Abstract
Serum amyloid A (SAA) is a high-density apolipoprotein whose plasma levels can increase more than 1000-fold during a severe acute-phase inflammatory response and are more modestly elevated in chronic inflammation. SAA is thought to play important roles in innate immunity, but its biological activities have not been completely delineated. We previously reported that SAA deficiency protects mice from developing abdominal aortic aneurysms (AAAs) induced by chronic angiotensin II (AngII) infusion. Here, we report that SAA is required for AngII-induced increases in interleukin-1β (IL-1β), a potent proinflammatory cytokine that is tightly controlled by the Nod-like receptor protein 3 (NLRP3) inflammasome and caspase-1 and has been implicated in both human and mouse AAAs. We determined that purified SAA stimulates IL-1β secretion in murine J774 and bone marrow–derived macrophages through a mechanism that depends on NLRP3 expression and caspase-1 activity, but is independent of P2X7 nucleotide receptor (P2X7R) activation. Inhibiting reactive oxygen species (ROS) by N-acetyl-l-cysteine or mito-TEMPO and inhibiting activation of cathepsin B by CA-074 blocked SAA–mediated inflammasome activation and IL-1β secretion. Moreover, inhibiting cellular potassium efflux with glyburide or increasing extracellular potassium also significantly reduced SAA–mediated IL-1β secretion. Of note, incorporating SAA into high-density lipoprotein (HDL) prior to its use in cell treatments completely abolished its ability to stimulate ROS generation and inflammasome activation. These results provide detailed insights into SAA–mediated IL-1β production and highlight HDL's role in regulating SAA's proinflammatory effects.
Keywords: inflammasome, high-density lipoprotein (HDL), NLRP3, interleukin 1 (IL-1), reactive oxygen species (ROS), Serum Amyloid A
Introduction
Interleukin-1β (IL-1β)2 is a key proinflammatory mediator in acute and chronic inflammation and a powerful inducer of the innate immune response (1). A growing body of evidence currently points to IL-1β as a major player in a wide variety of chronic diseases (2). Consistent with this concept, randomized clinical trials have shown that blocking IL-1β signaling leads to a sustained reduction in systemic inflammation and improvement in type 2 diabetes patients (3). More recently, the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), significantly lowers the rate of cancer mortality and recurrent cardiovascular events compared with placebo, independent of lipid lowering (4). However, there was also a significant increase in deaths from infection in patients who received canakinumab, leading to an overall neutral effect on mortality in the CANTOS trial. Thus, understanding the endogenous mechanisms leading to elevated IL-1β production in chronic inflammatory diseases are needed to develop effective strategies for anti-IL-1β therapy that avoid an overall suppression of immune responses.
The production of bioactive IL-1β is tightly controlled by the inflammasome, a multiprotein intracellular complex that serves as a platform for the proteolytic maturation of IL-1β. The well-studied of these complexes is the NLRP3 inflammasome, which is comprised of a scaffold protein, NLRP3; an adaptor protein, ASC; and the cysteine protease, pro-caspase-1. Two signals are required for NLRP3 inflammasome activation. The first signal requires a stimulus that induces the transcription of key components of the inflammasome including IL-1β and NLRP3. This “priming” signal typically involves nuclear factor-κB (NF-κB) activation induced by signaling through a Toll-like receptor or other pattern recognition receptors (5). Pathogen-associated or host-derived factors may prime the inflammasome in nonsterile and sterile inflammatory diseases, respectively (6). The second signal promotes the functional activity of the NLRP3 inflammasome. A variety of structurally diverse molecules, including bacterial toxins, ATP, uric acid crystals, silica, asbestos, alum, cholesterol crystals, and β-amyloid are known to activate the NLRP3 inflammasome (5). Interestingly, minimally modified LDL has been suggested to provide both signal 1 and signal 2 in macrophage foam cells, the latter most likely through the intracellular accumulation of cholesterol crystals (7).
Another danger-associated molecule capable of both priming and activating the NLRP3 inflammasome is serum amyloid A (SAA). SAA stimulates secretion of IL-1β in human and mouse macrophages, dendritic cells, and neutrophils (8–10). SAA represents a family of proteins with 103–104 amino acids that share high sequence homology that presumably arose from gene duplication. In humans, three SAA isoforms are expressed, SAA1, SAA2, and SAA4, which correspond to the highly homologous mouse SAA1.1, SAA2.1, and SAA4. Mice express a fourth SAA, SAA3, which is a pseudogene in humans due to a premature stop codon in the coding sequence (11). Whereas SAA4 is a constitutively expressed isoform that is present in plasma at relatively low concentrations (12), the other SAAs are acute-phase proteins that are highly induced during an acute inflammatory response (13). During severe inflammation such as sepsis, SAA can account for as much as 2.5% of hepatic protein production, with serum SAA levels increasing >1000-fold (13), suggesting an important role for SAA in the immediate response to tissue injury and infection. On the other hand, chronically elevated SAA is associated with a wide variety of chronic pathological conditions (14, 15). Although circulating SAA is thought to be primarily produced by hepatocytes, extra-hepatic synthesis of SAA has been reported in inflamed tissues (16, 17). Virtually all circulating SAA is bound to the surface of high-density lipoprotein (HDL) (18). SAA binds a wide range of molecules including heparan sulfate proteoglycans (19), extracellular matrix proteins (20), and pattern recognition receptors such as formyl peptide receptor-like 1 (FPRL-1) (21), FPRL-2 (22), TLR2 (23), TLR4 (24), SR-B1 (25), and CD36 (26). Despite accumulating evidence that SAA is a key mediator in innate immunity and is associated with numerous chronic inflammatory conditions, SAA's functions in immune responses and inflammatory diseases have not been clearly established.
In a previous publication, we reported that SAA deficiency attenuates abdominal aortic aneurysm (AAA) formation in the mouse angiotension II (AngII) infusion model (15). In the current study, we determined that reduced AngII-induced AAA in SAA-deficient mice is accompanied by significant reductions in IL-1β, suggesting that inflammasome activation in AngII-infused mice depends on SAA. This finding is notable given extensive evidence that IL-1β and NLRP3-mediated inflammasome activation promote experimental aortic aneurysms in mice (27–32) and the observation that circulating levels of IL-1β is elevated in patients with AAA (33). We also report that SAA activates the NLRP3 inflammasome through the generation of reactive oxygen species, cathepsin B activation, and potassium efflux. Notably, HDL blocks the ability of SAA to mediate NLRP3 activation. These data further our understanding of an endogenous factor that may mediate pathophysiological effects in the setting of chronic inflammation.
Results
SAA is required for angiotensin II–induced IL-1β production in a mouse model of abdominal aortic aneurysm
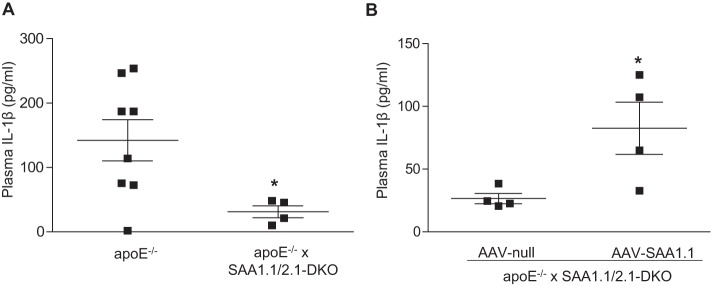
We previously reported that lack of endogenous acute-phase SAA1.1 and SAA2.1 protects apoE−/− mice from AngII-induced AAA formation (15). In a recent study, Usui et al. (29) determined that deficiency in either NLRP3 or caspase-1 prevents AngII-induced AAA in apoE−/− mice, establishing that activation of the NLRP3 inflammasome is a necessary event for AAA development in this animal model. Therefore, it was of interest to investigate whether SAA mediates inflammasome activation in AngII-infused apoE−/− mice. Accordingly, we assessed plasma IL-1β levels after 28-day AngII infusion in apoE−/− mice and apoE−/− mice lacking SAA1.1 and SAA2.1 (apoE−/− × SAA1.1/2.1-DKO mice). IL-1β was not detected in the plasma of either strain of mice prior to AngII infusion. Notably, the increase in plasma IL-1β levels observed in AngII-infused apoE−/− mice was significantly blunted in apoE−/− × SAA1.1/2.1-DKO mice (Fig. 1A). As a gain-of-function approach, we overexpressed SAA1.1 in apoE−/− × SAA1.1/2.1-DKO mice using adeno-associated virus (AAV)-mediated gene transfer. Fourteen days after AAV injections, the mice were infused with AngII for 28 days. Interestingly, there was a significant increase in plasma IL-1β in mice injected with AAV-SAA1.1 compared with mice injected with control AAV-null following AngII infusion (Fig. 1B). These results indicate that SAA plays a central role in AngII-induced IL-1β production, a key event in AngII-induced AAA.
Figure 1.
SAA mediates angiotensin II-induced IL-1β production in apoE−/− mice. A and B, male apoE−/− mice and apoE−/− mice lacking SAA 1.1 and SAA 2.1 (apoE−/− × SAA1.1/2.1-DKO) (A) apoE−/− × SAA1.1/2.1-DKO mice injected with a control AAV (AAV-null) or AAV expressing SAA1.1 (AAV-SAA1.1) (B) were infused with AngII (1000 ng/kg/min) for 28 days and plasma IL-1β levels were determined by ELISA. AAVs were injected (i.p.) 14 days prior to AngII infusion. IL-1β is not detectable in control, untreated mice. Data are presented as mean ± S.E. *, p < 0.05.
Mouse SAA induces IL-1β mRNA expression and protein secretion in macrophages
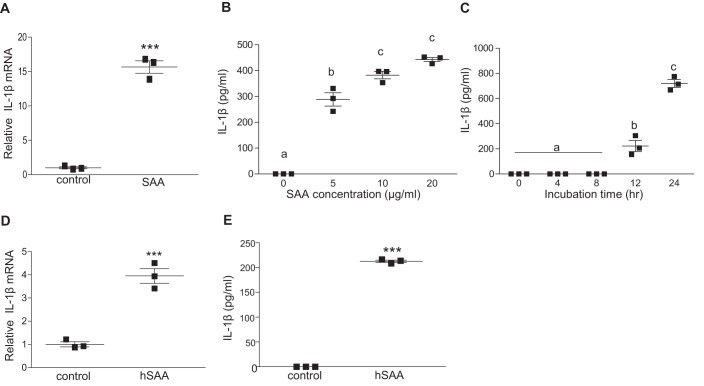
The observation that SAA is required for increased plasma IL-1β in mice infused with AngII prompted us to investigate whether SAA regulates IL-1β production in macrophages. J774 cells incubated with 50 μg/ml of purified mouse SAA (a mixture of SAA1.1 and SAA2.1, as well as small amounts of SAA3 (34) that has undetectable levels of endotoxin) for 8 h showed a significant 16-fold increase in pro-IL-1β mRNA (Fig. 2A). SAA also evoked a dose- and time-dependent increase in IL-1β secretion (Fig. 2, B and C). Human SAA (hSAA) isolated from the HDL fraction of cardiac surgery patients was also effective in inducing IL-1β mRNA expression (Fig. 2D) and IL-1β release (Fig. 2E) in J774 cells.
Figure 2.
SAA triggers inflammasome priming and activation in macrophages. A, J774 cells were incubated ±50 μg/ml of mouse SAA for 8 h prior to quantification of IL-1β mRNA by qRT-PCR. B, J774 cells were incubated with the indicated concentrations of mouse SAA for 24 h and IL-1β in the media was quantified by ELISA. C, J774 cells were incubated with 5 μg/ml of mouse SAA for the indicated times and IL-1β in the media was quantified by ELISA. D, IL-1β mRNA abundance was determined in untreated J774 cells (control), or cells treated for 8 h with 25 μg/ml of human SAA (hSAA) as indicated. E, IL-1β levels were determined by ELISA in conditioned media from J774 cells incubated for 24 h ±25 μg/ml of hSAA. Data are presented as mean ± S.E. ***, p < 0.001. Data that are not significantly different (p > 0.05) are indicated with the same letter.
SAA stimulates NLRP3 inflammasome-dependent IL-1β secretion in macrophages
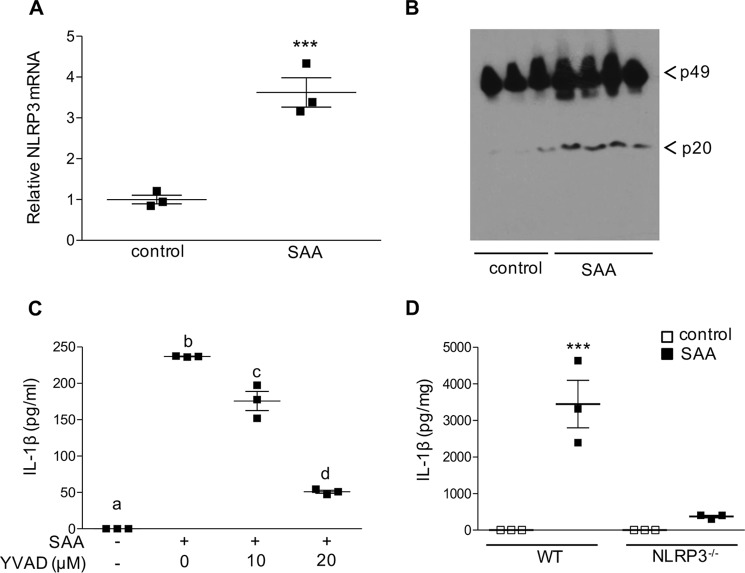
We next investigated the role of the NLRP3 inflammasome in SAA-mediated IL-1β release. Our results indicate SAA up-regulates NLRP3 mRNA expression ∼3-fold in J774 cells (Fig. 3A), consistent with inflammasome priming. The second “activation” step involves the intracellular assembly of the inflammasome complex, which in turn leads to the proteolytic activation of caspase-1. Immunoblot analysis showed increased cleavage of caspase-1 to its active p20 subunit in J774 cells incubated for 24 h with 50 μg/ml of mouse SAA compared with untreated control cells (Fig. 3B). SAA-induced IL-1β secretion was blocked when cells were treated with YVAD, a specific caspase-1 inhibitor (35) (Fig. 3C). To determine whether SAA-induced IL-1β secretion depends on NLRP3, bone marrow–derived macrophages (BMDMs) isolated from C57BL/6 (WT) and NLRP3−/− mice were treated with 5 μg/ml of mouse SAA for 24 h. As shown in Fig. 3D, IL-1β secretion induced by SAA was significantly lower (more than 10-fold) in BMDMs from NLRP3−/− mice compared with cells from WT mice. These results indicate that SAA stimulates IL-1β secretion in macrophages mainly through NLRP3 inflammasome-mediated caspase-1 activation.
Figure 3.
SAA stimulates NLRP3 inflammasome-dependent IL-1β secretion in macrophages. A, J774 cells were incubated with ±50 μg/ml of mouse SAA for 8 h prior to quantification of NLRP3 mRNA by qRT-PCR. B, J774 cells were incubated ±50 μg/ml of mouse SAA for 24 h and activation of caspase-1 was determined by immunoblot analysis; the migration of procaspase-1 (p49) and the active capase-1 (p20) cleavage product is indicated. C, IL-1β levels in conditioned media from J774 cells incubated for 24 h ±5 μg/ml of mouse SAA in the presence of varying concentrations of a caspase-1-specific inhibitor (Z-YVAD-fmk) were determined by ELISA. D, IL-1β levels in conditioned media from bone marrow–derived macrophages isolated from either WT or NLRP3-deficient (NLRP3−/−) mice treated ±5 μg/ml of mouse SAA for 24 h were determined by ELISA. Data are presented as mean ± S.E. ***, p < 0.001. Data that are significantly different (p > 0.05) are indicated with different letters.
SAA-mediated inflammasome activation involves alterations in cellular K+ flux that are independent of the P2X7 receptor
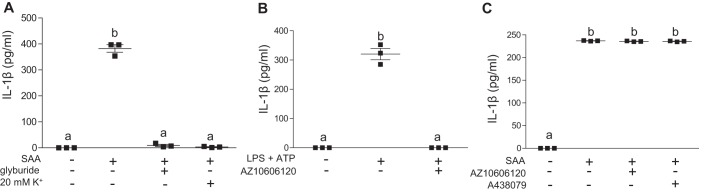
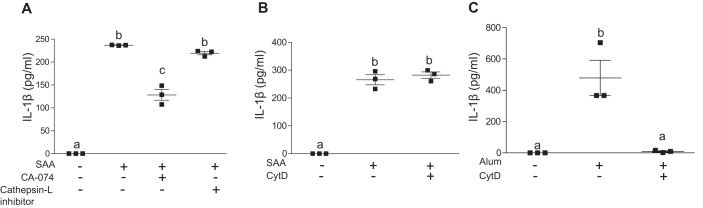
Potassium efflux and reduced intracellular K+ have been linked to NLRP3 inflammasome activation in monocyte/macrophages triggered by numerous known NLRP3 activators, such as ATP, nigericin, alum, and silica (5). Hence, we investigated whether an alteration in cellular K+ efflux was involved in SAA-mediated IL-1β release in J774 cells. Incubation of J774 cells with mouse SAA (5 μg/ml) along with a K+ channel blocker, glyburide (200 μm), or increased extracellular K+ concentration (20 mm), completely abolished SAA-mediated IL-1β release (Fig. 4A), demonstrating that SAA-mediated NLRP3 activation requires alterations in cellular K+ flux. One well-established pathway for activating NLRP3 through increased K+ efflux is by means of the trimeric ATP-gated cation channel, P2X7. The P2X7 receptor expressed by macrophages is activated by extracellular ATP to induce NLRP3 inflammasome assembly and release of IL-1β (36). To investigate whether the P2X7 receptor is required for the SAA-induced release of IL-1β, J774 cells were treated with purified SAA in the presence or absence of specific P2X7 receptor antagonists, AZ10606120 and A438079. The effectiveness of AZ10606120 to block NLRP3 inflammasome-mediated IL-1β release was confirmed in control experiments whereby J774 cells were primed with LPS and then stimulated with ATP (Fig. 4B). In contrast, both AZ10606120 and A438079 were completely ineffective in blocking the robust release of IL-1β produced in J774 cells after 24-h incubations with purified mouse SAA (5 μg/ml; Fig. 4C). Inhibition of P2X7 receptor signaling also failed to reduce SAA-induced conversion of pro-caspase-1 to the active form (Fig. S1). Taken together, our data indicate that SAA-mediated inflammasome activation depends on changes in intracellular K+ through a mechanism that is independent of the P2X7 receptor.
Figure 4.
SAA-mediated inflammasome activation involves cellular K+ efflux and is independent of P2X7R activation. A, IL-1β levels were determined for conditioned media from untreated J774 cells, or cells incubated with 5 μg/ml of SAA ±200 μm of a potassium-channel blocker, glyburide, or 20 mm K+ as indicated. B, IL-1β in conditioned media from J774 cells treated with 500 ng/ml of LPS for 3 h followed by incubation with 3 mm ATP for 45 min in the presence or absence of 10 μm P2X7R-specific antagonist AZ10606120 was determined by ELISA. C, IL-1β in conditioned media from J774 cells incubated ±5 μg/ml of SAA in the presence or absence of P2X7R-specific antagonists AZ10606120 (10 μm) or A438079 (25 μm) for 24 h was determined by ELISA. Data are presented as mean ± S.E. Data that are not significantly different (p > 0.05) are indicated with the same letter.
Generation of reactive oxygen species (ROS) is required for SAA-mediated activation of the NLRP3 inflammasome
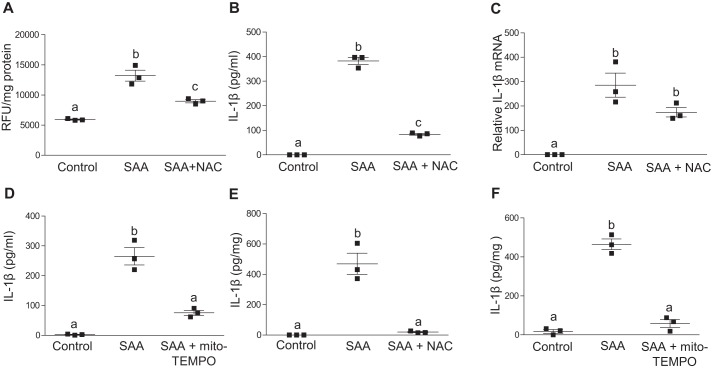
ROS have been shown to be essential for NLRP3 inflammasome activation (5). ROS generation is frequently accompanied by K+ efflux, although the interplay between these pathways is currently not clear, with low intracellular K+ concentration stimulating ROS production and vice versa (37). To investigate the possibility that SAA mediates activation of NLRP3 inflammasomes through ROS generation, J774 cells were treated with or without 5 μg/ml of mouse SAA for 24 h, and cellular ROS levels were quantified using a cell permeant fluorescent reagent, 2′,7′–dichlorofluorescin diacetate (DCFDA). ROS levels were significantly higher (2.2-fold) in J774 cells treated with SAA compared with untreated cells. Co-incubation with free-radical scavenger N-acetylcysteine (NAC) significantly reduced SAA-dependent ROS generation by 58% (Fig. 5A). This decrease in SAA-dependent ROS generation was accompanied by a 78% decrease in the amount of IL-1β released in SAA-stimulated cells (Fig. 5B). SAA-mediated priming, as evidenced by IL-1β mRNA levels in SAA-treated cells, was not significantly affected by NAC treatment (Fig. 5C). These results indicate that ROS generation is a prerequisite for SAA-mediated inflammasome activation. We next assessed SAA-mediated IL-1β release in the presence of mito-TEMPO, a scavenger specific for mitochondrial ROS. Interestingly, mito-TEMPO caused a significant inhibition of SAA-mediated IL-1β release (Fig. 5D). Similar to J774 cells, treatments with NAC or mito-TEMPO significantly suppressed SAA-mediated IL-1β release from C57BL/6 BMDMs (Fig. 5, E and F). These results indicate that SAA-mediated inflammasome activation depends on mitochondrial ROS generation.
Figure 5.
SAA triggers ROS production in macrophages and inhibition of ROS abrogates SAA-mediated IL-1β production. A, intracellular ROS in J774 cells incubated for 24 h with or without 5 μg/ml of SAA ± a free-radical scavenger, NAC (20 μm NAC) were quantified using cell permeant DCFDA reagent. B, IL-1β in conditioned media from J774 cells incubated for 24 h ± 5 μg/ml of SAA in the presence or absence of 20 μm NAC was determined by ELISA. C, J774 cells were incubated ±5 μg/ml of SAA for 8 h in the presence or absence of 20 μm NAC prior to quantification of IL-1β mRNA by qRT-PCR. D, IL-1β in conditioned media from J774 cells incubated ±5 μg/ml of SAA in the presence or absence of 500 μm mito-TEMPO for 24 h was determined by ELISA. E, IL-1β in the conditioned media from bone marrow–derived macrophages isolated from C57BL/6 mice incubated ±5 μg/ml of SAA in the presence or absence of 20 μm NAC for 24 h was determined by ELISA. F, IL-1β in conditioned media from bone marrow–derived macrophages isolated from C57BL/6 mice incubated ±5 μg/ml of SAA in the presence or absence of 500 μm mito-TEMPO for 24 h was determined by ELISA. Data are presented as mean ± S.E. Data that are not significantly different (p > 0.05) are indicated with the same letter.
SAA-mediated IL-1β release depends on cathepsin B activity
Disruption of lysosomal membranes caused by phagocytosis of particulate matter, live pathogens, or sterile lysosomal damage results in NLRP3 activation (5). The ensuing release of the lysosomal aspartyl protease cathepsin B into the cytoplasm triggers inflammasome activation either directly or indirectly through a poorly understood mechanism. Interestingly, we determined that incubations with CA-074-Me, a specific inhibitor of cathepsin B, resulted in a significant 46% decrease in IL-1β release induced by SAA (Fig. 6A). Inhibition of cathepsin L had no effect on SAA-induced IL-1β release. One mechanism for inducing lysosomal damage and subsequent cathepsin B release is through the phagocytosis of extracellular fibrils and crystals, such as β-amyloid and cholesterol crystals (38, 39). Because SAA is known to be capable of forming extracellular fibrils in AA amyloidosis (40), we considered the possibility that phagocytosis of SAA in the form of extracellular fibrils results in lysosomal damage and subsequent NLRP3 inflammasome activation. However, treatment with 3 μm cytochalasin D to block phagocytosis did not alter IL-1β release stimulated by SAA (Fig. 6B), whereas it effectively blocked alum-mediated IL-1β secretion in these cells (Fig. 6C).
Figure 6.
SAA-mediated IL-1β production depends on cathepsin B activity. A, IL-1β levels in conditioned media from untreated J774 cells or cells incubated for 24 h with 5 μg/ml of SAA in the presence or absence of specific inhibitors for cathepsin B (12.5 μm CA-074-Me) or cathepsin L inhibitor (15 μm), as indicated, were determined by ELISA. B, IL-1β levels in conditioned media from untreated J774 cells or cells incubated for 24 h with 5 μg/ml of SAA with and without 3 μm cytochalasin D (CytD) were determined by ELISA. C, IL-1β levels in conditioned media from J774 cells treated with 500 ng/ml of LPS for 3 h followed by a 4-h incubation with alum (200 μg/ml) in the presence or absence of 3 μm cytochalasin D were determined by ELISA. Data are presented as mean ± S.E. Data that are not significantly different (p > 0.05) are indicated with the same letter.
HDL inhibits SAA-induced IL-1β transcription and secretion
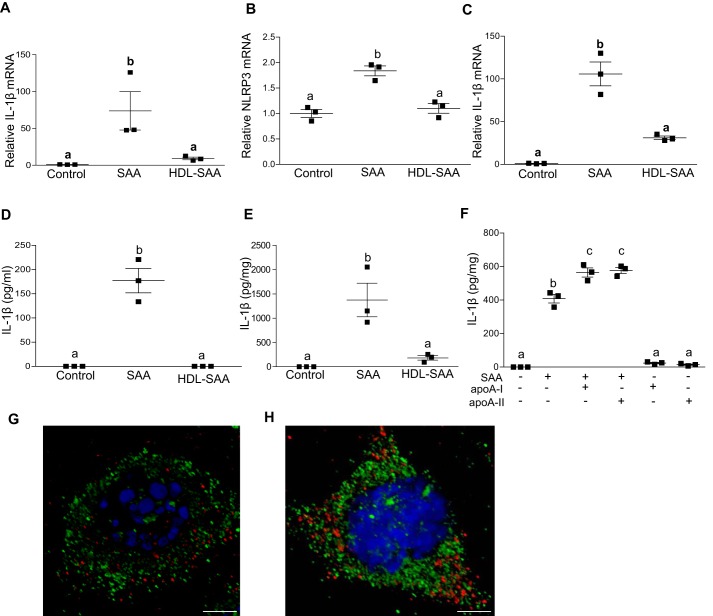
The vast majority of SAA secreted by the liver during an inflammatory response is associated with HDL in plasma (18). Our group previously reported that lipid-poor SAA, but not HDL-associated SAA, stimulates granulocyte colony stimulating factor and tumor necrosis factor-α production in macrophage cells (41). HDL suppresses inflammasome activation triggered by cholesterol crystals (42). It was therefore of interest to investigate whether HDL-associated SAA induces IL-1β secretion in J774 cells similarly to purified, lipid-poor SAA. Accordingly, J774 cells were incubated with 5 μg/ml of purified mouse SAA or 5 μg/ml of SAA associated with HDL (1:2.8 ratio of SAA protein to HDL protein). HDL-associated SAA was significantly reduced in its ability to induce IL-1β and NLRP3 mRNA expression in J774 cells (Fig. 7, A and B) and BMDMs (Fig. 7C), indicating that HDL does indeed block SAA-mediated inflammasome priming.
Figure 7.
HDL inhibits SAA-mediated IL-1β transcription and secretion in macrophages. IL-1β (A) and NLRP3 mRNA (B) abundance was quantified in untreated J774 cells (control) and J774 cells incubated for 8 h with 5 μg/ml of lipid-free SAA, or SAA bound to HDL (SAA:HDL protein ratio = 1:2.8). C, IL-1β mRNA abundance was quantified in BMDMs isolated from C57BL/6 mice and incubated for 8 h with 5 μg/ml of lipid-free SAA, or SAA bound to HDL. D, IL-1β levels in the conditioned media from untreated J774 cells (control) or cells incubated for 24 h with 5 μg/ml of lipid-free SAA, or SAA bound to HDL were determined by ELISA. E, IL-1β levels in conditioned media from BMDMs isolated from C57BL/6 mice, untreated (control) or treated with 5 μg/ml of lipid-free SAA, or SAA bound to HDL were determined. F, IL-1β levels were determined by ELISA in the conditioned media from untreated J774 cells (control) or cells treated with 5 μg/ml of SAA, 5 μg/ml of SAA + 14 μg/ml of apoA-I, 5 μg/ml of SAA + 14 μg/ml of apoA-II, 14 μg/ml of apoA-I or 14 μg/ml of apoA-II as indicated. G and H, confocal microscopy images of J774 cells incubated for 4 h with (G) ∼0.5 μg/ml of FITC-SAA (green) or (H) ∼0.5 μg/ml of FITC-SAA (green) complexed with 1.4 μg/ml of HDL. Lysosomes were stained with anti-LAMP-1 (red) and nuclei by DAPI (blue). Data are presented as mean ± S.E. Data that are not significantly different (p > 0.05) are indicated with the same letter. Scale bar: 5 μm.
IL-1β protein release by both J774 cells (Fig. 7D) and BMDMs (Fig. 7E) was also abrogated when SAA was bound to HDL. The two major apolipoproteins of HDL, apo-AI and apo-AII, were ineffective in blocking SAA-stimulated IL-1β secretion; indeed, IL-1β secretion by cells incubated with lipid-free SAA was modestly enhanced by the addition of apoA-I or apoA-II, whereas apoA-I and apoA-II alone were ineffective in inducing IL-1β secretion (Fig. 7F).
We investigated the possibility that HDL blocks SAA's activity by altering the cellular uptake of SAA. J774 cells were treated with 0.5 μg/ml of FITC-labeled SAA (green fluorescence) with or without HDL (1.4 μg/ml of protein) for 4 h, followed by immunocytochemical staining to visualize lysosomes with fluorescently-labeled anti-LAMP1 antibody (red fluorescence) and nuclei with DAPI (blue fluorescence). Imaging by confocal microscopy indicated that both lipid-free (Fig. 7G) and HDL-bound (Fig. 7H) SAA were readily taken up by J774 cells. Notably, there was no evidence of SAA co-localization with the lysosomal marker LAMP-1 for either experimental condition. These data suggest that the mechanism by which HDL suppresses SAA-mediated inflammasome activation does not involve alterations in cellular uptake or lysosomal accumulation of SAA.
HDL abrogates SAA-mediated inflammasome activation and ROS generation
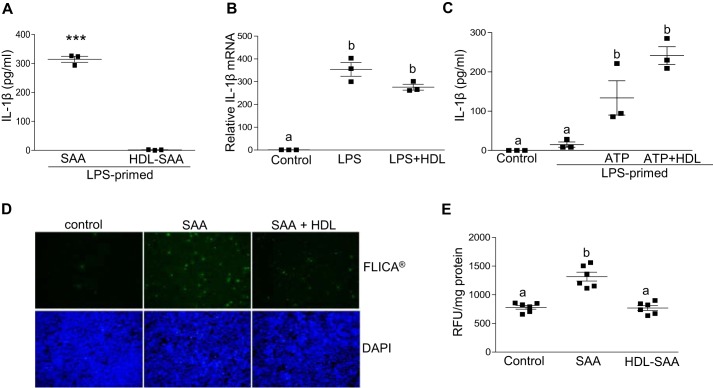
Several studies have established that HDL suppresses SAA signaling through TLR4 (41, 43, 44), suggesting that the lack of induction of IL-1β secretion by cells treated with HDL-associated SAA may merely reflect the absence of SAA-induced IL-1β mRNA expression (i.e. inflammasome priming). Thus, it was of interest to determine whether HDL prevents SAA-mediated inflammasome priming, or both priming and activation. To specifically address this question, J774 cells were pre-treated with 0.5 μg/ml of LPS to induce IL-1β and NLRP3 expression, and then washed to remove the LPS. The primed cells were then treated with 5 μg/ml of lipid-free mouse SAA or SAA associated with HDL. In contrast to lipid-free SAA, HDL-associated SAA was ineffective in inducing IL-1β release even after priming the cells with LPS (Fig. 8A), indicating that HDL masks not only SAA-mediated priming, but also SAA-mediated inflammasome activation.
Figure 8.
HDL abrogates SAA-mediated inflammasome activation and ROS generation. A, IL-1β levels in conditioned media from J774 cells primed with 0.5 μg/ml of LPS for 3 h prior to 24-h incubations with 5 μg/ml of SAA ± 14 μg/ml of HDL were determined by ELISA. B, IL-1β mRNA abundance was determined for untreated J774 cells (control), or cells treated for 3 h with 0.5 μg/ml of LPS ± 70 μg/ml of HDL, as indicated. C, IL-1β levels in conditioned media from untreated J774 cells (control), cells primed with 0.5 μg/ml of LPS alone, or LPS followed by 3 mm ATP ± 70 μg of HDL for 45 min were determined by ELISA. D, J774 cells were incubated ±5 μg/ml of SAA for 24 h and activation of caspase-1 was determined by FLICA, with activated caspase-1 visualized as green fluorescence. E, intracellular ROS levels were quantified using DCFDA reagent in J774 cells that were left untreated (control) or treated with 5 μg/ml of SAA ± 14 μg/ml of HDL. Data are presented as mean ± S.E. ***, p < 0.001. Data that are not significantly different (p > 0.05) are indicated with the same letter.
We considered the possibility that the ability of HDL to interfere with SAA's effects may be due to a general effect of HDL to impede inflammatory responses in cells. J774 cells were sequentially incubated with LPS (0.5 μg/ml) for 3 h followed by 3 mm ATP for 45 min in the absence and presence of 70 μg/ml of HDL. Interestingly, HDL did not significantly impede LPS-mediated priming (Fig. 8B) or ATP-mediated activation of inflammasomes (Fig. 8C), as assessed by the induction of IL-1β mRNA expression and protein secretion, respectively.
As an alternate analysis of inflammasome activation, J774 cells were treated with or without 5 μg/ml of lipid-free or HDL-associated mouse SAA for 24 h and then labeled with FAM-YVAD-fmk (FLICA®), a fluorescent caspase-1 inhibitor that binds activated caspase-1 but not pro-caspase-1 (45). Fluorescence microscopy showed increased FLICA® staining for cells treated with lipid-free SAA, but not SAA bound to HDL, compared with control untreated cells (Fig. 8D). Notably, the ability of HDL to interfere with SAA-induced caspase-1 activation and IL-1β secretion was associated with a significant effect on SAA-mediated ROS generation in J774 cells (Fig. 8E).
Discussion
In this study, we determined that SAA is required for the increased IL-1β production that occurs in mice chronically infused with AngII. This finding has potential clinical implications, given the accumulating evidence that IL-1β and NLRP3 inflammasome activation play a critical role in the development of human and mouse AAA (27–32) and our previous report that SAA mediates AngII–induced AAA in mice (15). Circulating SAA is elevated in a number of chronic inflammatory diseases including obesity, type 2 diabetes, rheumatic diseases, cancer, and cardiovascular diseases where IL-1β is thought to play a pathological role. Thus, understanding the pathways by which SAA stimulates IL-1β production may provide novel insights into chronic disease mechanisms and hence was the focus of our current studies.
Other groups have reported that SAA stimulates dendritic cells, macrophages, and neutrophils to secrete IL-1β by activating the NLRP3 inflammasome (8–10). However, these studies utilized a recombinant SAA protein that has 2 amino acid substitutions (at positions 61 and 72) when compared with native SAA1, and recent evidence suggests that this recombinant form of SAA may exert activities not shared by mouse or human SAA (46, 47). In our studies investigating SAA-mediated IL-β expression and secretion we utilized SAA isolated from acute-phase mouse or human plasma to facilitate interpretation of results. The purified SAA preparations contained a mixture of the acute-phase SAA isoforms, namely SAA1 and SAA2 in humans, and SAA1.1, SAA2.1, and to a lesser extent, SAA3 in mice. Although mouse SAA1.1 and SAA2.1 are highly homologous (91% amino acid conservation) and are coordinately regulated, functional differences have been identified. Most notably, SAA1.1, and not SAA2.1, has a propensity to be deposited extracellularly as insoluble amyloid fibrils (48). Modest differences in the capacity to promote cellular cholesterol efflux and interact with pattern recognition receptors have also been noted for the two isoforms (49, 50). Whether individual acute-phase SAA isoforms differ in their ability to trigger IL-1β secretion merits future study.
SAA's ability to activate NF-κB pathways (the priming step) by signaling through multiple pattern recognition receptors is widely recognized. Thus, the induction of IL-1β and NLRP3 mRNA expression in J774 macrophage-like cells incubated with SAA was not unexpected. The ability of SAA to both prime and activate the NRLP3 inflammasome to stimulate IL-1β secretion in J774 cells distinguishes it from the myriad of compounds such as ATP, pore-forming toxins (51), β-amyloid (14, 38), and cholesterol crystals (7) that are incapable of inducing IL-1β secretion in the absence of a priming stimulus. Thus, SAA seems to constitute an endogenous “danger” signal with the unique ability of stimulating both essential steps of NLRP3 inflammasome-mediated IL-1β secretion.
Although the precise mechanism by which the NLRP3 inflammasome is activated in cells remains unknown, several stress-related cellular processes, including cytosolic depletion of potassium, lysosome disruption, mitochondrial damage, or generation of ROS have been proposed to be involved (5). For example, extracellular ATP released by damaged cells binds the ATP-gated P2X7 receptor and activates the NLRP3 inflammasome through the rapid production of ROS (36). In an earlier report, inhibitors of P2X7 receptor signaling significantly abrogated IL-1β release by human monocyte-derived macrophages treated with recombinant human SAA (9). Based on this finding, the authors proposed that SAA directly interacts with this receptor to stimulate NLRP3 activation (9). However, in the present study with endogenous, purified mouse SAA, we did not observe an inhibition of SAA-mediated IL-1β release with P2X7 receptor antagonists. The discrepancy with the previous report may be due to differences in properties between recombinant and purified native SAA. SAA is known to mediate ROS generation in neutrophils and Swiss 3T3 fibroblast cells (52, 53). A previous study indicated that mitochondrial ROS-dependent and -independent mechanisms play a part in SAA-mediated inflammasome activation (54). In the current study, we demonstrate that SAA enhances ROS generation in J774 cells and that inhibition of ROS by the cytosolic ROS scavenger, NAC, and mitochondrial ROS scavenger, mito-TEMPO cause a significant decrease in SAA-mediated IL-1β release (Fig. 5). Inhibiting cathepsin B activity potently abrogated IL-1β release by cells incubated with purified mouse SAA, consistent with previous results with recombinant SAA (9). These data suggest that SAA mediates NLRP3 inflammasome activation through a mechanism involving lysosomal degradation and protease release (5). Interestingly, cathepsin B is known to be involved in the proteolytic processing of SAA resulting in AA amyloid proteins (55). Whether cathepsin B-mediated proteolytic processing of SAA leads to NLRP3 inflammasome activation needs to be investigated.
We report a role for K+ efflux in SAA-mediated IL-1β release. Potassium efflux has been shown to be required for NLRP3 activation by various inflammasome stimuli (56, 57). Thus, it appears that SAA mediates inflammasome activation through multiple pathways. Whether these processes are interlinked or act independently is currently unclear. Evidence suggests that other NLRP3 activators induce lysosomal disruption, ROS generation, and K+ efflux, which apparently act in concert to ultimately lead to caspase-1 activation and IL-1β maturation (5). For example, in a study of the pathogenesis of osteoarthritis, hydroxyapatite crystal-stimulated IL-1β release was shown to require ROS generation, lysosomal protease release, and K+ efflux (58).
The majority of SAA in plasma is associated with HDL, a lipoprotein fraction generally thought to be anti-inflammatory (59, 60). During an acute inflammatory response SAA can be the major apolipoprotein on HDL (61). The relationship between the respective functions of SAA and HDL is complex. Although HDL enriched in SAA is thought to be impaired in its anti-inflammatory properties (62, 63), many of the proinflammatory effects of SAA have been attributed to lipid-free, and not HDL-associated SAA (41). In our study, we report that HDL completely masks SAA-mediated NLRP3 inflammasome priming and activation. HDL is known to inhibit inflammasome activation by other stimuli including cholesterol crystals, ATP, nigericin, silica, and alum (42) in THP-1 cells, a monocytic cell line that requires no priming for inflammasome activation. On the other hand, in our studies, HDL did not alter LPS/ATP-induced IL-1β secretion, even though there was a trend for HDL to decrease LPS-mediated IL-1β mRNA expression (Fig. 8, B and C). Thus, HDL does not appear to have a global effect on the ability of cells to respond to stimuli that provoke IL-1β release. Although the precise mechanism by which HDL protects cells from inflammasome activation has not been clearly delineated, we noted that the effect of HDL to suppress SAA-stimulated IL-1β release was accompanied by a significant reduction in ROS (Fig. 8E). The major apolipoproteins of HDL, apoA-I and apoA-II, were ineffective in blocking SAA's effects (Fig. 7F). Lipid-free apoA-I and apoA-II were also incapable of mimicking SAA's ability to trigger IL-1β release (Fig. 7F).
At this time it is unclear how HDL masks SAA's biological effects. One possibility is there are bioactive motifs on SAA that are masked when SAA is bound to lipoprotein particles. Alternatively, differences in the conformation of SAA in the lipid-free versus HDL-bound form may be critical for its biological activity. Lipid-free SAA possesses a random coil-like conformation at 37 °C (64, 65), whereas HDL binding stabilizes SAA into an α-helical conformation (64–66). SAA-induced signaling via the formyl peptide (67), CD36 (26), and Toll-like receptors (41) is apparent only with lipid-free SAA, whereas both lipid-free and HDL-bound SAA interact with SR-BI (25). It is possible that once liberated from HDL, SAA undergoes structural changes or becomes susceptible to limited proteolysis and the modified protein triggers inflammasome activation. Our study, consistent with other reports (68, 69), indicates that HDL does not prevent cellular uptake of SAA (Fig. 7, G and H). According to one report, HDL-SAA is internalized through a clathrin-dependent endocytic pathway and then trafficked to lysosomes where it accumulates as SAA aggregates or amyloid deposits (69). We did not observe localization of SAA in lysosomes in our studies, which involved 4-h treatments (Fig. 7, G and H). Amyloid fibril formation is believed to occur when the influx of SAA into cells exceeds their proteolytic capacity (69). Amyloid fibrils can cause disruption of lysosomal membranes (69), which may trigger inflammasome activation. However, it seems unlikely that SAA fibril formation is responsible for triggering inflammasome activation in our study, as HDL-bound SAA is capable of forming intracellular fibrils (68, 69).
Under homeostatic conditions, liver-derived SAA is unlikely to trigger inflammasome activation because virtually all of it is bound to HDL. Indeed, transgenic mice with inducible, liver-specific SAA expression do not exhibit increased inflammation despite very high levels of plasma SAA (>1 mg/ml) (70). However, in the right context SAA might be released from HDL in tissues to exert local pro-inflammatory effects. Our finding that AngII-induced increases in IL-1β are significantly blunted in mice lacking acute-phase SAAs underscores the fact that SAA has pro-inflammatory effects in pathological settings, such as experimental AAA. SAA concentrations can be dramatically elevated in tissues due to local injury, infection, or inflammation (15, 71–74). The relative contribution of locally produced SAA versus HDL-bound SAA that has deposited in tissues at the site of injury or inflammation is not known, and merits further investigation. Although inflammasome activation represents a host defense to pathogens and host-derived danger signals, inappropriate or excessive activation results in tissue injury (75). Thus, to protect the host from widespread tissue damage, HDL may serve as a transporter and shield to prevent excessive SAA-mediated systemic inflammasome activation.
Materials and methods
Animals
Targeted deletion of the saa1.1 and saa2.1 genes in C57BL/6 mice was performed by InGenious Targeting Laboratory, Inc. as described earlier (76). The mice were then crossed with apoE−/− mice to generate apoE−/− mice lacking acute-phase SAAs (apoE−/− × SAA1.1/2.1-DKO) (15). For AngII infusion studies, animals were housed in microisolator cages and provided normal rodent diet and water ad libitum. AngII (1,000 ng kg−1 min−1; Sigma) or saline was administered via Alzet osmotic minipumps (model 2004; Durect Corporation) to 12–14–week-old male mice anesthetized with 50 μl of ketamine/xylazine mixture, 90 and 10 mg/ml, respectively (Ketamine, Butler Schein; Xylazine, Lloyd Laboratories) as previously described (77). Plasma was collected 28 days after pump implantation for IL-1β determinations. AAV vectors (serotype 8) were produced by the Viral Vector Core at the University of Pennsylvania. The AAV-SAA1.1 vector contains an insert encoding mouse SAA1.1 (GenBankTM accession NM_011314). Empty AAV vector (AAV-null) was used as control. For the AAV study, apoE−/− × SAA1.1/2.1 DKO mice were injected i.p. with 1 × 10e11 particles of AAV-SAA1.1 or AAV-null in a total volume of 200 μl of sterile saline. Blood samples were collected from the retroorbital sinus at baseline (prior to AAV injections) and 14 days later to confirm that blood SAA levels were elevated in AAV-SAA1.1-treated mice (typically 300–500 μg/ml). AngII pumps were implanted on day 15 as described above. Plasma IL-1β was determined after 28-day AngII infusion. All studies were performed with the approval of the University of Kentucky Institutional Animal Care and Use Committee.
HDL isolation
HDLs (d = 1.063–1.21 g/ml) were isolated from C57BL/6 mice and healthy human volunteers by density gradient ultracentrifugation, dialyzed against 150 mmol/liter of NaCl, 0.01% (w/v) EDTA, sterile filtered, and stored under argon gas at 4 °C (18). Protein concentrations were determined by the method of Lowry et al. (78). The human plasma was collected under an IRB-approved protocol.
SAA purification
hSAA and mouse SAA were purified as described earlier (79). Briefly, human and mouse HDL were isolated by serial density gradient ultracentrifugation from plasma of patients 24 h after cardiac surgery or mice injected with lipopolysaccharide (50 μg/mouse), respectively. The human plasma was collected under an IRB-approved protocol. The HDLs (∼20 mg of protein) were then delipidated, and the delipidated proteins were separated by gel filtration on a Sephacryl S-200 column in a buffer containing 7 mol/liter of urea, 20 mmol/liter of Tris, 150 mmol/liter of NaCl, 1 mmol/liter of EDTA, pH 8. SAA-containing fractions were identified by SDS-PAGE, pooled, and dialyzed against 2 mmol/liter of Tris, 15 mmol/liter of NaCl, and 0.1 mmol/liter of EDTA, pH 8.4, prior to 10-fold concentration. LPS contamination in purified SAA preparations was below the level of detection (<0.075 EU/μg; ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit, catalog number L00350C, GenScript).
Cell cultures and treatments
Murine J774.2 macrophage-like cells were obtained from Sigma and maintained in complete medium (Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum, 100 units/ml of penicillin/streptomycin, 2 mm l-glutamine). For experiments with SAA, J774.2 cells were incubated with 5–50 μg/ml of purified mouse or human SAA in serum-free DMEM containing 0.2% fatty acid-free BSA (SFM) for 8–24 h. For experiments with SAA and HDL, SAA and HDL were co-incubated at a ratio of 1:2.8 (protein:protein) for 1 h at room temperature prior to treating the cells. For LPS (Innaxon, catalog number IAX-100-012-5001) and ATP (Sigma) treatment, the cells were incubated with LPS (0.5 μg/ml) for 3 h in SFM followed by treatment with ATP (3 mm) for 45 min. For LPS and alum (Imject alum adjuvant; a mixture of aluminum hydroxide and magnesium hydroxide, Pierce) treatment, the cells were incubated with LPS (0.5 μg/ml) for 3 h in SFM followed by treatment with alum (200 μg/ml) for 4 h. Cathepsin B inhibitor, CA-074-Me (Sigma), caspase-1 inhibitor, Z-YVAD-fmk (Vergent Bioscience), cathepsin L inhibitor (Calbiochem), cytochalasin D (Sigma), glyburide (Sigma), and the P2X7R antagonists, A-438079 hydrochloride (Sigma) and AZ-10606120 (TOCRIS) were dissolved in DMSO, and used at the indicated concentrations. Mito-TEMPO (Santa Cruz Biotechnology) was dissolved in water and used at the indicated concentration. NAC (Sigma) and ATP were dissolved directly in SFM, and the pH was adjusted to 7.4 before treating the cells.
Isolation and preparation of bone marrow–derived macrophages
BMDMs were isolated and cultured as previously described (80). Briefly, femurs and tibias were removed from mice, cleaned of surrounding muscles, and then washed in PBS followed by complete DMEM. After cutting through the epiphysis at both ends, the femur and tibia were slowly flushed with complete DMEM using a 23-gauge needle and 5-ml syringe, which was then passed through a 70-μm cell strainer. After dispersing the cells by repeatedly passing through an 18-gauge needle, the cells were centrifuged at 850 rpm for 5 min. Cell pellets were re-suspended in complete DMEM containing 25 ng/ml of macrophage colony-stimulating factor (PeproTech) and plated in a 24-well dish. After 3 days, media was changed to fresh complete DMEM with 25 ng/ml of macrophage colony-stimulating factor; the differentiated cells were used after 6 additional days of culture.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from cultured cells according to the manufacturer's instructions (RNeasy® Mini Kit, Qiagen). RNA samples were incubated with DNase I (Qiagen) for 15 min at room temperature prior to reverse transcription. RNA from cultured cells (0.2–0.5 μg) was reverse transcribed into cDNA using the Reverse Transcription System (Applied Biosystems). After 4-fold dilution, 5 μl was used as a template for real-time RT-PCR. Amplification was done for 40 cycles using Power SYBR Green PCR master mix kit (Applied Biosystems). Quantification of mRNA was performed using the ΔΔCT method and normalized to GAPDH. Primer sequences are as follows: GAPDH (NM_008084), 5′-CTCATGACCACAGTCCATGCCA-3′, 5′-GGATGACCTTGCCCACAGCCTT-3′; IL-1β (NM 008361), 5′-GTCACAAGAAACCATGGCACAT-3′, 5′-GCCCATCAGAGGCAAGGA-3′; and NLRP3 (XR_388400), 5′-TGCTCTTCACTGCTATCAAGCCCT-3′, 5′-ACAAGCCTTTGCTCCAGACCCTAT-3′.
Fluorescent microscopy
FITC-labeled SAA was prepared according to the manufacturer's instructions (Invitrogen) using 50 μg each of recombinant mouse SAA1.1 and -2.1 (R&D Systems) reconstituted in 100 μl of PBS. Unbound FITC was removed by washing the bound material with cold PBS on a 3K concentrator (Millipore). J774 cells were seeded on glass coverslips and grown until confluent. Cells were then incubated with FITC-labeled free SAA (0.5 μg/ml) or FITC-labeled SAA bound to HDL (1:2.8 SAA to HDL) in SFM for 40 min. The cells were fixed for 30 min with 4% (v/v) paraformaldehyde, followed by incubation for 2 h at 25 °C with primary antibody for lysosomal marker anti-mouse LAMP-1 (CD107a- eBioscience) and Alexa 568-labeled anti-mouse secondary antibody (Thermo Fisher Scientific). The cells were washed extensively and mounted on slides using Vectashield mounting medium with DAPI (Vector Laboratories). Confocal microscopy was performed at the University of Kentucky Imaging Facility using A1R+ Resonant Scanning Confocal microscope (Nikon).
Western blotting
Cell lysates were prepared from J774 cells after treatment with or without SAA (50 μg/ml). Aliquots corresponding to 10 μg of protein were separated on a 4–20% polyacrylamide gradient gel (Bio-Rad) and immunoblotted with anti-caspase-1 (p20) mouse antibody (Adipogen Life Sciences).
SAA and IL-1β measurements
Plasma SAA concentrations were determined using a mouse ELISA kit (Tridelta Development Ltd.). Concentrations of IL-1β in plasma and cell culture media were determined using a mouse IL-1β ELISA kit (R&D Systems). For BMDMs, IL-1β in conditioned media was normalized to total cell protein to account for potential differences in seeding density.
Intracellular caspase-1 activation assay
Active caspase-1 was visualized using a FAM-FLICA capase-1 assay kit (FLICA®; ImmunoChemistry Technologies) according to the manufacturer's guidelines. Stained cells were visualized by fluorescent microscopy (Nikon Eclipse 80i microscope, Nikon Instruments).
Cellular ROS assay
Cellular ROS levels were determined using a cell permeant reagent DCFDA, a fluorogenic dye that measures hydroxyl, peroxyl, and other ROS activity within the cell (Abcam) following the manufacturer's guidelines.
Statistical analysis
Data are expressed as mean ± S.E. Results were analyzed by Student's t test or one-way analysis of variance followed by Bonferroni's post test. Values of p < 0.05 were considered statistically significant.
Author contributions
P. S. and N. R. W. conceptualization; P. S. data curation; P. S. and M. C. D. B. formal analysis; P. S. and N. R. W. validation; P. S. visualization; P. S. and M. C. D. B. methodology; P. S. writing-original draft; P. S. project administration; P. S., M. C. D. B., and N. R. W. writing-review and editing; M. C. D. B. and N. R. W. resources; N. R. W. supervision; N. R. W. funding acquisition; N. R. W. investigation.
Supplementary Material
Acknowledgments
We thank Dr. Frederick C. De Beer for helpful discussions and Andrea Trumbauer and Victoria Noffsinger for excellent technical assistance.
This work was supported by National Institutes of Health Grant HL134731 (to N. R. W) and Institutional Development Award (IDeA), National Institute of Health NIGMS Grant P20 GM103527. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- IL-1β
- interleukin-1β
- NF-κB
- nuclear factor κB
- NLRP3
- Nod-like receptor protein 3
- hSSA
- human SAA
- SAA
- serum amyloid A
- HDL
- high-density lipoprotein
- AngII
- angiotension II
- AAV
- adeno-associated virus
- BMDM
- bone marrow–derived macrophage
- ROS
- reactive oxygen species
- DCFDA
- 2′,7′-dichlorofluorescein diacetate
- NAC
- N-acetylcysteine
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- LPS
- lipopolysaccharide
- DMEM
- Dulbecco's modified Eagle's medium
- qRT
- quantitative RT
- mito-TEMPO
- (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride
- SFM
- serum-free medium.
References
- 1. Dinarello C. A., Simon A., and van der Meer J. W. (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug. Discov. 11, 633–652 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo H., Callaway J. B., and Ting J. P. (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larsen C. M., Faulenbach M., Vaag A., Vølund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., and Donath M. Y. (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 10.1056/NEJMoa065213 [DOI] [PubMed] [Google Scholar]
- 4. Ridker P. M., Everett B. M., Thuren T., MacFadyen J. G., Chang W. H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S. D., Kastelein J. J. P., Cornel J. H., Pais P., Pella D., Genest J., et al. (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 5. Jo E. K., Kim J. K., Shin D. M., and Sasakawa C. (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol. Immunol. 13, 148–159 10.1038/cmi.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel M. N., Carroll R. G., Galván-Pena S., Mills E. L., Olden R., Triantafilou M., Wolf A. I., Bryant C. E., Triantafilou K., and Masters S. L. (2017) Inflammasome priming in sterile inflammatory disease. Trends Mol. Med. 23, 165–180 10.1016/j.molmed.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 7. Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., et al. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ather J. L., Ckless K., Martin R., Foley K. L., Suratt B. T., Boyson J. E., Fitzgerald K. A., Flavell R. A., Eisenbarth S. C., and Poynter M. E. (2011) Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol. 187, 64–73 10.4049/jimmunol.1100500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niemi K., Teirilä L., Lappalainen J., Rajamäki K., Baumann M. H., Öörni K., Wolff H., Kovanen P. T., Matikainen S., and Eklund K. K. (2011) Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J. Immunol. 186, 6119–6128 10.4049/jimmunol.1002843 [DOI] [PubMed] [Google Scholar]
- 10. Migita K., Izumi Y., Jiuchi Y., Kozuru H., Kawahara C., Nakamura M., Nakamura T., Agematsu K., Masumoto J., Yasunami M., Kawakami A., and Eguchi K. (2014) Serum amyloid A induces NLRP-3-mediated IL-1β secretion in neutrophils. PLoS ONE 9, e96703 10.1371/journal.pone.0096703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kluve-Beckerman B., Drumm M. L., and Benson M. D. (1991) Nonexpression of the human serum amyloid A three (SAA3) gene. DNA Cell Biol. 10, 651–661 10.1089/dna.1991.10.651 [DOI] [PubMed] [Google Scholar]
- 12. de Beer M. C., Yuan T., Kindy M. S., Asztalos B. F., Roheim P. S., and de Beer F. C. (1995) Characterization of constitutive human serum amyloid A protein (SAA4) as an apolipoprotein. J. Lipid Res. 36, 526–534 [PubMed] [Google Scholar]
- 13. Uhlar C. M., and Whitehead A. S. (1999) Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 265, 501–523 10.1046/j.1432-1327.1999.00657.x [DOI] [PubMed] [Google Scholar]
- 14. Eklund K. K., Niemi K., and Kovanen P. T. (2012) Immune functions of serum amyloid A. Crit. Rev. Immunol. 32, 335–348 10.1615/CritRevImmunol.v32.i4.40 [DOI] [PubMed] [Google Scholar]
- 15. Webb N. R., De Beer M. C., Wroblewski J. M., Ji A., Bailey W., Shridas P., Charnigo R. J., Noffsinger V. P., Witta J., Howatt D. A., Balakrishnan A., Rateri D. L., Daugherty A., and De Beer F. C. (2015) Deficiency of endogenous acute-phase serum amyloid A protects apoE−/− mice from angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 35, 1156–1165 10.1161/ATVBAHA.114.304776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meek R. L., Urieli-Shoval S., and Benditt E. P. (1994) Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc. Natl. Acad. Sci. U.S.A. 91, 3186–3190 10.1073/pnas.91.8.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connolly M., Marrelli A., Blades M., McCormick J., Maderna P., Godson C., Mullan R., FitzGerald O., Bresnihan B., Pitzalis C., Veale D. J., and Fearon U. (2010) Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J. Immunol. 184, 6427–6437 10.4049/jimmunol.0902941 [DOI] [PubMed] [Google Scholar]
- 18. Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., and de Beer F. C. (1986) Serum amyloid A-containing human high density lipoprotein 3: density, size, and apolipoprotein composition. J. Biol. Chem. 261, 9644–9651 [PubMed] [Google Scholar]
- 19. Ancsin J. B., and Kisilevsky R. (1999) The heparin/heparan sulfate-binding site on apo-serum amyloid A: implications for the therapeutic intervention of amyloidosis. J. Biol. Chem. 274, 7172–7181 10.1074/jbc.274.11.7172 [DOI] [PubMed] [Google Scholar]
- 20. Preciado-Patt L., Levartowsky D., Prass M., Hershkoviz R., Lider O., and Fridkin M. (1994) Inhibition of cell adhesion to glycoproteins of the extracellular matrix by peptides corresponding to serum amyloid A: toward understanding the physiological role of an enigmatic protein. Eur. J. Biochem. 223, 35–42 10.1111/j.1432-1033.1994.tb18963.x [DOI] [PubMed] [Google Scholar]
- 21. Lee H. Y., Kim S. D., Shim J. W., Lee S. Y., Lee H., Cho K. H., Yun J., and Bae Y. S. (2008) Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J. Immunol. 181, 4332–4339 10.4049/jimmunol.181.6.4332 [DOI] [PubMed] [Google Scholar]
- 22. Lee H. Y., Kim S. D., Shim J. W., Kim H. J., Yun J., Baek S. H., Kim K., and Bae Y. S. (2010) A pertussis toxin sensitive G-protein-independent pathway is involved in serum amyloid A-induced formyl peptide receptor 2-mediated CCL2 production. Exp. Mol. Med. 42, 302–309 10.3858/emm.2010.42.4.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng N., He R., Tian J., Ye P. P., and Ye R. D. (2008) Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 181, 22–26 10.4049/jimmunol.181.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandri S., Rodriguez D., Gomes E., Monteiro H. P., Russo M., and Campa A. (2008) Is serum amyloid A an endogenous TLR4 agonist? J. Leukoc. Biol. 83, 1174–1180 10.1189/jlb.0407203 [DOI] [PubMed] [Google Scholar]
- 25. Cai L., de Beer M. C., de Beer F. C., and van der Westhuyzen D. R. (2005) Serum amyloid A is a ligand for scavenger receptor class B type I and inhibits high density lipoprotein binding and selective lipid uptake. J. Biol. Chem. 280, 2954–2961 10.1074/jbc.M411555200 [DOI] [PubMed] [Google Scholar]
- 26. Baranova I. N., Bocharov A. V., Vishnyakova T. G., Kurlander R., Chen Z., Fu D., Arias I. M., Csako G., Patterson A. P., and Eggerman T. L. (2010) CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J. Biol. Chem. 285, 8492–8506 10.1074/jbc.M109.007526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnston W. F., Salmon M., Pope N. H., Meher A., Su G., Stone M. L., Lu G., Owens G. K., Upchurch G. R. Jr., and Ailawadi G. (2014) Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130, S51–S59 10.1161/CIRCULATIONAHA.113.006800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston W. F., Salmon M., Su G., Lu G., Stone M. L., Zhao Y., Owens G. K., Upchurch G. R. Jr., and Ailawadi G. (2013) Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 33, 294–304 10.1161/ATVBAHA.112.300432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Usui F., Shirasuna K., Kimura H., Tatsumi K., Kawashima A., Karasawa T., Yoshimura K., Aoki H., Tsutsui H., Noda T., Sagara J., Taniguchi S., and Takahashi M. (2015) Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 35, 127–136 10.1161/ATVBAHA.114.303763 [DOI] [PubMed] [Google Scholar]
- 30. Sun W., Pang Y., Liu Z., Sun L., Liu B., Xu M., Dong Y., Feng J., Jiang C., Kong W., and Wang X. (2015) Macrophage inflammasome mediates hyperhomocysteinemia-aggravated abdominal aortic aneurysm. J. Mol. Cell Cardiol. 81, 96–106 10.1016/j.yjmcc.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 31. Roberts R. L., Van Rij A. M., Phillips L. V., Young S., McCormick S. P., Merriman T. R., and Jones G. T. (2011) Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis 218, 123–126 10.1016/j.atherosclerosis.2011.04.043 [DOI] [PubMed] [Google Scholar]
- 32. Wu D., Ren P., Zheng Y., Zhang L., Xu G., Xie W., Lloyd E. E., Zhang S., Zhang Q., Curci J. A., Coselli J. S., Milewicz D. M., Shen Y. H., and LeMaire S. A. (2017) NLRP3 (nucleotide oligomerization domain-like receptor family, pyrin domain containing 3)-caspase-1 inflammasome degrades contractile proteins: implications for aortic biomechanical dysfunction and aneurysm and dissection formation. Arterioscler. Thromb. Vasc. Biol. 37, 694–706 10.1161/ATVBAHA.116.307648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Juvonen J., Surcel H. M., Satta J., Teppo A. M., Bloigu A., Syrjälä H., Airaksinen J., Leinonen M., Saikku P., and Juvonen T. (1997) Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 17, 2843–2847 10.1161/01.ATV.17.11.2843 [DOI] [PubMed] [Google Scholar]
- 34. Tannock L. R., De Beer M. C., Ji A., Shridas P., Noffsinger V. P., den Hartigh L., Chait A., De Beer F. C., and Webb N. R. (2017) Serum amyloid A3 is a high-density lipoprotein-associated acute phase protein. J. Lipid Res. 59, 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia-Calvo M., Peterson E. P., Leiting B., Ruel R., Nicholson D. W., and Thornberry N. A. (1998) Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273, 32608–32613 10.1074/jbc.273.49.32608 [DOI] [PubMed] [Google Scholar]
- 36. Gombault A., Baron L., and Couillin I. (2012) ATP release and purinergic signaling in NLRP3 inflammasome activation. Front. Immunol. 3, 414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tschopp J., and Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 10.1038/nri2725 [DOI] [PubMed] [Google Scholar]
- 38. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., and Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 10.1038/ni.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rajamäki K., Lappalainen J., Oörni K., Välimäki E., Matikainen S., Kovanen P. T., and Eklund K. K. (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 10.1371/journal.pone.0011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kluve-Beckerman B., Liepnieks J. J., Wang L., and Benson M. D. (1999) A cell culture system for the study of amyloid pathogenesis: amyloid formation by peritoneal macrophages cultured with recombinant serum amyloid A. Am. J. Pathol 155, 123–133 10.1016/S0002-9440(10)65107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim M. H., de Beer M. C., Wroblewski J. M., Webb N. R., and de Beer F. C. (2013) SAA does not induce cytokine production in physiological conditions. Cytokine 61, 506–512 10.1016/j.cyto.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thacker S. G., Zarzour A., Chen Y., Alcicek M. S., Freeman L. A., Sviridov D. O., Demosky S. J. Jr., and Remaley A. T. (2016) High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 149, 306–319 10.1111/imm.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Witting P. K., Song C., Hsu K., Hua S., Parry S. N., Aran R., Geczy C., and Freedman S. B. (2011) The acute-phase protein serum amyloid A induces endothelial dysfunction that is inhibited by high-density lipoprotein. Free Radic. Biol. Med. 51, 1390–1398 10.1016/j.freeradbiomed.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 44. Song C., Hsu K., Yamen E., Yan W., Fock J., Witting P. K., Geczy C. L., and Freedman S. B. (2009) Serum amyloid A induction of cytokines in monocytes/macrophages and lymphocytes. Atherosclerosis 207, 374–383 10.1016/j.atherosclerosis.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 45. Amstad P. A., Yu G., Johnson G. L., Lee B. W., Dhawan S., and Phelps D. J. (2001) Detection of caspase activation in situ by fluorochrome-labeled caspase inhibitors. BioTechniques 31, 608–610, 612, 614, passim [DOI] [PubMed] [Google Scholar]
- 46. van den Brand B., Waterborg C., van den Berg W., and van de Loo F. (2013) Is the serum amyloid A we use really serum amyloid A? Comment on the article by Connolly et al. Arthritis Rheum. 65, 283–284 10.1002/art.37737 [DOI] [PubMed] [Google Scholar]
- 47. Björkman L., Raynes J. G., Shah C., Karlsson A., Dahlgren C., and Bylund J. (2010) The proinflammatory activity of recombinant serum amyloid A is not shared by the endogenous protein in the circulation. Arthritis Rheum. 62, 1660–1665 10.1002/art.27440 [DOI] [PubMed] [Google Scholar]
- 48. Srinivasan S., Patke S., Wang Y., Ye Z., Litt J., Srivastava S. K., Lopez M. M., Kurouski D., Lednev I. K., Kane R. S., and Colón W. (2013) Pathogenic serum amyloid A 1.1 shows a long oligomer-rich fibrillation lag phase contrary to the highly amyloidogenic non-pathogenic SAA2.2. J. Biol. Chem. 288, 2744–2755 10.1074/jbc.M112.394155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tam S. P., Flexman A., Hulme J., and Kisilevsky R. (2002) Promoting export of macrophage cholesterol: the physiological role of a major acute-phase protein, serum amyloid A 2.1. J. Lipid Res. 43, 1410–1420 10.1194/jlr.M100388-JLR200 [DOI] [PubMed] [Google Scholar]
- 50. Chen M., Zhou H., Cheng N., Qian F., and Ye R. D. (2014) Serum amyloid A1 isoforms display different efficacy at Toll-like receptor 2 and formyl peptide receptor 2. Immunobiology 219, 916–923 10.1016/j.imbio.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., and Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Björkman L., Karlsson J., Karlsson A., Rabiet M. J., Boulay F., Fu H., Bylund J., and Dahlgren C. (2008) Serum amyloid A mediates human neutrophil production of reactive oxygen species through a receptor independent of formyl peptide receptor like-1. J. Leukoc. Biol. 83, 245–253 10.1189/jlb.0607-408 [DOI] [PubMed] [Google Scholar]
- 53. Hatanaka E., Dermargos A., Armelin H. A., Curi R., and Campa A. (2011) Serum amyloid A induces reactive oxygen species (ROS) production and proliferation of fibroblast. Clin. Exp. Immunol. 163, 362–367 10.1111/j.1365-2249.2010.04300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jabaut J., Ather J. L., Taracanova A., Poynter M. E., and Ckless K. (2013) Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1β secretion in association with alterations in cellular redox and energy status. Free Radic. Biol. Med. 60, 233–245 10.1016/j.freeradbiomed.2013.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rocken C., Menard R., Bühling F., Vöckler S., Raynes J., Stix B., Krüger S., Roessner A., and Kähne T. (2005) Proteolysis of serum amyloid A and AA amyloid proteins by cysteine proteases: cathepsin B generates AA amyloid proteins and cathepsin L may prevent their formation. Ann. Rheum. Dis. 64, 808–815 10.1136/ard.2004.030429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., and Tschopp J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 10.1038/sj.cdd.4402195 [DOI] [PubMed] [Google Scholar]
- 57. Franchi L., Kanneganti T. D., Dubyak G. R., and Núñez G. (2007) Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 282, 18810–18818 10.1074/jbc.M610762200 [DOI] [PubMed] [Google Scholar]
- 58. Jin C., Frayssinet P., Pelker R., Cwirka D., Hu B., Vignery A., Eisenbarth S. C., and Flavell R. A. (2011) NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. U.S.A. 108, 14867–14872 10.1073/pnas.1111101108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., and Fogelman A. M. (2004) Antiinflammatory properties of HDL. Circ. Res. 95, 764–772 10.1161/01.RES.0000146094.59640.13 [DOI] [PubMed] [Google Scholar]
- 60. Navab M., Yu R., Gharavi N., Huang W., Ezra N., Lotfizadeh A., Anantharamaiah G. M., Alipour N., Van Lenten B. J., Reddy S. T., and Marelli D. (2007) High-density lipoprotein: antioxidant and anti-inflammatory properties. Curr. Atheroscler. Rep. 9, 244–248 10.1007/s11883-007-0026-3 [DOI] [PubMed] [Google Scholar]
- 61. Lindhorst E., Young D., Bagshaw W., Hyland M., and Kisilevsky R. (1997) Acute inflammation, acute phase serum amyloid A and cholesterol metabolism in the mouse. Biochim. Biophys. Acta 1339, 143–154 10.1016/S0167-4838(96)00227-0 [DOI] [PubMed] [Google Scholar]
- 62. Han C. Y., Tang C., Guevara M. E., Wei H., Wietecha T., Shao B., Subramanian S., Omer M., Wang S., O'Brien K. D., Marcovina S. M., Wight T. N., Vaisar T., de Beer M. C., de Beer F. C., et al. (2016) Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Invest. 126, 796 10.1172/JCI86401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tölle M., Huang T., Schuchardt M., Jankowski V., Prüfer N., Jankowski J., Tietge U. J., Zidek W., and van der Giet M. (2012) High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc. Res. 94, 154–162 10.1093/cvr/cvs089 [DOI] [PubMed] [Google Scholar]
- 64. Patke S., Srinivasan S., Maheshwari R., Srivastava S. K., Aguilera J. J., Colón W., and Kane R. S. (2013) Characterization of the oligomerization and aggregation of human serum amyloid A. PLoS ONE 8, e64974 10.1371/journal.pone.0064974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jayaraman S., Haupt C., and Gursky O. (2015) Thermal transitions in serum amyloid A in solution and on the lipid: implications for structure and stability of acute-phase HDL. J. Lipid Res. 56, 1531–1542 10.1194/jlr.M059162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McCubbin W. D., Kay C. M., Narindrasorasak S., and Kisilevsky R. (1988) Circular-dichroism studies on two murine serum amyloid A proteins. Biochem. J. 256, 775–783 10.1042/bj2560775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. He R., Sang H., and Ye R. D. (2003) Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 101, 1572–1581 10.1182/blood-2002-05-1431 [DOI] [PubMed] [Google Scholar]
- 68. Kluve-Beckerman B., Manaloor J., and Liepnieks J. J. (2001) Binding, trafficking and accumulation of serum amyloid A in peritoneal macrophages. Scand. J. Immunol. 53, 393–400 10.1046/j.1365-3083.2001.00879.x [DOI] [PubMed] [Google Scholar]
- 69. Claus S., Meinhardt K., Aumüller T., Puscalau-Girtu I., Linder J., Haupt C., Walther P., Syrovets T., Simmet T., and Fändrich M. (2017) Cellular mechanism of fibril formation from serum amyloid A1 protein. EMBO Rep. 18, 1352–1366 10.15252/embr.201643411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simons J. P., Al-Shawi R., Ellmerich S., Speck I., Aslam S., Hutchinson W. L., Mangione P. P., Disterer P., Gilbertson J. A., Hunt T., Millar D. J., Minogue S., Bodin K., Pepys M. B., and Hawkins P. N. (2013) Pathogenetic mechanisms of amyloid A amyloidosis. Proc. Natl. Acad. Sci. U.S.A. 110, 16115–16120 10.1073/pnas.1306621110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anderberg R. J., Meek R. L., Hudkins K. L., Cooney S. K., Alpers C. E., Leboeuf R. C., and Tuttle K. R. (2015) Serum amyloid A and inflammation in diabetic kidney disease and podocytes. Lab. Invest. 95, 250–262 10.1038/labinvest.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ren S. W., Qi X., Jia C. K., and Wang Y. Q. (2014) Serum amyloid A and pairing formyl peptide receptor 2 are expressed in corneas and involved in inflammation-mediated neovascularization. Int. J. Ophthalmol. 7, 187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. López-Campos J. L., Calero C., Rojano B., López-Porras M., Sáenz-Coronilla J., Blanco A. I., Sánchez-Lopez V., Tobar D., Montes-Worboys A., and Arellano E. (2013) C-reactive protein and serum amyloid a overexpression in lung tissues of chronic obstructive pulmonary disease patients: a case-control study. Int. J. Med. Sci. 10, 938–947 10.7150/ijms.6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Calero C., Arellano E., Lopez-Villalobos J. L., Sánchez-López V., Moreno-Mata N., and López-Campos J. L. (2014) Differential expression of C-reactive protein and serum amyloid A in different cell types in the lung tissue of chronic obstructive pulmonary disease patients. BMC Pulm. Med. 14, 95 10.1186/1471-2466-14-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Man S. M., and Kanneganti T. D. (2015) Regulation of inflammasome activation. Immunol. Rev. 265, 6–21 10.1111/imr.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Beer M. C., Webb N. R., Wroblewski J. M., Noffsinger V. P., Rateri D. L., Ji A., van der Westhuyzen D. R., and de Beer F. C. (2010) Impact of serum amyloid A on high density lipoprotein composition and levels. J. Lipid Res. 51, 3117–3125 10.1194/jlr.M005413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Daugherty A., and Cassis L. (1999) Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann. N.Y. Acad. Sci. 892, 108–118 10.1111/j.1749-6632.1999.tb07789.x [DOI] [PubMed] [Google Scholar]
- 78. Lowry O. H., Rosebrough N. J., Farr A. L., and Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 79. Strachan A. F., Shephard E. G., Bellstedt D. U., Coetzee G. A., van der Westhuyzen D. R., and de Beer F. C. (1989) Human serum amyloid A protein: behaviour in aqueous and urea-containing solutions and antibody production. Biochem. J. 263, 365–370 10.1042/bj2630365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pannell M., Labuz D., Celik M. Ö., Keye J., Batra A., Siegmund B., and Machelska H. (2016) Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflammation 13, 262 10.1186/s12974-016-0735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.