Abstract
The antimalarial activity of chemically diverse compounds, including the clinical candidate cipargamin, has been linked to the ATPase PfATP4 in the malaria-causing parasite Plasmodium falciparum. The characterization of PfATP4 has been hampered by the inability thus far to achieve its functional expression in a heterologous system. Here, we optimized a membrane ATPase assay to probe the function of PfATP4 and its chemical sensitivity. We found that cipargamin inhibited the Na+-dependent ATPase activity present in P. falciparum membranes from WT parasites and that its potency was reduced in cipargamin-resistant PfATP4-mutant parasites. The cipargamin-sensitive fraction of membrane ATPase activity was inhibited by all 28 of the compounds in the “Malaria Box” shown previously to disrupt ion regulation in P. falciparum in a cipargamin-like manner. This is consistent with PfATP4 being the direct target of these compounds. Characterization of the cipargamin-sensitive ATPase activity yielded data consistent with PfATP4 being a Na+ transporter that is sensitive to physiologically relevant perturbations of pH, but not of [K+] or [Ca2+]. With an apparent Km for ATP of 0.2 mm and an apparent Km for Na+ of 16–17 mm, the protein is predicted to operate at below its half-maximal rate under normal physiological conditions, allowing the rate of Na+ efflux to increase in response to an increase in cytosolic [Na+]. In membranes from a cipargamin-resistant PfATP4-mutant line, the apparent Km for Na+ is slightly elevated. Our study provides new insights into the biochemical properties and chemical sensitivity of an important new antimalarial drug target.
Keywords: malaria, membrane transport, drug action, drug resistance, ATPase, antiplasmodial drug, cipargamin, PfATP4, Plasmodium falciparum, sodium transporter, antiplasmodial drug
Introduction
With the emergence and spread of malaria parasites that are resistant to most of the antimalarial drugs registered for use, there is an urgent need for the development of new antimalarials. Cipargamin (previously known as KAE609 and NITD609) is a new antimalarial currently undergoing clinical trials. In an initial Phase 2 trial, cipargamin administered at a dose of 30 mg daily for 3 days cleared parasitemia rapidly in adults with uncomplicated Plasmodium falciparum or Plasmodium vivax malaria (1).
Cipargamin is a synthetic spiroindolone, first identified as an antimalarial in a whole-cell screen of a chemical library composed of some 12,000 compounds (2). Long-term in vitro exposure of P. falciparum cultures to sub-lethal concentrations of the compound led to the emergence of parasites showing low-level resistance to cipargamin (2). These parasites were characterized by mutations in PfATP4, a plasma membrane type II P-type ATPase reported to belong to a subclass unique to apicomplexan parasites (3).
During the disease-causing stage of its life cycle, the P. falciparum parasite resides in a parasitophorous vacuole within the human erythrocyte. Like other cell types, erythrocytes normally maintain a low internal Na+ concentration. However, as the parasite matures into a trophozoite, it induces an increase in the permeability of the erythrocyte plasma membrane (4, 5), allowing a net influx of Na+ that results in the erythrocyte's cytosolic Na+ concentration increasing to a level approaching that in the extracellular medium (6–9). The parasitophorous vacuole membrane that encloses the mature intraerythrocytic trophozoite is believed to be freely permeable to low molecular weight solutes and is not expected to present a barrier to the movement of ions (10, 11). Thus, despite its intracellular location, the mature trophozoite finds itself in a high-Na+ environment and must efflux Na+ ions across its plasma membrane to maintain a low cytosolic Na+ concentration ([Na+]cyt).
There is functional evidence that PfATP4 serves as the parasite's primary Na+-efflux pump. On addition of cipargamin or related antimalarial spiroindolones to trophozoite-stage parasites, there is an increase in the parasite's Na+ content (9, 12) and [Na+]cyt (13–15). The data are consistent with the spiroindolones inhibiting Na+ extrusion via PfATP4, resulting in a net uptake of Na+ as the ion moves into the parasite, down its electrochemical gradient. The increase in [Na+]cyt is accompanied by an increase in cytosolic pH (pHcyt), and it has been proposed that PfATP4 imports H+ into the parasite while extruding Na+ (15), with inhibition of the protein resulting in a cessation of H+ import and hence a cytosolic alkalinization.
To date, it has not been possible to confirm the function of PfATP4 or to study its transport properties in a heterologous system. In the initial description of PfATP4, it was reported that expression of PfATP4 in Xenopus laevis oocytes gave rise to increased Ca2+-dependent ATPase activity (3); however, at least one other laboratory has been unable to reproduce this finding or to achieve functional expression of the transporter (2).
In the last few years, numerous chemically diverse compounds have been shown to disrupt parasite ion regulation in the same manner as that seen for the antimalarial spiroindolones (13, 14, 16–19), including 28 compounds from the Medicines for Malaria Venture's (MMV's)3 “Malaria Box” (18) and 11 compounds from the MMV's “Pathogen Box” (13). A number of these compounds have been used to select for resistant parasites, and in each case resistance was associated with one or two mutations in PfATP4 (14, 17–19).
With only indirect assays available, and with an increasingly large number of diverse chemotypes displaying “PfATP4-associated” activity, the question arises of whether PfATP4 is the direct target of all of the PfATP4-associated compounds (20). It is conceivable that certain compounds could perturb cellular physiology or other proteins in such a way as to inhibit PfATP4 indirectly. The most direct evidence for inhibition of PfATP4 by an antiplasmodial compound comes from studies with membrane preparations. Spillman et al. (15) prepared membranes from isolated asexual blood-stage parasites and demonstrated the presence in this preparation of a Na+-dependent ATPase activity that was inhibited by the antimalarial spiroindolone NITD246. The sensitivity of this ATPase activity to inhibition by NITD246 was reduced in membranes prepared from spiroindolone-resistant parasites carrying mutations in PfATP4, consistent with the ATPase activity being associated with PfATP4. Recently, the 11 PfATP4-associated Pathogen Box compounds have also been shown to inhibit Na+-dependent ATPase activity in parasite membrane preparations (13).
In this study, we have carried out a detailed biochemical characterization of the PfATP4-associated ATPase activity in membranes of blood-stage P. falciparum parasites, and we investigated whether this activity is inhibited by the diverse Malaria Box compounds shown previously (18) to perturb Na+ and pH homeostasis in the parasite.
Results
Inhibition of Na+-dependent ATPase activity in parasite membranes by cipargamin
In the initial experiments of this study, the lead spiroindolone cipargamin was tested (at a concentration of 500 nm) for its effect on the time course of ATP hydrolysis by isolated parasite membranes, measured both in the presence of a high (152 mm) and low (2 mm) concentration of Na+. The organic cation choline was used to replace Na+ in the low-[Na+] medium. By adding ATP to initiate the reaction, it was necessary to use Na2ATP as we found that the commercially available K+ and Mg2+ salts of ATP both had significant Pi contamination (as reported previously (15)). It was therefore not possible to perform measurements in the complete absence of Na+.
As is illustrated in Fig. 1a, the amount of Pi produced as a result of ATP hydrolysis increased approximately linearly with time for the first 10 min under all the conditions tested, before starting to slow. The Pi production measured in the high-[Na+] medium in the absence of cipargamin was higher than that measured under any of the other conditions tested. At the 10-min time point, Pi production in the low-[Na+] condition in the presence of solvent alone (0.4% v/v DMSO) was 78.1 ± 0.5% (mean ± S.D.; n = 3) of that seen in the high-[Na+] condition (p < 0.001, paired t test). This value is very similar to that reported previously (76% (15)). Cipargamin (tested at 500 nm) decreased Pi production in the high-[Na+] condition to 72.8 ± 0.6% (mean ± S.D.; n = 3) of the control value (p < 0.001, paired t test). Cipargamin also caused a slight but statistically significant decrease in Pi production in the low [Na+] condition (p = 0.03, paired t test); this most likely results from inhibition of the residual Na+-ATPase activity that is present in the 2 mm Na+ medium. The ATPase activities measured in the high- and low-[Na+] media in the presence of cipargamin were not significantly different from one another (p = 0.3, paired t test), consistent with 500 nm cipargamin having eliminated 100% of the Na+-ATPase activity (i.e. there is no Na+-dependent component to the ATPase activity when 500 nm cipargamin is present).
Figure 1.
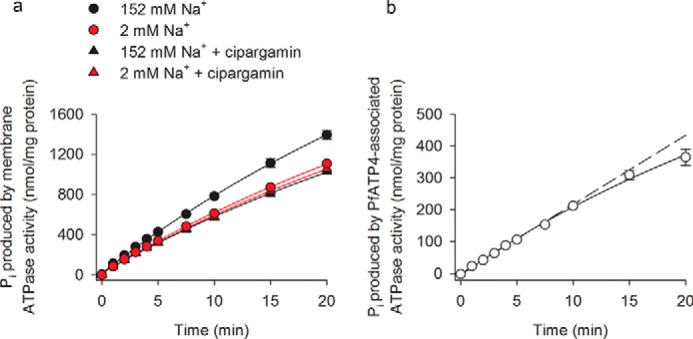
Time course for Pi production by ATPases in P. falciparum membranes. a, amount of Pi produced by P. falciparum membranes was measured under high-[Na+] (152 mm) and low-[Na+] (2 mm) conditions in the presence and absence of 500 nm cipargamin. b, amount of Pi produced in the high-[Na+] condition in the presence of cipargamin was subtracted from that obtained in the high-[Na+] condition in the absence of cipargamin to determine the “PfATP4-associated ATPase activity.” The dashed line indicates the amount of Pi that would be produced if PfATP4-associated ATPase activity continued at the initial rate for the duration of the experiment. The data were obtained with Dd2 parasites and are shown as the mean (± S.D.) from three independent experiments, each performed on different days with different membrane preparations. Where not shown, the error bars fall within the symbols. The ATP concentration was 1 mm.
PfATP4 has been proposed to function as a Na+ efflux transporter and to be the target of antimalarial spiroindolones (15). Henceforth, in this study, the cipargamin-sensitive Na+-ATPase activity in P. falciparum membranes (calculated by subtracting the amount of Pi produced in the presence of 500 nm cipargamin from that obtained in its absence) is used as a proxy for PfATP4 activity and is referred to as “PfATP4-associated ATPase activity.”
Under the conditions of our experiments, the amount of Pi produced by PfATP4-associated ATPase activity increased approximately linearly with time for the first 15 min before starting to slow (Fig. 1b). In subsequent experiments, the rate of Pi production was estimated using a single time point. For those experiments in which the effects of Malaria Box compounds on PfATP4-associated ATPase activity were investigated, we used a time point of 15 min. For those experiments in which it was essential that PfATP4-associated ATPase activity was measured at its maximal rate (e.g. kinetic measurements), we used a time point of 10 min.
Inhibition of Na+-dependent ATPase activity in parasite membranes by diverse Malaria Box compounds
We investigated the effects on the parasite's Na+-dependent ATPase activity of 28 compounds from the Malaria Box shown previously (18) to give rise to the “ionic signature” of PfATP4 inhibition (i.e. an increase in both [Na+]cyt and pHcyt and a reduction in the level of cytosolic acidification seen following inhibition of the V-type H+-ATPase with concanamycin A). Like cipargamin, each of the 28 PfATP4-associated compounds from the Malaria Box (tested at 4 μm) significantly inhibited Pi production in the high-[Na+] condition; at the concentrations tested here only two of the compounds (MMV000642 and MMV008455) gave rise to statistically significant effects in the low-[Na+] condition (Fig. S1). Fig. 2 shows the effect of each compound on the Na+-dependent ATPase activity of parasite membranes (calculated by subtracting the amount of Pi produced in the low-[Na+] medium from that produced in the high-[Na+] medium). Each of the 28 PfATP4-associated Malaria Box compounds (tested at 4 μm) effectively eliminated the Na+-dependent ATPase activity in parasite membranes (Fig. 2). Two Malaria Box compounds (MMV007839 and MMV000972) that do not display the ionic signature of PfATP4 inhibition and that have been found to inhibit the unrelated (non-ATP-hydrolyzing) P. falciparum lactate/H+ transporter PfFNT (21) were included as controls. Neither of these compounds (each tested at 4 μm) affected Na+-dependent ATPase activity (Fig. 2).
Figure 2.
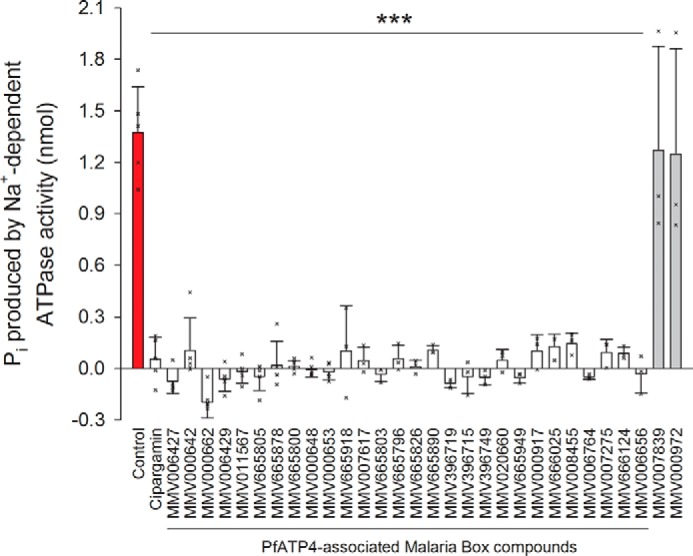
Effects of various compounds on P. falciparum Na+-dependent ATPase activity. The data were obtained by subtracting the amount of Pi produced in the low-[Na+] (0.5 mm) medium from that produced in the high-[Na+] (125.5 mm) medium. Cipargamin was tested at a concentration of 500 nm; the other compounds (all from the Malaria Box, with 28 of them (white bars) having PfATP4-associated activity and two of them, MMV007839 and MMV000972 (gray bars), having activity against an unrelated transport protein) were tested at a concentration of 4 μm. The crosses indicate the data from the individual independent biological replicates. The ATP concentration was 0.25 mm; the pH was 7.4; and the duration of the reaction was 15 min. The data were obtained with Dd2 parasites and are shown as the mean (+ or − S.D.) from three to five independent experiments, each performed on different days with different membrane preparations. The protein content of the membrane preparations was not quantified in these experiments; each reaction well contained membrane from 3 × 106 cells. For each compound, the data were tested for statistical significance compared with the control (red bar; 0.4% v/v DMSO) using a one-way ANOVA (which yielded an F probability <0.001) followed by a post hoc least significant difference test; ***, p < 0.001.
We also tested each of the 28 PfATP4-associated Malaria Box compounds, as well as the control compounds MMV007839 and MMV000972, for their effects on ATPase activity when added to membrane preparations that were simultaneously exposed to 500 nm cipargamin (a concentration that completely eliminates Na+-ATPase activity; Fig. 1). The majority of the PfATP4-associated Malaria Box compounds did not cause any further inhibition of ATPase activity when tested in the presence of 500 nm cipargamin (Fig. S1), consistent with their effects on Pi production (as measured in the absence of cipargamin; Fig. 2) being due wholly to inhibition of the same ATPase as is targeted by cipargamin. The PfATP4-associated Malaria Box compounds MMV000642 and MMV008455 were exceptions, decreasing Pi production significantly in the presence of cipargamin under both high-[Na+] and low-[Na+] conditions (Fig. S1). This suggests that these compounds inhibit one or more Na+-independent ATPases in addition to inhibiting Na+-dependent ATPase activity. Three other PfATP4-associated Malaria Box compounds (MMV006427, MMV000662, and MMV396719) caused minor decreases in ATPase activity in the presence of 500 nm cipargamin, with these decreases attaining statistical significance in the high-[Na+] condition but not in the low-[Na+] condition (Fig. S1). These minor effects were not investigated further. The control (non-PfATP4-associated) compounds MMV007839 and MMV000972 had no effect on ATPase activity under any of the conditions tested (Fig. S1).
Estimation of the affinity of PfATP4 for ATP
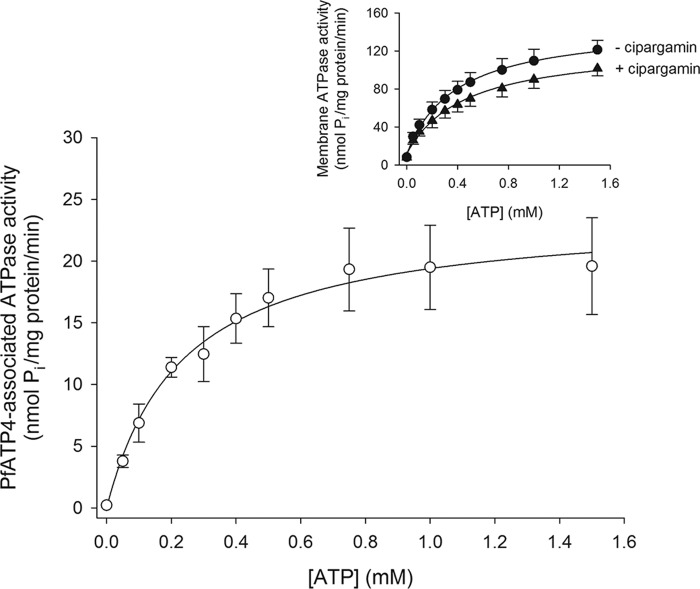
The ATP concentration dependence of total membrane ATPase activity (Fig. 3, inset) and PfATP4-associated ATPase activity (Fig. 3) was measured over 10 min in media containing high [Na+] (150–153 mm depending on the concentration of Na2ATP). Both the total membrane ATPase activity and the PfATP4-associated ATPase activity increased with increasing [ATP], with both reaching a maximal rate at an [ATP] of ∼1 mm (Fig. 3). Fitting the Michaelis-Menten equation to the data for PfATP4-associated ATPase activity (Fig. 3) yielded a Km for ATP (Km(ATP)) of 0.23 ± 0.06 mm (mean ± S.D.; n = 4). The Vmax for PfATP4-associated Pi production under the conditions tested was 24 ± 5 nmol/min/mg of protein (noting that the protein measurement includes all protein present in the membrane preparation).
Figure 3.
[ATP] dependence of PfATP4-associated ATPase activity under high-[Na+] (150–153 mm) conditions. The PfATP4-associated ATPase activity (white circles) was calculated by subtracting the total ATPase activity measured in the presence of 500 nm cipargamin (inset, black triangles) from that measured in the absence of cipargamin (inset, black circles). The data were obtained with Dd2 parasites and are shown as the mean (± S.D.) from four independent experiments, each performed on different days with different membrane preparations. For reasons of clarity, only upward error bars are shown for the data obtained in the absence of cipargamin (inset), and only downward error bars are shown for the data obtained in the presence of cipargamin (inset). Where not shown, the error bars fall within the symbols. The addition of ATP to the reaction mixtures was found to give rise to minor effects on pH, with the pH varying from 7.4 in the absence of ATP to 7.2 in the presence of the highest [ATP] tested. The PfATP4-associated ATPase activity data were fitted with the Michaelis-Menten equation (PfATP4-associated ATPase activity = Vmax × [ATP]/([ATP] + Km(ATP))), yielding a Km(ATP) of 0.23 ± 0.06 mm and a Vmax of 24 ± 5 nmol/min/mg of protein (mean ± S.D.).
All subsequent experiments for which results are reported below were performed over 10 min with an [ATP] of 1 mm. The use of 1 mm ATP led to a concentration of 2 mm Na+ in the low-[Na+] measurements. The pH of the reaction solutions (after the addition of 1 mm ATP) was 7.2, which corresponds to the estimated pHcyt in intraerythrocytic P. falciparum parasites (22).
Estimation of the affinity of PfATP4 for Na+
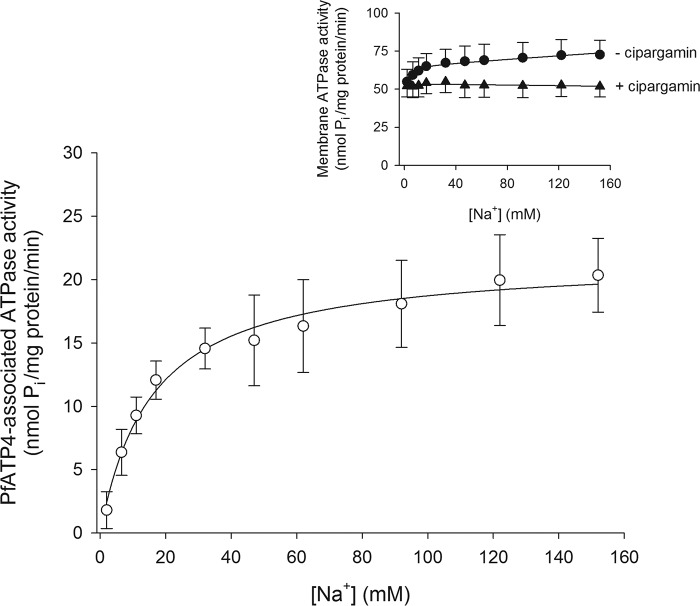
We next sought to determine the affinity of PfATP4 for Na+ by measuring PfATP4-associated ATPase activity in media having a range of [Na+]. Both the total membrane ATPase activity (Fig. 4, inset) and the PfATP4-associated ATPase activity (Fig. 4) increased with increasing [Na+]. In the presence of 500 nm cipargamin, there was little effect of [Na+] on the remaining fraction of membrane ATPase activity (Fig. 4, inset). Fitting a Michaelis-Menten curve to the data for PfATP4-associated ATPase activity yielded a Km for Na+ (Km(Na+)) of 16.1 ± 3.8 mm (mean ± S.D.; n = 8). The Vmax under the conditions of these experiments was 21.8 ± 3.8 nmol of Pi per mg of (total) protein per min.
Figure 4.
[Na+] dependence of PfATP4-associated ATPase activity. The PfATP4-associated ATPase activity (white circles) was calculated as described under “Experimental procedures” using the total ATPase activity measured in the presence of 500 nm cipargamin (inset, black triangles) and that measured in the absence of cipargamin (inset, black circles). The data were obtained with Dd2 parasites and are shown as the mean (± S.D.) from eight independent experiments, each performed on different days with different membrane preparations. For reasons of clarity, only upward error bars are shown for the data obtained in the absence of cipargamin (inset), and only downward error bars are shown for the data obtained in the presence of cipargamin (inset). The PfATP4-associated ATPase activity data were fitted with the Michaelis-Menten equation (PfATP4-associated ATPase activity = Vmax × [Na+]/([Na+] + Km(Na+))), yielding a Km(Na+) of 16.1 ± 3.8 mm and a Vmax of 21.8 ± 3.8 nmol/min/mg of protein (mean ± S.D.).
We investigated the Na+ dependence of PfATP4-associated ATPase activity over a range of different [ATP] (Fig. S2). Consistent with our findings with membranes in a high-[Na+] medium (Fig. 3), PfATP4-associated ATPase activity decreased as the [ATP] was reduced below 1 mm at each of the [Na+] tested (Fig. S2). The Km(Na+) at the lowest [ATP] (0.25 mm) was 1.4-fold higher than that at the highest [ATP] tested (1 mm; Fig. S2).
Effect of pH, [K+], and [Ca2+] on PfATP4-associated ATPase activity
The effect of pH, [K+], and [Ca2+] on total P. falciparum membrane ATPase activity and on PfATP4-associated ATPase activity was investigated, with measurements performed under both high-[Na+] and low-[Na+] conditions in the presence and absence of cipargamin (500 nm). The data are shown in Fig. 5, a–f; the p values cited in this section are from the least significant difference tests that proceeded one-way ANOVAs performed with all the data contained within each panel (the ANOVAs yielded F probabilities <0.05 for Fig. 5, a–c, e, and f).
Figure 5.
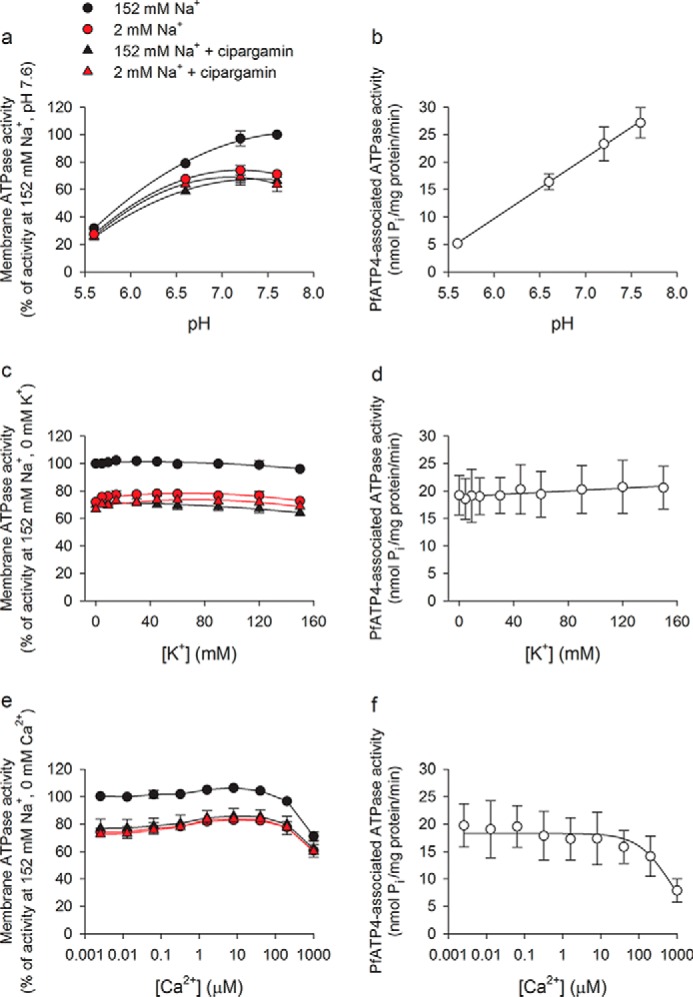
Effect of pH (a and b), [K+] (c and d), and [Ca2+] (e and f) on membrane ATPase activity and PfATP4-associated ATPase activity. Total ATPase activity (a, c, and e) was measured under high-[Na+] (black) and low-[Na+] (red) conditions and in the presence (triangles) and absence (circles) of cipargamin (500 nm). PfATP4-associated ATPase activity (b, d, and f) was calculated by subtracting the (pre-normalized) data obtained in the presence of cipargamin (and a high [Na+]) from those obtained in the high-[Na+] condition in the absence of cipargamin. The data were obtained with Dd2 parasites and are shown as the mean (± S.D.) from three independent experiments, each performed on different days with different membrane preparations. Where not shown, the error bars fall within the symbols. In the experiments in which [K+] was varied, [choline] was also varied such that [KCl] + [choline chloride] = 150 mm in the high-[Na+] condition. Choline was also used to replace Na+ in the low-[Na+] condition. e and f, [Ca2+] indicated corresponds to free [Ca2+] and was determined by adding 1 mm EGTA to the reaction mixture as well as sufficient CaCl2 to achieve the desired free [Ca2+] (Ca-EGTA Calculator v1.3).
ATPase activity was measured at four different pH values: pH 5.6, 6.6, 7.2, and 7.6. Between pH 5.6 and 7.2, total membrane ATPase activity increased with increasing pH values under each of the conditions tested (p < 0.001, compared with the activity at pH 5.6; Fig. 5a). In the high-[Na+] condition in the absence of cipargamin, membrane ATPase activity increased further when the pH was increased from 7.2 to 7.6, although the difference was not significant (p > 0.05; Fig. 5a). In contrast, membrane ATPase activity measured in the presence of cipargamin and in the low-[Na+] conditions decreased slightly (but not significantly; p > 0.05) when the pH was increased from 7.2 to 7.6 (Fig. 5a).
As shown in Fig. 5b, the PfATP4-associated ATPase activity, calculated by subtracting the total ATPase activity measured in the presence of 500 nm cipargamin from that measured in the absence of cipargamin, increased with increasing pH values over the full pH range tested. The increase was statistically significant (p < 0.01) for all pairwise comparisons except for the pH 7.2 versus 7.6 comparison (p > 0.05).
As well as measuring the pH dependence of ATPase activity under high- and low-[Na+] conditions (Fig. 5a), we investigated the Na+ dependence of PfATP4-associated ATPase activity over a range of different pH values (Fig. S3). The Km(Na+) decreased with increasing pH values (in the range 5.6 to 7.6), consistent with the affinity of PfATP4 for Na+ increasing with increasing pH values over this range (Fig. S3). The Vmax for PfATP4-associated ATPase activity also increased with increasing pH values (Fig. S3).
Experiments in which [K+] was varied over the range 0–150 mm revealed that [K+] had little effect on total membrane ATPase activity under any of the conditions tested (Fig. 5c). There was a slight but significant increase in ATPase activity (relative to that observed in the absence of K+) in the low-[Na+] conditions in the presence of 15–120 mm K+ (p < 0.05, compared with the activity with K+ = 0 mm). The PfATP4-associated ATPase activity was unaffected by [K+] over the full range of concentrations tested (Fig. 5d; p > 0.05).
In experiments in which free [Ca2+] was varied between 0 and 1 mm, there were two effects of [Ca2+] on the total ATPase activity associated with membranes isolated from P. falciparum parasites. First, there was a very slight but statistically significant increase in ATPase activity in the presence of 1.6–40 μm Ca2+ (p < 0.05 for all conditions, compared with the activity at [Ca2+] = 0 mm). This was seen under both high- and low-[Na+] conditions and suggests that there may be at least one functional Ca2+-dependent ATPase in the membrane preparation. Second, at the highest concentration tested (1 mm), Ca2+ had an inhibitory effect on ATPase activity under all of the conditions tested (Fig. 5e; p < 0.001, compared with the activity at [Ca2+] = 0 mm). The PfATP4-associated ATPase activity showed just the second of the two effects (Fig. 5f); Ca2+ inhibited PfATP4-associated ATPase activity significantly at the highest concentration tested (1 mm; p < 0.01), but it had no significant effect on PfATP4-associated ATPase activity at concentrations ≤200 μm (p > 0.05). The PfATP4-associated ATPase activity is therefore not dependent on Ca2+ but is susceptible to inhibition by Ca2+ at high concentrations.
Sensitivity of PfATP4-associated ATPase activity to cipargamin in WT and PfATP4-mutant parasites
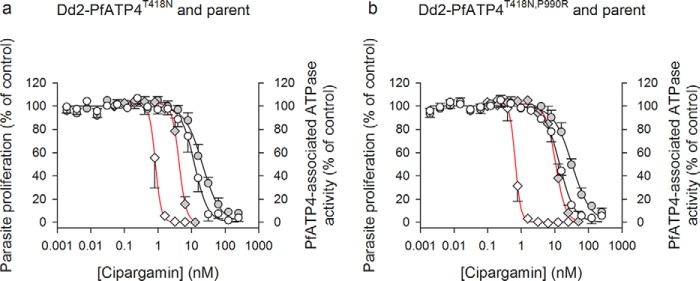
We investigated the concentration dependence of the inhibition of PfATP4-associated ATPase activity by cipargamin in two different pairs of parasite lines: (i) a Dd2 mutant parasite line with a T418N mutation in PfATP4 (Dd2-PfATP4T418N), generated by selection with the PfATP4-associated Malaria Box compound MMV007275 (18) and then cloned by limiting dilution, together with its Dd2 parental line; and (ii) the spiroindolone-selected line NITD609-RDd2-clone 2 (from Ref. 2 with T418N and P990R mutations in PfATP4 and referred to here as Dd2-PfATP4T418N,P990R) together with its Dd2 parental line. The IC50 for growth inhibition by cipargamin was 5-fold higher in Dd2-PfATP4T418N parasites than in their parental parasites and 16-fold higher in Dd2-PfATP4T418N,P990R parasites than in their parental parasites (Table 1; Fig. 6).
Table 1.
Potency of cipargamin against parasite proliferation and PfATP4-associated ATPase activity in two PfATP4-mutant lines and their parental lines
The data presented are the mean IC50 ± S.D. from the number of experiments indicated in parentheses. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t-tests compared with matched parental line, paired or unpaired as appropriate).
| Cipargamin IC50 (nm) |
||
|---|---|---|
| Parasite proliferation | PfATP4-associated ATPase activity | |
| Parent of Dd2-PfATP4T418N | 0.84 ± 0.17 (n = 6) | 12.3 ± 3.3 (n = 4) |
| Dd2-PfATP4T418N | 4.3 ± 0.5 (n = 6)*** | 21.1 ± 3.0 (n = 5)** |
| Parent of Dd2-PfATP4T418N,P990R | 0.66 ± 0.09 (n = 3) | 13.9 ± 1.4 (n = 3) |
| Dd2-PfATP4T418N,P990R | 10.6 ± 2.2 (n = 3)* | 32.5 ± 3.2 (n = 3)** |
Figure 6.
Potency of cipargamin against parasite growth (red curves) and PfATP4-associated ATPase activity (black curves) in PfATP4-mutant lines and their parents. The data for Dd2-PfATP4T418N and its parent (a) and Dd2-PfATP4T418N,P990R and its parent (b) were obtained from the number of independent experiments indicated in Table 1, and they are shown as the mean ± S.D. Where not shown, the error bars fall within the symbols. Parasite proliferation data for the PfATP4-mutant lines (gray symbols) and their parental lines (white symbols) are shown with diamonds and red lines, and data for PfATP4-associated ATPase activity are shown with circles and black lines. For clarity, only upward error bars are shown for data obtained with the PfATP4-mutant lines, and only downward error bars are shown for data obtained with the parental lines. ATPase activity was determined at pH 7.2 in the presence of 152 mm Na+ and 1 mm ATP. In each case the data were fit to a sigmoidal curve: y = a/(1 + ([cipargamin]/IC50)b, where a is the maximum y value, and b is a fitted constant.
In membranes prepared from Dd2 parental parasites, cipargamin inhibited PfATP4-associated ATPase activity with an IC50 of 12.3 ± 3.3 nm for the parent of Dd2-PfATP4T418N and 13.9 ± 1.4 nm for the parent of Dd2-PfATP4T418N,P990R (Table 1; Fig. 6). These values are 15–21-fold higher than the IC50 values for cipargamin in parasite proliferation assays with these lines (Table 1). The potency with which cipargamin inhibited PfATP4-associated ATPase activity was decreased by 1.7-fold in membranes prepared from Dd2-PfATP4T418N parasites relative to their parental parasites and by 2.3-fold in membranes prepared from Dd2-PfATP4T418N,P990R parasites relative to their parental parasites (both decreases were statistically significant; Table 1; Fig. 6).
Cipargamin therefore inhibits parasite growth somewhat more potently than it inhibits PfATP4-associated ATPase activity in the isolated membrane assay used here, and a decrease in parasite susceptibility to cipargamin is associated with a reduced sensitivity of PfATP4-associated ATPase activity to the drug. The higher level of resistance to inhibition of parasite proliferation by cipargamin in Dd2-PfATP4T418N,P990R parasites relative to Dd2-PfATP4T418N parasites was associated with a significantly higher level of resistance of PfATP4-associated ATPase activity to cipargamin in the former relative to the latter line (p = 0.002, unpaired t test; Table 1). This provides further evidence that the PfATP4-associated ATPase activity measured here corresponds to the activity of the PfATP4 protein.
Affinity of PfATP4 for Na+ in WT and cipargamin-resistant parasite lines
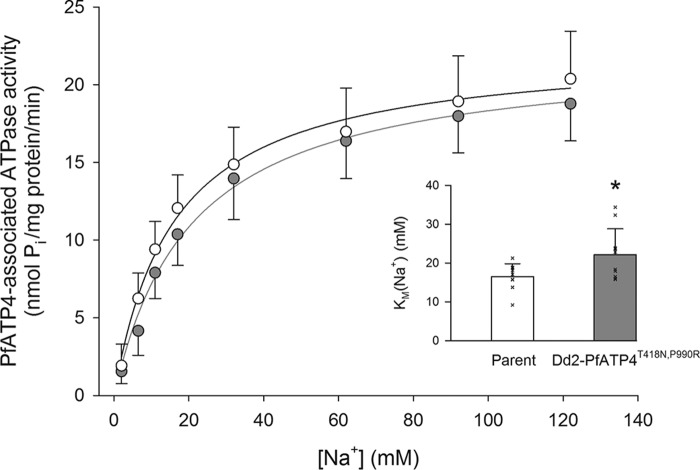
The affinity of PfATP4 for Na+ was investigated in Dd2-PfATP4T418N,P990R parasites and their matched parental parasites. In Dd2-PfATP4T418N,P990R parasites, we estimated a Km(Na+) of 22 ± 7 mm (mean ± S.D.), significantly higher than that measured with the Dd2 parental parasites (16.5 ± 3.3 mm; p = 0.04, paired t test; Fig. 7). These data are consistent with the combination of the T418N and P990R mutations in PfATP4 reducing the affinity of the transporter for Na+. The Vmax for PfATP4-associated ATPase activity was not significantly different between the two lines: 22.5 ± 3.2 nmol of Pi per mg of (total) protein per min (mean ± S.D.) in the parental parasites and 22.3 ± 2.6 nmol of Pi per mg of (total) protein per min in the Dd2-PfATP4T418N,P990R parasites (p = 0.87, paired t test).
Figure 7.
Effect of [Na+] on PfATP4-associated ATPase activity in Dd2-PfATP4T418N,P990R parasites (gray) and their parental parasites (white). The data are shown as the mean (+ or − S.D.) from 10 independent experiments, each performed on different days with different membrane preparations. The curves were drawn using the Michaelis-Menten equation (PfATP4-associated ATPase activity = Vmax × [Na+]/([Na+] + Km(Na+))) fitted to the data. The Km(Na+) in the two lines (shown as the mean ± S.D.) from the same experiments are shown as an inset, with Km values from each individual experiment shown with a cross. *, p < 0.05, paired t test.
Discussion
The finding that the activity of numerous diverse antiplasmodial chemotypes can be linked to one protein, PfATP4, is unexplained. To date, there is no system for studying PfATP4 activity in isolation, and it has not been possible to determine whether the numerous PfATP4-associated compounds that have been shown to disrupt Na+ and pH regulation in parasites and to be rendered less potent by mutations in PfATP4 act via direct inhibition of PfATP4. The lack of a direct assay to study PfATP4 has also hampered investigations into its transport characteristics. Here, we have optimized a cell-free assay in which the ATPase activity present in P. falciparum membrane preparations is measured under varying conditions and, using the cipargamin-sensitive fraction of ATPase activity as a proxy for PfATP4 activity, have characterized the biochemical properties of the protein.
Approximately one-quarter of the ATPase activity measured in P. falciparum membranes was Na+-dependent. This fraction was inhibited by the spiroindolone cipargamin, the best-studied and most clinically advanced of the PfATP4-associated compounds. These data are consistent with those obtained previously with spiroindolones (13, 15) and suggest that the majority, if not all, of the Na+-dependent ATPase activity in P. falciparum membranes is contributed by PfATP4. Of note, cipargamin has been found to inhibit directly the ATPase activity of a H+-transporting P-type ATPase from Saccharomyces cerevisiae (23).
The IC50 value for cipargamin against PfATP4-associated ATPase activity in isolated membranes was somewhat higher than its IC50 value for parasite killing (3–21-fold higher in our study, depending on the parasite line). This is in line with a previous study (15), which found that a different spiroindolone, NITD246, was more potent against parasite growth than against the parasite's Na+-dependent ATPase activity, although in the previous study the fold differences were lower (< 2-fold). There are many potential explanations for a difference in the potency of a compound against its target and against a whole organism. It is possible that a relatively small reduction in PfATP4 activity might have a relatively large effect on parasite growth. It is also possible that in the parasite growth experiments cipargamin accumulates at its site of action to concentrations higher than those present in the extracellular media. It should also be noted that in our experiments inhibition of the Na+-dependent ATPase activity was measured under high (152 mm) [Na+] conditions and that this may have resulted in higher IC50 values than would be the case if inhibition were to be measured under the lower [Na+] conditions that prevail in the cytosol of the parasite under resting conditions (7–15 mm).
Consistent with a previous finding with NITD246 (15), the potency of cipargamin against PfATP4-associated ATPase activity was reduced in PfATP4-mutant parasites. The double mutant line (Dd2-PfATP4T418N,P990R) was more resistant to growth inhibition by cipargamin than the single mutant line (Dd2-PfATP4T418N) and also displayed a higher level of resistance to inhibition of PfATP4-associated ATPase activity by cipargamin. The fold-increase in the IC50 for cipargamin against PfATP4-associated ATPase activity that was conferred by the PfATP4 mutation(s) (1.7–2.3-fold) was lower than that observed for growth inhibition (5–16-fold). It is possible that the high [Na+] conditions may have masked differences in cipargamin sensitivity between WT and mutant proteins that exist under physiological conditions. Alternatively, it is possible that factors other than a reduced interaction of PfATP4 and cipargamin contribute to parasite resistance in the PfATP4-mutant lines investigated.
The Na+-dependent fraction of P. falciparum membrane ATPase activity was inhibited by all 28 of the Malaria Box compounds that had previously been shown to disrupt ion homeostasis in P. falciparum in a spiroindolone-like manner (18). In light of this finding, certain scenarios (20) that might have explained the activity of the compounds can now be excluded. Specifically, our data are not consistent with models whereby the compounds act by: (i) interfering with PfATP4 trafficking or localization to the plasma membrane; (ii) inhibiting a non-membrane-associated protein that modulates PfATP4 activity; (iii) altering cell pH by a mechanism not involving PfATP4 (with a change in PfATP4 activity being a downstream consequence); and (iv) rendering the parasite plasma membrane permeable to Na+ (i.e. behaving as a Na+ ionophore, with a change in pH being a downstream consequence, and mutations in PfATP4 having a compensatory function in resistant parasites). Our data are consistent with the simplest model, whereby the PfATP4-associated compounds bind directly to PfATP4 and inhibit its normal function. However, our data do not rule out the possibility that some of the compounds affect PfATP4 activity by inhibiting a membrane-associated regulator of its function or by altering the physical properties of the membrane in such a way as to alter PfATP4 function.
Our optimized assay enabled a characterization of the kinetic properties and ion dependence of PfATP4-associated ATPase activity. Our finding that PfATP4-associated ATPase activity increased with increasing [Na+] is consistent with other lines of evidence (9, 15) that PfATP4 serves as a Na+ pump. We estimated the affinity of PfATP4 for Na+ by measuring the cipargamin-sensitive fraction of ATPase activity at a range of [Na+] and fitting a Michaelis-Menten curve to the data. Under the conditions of our experiments, pH 7.2, we estimated that WT PfATP4 (as measured in two Dd2 clones originating from different laboratories) has a Km(Na+) of 16–17 mm. The resting [Na+]cyt in Dd2 parasites is ∼7 mm (15). Thus, under resting conditions the ATPase is predicted to be operating at ∼30% of its maximum activity and therefore to have the capacity to increase its activity (i.e. increase the rate of Na+ efflux) by up to 3.2-fold in response to an increase in [Na+]cyt. The Na+ Km value determined here for PfATP4 is within the range of Na+ Km values that have been reported for different isoforms of the Na+/K+ pump that control [Na+]cyt in human cells (8–33 mm (24, 25)) and is slightly lower than that estimated for a P-type Na+-ATPase in Leishmania amazonensis parasites (29 mm) (26).
Our investigation of the Na+ dependence of PfATP4-associated ATPase activity with cipargamin-resistant parasites yielded evidence that the resistance-associated double-mutant (T418N,P990R) variant of PfATP4 has a reduced affinity for Na+ (22 mm) compared with WT PfATP4, with no discernible effect of the mutations on the Vmax of the transporter. This provides a potential explanation for the observation that [Na+]cyt is elevated in Dd2-PfATP4T418N,P990R parasites relative to their parental parasites (15). The [Na+]cyt measured in Dd2-PfATP4T418N,P990R was 15 mm, compared with an estimated value of 7 mm in the parental parasites (15).
The Km(ATP) estimated for PfATP4 (0.2 mm) is much lower than typical intracellular ATP concentrations, including the intracellular ATP concentration that has been estimated for P. falciparum parasites (2.5 mm) (27). Thus, the ATP concentration inside the parasite should not be limiting for PfATP4 activity, and fluctuations in [ATP] within the physiological range will have little effect on its transport rate.
Previous studies have shown that antiplasmodial spiroindolones and other PfATP4-associated compounds increase pHcyt in P. falciparum (i.e. increase the pH gradient across the parasite plasma membrane) (15–19), prompting the suggestion that PfATP4 imports H+ while exporting Na+ (15). pH can affect the activity of proteins in a variety of ways through its effects on protonatable residues and in some cases also by H+ (or OH−) being a substrate and/or allosteric activator or inhibitor. Thus, calculating a Km value for H+ transport is not straightforward and was not attempted here. Nevertheless, our finding that PfATP4 activity increased with increasing pH raises the possibility that the protein is regulated by pH; if the region of the protein responsible for the pH dependence of PfATP4-associated ATPase activity seen here is exposed to the parasite cytosol, the protein could respond to a cytosolic alkalinization by undergoing an increase in activity, importing H+ into the parasite at an increased rate.
PfATP4 was originally annotated as a Ca2+ transporter, and its expression in Xenopus oocytes was reported to increase the Ca2+-dependent ATPase activity present in oocyte membranes (3). However, others were not able to observe a Ca2+-dependent ATPase activity when attempting to reproduce this result (2), and the addition of an antiplasmodial spiroindolone to isolated parasites was without effect on [Ca2+]cyt (15). Our finding that Ca2+ did not stimulate the parasite's cipargamin-sensitive ATPase activity provides further evidence that PfATP4 is not a Ca2+ transporter. There was a slight stimulation of cipargamin-insensitive ATPase activity at [Ca2+] between 1.6 and 40 μm, consistent with there being one or more Ca2+-ATPases in parasite membranes that could be characterized using a similar approach to that used here to study PfATP4. At the highest concentration tested (1 mm), Ca2+ was found to inhibit PfATP4-associated ATPase activity as well as the activity of other (Na+-independent, cipargamin-insensitive) ATPases. Inhibition of various ATPases by millimolar [Ca2+] has been reported in previous studies (28, 29), and it may be explained at least in part by Ca2+ competing with Mg2+ for protein binding (28).
K+ was without effect on PfATP4-associated ATPase activity when present at concentrations ranging from 0 to 150 mm. The slight stimulation of membrane ATPase activity in low [Na+] conditions by [K+] of 15–120 mm suggests that there may be an active K+-dependent ATPase in parasite membranes.
The system described here to study PfATP4 is more direct than whole-cell approaches, and it enables the precise control of experimental conditions required to characterize PfATP4 function and drug interactions. The assay will enable an evaluation of the function of mutant forms of the PfATP4 protein that arise in the laboratory or the field and the determination of whether (and how potently) antiplasmodial compounds inhibit different variants of the transporter.
Experimental procedures
Ethics statement
The use of human blood in this study was approved by the Australian National University Human Research Ethics Committee. The blood was provided by the Australian Red Cross Blood Service without revealing the identities of the donors. The donors signed an agreement with the Blood Service granting permission for their blood to be used for medical research.
Parasite culture and isolation
Unless stated otherwise, a cloned Dd2 line (the parent of Dd2-PfATP4T418N, generated from the Dd2 strain by limiting dilution (30)) was used throughout this study. In a previous study (18), the cloned Dd2 parasites were exposed to the PfATP4-associated Malaria Box compound MMV007275, yielding MMV007275-resistant parasites (18). The Dd2-PfATP4T418N parasite line for which data are shown in Fig. 6 and Table 1 was obtained from the MMV007275-resistant bulk culture by limiting dilution for use in this study. The Dd2-PfATP4T418N,P990R parasites and their matched parental parasites (from Ref. 2; for which data are shown in Table 1 and Figs. 6 and 7) were generously provided by Elizabeth Winzeler.
Parasites were cultured in human erythrocytes (31) with continuous shaking (32) and were synchronized by exposure of cultures to sorbitol (33). The culture medium was RPMI 1640 medium containing 25 mm HEPES (Gibco) supplemented with 11 mm additional glucose, 0.2 mm hypoxanthine, 20 μg/ml gentamicin sulfate, and 3 g/liter Albumax II.
Prior to preparing membranes, mature trophozoite-stage parasites (∼34–40 h post-invasion) were functionally isolated from their host erythrocytes by brief exposure (of cultures at ∼4% hematocrit) to saponin (0.05% w/v, of which ≥10% was the active agent sapogenin) (34). The parasites were then washed several times in bicarbonate-free RPMI 1640 medium supplemented with 11 mm additional glucose, 0.2 mm hypoxanthine, and 25 mm HEPES, pH 7.10.
Preparation of parasite membranes
Membranes were prepared from saponin-isolated trophozoite-stage parasites essentially as described previously (15). Briefly, parasites were sedimented by centrifugation (12,000 × g, 30 s) and then lysed in ice-cold deionized water containing protease inhibitors (a 1:500 dilution of Protease Inhibitor Mixture Set III; Merck Millipore). The membrane preparation was then washed three times in ice-cold deionized water (14,000 × g for 10 min at 4 °C, with protease inhibitors present for the first two washes). The protein content in the membrane preparation was measured using a modified Bradford assay (35). The membrane preparation is likely to contain membrane from the host erythrocyte and the parasite's intracellular organelles, in addition to parasite plasma membrane.
ATPase assays
ATPase activity was quantified by measuring the production of Pi from the hydrolysis of ATP, using the PiColorLock Gold Phosphate Detection System (Innova Biosciences). Unless stated otherwise in the relevant figure legends, the duration of the reactions was 10 min, and the final reaction mixtures had a pH of 7.2 and consisted of 20 mm KCl, 2 mm MgCl2, 50 mm Tris, NaCl and/or choline chloride ([NaCl] + [choline chloride] = 150 mm), parasite membrane (final concentration of total protein = 50 μg/ml), and 1 mm ATP (Na2ATP·3H2O; MP Biomedicals; added last to initiate the reaction).
To terminate the reaction, 100 μl of each reaction mixture was added (in duplicate) to 25 μl of “Gold mix” in a 96-well plate. “Stabilizer” was added 2–3 min later, and the plate was incubated for 1 h before absorbance was measured at 635 nm. Blank values (from wells containing only water, Gold mix, and stabilizer) and control values (from wells containing all components, but to which ATP was not added until after the membrane was exposed to Gold mix) were subtracted from the data. PfATP4-associated ATPase activity was determined for each condition by subtracting the data obtained in the presence of 500 nm cipargamin from that obtained in the absence of cipargamin. For experiments in which a range of [Na+] were tested, a line was fitted to the data obtained over the range of [Na+] in the presence of cipargamin, and for each [Na+] the ATPase activity estimated from the fitted line (i.e. the ATPase activity in the presence of cipargamin) was subtracted from the ATPase activity measured at the corresponding [Na+] in the absence of cipargamin to yield the PfATP4-associated ATPase activity.
Parasite proliferation assays
Parasite proliferation assays were commenced with P. falciparum cultures adjusted to 1% parasitemia (consisting of predominantly ring-stage parasites) and 1% hematocrit. Parasite proliferation was determined using a DNA-intercalating fluorescent dye (36) after a 72-h incubation with the compound of interest, using a method detailed previously (37).
Statistics and fitted curves
Statistical comparisons were performed using either t-tests or one-way ANOVAs (as stated in the relevant sections). ANOVAs were performed in GenStat (15th edition). In the case of data that are presented as a percentage of a control value (Fig. 5, a, c, and e, and Fig. S1) ANOVAs were performed with the pre-normalized data, and the “experiment” was nominated as a “blocking factor.” The least significant difference test was used for post hoc comparisons. p values < 0.05 indicate statistical significance.
In those cases in which equations were fitted to data to yield estimates of fitted constants the relevant equations are specified. In those cases in which curves are fitted simply to serve as illustrative trend lines, the underlying (arbitrary) equations are not specified.
Author contributions
J. E. O. R., K. K., and A. M. L. conceptualization; J. E. O. R., M. C. R., and A. M. L. formal analysis; J. E. O. R. and M. C. R. investigation; J. E. O. R., R. L. S., K. K., and A. M. L. methodology; J. E. O. R., M. C. R., R. L. S., and K. K. writing-review and editing; K. K. and A. M. L. resources; K. K. and A. M. L. supervision; K. K. and A. M. L. funding acquisition; K. K. and A. M. L. project administration; A. M. L. writing-original draft.
Supplementary Material
Acknowledgments
We are grateful to MMV for providing cipargamin, to Elizabeth Winzeler for providing the Dd2-PfATP4T418N,P990R line and its parent, and to the Canberra Branch of the Australian Red Cross Blood Service for the provision of blood.
This work was supported by an Australian Research Council Discovery Early Career Researcher Award (DE160101035 to A. M. L.), an Australian Research Council Linkage Project Grant (LP150101226 to K. K.), and a National Health and Medical Research Council Project Grant (1042272 to K. K.). The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as one of our Editors' Picks.
- MMV
- Medicines for Malaria Venture
- ANOVA
- analysis of variance.
References
- 1. White N. J., Pukrittayakamee S., Phyo A. P., Rueangweerayut R., Nosten F., Jittamala P., Jeeyapant A., Jain J. P., Lefèvre G., Li R., Magnusson B., Diagana T. T., and Leong F. J. (2014) Spiroindolone KAE609 for falciparum and vivax malaria. N. Engl. J. Med. 371, 403–410 10.1056/NEJMoa1315860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rottmann M., McNamara C., Yeung B. K., Lee M. C., Zou B., Russell B., Seitz P., Plouffe D. M., Dharia N. V., Tan J., Cohen S. B., Spencer K. R., González-Páez G. E., Lakshminarayana S. B., Goh A., et al. (2010) Spiroindolones, a potent compound class for the treatment of malaria. Science 329, 1175–1180 10.1126/science.1193225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krishna S., Woodrow C., Webb R., Penny J., Takeyasu K., Kimura M., and East J. M. (2001) Expression and functional characterization of a Plasmodium falciparum Ca2+-ATPase (PfATP4) belonging to a subclass unique to apicomplexan organisms. J. Biol. Chem. 276, 10782–10787 10.1074/jbc.M010554200 [DOI] [PubMed] [Google Scholar]
- 4. Ginsburg H., Kutner S., Krugliak M., and Cabantchik Z. I. (1985) Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 14, 313–322 10.1016/0166-6851(85)90059-3 [DOI] [PubMed] [Google Scholar]
- 5. Kirk K., Horner H. A., Elford B. C., Ellory J. C., and Newbold C. I. (1994) Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem. 269, 3339–3347 [PubMed] [Google Scholar]
- 6. Staines H. M., Ellory J. C., and Kirk K. (2001) Perturbation of the pump-leak balance for Na+ and K+ in malaria-infected erythrocytes. Am. J. Physiol. Cell Physiol. 280, C1576–C1587 10.1152/ajpcell.2001.280.6.C1576 [DOI] [PubMed] [Google Scholar]
- 7. Lee P., Ye Z., Van Dyke K., and Kirk R. G. (1988) X-ray microanalysis of Plasmodium falciparum and infected red blood cells: effects of qinghaosu and chloroquine on potassium, sodium, and phosphorus composition. Am. J. Trop. Med. Hyg. 39, 157–165 10.4269/ajtmh.1988.39.157 [DOI] [PubMed] [Google Scholar]
- 8. Mauritz J. M., Seear R., Esposito A., Kaminski C. F., Skepper J. N., Warley A., Lew V. L., and Tiffert T. (2011) X-ray microanalysis investigation of the changes in Na, K, and hemoglobin concentration in Plasmodium falciparum-infected red blood cells. Biophys. J. 100, 1438–1445 10.1016/j.bpj.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winterberg M., and Kirk K. (2016) A high-sensitivity HPLC assay for measuring intracellular Na+ and K+ and its application to Plasmodium falciparum infected erythrocytes. Sci. Rep. 6, 29241 10.1038/srep29241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai S. A., and Rosenberg R. L. (1997) Pore size of the malaria parasite's nutrient channel. Proc. Natl. Acad. Sci. U.S.A. 94, 2045–2049 10.1073/pnas.94.5.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai S. A., Krogstad D. J., and McCleskey E. W. (1993) A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362, 643–646 10.1038/362643a0 [DOI] [PubMed] [Google Scholar]
- 12. Dennis A. S. M., Lehane A. M., Ridgway M. C., Holleran J. P., and Kirk K. (2018) Cell swelling induced by the antimalarial KAE609 (cipargamin) and other PfATP4-associated antimalarials. Antimicrob. Agents Chemother. 62, e00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dennis A. S. M., Rosling J. E. O., Lehane A. M., and Kirk K. (2018) Diverse antimalarials from whole-cell phenotypic screens disrupt malaria parasite ion and volume homeostasis. Sci. Rep. 8, 8795 10.1038/s41598-018-26819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flannery E. L., McNamara C. W., Kim S. W., Kato T. S., Li F., Teng C. H., Gagaring K., Manary M. J., Barboa R., Meister S., Kuhen K., Vinetz J. M., Chatterjee A. K., and Winzeler E. A. (2015) Mutations in the P-type cation-transporter ATPase 4, PfATP4, mediate resistance to both aminopyrazole and spiroindolone antimalarials. ACS Chem. Biol. 10, 413–420 10.1021/cb500616x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spillman N. J., Allen R. J., McNamara C. W., Yeung B. K., Winzeler E. A., Diagana T. T., and Kirk K. (2013) Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13, 227–237 10.1016/j.chom.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hewitt S. N., Dranow D. M., Horst B. G., Abendroth J. A., Forte B., Hallyburton I., Jansen C., Baragaña B., Choi R., Rivas K. L., Hulverson M. A., Dumais M., Edwards T. E., Lorimer D. D., Fairlamb A. H., et al. (2017) Biochemical and structural characterization of selective allosteric inhibitors of the Plasmodium falciparum drug target, prolyl-tRNA-synthetase. ACS Infect. Dis. 3, 34–44 10.1021/acsinfecdis.6b00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jimenez-Diaz M. B., Ebert D., Salinas Y., Pradhan A., Lehane A. M., Myrand-Lapierre M. E., O'Loughlin K. G., Shackleford D. M., Justino de Almeida M., Carrillo A. K., Clark J. A., Dennis A. S., Diep J., Deng X., Duffy S., et al. (2014) (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. U.S.A. 111, E5455–E5462 10.1073/pnas.1414221111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehane A. M., Ridgway M. C., Baker E., and Kirk K. (2014) Diverse chemotypes disrupt ion homeostasis in the malaria parasite. Mol. Microbiol. 94, 327–339 10.1111/mmi.12765 [DOI] [PubMed] [Google Scholar]
- 19. Vaidya A. B., Morrisey J. M., Zhang Z., Das S., Daly T. M., Otto T. D., Spillman N. J., Wyvratt M., Siegl P., Marfurt J., Wirjanata G., Sebayang B. F., Price R. N., Chatterjee A., Nagle A., et al. (2014) Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nat. Commun. 5, 5521 10.1038/ncomms6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spillman N. J., and Kirk K. (2015) The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int. J. Parasitol. Drugs Drug Resist. 5, 149–162 10.1016/j.ijpddr.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hapuarachchi S. V., Cobbold S. A., Shafik S. H., Dennis A. S., McConville M. J., Martin R. E., Kirk K., and Lehane A. M. (2017) The malaria parasite's lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog. 13, e1006180 10.1371/journal.ppat.1006180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhn Y., Rohrbach P., and Lanzer M. (2007) Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell. Microbiol. 9, 1004–1013 10.1111/j.1462-5822.2006.00847.x [DOI] [PubMed] [Google Scholar]
- 23. Goldgof G. M., Durrant J. D., Ottilie S., Vigil E., Allen K. E., Gunawan F., Kostylev M., Henderson K. A., Yang J., Schenken J., LaMonte G. M., Manary M. J., Murao A., Nachon M., Stanhope R., et al. (2016) Comparative chemical genomics reveal that the spiroindolone antimalarial KAE609 (Cipargamin) is a P-type ATPase inhibitor. Sci. Rep. 6, 27806 10.1038/srep27806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crambert G., Hasler U., Beggah A. T., Yu C., Modyanov N. N., Horisberger J. D., Lelièvre L., and Geering K. (2000) Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem. 275, 1976–1986 10.1074/jbc.275.3.1976 [DOI] [PubMed] [Google Scholar]
- 25. Zahler R., Zhang Z. T., Manor M., and Boron W. F. (1997) Sodium kinetics of Na,K-ATPase α isoforms in intact transfected HeLa cells. J. Gen. Physiol. 110, 201–213 10.1085/jgp.110.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Almeida-Amaral E. E., Caruso-Neves C., Pires V. M., and Meyer-Fernandes J. R. (2008) Leishmania amazonensis: characterization of an ouabain-insensitive Na+-ATPase activity. Exp. Parasitol. 118, 165–171 10.1016/j.exppara.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 27. Saliba K. J., and Kirk K. (1999) pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H+ extrusion via a V-type H+-ATPase. J. Biol. Chem. 274, 33213–33219 10.1074/jbc.274.47.33213 [DOI] [PubMed] [Google Scholar]
- 28. Robinson J. D. (1981) Effect of cations on (Ca2+ + Mg2+)-activated ATPase from rat brain. J. Neurochem. 37, 140–146 10.1111/j.1471-4159.1981.tb05301.x [DOI] [PubMed] [Google Scholar]
- 29. Winkler B. S., and Riley M. V. (1980) Influence of calcium on retinal ATPases. Invest. Ophthalmol. Vis. Sci. 19, 562–564 [PubMed] [Google Scholar]
- 30. Adjalley S. H., Lee M. C., and Fidock D. A. (2010) A method for rapid genetic integration into Plasmodium falciparum utilizing mycobacteriophage Bxb1 integrase. Methods Mol. Biol. 634, 87–100 10.1007/978-1-60761-652-8_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trager W., and Jensen J. B. (1976) Human malaria parasites in continuous culture. Science 193, 673–675 10.1126/science.781840 [DOI] [PubMed] [Google Scholar]
- 32. Allen R. J., and Kirk K. (2010) Plasmodium falciparum culture: the benefits of shaking. Mol. Biochem. Parasitol. 169, 63–65 10.1016/j.molbiopara.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 33. Lambros C., and Vanderberg J. P. (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418–420 10.2307/3280287 [DOI] [PubMed] [Google Scholar]
- 34. Saliba K. J., Horner H. A., and Kirk K. (1998) Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem. 273, 10190–10195 10.1074/jbc.273.17.10190 [DOI] [PubMed] [Google Scholar]
- 35. Ernst O., and Zor T. (2010) Linearization of the Bradford protein assay. J. Vis. Exp. 2010, 1918 10.3791/1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smilkstein M., Sriwilaijaroen N., Kelly J. X., Wilairat P., and Riscoe M. (2004) Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48, 1803–1806 10.1128/AAC.48.5.1803-1806.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spry C., Macuamule C., Lin Z., Virga K. G., Lee R. E., Strauss E., and Saliba K. J. (2013) Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. PLoS ONE 8, e54974 10.1371/journal.pone.0054974 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.