiovasc Surg 1983;86:400–8. [PubMed] [Google Scholar]
- 6. Sörelius K, Mani K, Björck M, Sedivy P, Wahlgren CM, Taylor P, et al. Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation 2014;130:2136–42. [DOI] [PubMed] [Google Scholar]
- 7. Aoki C, Fukuda W, Kondo N, Minakawa M, Taniguchi S, Daitoku K, et al. Surgical management of mycotic aortic aneurysms. Ann Vasc Dis 2017;10:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]