Abstract
Mice harboring a particular allele of the human brain-derived neurotropic factor (BDNFM/M mice) develop extreme obesity and insulin resistance when fed a high-fat diet. The underlying mechanisms of this genetic risk factor for obesity are unclear. In the current issue of JBC, Yang et al. report that pharmacological inhibition of integral membrane protein CD36 significantly reduces body weight gain and improves glucose tolerance in BDNFM/M mice. Targeting CD36 may therefore be a promising strategy to improve metabolic dysfunctions and normalize risk factors in obese individuals.
Introduction
Obesity is a major health risk that can contribute to life-threatening metabolic complications including dyslipidemia and type 2 diabetes (1). The pathogenesis of obesity involves complex interactions between environmental and genetic factors, leading to dysregulated energy balance. Genetic variants, including single-nucleotide polymorphisms (SNPs),2 account for 40–70% of the heritability of body mass index (BMI) (2). One such SNP that is associated with body weight regulation is a mutation in the gene for brain-derived neurotrophic factor (BDNF).
BDNF plays a critical role in nervous system development and function, including the regulation of energy intake and expenditure (3). One common polymorphism in the BDNF gene is a substitution of a valine with a methionine at codon 66 (Val66Met SNP) (4). The Val66Met allele has been associated with several disorders, including early seizures, obsessive-compulsive disorder, eating disorders, and obesity in humans (5).
Yang et al. (6) used a unique mouse model containing the human BDNF Val66Met variant and found that, consistent with observations in humans, BDNFM/M mice fed a normal diet had significantly increased body weight relative to BDNFV/V controls by 6 weeks of age. When subsequently fed a high-fat diet for 8 weeks, the BDNFM/M mice became extremely obese with elevated fasting glucose levels and impaired glucose tolerance, implying the development of insulin resistance. The authors specifically investigated the connection between these phenotypes and the expression of integral membrane protein CD36, a multifunctional receptor with documented links to obesity-associated metabolic disorders. They found that, compared with controls, BDNFM/M mice had significantly increased levels of CD36 mRNA and protein, including the soluble form (sCD36), which is a marker of insulin resistance in diabetes (7). Thus, changes in BDNF were correlated with changes in CD36 and associated metabolic phenotypes.
CD36, a class B scavenger receptor, functions as a fatty acid transporter to promote fatty acid uptake. CD36 is thought to mediate cross-talk between adipocytes and macrophages in obese mice by facilitating cytokine secretion from macrophages (8). To identify the role of CD36 in the development of obesity in BDNFM/M mice, Yang et al. (6) used salvianolic acid B (SAB), a specific CD36 antagonist, in experiments both in vitro and in vivo (9). Although chronic administration of SAB into diet-induced obese (DIO) WT mice had no effect on total body weight during the experimental period (8 weeks), it significantly reduced the accumulation of visceral fat and improved glucose tolerance relative to DIO mice receiving the vehicle. Importantly, these beneficial effects were abolished in CD36 knockout mice, further confirming that SAB counteracts insulin resistance and visceral obesity through its effect on CD36 function.
Yang et al. (6) then asked whether the inhibition of CD36 by SAB would provide greater benefits in the obesity-prone BDNFM/M mice. As predicted, relative to what occurred in vehicle-treated DIO BDNFV/V mice, chronic SAB infusion significantly decreased weight gain, reduced lipid accumulation in both adipose tissue and liver, and improved insulin sensitivity in BDNFM/M mice. SAB treatment also significantly decreased the expression of genes for CD36, F4/80, and monocyte chemoattractant protein-1 (MCP-1) in adipose tissue of the DIO BDNFM/M mice, suggesting that SAB treatment counteracts obesity-induced macrophage infiltration and inflammation in adipose tissue.
These results are very informative and raise a number of questions. First, although Yang et al. (6) clearly documented increased CD36 gene expression in BDNFM/M mice, what is the molecular mechanism mediating this effect of the BDNF variant? Second, because BDNF exerts its catabolic actions mainly within the hypothalamus, where CD36 is also expressed (10), is the influence of the BDNF variant on adiposity mediated through a central or peripheral action? Further investigation is needed to determine if central CD36 is involved in the development of obesity induced by genetic BDNF variability. Finally, how can this knowledge be exploited for the treatment of obesity and insulin resistance?
Yang's innovative studies lay the groundwork for furthering our understanding of the connection between a common BDNF variant and CD36. BDNFM/M stimulates CD36 gene expression, and the consequently increased level of CD36 underlies the progression of obesity-associated metabolic dysfunctions through increasing lipid accumulation and inflammation (Fig. 1). These experiments also clearly make the case that CD36 is a key player in the development of high-fat diet–induced obesity and type 2 diabetes.
Figure 1.
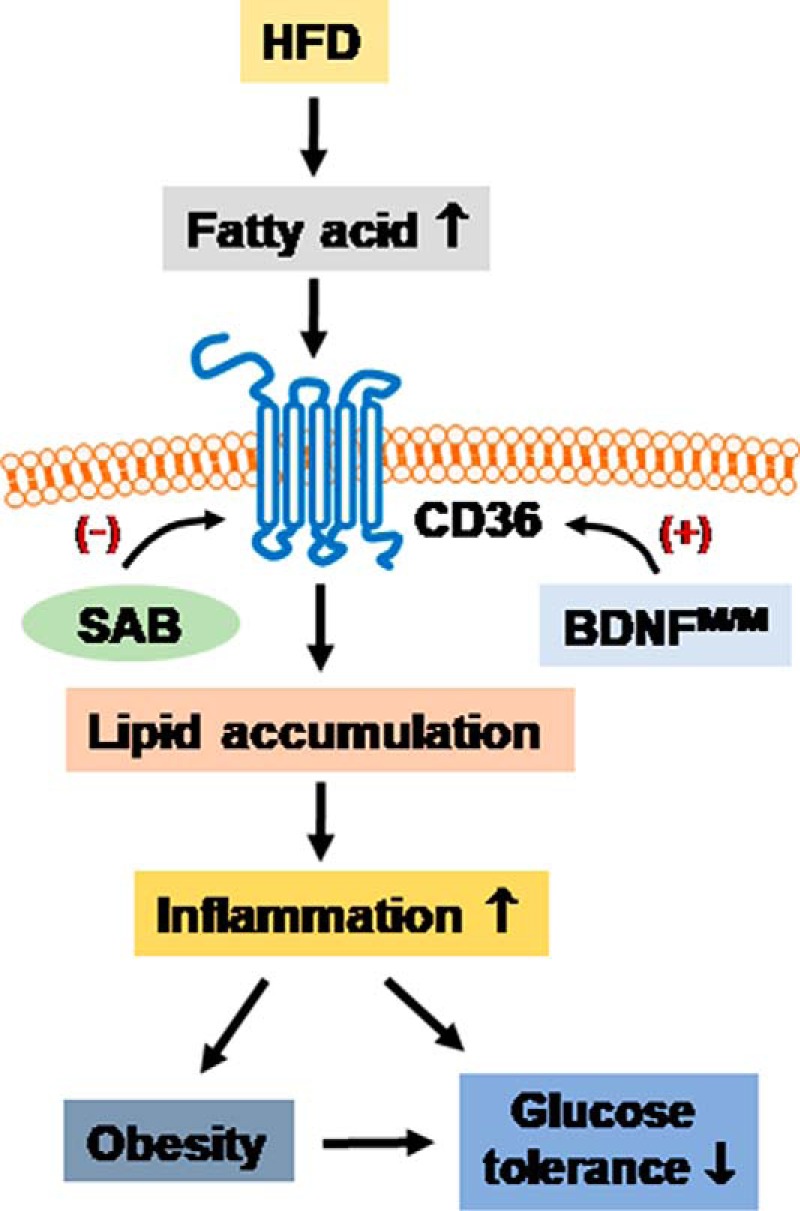
BDNFM/M mice fed a high-fat diet have extreme obesity with increased CD36 gene and protein levels in macrophages. Chronic infusion of a CD36 inhibitor leads to decreased fatty acid uptake, consequently reduces intracellular lipid accumulation and inflammation, and improves insulin sensitivity.
This work was supported by NIDDK, National Institutes of Health Grants R01 DK 92779 and R01 DK 95440. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- SNP
- single-nucleotide polymorphism
- BDNF
- brain-derived neurotropic factor
- SAB
- salvianolic acid B
- DIO
- diet-induced obese.
References
- 1. Kahn B. B., and Flier J. S. (2000) Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 10.1172/JCI10842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El-Sayed Moustafa J. S., and Froguel P. (2013) From obesity genetics to the future of personalized obesity therapy. Nat. Rev. Endocrinol. 9, 402–413 10.1038/nrendo.2013.57 [DOI] [PubMed] [Google Scholar]
- 3. Xu B., and Xie X. (2016) Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 17, 282–292 10.1038/nrn.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., and Weinberger D. R. (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 10.1016/S0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- 5. Rosas-Vargas H., Martínez-Ezquerro J. D., and Bienvenu T. (2011) Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch. Med. Res. 42, 482–494 10.1016/j.arcmed.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 6. Yang J., Park K. W., and Cho S. (2018) Inhibition of the CD36 receptor reduces visceral fat accumulation and improves insulin resistance in obese mice carrying the BDNF-Val66Met variant. J. Biol. Chem. 293, 13338–13348 10.1074/jbc.RA118.002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Handberg A., Norberg M., Stenlund H., Hallmans G., Attermann J., and Eriksson J. W. (2010) Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J. Clin. Endocrinol. Metab. 95, 1939–1946 10.1210/jc.2009-2002 [DOI] [PubMed] [Google Scholar]
- 8. Kennedy D. J., Kuchibhotla S., Westfall K. M., Silverstein R. L., Morton R. E., and Febbraio M. (2011) A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 89, 604–613 10.1093/cvr/cvq360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bao Y., Wang L., Xu Y., Yang Y., Wang L., Si S., Cho S., and Hong B. (2012) Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 223, 152–159 10.1016/j.atherosclerosis.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moullé V. S., Le Foll C., Philippe E., Kassis N., Rouch C., Marsollier N., Bui L.-C., Guissard C., Dairou J., Lorsignol A., Pénicaud L., Levin B. E., Cruciani-Guglielmacci C., and Magnan C. (2013) Fatty acid transporter CD36 mediates hypothalamic effect of fatty acids on food intake in rats. PLoS ONE 8, e74021 10.1371/journal.pone.0074021 [DOI] [PMC free article] [PubMed] [Google Scholar]


