Abstract
The Arc (anoxic redox control) two-component system of Escherichia coli, comprising ArcA as the response regulator and ArcB as the sensor histidine kinase, modulates the expression of numerous genes in response to respiratory growth conditions. Under reducing growth conditions, ArcB autophosphorylates at the expense of ATP, and transphosphorylates ArcA via a His292 → Asp576 → His717 → Asp54 phosphorelay, whereas under oxidizing growth conditions, ArcB catalyzes the dephosphorylation of ArcA-P by a reverse Asp54 → His717 → Asp576 → Pi phosphorelay. However, the exact phosphoryl group transfer routes and the molecular mechanisms determining their directions are unclear. Here, we show that, during signal propagation, the His292 → Asp576 and Asp576 → His717 phosphoryl group transfers within ArcB dimers occur intra- and intermolecularly, respectively. Moreover, we report that, during signal decay, the phosphoryl group transfer from His717 to Asp576 takes place intramolecularly. In conclusion, we present a mechanism that dictates the direction of the phosphoryl group transfer within ArcB dimers and that enables the discrimination of the kinase and phosphatase activities of ArcB.
Keywords: bacteria, histidine kinase, bacterial signal transduction, phosphorylation, phosphoryl transfer, ArcB/A two-component system, sensor kinase, phosphorelay, intramolecular phosphotransfer, intermolecular phosphotransfer, conformation
Introduction
The Arc two-component signal transduction system plays an important role in the transcriptional regulatory network that allows facultative anaerobic bacteria, such as Escherichia coli, to sense and signal changes in respiratory growth conditions and to adapt their gene expression accordingly (1–4). This system consists of the cytoplasmic response regulator ArcA, and the membrane-anchored sensor kinase ArcB (5, 6). ArcA is a typical response regulator, possessing an N-terminal receiver domain with a phosphoryl group–accepting Asp residue at position 54 and a C-terminal helix-turn-helix DNA-binding domain. In contrast, ArcB is an unorthodox sensor kinase that contains three catalytic cytosolic domains: a transmitter domain (H1) with a conserved His292 residue, a central receiver domain (D1) with a conserved Asp576 residue, and a C-terminal histidine phosphotransfer domain (H2) with a conserved His717 residue (6, 7). In addition, the ArcB protein contains a functional leucine zipper (8) and a PAS domain (9), both located in the linker region, which is the segment connecting the transmembrane domain with the transmitter domain.
Under reducing growth conditions, ArcB autophosphorylates in an ATP-dependent manner, a process that is enhanced by certain anaerobic metabolites, such as d-lactate, acetate, and pyruvate (10, 11), and transphosphorylates ArcA via a His292 → Asp576 → His717 → Asp54 phosphorelay (12, 13). Phosphorylated ArcA (ArcA-P),2 in turn, represses the expression of many operons involved in respiratory metabolism and activates some others encoding proteins involved in fermentative metabolism (14–17). Under oxic growth conditions, ArcB acts as a specific ArcA-P phosphatase, catalyzing the dephosphorylation of ArcA-P by a reverse Asp54 → His717 → Asp576 → Pi phosphorelay (18, 19). The catalytic activity of ArcB has been shown to be set by rotational movements that alter the orientation of the cytosolic portion of ArcB (20). Moreover, the molecular event for ArcB regulation involves the oxidation or reduction of two cytosol-located redox-active cysteine residues that participate in intermolecular disulfide bond formation, a reaction in which the quinol/quinone electron carriers act as the direct oxidants or reductants (21–24).
In general, histidine sensor kinases act as homodimers (25–28) and autophosphorylate by either an inter- or intramolecular reaction (29–35). Also, studies on the subsequent phosphoryl group (∼P) transfer steps (i.e. from H1 to D1 and from D1 to H2) in various hybrid histidine kinases indicate that they can occur either intra- or intermolecularly, depending on the particular sensor kinase protein and on the specific ∼P transfer step (36–38). In contrast, the ∼P transfer step from H2 to D1 in the reverse phosphorelay, responsible for the phosphatase activity of these hybrid sensor kinases, has yet to be addressed.
In the case of ArcB, the autophosphorylation reaction appears to occur intramolecularly, as the γ-phosphoryl group of ATP bound to one monomer in the homodimer was shown to be transferred to the histidine of the same monomer (35, 38). In contrast, the mode of ∼P transfer in the two subsequent steps in ArcB signaling remains ambiguous, because two independent studies reached dissimilar conclusions (38, 39). On one hand, in a report based on in vivo experiments analyzed with mathematical and statistical models, it was suggested that a bimolecular or allosteric mechanism, in which the integrity of all phospho-accepting/donating sites is required and in which no exclusive intra- or intermolecular ∼P transfer mechanisms are identifiable, may be operating during ArcB signaling (39). On the other hand, in a later study based on in vitro complementation analyses, it was proposed that the ∼P transfers from H1 to D1 and from D1 to H2 occur intermolecularly (38).
Here, we present results from in vitro and in vivo experiments addressing the above mentioned discrepancy and extend these studies to include the characterization of the H2 to D1 ∼P transfer step, involved in ArcA-P dephosphorylation during signal decay. Our results demonstrate that the ∼P transfer from H1 to D1 occurs exclusively intramolecularly, whereas the following step (i.e. from D1 to H2) occurs preferentially intermolecularly. Finally, the H2 to D1 ∼P transfer, responsible for signal decay, shows a clear preference for the intramolecular reaction. The consequences of the proposed ∼P transfers between the various ArcB modules for the regulation of signal transmission and signal decay of the Arc two-component system are discussed.
Results
Probing the mode of phosphoryl group transfer from the transmitter domain to the receiver domain of ArcB
Autophosphorylation of the tripartite sensor kinase ArcB was shown to be an intramolecular reaction (35, 38), whereas contradictory results were reported for the mode of ∼P transfer in the two subsequent steps (38, 39). Therefore, we attempted to probe the mode of these ∼P transfer steps by an approach different from the ones used previously.
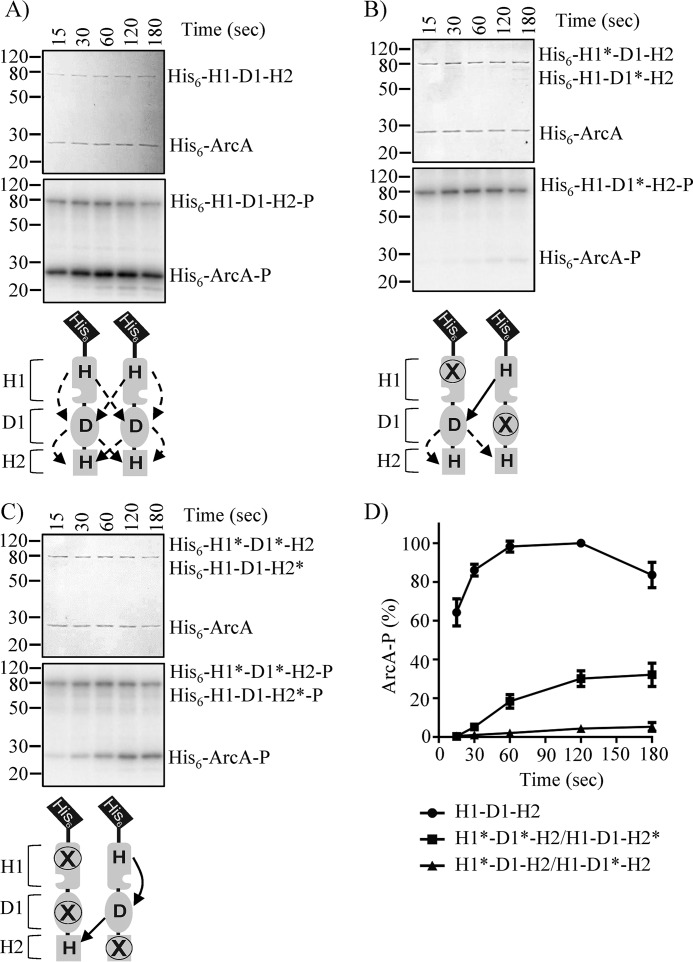
To this end, we generated N-terminal His6-tagged cytosolic WT and mutant ArcB variants (hereafter referred to as His6-H1-D1-H2), carrying single or multiple punctual mutations of the conserved phosphorylation sites. In other words, the conserved histidine 292 in H1 was replaced by glutamine (hereafter referred to as H1*), the conserved aspartate 576 in D1 was replaced by alanine (hereafter referred to as D1*), and the conserved histidine 717 in H2 was replaced by glutamine (hereafter referred to as H2*). WT and single- and multiple-mutant His6-H1-D1-H2 proteins were purified, and their ability to autophosphorylate and transphosphorylate His6-ArcA (hereafter referred to as ArcA) was tested. ArcA was rapidly phosphorylated by His6-H1-D1-H2 (Fig. 1A), but not by His6-H1*-D1-H2, His6-H1-D1*-H2, His6-H1-D1-H2*, or His6-H1*-D1*-H2 mutant proteins (Fig. S1, A–D), in agreement with previous reports (12, 13). The ability of combinations of mutant His6-H1-D1-H2 variants to transphosphorylate ArcA was then evaluated. No ArcA phosphorylation was observed in the reaction mixture containing His6-H1*-D1-H2 and His6-H1-D1*-H2, in which only intermolecular H1 to D1 phosphotransfer is allowed (Fig. 1, B and D). On the other hand, a weak ArcA phosphorylation, ∼30% of that observed by the WT His6-H1-D1-H2, was obtained in the reaction mixture containing His6-H1*-D1*-H2 and His6-H1-D1-H2*, in which only the intramolecular H1 to D1 ∼P transfer is permitted (Fig. 1, C and D), indicating that the ∼P transfer from H1 to D1 occurs intramolecularly. However, this conclusion is hampered by the fact that none of the combinations was able to restore the phosphorelay at WT ArcB levels, most likely due to the insufficient heterodimer formation in the reaction mixture.
Figure 1.
ArcA phosphorylation by His6-ArcB78–778 and by combinations of His6-ArcB78–778 phosphorelay mutants. Purified ArcA was incubated in a 30-μl reaction mixture with [γ-32P]ATP and His6-H1-D1-H2 (A), His6-H1*D1-H2 and His6-H1-D1*-H2 (B), or His6-H1*-D1*-H2 and His6-H1-D1-H2* (C). At the indicated time intervals, 5-μl samples were withdrawn and subjected to SDS-PAGE analysis. Coomassie Blue-stained gels revealing protein bands (top panels), corresponding autoradiograms (middle panels), and schemes showing the permitted ∼P transfers into ArcB dimers (bottom panels) are presented. The molecular mass standard values (kDa) are shown on the left, and the position of each polypeptide in the gel is indicated on the right of each panel. D, relative amount of ArcA-P formed, as quantified by densitometric analysis of the autoradiogams. Data represent the averages from at least three independent experiments, and S.D. values (error bars) are indicated.
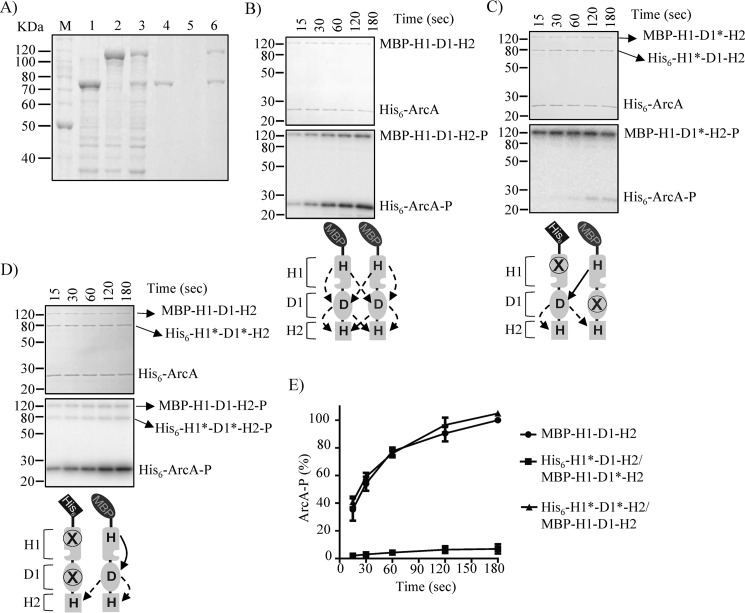
To ensure heterodimer formation, a set of plasmids carrying arabinose-inducible MBP-tagged H1-D1-H2 variants were constructed and transformed in strains harboring plasmids that express complementary His6-tagged H1-D1-H2 mutant proteins. The desired combinations of MBP-tagged and His6-tagged protein versions were simultaneously overexpressed and purified under native conditions, using Ni-NTA affinity chromatography. According to this approach, only His6-tagged/MBP-tagged protein heterodimers and His6-tagged protein homodimers should be purified, and MBP-tagged protein homodimers should not be. An example of this approach is presented in Fig. 2A. The obtained results demonstrate that His6-H1*-D1-H2 and MBP-H1-D1*-H2 were readily overexpressed (Fig. 2A, lanes 2 and 3) and that only His6-H1*-D1-H2, and not MBP-H1-D1*-H2, was retained by and eluted from the nickel-nitrilotriacetic acid resin (Fig. 2A, lanes 5 and 6). However, when both proteins were co-expressed (Fig. 2A, lane 4), the MBP-H1-D1*-H2 protein co-eluted with His6-H1*-D1-H2 to almost stoichiometric amounts (Fig. 2A, lane 7), ensuring heterodimer formation.
Figure 2.
Probing the mode of H1 to D1 ∼P group transfer by in vitro phosphorelay complementation assays using His6-ArcB78–778*/MBP-ArcB78–778* dimers. A, overexpression of His6-H1*-D1-H2 (lane 1), MBP-H1-D1*-H2 (lane 2), and His6-H1*-D1-H2/MBP-H1-D1*-H2 (lane 3) was analyzed by SDS-PAGE and Coomassie Blue staining. Overexpressed proteins were purified by Ni-NTA affinity chromatography, and the elution fractions of His6-H1*-D1-H2 (lane 4), MBP-H1-D1*-H2 (lane 5), and His6-H1*-D1-H2/MBP-H1- D1*-H2 (lane 6) were analyzed by SDS-PAGE and visualized by Coomassie Blue staining. Purified ArcA was incubated in a 30-μl reaction mixture with [γ-32P]ATP and purified/co-eluted MBP-H1-D1-H2 (B), His6-H1*-D1-H2/MBP-H1-D1*-H2 (C), or His6-H1*-D1*-H2/MBP-H1-D1-H2 (D). At the indicated time intervals, 5-μl samples were withdrawn and subjected to SDS-PAGE analysis. Coomassie Blue-stained gels revealing protein bands (top panels), the corresponding autoradiograms (middle panels), and schemes showing the permitted ∼P transfers (bottom panels) are presented. The molecular mass standard values (kDa) are shown on the left, and the position of each polypeptide in the gel is indicated on the right of each panel. E, relative amount of ArcA-P formed, as quantified by densitometric analysis of the autoradiograms. Data represent the averages from at least three independent experiments, and the S.D. values (error bars) are indicated.
Single MBP-tagged proteins, used for heterodimer formation, were purified and subjected to in vitro phosphorylation assays with [γ-32P]ATP and His6-ArcA. Only the MBP-tagged proteins having a WT H1 were able to autophosphorylate (Fig. S1E), and only the MBP-H1-D1-H2 was able to transphosphorylate ArcA (Fig. 2B), indicating that the purified proteins have the expected activities and that the N-terminal MBP tag does not interfere with the activity of ArcB. Subsequently, the ability of the His6-H1*-D1-H2/MBP-H1-D1*-H2 heterodimer, in which only intermolecular phosphoryl group transfer from H1 to D1 is permitted, to functionally complement and restore the phosphorelay to ArcA was tested (Fig. 2C). Although the MBP-H1-D1*-H2 protein was rapidly autophosphorylated, no His6-H1*-D1-H2-P was observed, and almost no ArcA-P was formed (Fig. 2, C and E), suggesting that the H1 to D1 phosphotransfer does not occur intermolecularly. In contrast, the His6-H1*-D1*-H2/MBP-H1-D1-H2 heterodimer was found to readily transphosphorylate ArcA (Fig. 2, D and E), indicating that the phosphoryl group transfer from H1 to D1 occurs intramolecularly, at least in vitro. It has to be noted that phosphorylation of His6-H1*-D1*-H2 was also observed, most likely due to intermolecular phosphoryl group transfer from D1 to H2 or from ArcA to H2.
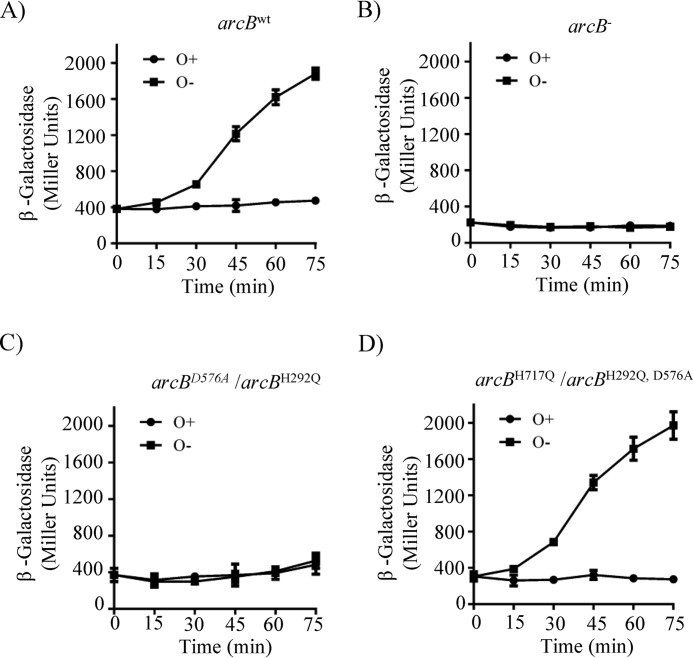
To verify the in vitro results by in vivo experiments, low-copy number plasmids carrying the arcBH292Q,D576A (pEXT22 CmArcBH292Q,D576A) and arcBH292Q (pEXT22CmArcBH292Q) mutant alleles were generated and transformed, respectively, into the arcBH717Q (IFC2002) and arcBD576A (IFC2001) mutant strains, both carrying a λΦ(cydA′-lacZ) reporter (40). Plasmid pEXT22, which was reported to have 1–2 copies/cell (41), and arcB mutant alleles under the control of the native arcB promoter were chosen to avoid the overexpression of the sensor kinase. This is because distortion of the ArcB/ArcA concentration balance has been reported to produce unpredictable effects on reporter expression (42). Indeed, Western blot analysis using ArcB polyclonal antibodies, raised against purified His6-ArcB78–520 (40), revealed that all plasmid-borne arcB alleles produce similar to WT levels of ArcB protein (Fig. S2E). Subsequently, the phenotypic consequences of the above described strains were analyzed by changes in the in vivo levels of phosphorylated ArcA, as indicated by the expression of the ArcA-P–activatable λΦ(cydA′-lacZ) reporter (Fig. 3). As expected, arcB-null phenotypes were found for strains IFC2002 (arcBH717Q) and IFC2001 (arcBD576A) and when the plasmid-borne arcBH717Q,D576A and arcBH292A were expressed in the ΔarcB mutant strain ECL5004 (Fig. S2, A–D), in agreement with the in vitro results and previous reports (12). Moreover, expression of the plasmid-borne arcBH292Q in IFC2001 (arcBD576A), where only intermolecular ∼P transfer from H1 to D1 is permitted, resulted in an arcB-null phenotype (Fig. 3, B and C). In contrast, expression of the plasmid-borne arcBH292Q,D576A in IFC2002 (arcBH717Q), where only intramolecular phosphoryl group transfer from H1 to D1 is permitted, resulted in WT levels of reporter expression (Fig. 3, A and D), in accordance with the above presented in vitro results. Therefore, it appears reasonable to conclude that an intramolecular mode of ∼P transfer operates from H1 to D1. This result also suggests that the in vivo ∼P transfer from D1 to H2 occurs intermolecularly, although the intramolecular mode cannot be excluded. Taken together, the above results from the in vitro and in vivo experiments provide strong evidence that the phosphorelay along ArcB comprises an intramolecular H1 to D1 ∼P transfer.
Figure 3.
Co-expression of arcBH717Q and arcBH292Q,D576A restores ArcB activity and regulation of reporter expression. Cultures of strain ECL5003 (arcBwt) (A) and its isogenic strains ECL5004 (arcB−) (B) and IFC2001 (arcBD576A) (C) harboring plasmid pEXT22CmArcBH292Q and IFC2002 (arcBH717Q) (D) harboring plasmid pEXT22CmArcBH292Q,D576A, all carrying the ArcA-P–activatable λΦ(cydA′-lacZ) reporter, were grown aerobically in LB buffered with 0.1 m MOPS (pH 7.4) and supplemented with 20 mm d-xylose. At an A600 of 0.2, one aliquot was withdrawn, to measure the β-gal activity (depicted as 0 min), and the rest of the culture was divided in two. One part was kept under aerobic conditions (circles) as a control, whereas the other was shifted to anaerobiosis (squares), and the time course of the β-gal activity was followed. The data represent the averages from three independent experiments, and the S.D. values (error bars) are indicated.
Probing the mode of ∼P transfer from D1 to H2 within the ArcB dimer
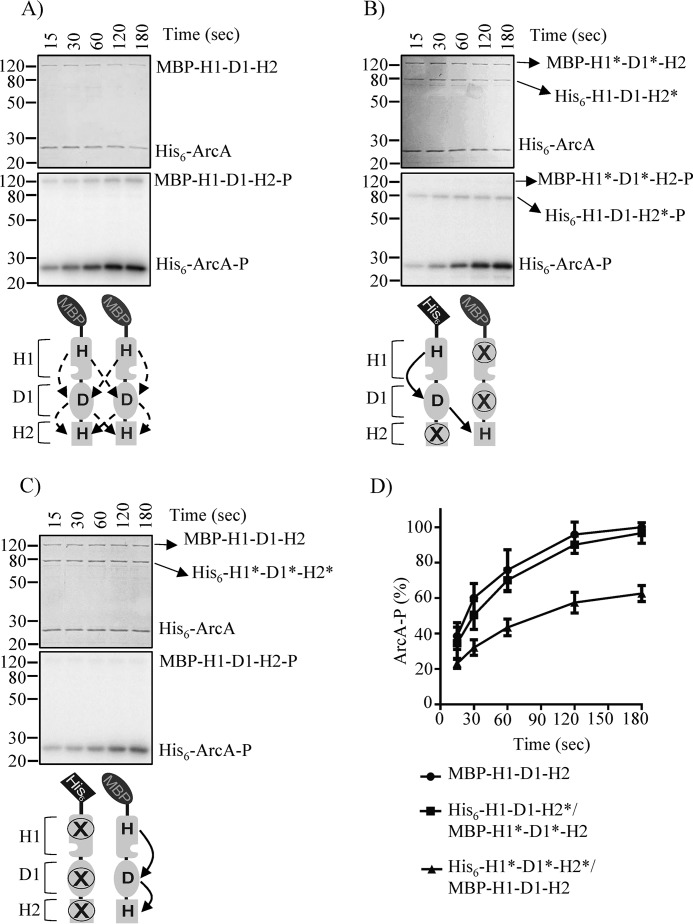
We then asked whether the D1 to H2 ∼P transfer step within ArcB dimers occurs intra- or intermolecularly. To this end, the His6-H1-D1-H2*/MBP-H1*-D1*-H2 heterodimer, permitting intermolecular ∼P transfer, and His6-H1*-D1*-H2*/MBP-H1-D1-H2 heterodimer, permitting intramolecular ∼P transfer, were obtained, and their ability to complete the phosphorelay and transphosphorylate ArcA was evaluated. His6-H1-D1-H2*/MBP-H1*-D1*-H2 was found to phosphorylate ArcA at WT ArcB levels, whereas the His6-H1*-D1*-H2*/MBP-H1-D1-H2 heterodimer phosphorylated ArcA at a significantly lower rate (Fig. 4). In contrast, homodimers composed by any of the ArcB mutant variants were unable to phosphorylate ArcA (Fig. S1). These results indicate that the D1 to H2 ∼P transfer step occurs preferentially intermolecularly in vitro, in agreement with a previous study (38). Indeed, in the above presented in vivo complementation assay, co-expression of the arcBH717Q and arcBH292Q,D576A mutant alleles resulted in WT levels of reporter expression (Fig. 3D), indicating that an intermolecular D1 to H2 phosphotransfer does occur during in vivo ArcB signaling. The direct evaluation of an intramolecular ∼P transfer from D1 to H2 in vivo, requiring the expression of ArcBD576A,H717Q and ArcBH292A, however, is unattainable because the ∼P transfer step from H1 to D1 is exclusively intramolecular, rendering the use of a WT ArcB mandatory, which, in turn, impedes the discrimination between the two modes of ∼P transfer. To circumvent this problem, we reasoned that expression of increasing amounts of arcBD576A,H717Q in an arcBwt background strain should favor ArcBD576A,H717Q/ArcBwt heterodimer formation. Because such heterodimers allow only intramolecular D1 to H2 phosphotransfer, a reduction of the ArcA-P levels should be obtained if an intermolecular D1 to H2 ∼P transfer were to be favored. Because overexpression of a sensor kinase may have unexpected effects on its signaling, increased concentrations of arcBH292Q,D576A in the arcBwt background, favoring the formation of ArcBH292Q,D576A/ArcBwt heterodimers that permit both inter- and intramolecular D1 to H2 phosphotransfer, was used as a control.
Figure 4.
Probing the mode of ∼P group transfer from D1 to H2 in vitro. Purified ArcA was incubated in a 30-μl reaction mixture, in the presence of [γ-32P]ATP, with purified MBP-H1-D1-H2 (A), co-purified His6-H1-D1-H2*/MBP-H1*-D1*-H2 (B), or co-purified His6-H1*-D1*-H2*/MBP-H1-D1-H2 (C). At the indicated time intervals, 5-μl samples were withdrawn and subjected to SDS-PAGE analysis. Coomassie Blue–stained gels revealing protein bands (top panels), the corresponding autoradiograms (middle panels), and schemes showing the permitted ∼P transfers into ArcB dimers (bottom panels) are presented. The molecular mass standard values (kDa) are shown on the left, and the position of each polypeptide in the gel is indicated on the right of each panel. D, relative amount of ArcA-P formed, as quantified by densitometric analysis of the autoradiograms. Data represent the averages from at least three independent experiments, and the S.D. values (error bars) are indicated.
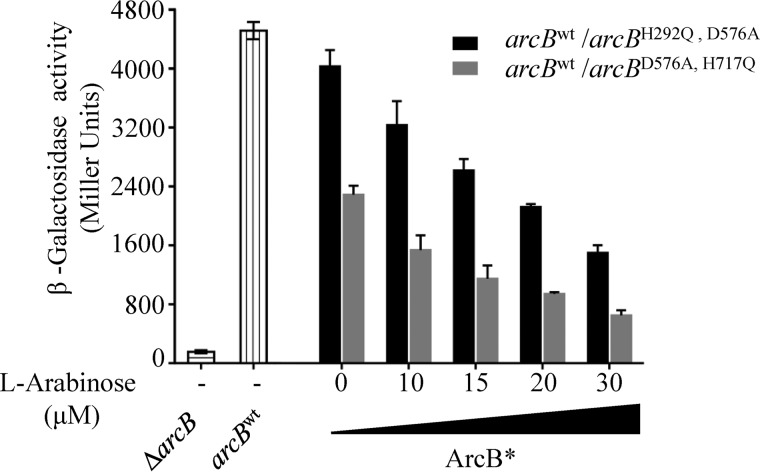
To this end, plasmids pBADArcBH292Q,D576A and pBAD ArcBD576D,H717A, carrying, respectively, the arcBH292Q,D576A and arcBD576A,H717Q mutant alleles under the control of the inducible arabinose promoter were constructed and transformed into the ECL5003 strain, which carries the ArcA-P–activatable λΦ(cydA′-lacZ) reporter (12). The transformants were grown anaerobically in the presence of various concentrations of arabinose (0–30 μm), and at midexponential phase of growth, A600 of ∼0.5, the β-gal activities were determined (Fig. 5). Increasing amounts of inductor in the growth medium of the strain carrying the ArcBH292Q,D576A-expressing plasmid resulted in decreasing levels of reporter expression (Fig. 5). Because the intramolecular H1 to D1 phosphotransfer and both modes of phosphotransfer from D1 to H2 are permitted within the ArcBH292Q,D576A/ArcBwt heterodimer, a possible explanation could be that the increasing amount of ArcBH292Q,D576A homodimers may lead to ∼P transfer from ArcA-P to H2, as has been shown earlier (19), thereby lowering its regulatory activity. Nevertheless, the strain carrying the ArcBD576A,H717Q-expressing plasmid exhibited a more notorious effect. A 40% reduction of reporter expression as compared with that of the ArcBH292Q,D576A-carrying strain was observed under all tested conditions (Fig. 5). It thus appears that, in agreement with the in vitro results, the ∼P transfer from D1 to H2 has a clear preference for the intermolecular reaction in vivo.
Figure 5.
Effect of increasing concentrations of ArcBH292Q,D576A or ArcBH576A,H717Q in an arcBwt WT strain on the anaerobic ArcA phosphorylation in vivo. Strain ECL5003 (arcBwt), carrying plasmid pBADArcBH292Q,D576A (black bars) or pBADArcBD576D,H717A (gray bars) was grown aerobically in LB buffered with 0.1 m MOPS (pH 7.4) and supplemented with 20 mm d-xylose to an A600 of 0.1. The culture was split to five screw-cap tubes (filled to the rim) for anaerobic growth, and l-arabinose was added at the indicated concentrations. At the mid-exponential phase of growth (A600 of 0.5) β-gal activity was assayed. For comparison purposes, β-gal activities of ECL5003 (bars with vertical lines) and ECL5004 (arcB−) (bars with horizontal lines) anaerobic cultures are indicated. The data are averages from three independent experiments, and the S.D. values (error bars) are indicated.
Probing the mode of the H2 to D1 ∼P transfer for ArcA-P dephosphorylation
The amplitude and duration of an adaptive response depend on the balance between the rates of phosphorylation and dephosphorylation of the response regulator. The sensor kinase ArcB is a bifunctional enzyme that phosphorylates ArcA under stimulatory conditions, and it also dephosphorylates ArcA-P under nonstimulatory conditions (12, 13, 18, 19). The fact that the conserved His717 and Asp576 are required for both opposing activities of ArcB (18, 19) raises the intriguing possibility that the forward and the reverse phosphorelay do not have the same pattern with respect to inter- and intramolecular phosphoryl group transfers.
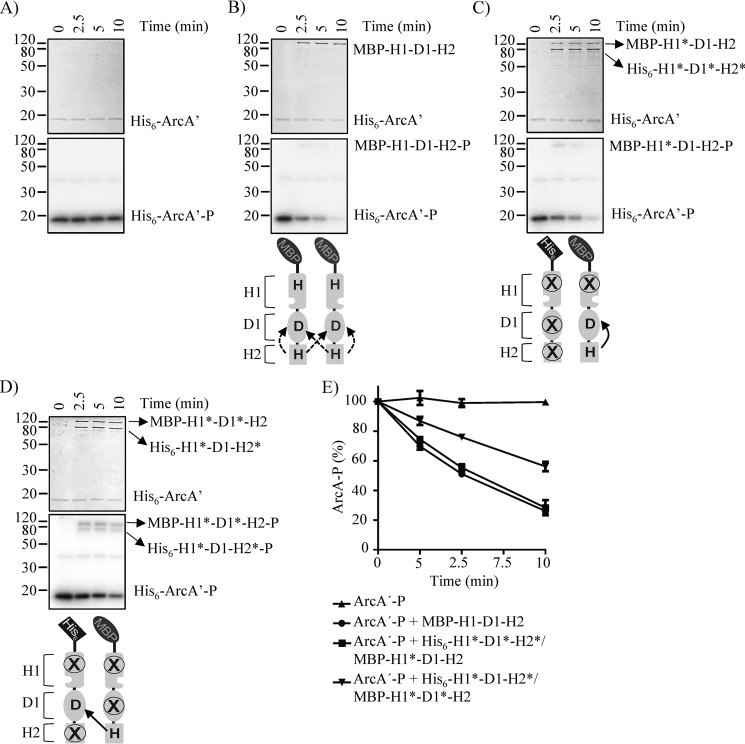
To test this, His6-ArcA1–136 (hereafter referred to as ArcA′), containing the receiver domain but lacking the DNA-binding domain, was purified. ArcA′ was used instead of the full-length His6-ArcA due to the tendency of His6–ArcA-P to aggregate during its purification process (18). The purified protein was incubated with MBP-H1-D1-H2 in the presence of [γ-32P]ATP to generate ArcA′-P. The product, ArcA′-P, was separated from MBP-H1-D1-H2 and [γ-32P]ATP and was used as substrate in phosphatase assays. As observed previously, ArcA′-P was stable, and it maintained in its phosphorylated state during the experiment (Fig. 6A) (18). By contrast, both MBP-H1-D1-H2 and MBP-H1*-D1-H2 were shown to rapidly dephosphorylate ArcA-P (Fig. 6B and Fig. S3A), whereas the His6-H1*-D1-H2* and His6-H1*-D1*-H2* were without effect (Fig. S3, B and C), in agreement with previous reports (18). On the other hand, MBP-H1*-D1*-H2 was able to receive the ∼P from ArcA′-P, but it was unable to release it and, as a consequence, failed to properly dephosphorylate ArcA-P (Fig. S3D), as observed previously (18). In contrast, the His6-H1*-D1*-H2*/MBP-H1*-D1-H2 heterodimer, which only permits the intramolecular H2 to D1 ∼P transfer, dephosphorylated ArcA′-P at levels similar to those exerted by the WT ArcB, whereas the His6-H1*-D1-H2*/MBP-H1*-D1*-H2 heterodimer, which allows only intermolecular ∼P transfer from H2 to D1, dephosphorylated ArcA′-P with a significantly lower rate (Fig. 6, C–E). It thus appears that the reverse H2 to D1 ∼P transfer occurs preferably intramolecularly.
Figure 6.
Testing the ArcA′-P dephosphorylation by MBP-H1-D1-H2 and His6-ArcB78–778*/MBP-ArcB78–778* dimers. Purified ArcA′-P was incubated alone (A) or with MBP-H1-D1-H2 (B), His6-H1*-D1*-H2*/MBP-H1*-D1-H2 (C), or His6-H1*-D1-H2*/MBP-H1*-D1*-H2 (D) in 25-μl reaction mixtures. At the indicated time points, 5-μl samples were withdrawn for SDS-PAGE analysis. Coomassie Blue–stained gels revealing protein bands (top panels), the corresponding autoradiograms (middle panels), and schemes showing the permitted ∼P transfers into ArcB dimers (bottom panels) are presented. The molecular mass standard values (kDa) are shown on the left, and the position of each polypeptide in the gel is indicated on the right of each panel. E, relative amount of ArcA-P formed, as quantified by densitometric analysis of the autoradiograms. Data represent the averages from at least three independent experiments, and the S.D. values (error bars) are indicated.
The mode of ∼P transfer from H2 to D1 was then probed in vivo, by examining the time lag of ArcA-P dephosphorylation after a shift from anaerobic to aerobic growthconditions. To this end, strain ECL5032 (arcBH171Q) harboring the ArcBH292Q,D576A-expressing plasmid (pEXT22CmArcBH292Q,D576A) and the isogenic WT strain ECL5002, both carrying the ArcA-P–repressible λΦ(lldP′-lacZ) reporter (12, 40), were grown anaerobically in lysogeny broth (LB) supplemented with 20 mm l-lactate to induce expression of the reporter (43). At an A600 of ∼0.2, a sample was withdrawn, and the expression of the reporter was determined (depicted as 0 min in Fig. 7). As expected, in both strains, the expression of the reporter was very low (Fig. 7), indicative of ArcA-P–dependent reporter repression. This result demonstrates that co-expression of the arcBH717Q and arcBH292Q,D576A mutant alleles fully restores ArcB kinase activity and thus proper ArcBH717Q/ArcBH292Q,D576A heterodimer formation. Subsequently, the cultures were shifted to aerobiosis, and the time course of the β-gal activity was followed. An almost immediate increase of reporter expression (Fig. 7) was observed for the strain carrying the WT arcB allele, indicating ArcA-P dephosphorylation and release of reporter repression. On the other hand, a retarded and scarce increase of reporter expression was noticed for the strain carrying the arcBH717Q and arcBH292Q,D576A mutant alleles (Fig. 7). Taken together, these results strongly suggest that the reverse ∼P transfer during signal decay (i.e. from H2 to D1) operates intramolecularly.
Figure 7.
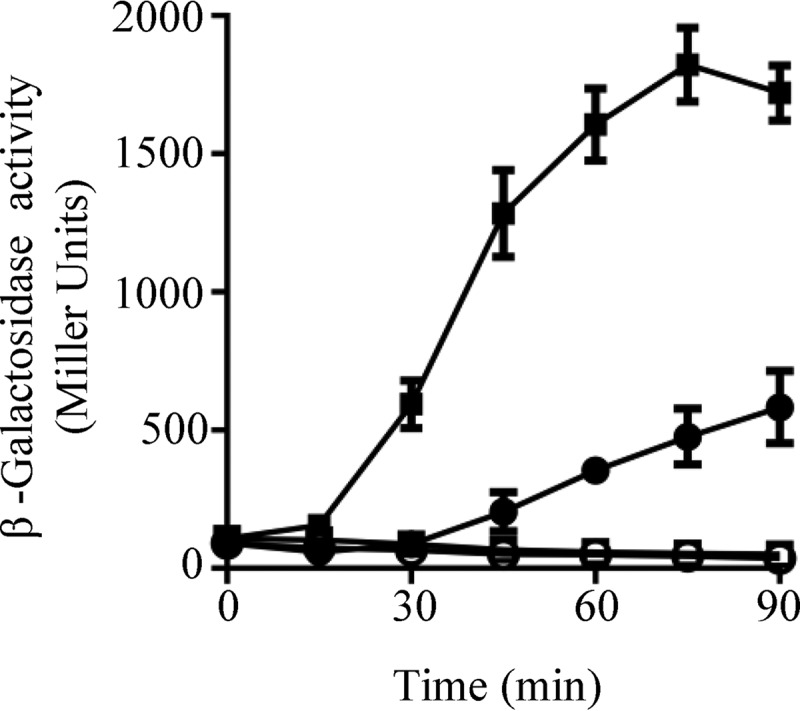
Testing the time lag for ArcA-P dephosphorylation (signal decay) after a shift to nonstimulating conditions. Strain ECL5002 (squares) and its isogenic ECL5032 (arcBH171Q) strain harboring a plasmid-born arcBH292Q,D576A allele (circles), both carrying the λΦ(lldP′-lacZ) reporter, were grown anaerobically in LB buffered with 0.1 m MOPS (pH 7.4) and supplemented with 20 mm d-xylose and 20 mm l-lactate. At an A600 of 0.2, one aliquot was withdrawn, to measure the β-gal activity (depicted as 0 min), and the rest of the cultures were divided into two: one of the subcultures was kept under anaerobiosis (open symbols), serving as control, whereas the other one was shifted to aerobiosis (filled symbols), and the time course of the β-gal activity was followed. Data represent the averages from three independent experiments, and the S.D. values (error bars) are indicated.
Thus, it seems reasonable to conclude that conformational changes of ArcB may affect the mode of ∼P transfer between the D1 and H2 domains, thereby discriminating between the two opposing activities (i.e. between signal transmission and signal decay).
Discussion
In this study, results from in vitro and in vivo experiments demonstrate that the His292 to Asp576 ∼P transfer within the ArcB dimer occurs exclusively intramolecularly, whereas the ∼P transfer from Asp576 to His717 occurs preferentially intermolecularly. Finally, the reverse ∼P transfer responsible for signal decay, namely from His717 to Asp576, appears to have a clear preference for the intramolecular reaction. It is therefore tempting to propose a model in which, during signal propagation, the physiological mode of ∼P transfers between the individual ArcB modules occurs intramolecularly from H1 to D1 and intermolecularly from D1 to H2, whereas the reverse ∼P transfer during signal decay (i.e. from H2 to D1) occurs intramolecularly (Fig. 8).
Figure 8.
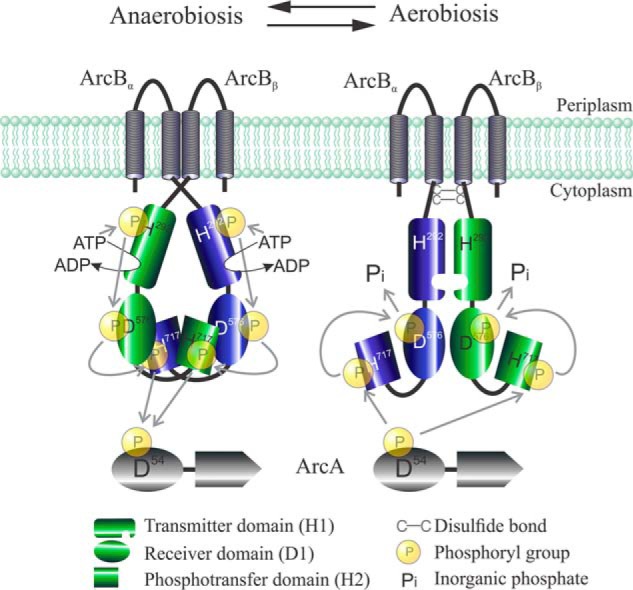
Graphic representation of the ArcB/A two-component system showing the proposed modes of ∼P group transfer during signal propagation (anaerobiosis) and signal decay (aerobiosis). Under anoxic growth conditions, the ATP-dependent intramolecular autophosphorylation in H1 is followed by a phosphorelay that involves an intramolecular H1 to D1 phosphotransfer and an intermolecular D1 to H2 phosphotransfer. Under oxic growth conditions, disulfide bond–dependent conformational adjustments of the ArcB modules enforce an intramolecular phosphotransfer from H2 to D1 followed by release of ∼P as Pi, resulting in ArcA-P dephosphorylation and signal decay.
This model differs from that of a previous study, based on in vivo experiments analyzed with mathematical and statistical models, where it was suggested that the mechanism operating in ArcB ∼P transfers is dictated by a cooperative or bimolecular mechanism in which all phosphorylation sites of both monomers appear to be required for proper functioning of the ArcB phosphorelay (39). It is worth noting that in these in vivo experiments, the ArcB variants were expressed from medium to high copy number plasmids under the control of IPTG-inducible promoters and with the addition of 0.1 mm IPTG. Surprisingly, all combinations, including those in which a WT ArcB was co-expressed with mutant ArcB variants, failed to fully restore a functional phosphorelay. Therefore, it was suggested that all domains and phosphorylation sites in both monomers have to be present if a functional phosphorelay is to be allowed. However, in our hands, co-expression of a chromosomal arcBH717Q mutant and a low-copy number plasmid-borne arcBH292Q,D576A mutant fully restored the kinase activity of ArcB (Fig. 3D), indicating that the mutant proteins do complement not only the WT ArcB activity but also its regulation. Therefore, we could only speculate that, in the earlier study, a possible overproduction of the ArcB mutant variants might have been the cause of the lower ArcA activation. Indeed, it has been demonstrated previously that significant overexpression of proteins can be achieved with plasmid pCA24N, which was used in the above study, with as little as 50 μm IPTG (44). In fact, we have observed that overexpression of a mutant version of ArcB in a WT strain results in a drastic reduction of ArcA-P formation and, thereby, reporter expression (Fig. 5). This, most probably, could be caused by the alteration of the sensor/response regulator concentration balance, in accordance with previous observations for other two-component signal transduction systems (42, 45). Moreover, an intermolecular ∼P transfer from H1 to D1 of ArcB, in contrast to our result, was reported in an earlier study. In that study, the mode of ∼P transfers involved in the phosphorelay of ArcB was analyzed by complementation of single-phosphorelay mutant proteins in in vitro phosphorylation assays, followed by separation of phosphorylated proteins by Phos-Tag SDS-PAGE and detection by Coomassie Blue staining (38). However, the high protein concentrations needed for detection of phosphorylated forms of proteins by Coomassie Blue after Phos-Tag SDS-PAGE separation could increase the incidence of nonspecific phosphorylation and, therefore, weaken the resulting conclusions. In contrast, our in vitro assays, using ArcB mutant heterodimers, in addition to the in vivo complementation experiments, clearly discard intermolecular H1 to D1 phosphotransfer and demonstrate that only the intramolecular transfer is allowed.
At any rate, the model proposed herein for ArcB (Fig. 8) appears appealing if we consider that the spatio-steric constraints of a given assembly of an ArcBα/ArcBβ homodimer should allow only Asp576α or Asp576β to be in the vicinity of His292α, permitting either an inter- or intramolecular ∼P transfer. Likewise, only one His717, the one of either ArcBα or ArcBβ, could be in the vicinity of Asp576α, permitting only one mode of ∼P transfer. Thus, under anoxic growth conditions, the assembly of the ArcBα/ArcBβ dimer would permit the intramolecular ∼P transfer from H1 to D1 (His292α → Asp576α and His292β → Asp576β) and the intermolecular ∼P transfer from D1 to H2 (Asp576α → His717β and Asp576β → His717α), favoring the kinase activity of ArcB. On the other hand, under oxic growth conditions, redox-dependent conformational adjustments of the individual modules within the ArcB dimer would permit the intramolecular ∼P transfer from H2 to D1 (His717α → Asp576α and His717β → Asp576β), favoring the phosphatase activity of ArcB. Such a model provides some insights within the biochemical mechanism that dictates the direction of ∼P transfer, thereby differentiating the kinase and phosphatase activities of ArcB. A similar modus operandi may also apply to other two-component systems comprising a tripartite sensor kinase. Nevertheless, the attainment and analysis of the ArcB crystallographic structure should be needed to elucidate the conformational adjustments that occur upon changes in the redox conditions and to completely understand the bases of the ArcB ∼P transfer pattern.
Experimental procedures
Bacterial strains, plasmids, and growth conditions
The strains and plasmids used in this work are listed in Table S1. Strains IFC2001 and IFC2002 were constructed by P1vir transduction of the fnr::Tn10 allele from strain ECL556 (46) into strains ECL5023 and ECL5024 (12), respectively. Plasmids pQE30ArcB78–778, pQE30ArcB78–778,H292Q (pMX028), pQE30ArcB78–778,D576A,H717Q, pQE30ArcB78–661,D576A, pQE30ArcB521–778,H717Q, pQE30ArcA, and pQE30ArcA1–136, used for cloning and/or for expression of His6-ArcB and His6-ArcA derivatives, have been described previously (10, 13, 16, 18, 35). Plasmid pQE30ArcB78–778,D576A was constructed by replacing the BamHI-EcoRV fragment of plasmid pQE30ArcB78–778 with the corresponding restriction fragment of pQE30ArcB78–661,D576A. Plasmids pQE30ArcB78–778,H717Q and pQE30ArcB78–778,H292Q,H717Q were constructed by replacing the EcoRV-HindIII fragment of pQE30ArcB78–778 and pQE30ArcB78–778,H292Q, respectively, with the corresponding fragment of pQE30ArcB521–778,H717Q. Plasmid pQE30ArcB78–778,H292Q,D576A was constructed by replacing the MluI-EcoRV fragment of plasmid pQE30ArcB78–778,H292Q with the corresponding MluI-EcoRV fragment of pQE30ArcB78–778,D576A. Plasmid pQE30ArcB78–778,H292Q,D576A,H717Q was created by replacing the MluI-HindIII fragment of plasmid pQE30ArcB78–778,H292Q with the corresponding fragment of plasmid pQE30ArcB78–778,D576A,H717Q. Plasmids pBADHis-ArcB78–778* (where the asterisk refers to specific amino acid replacements; see Table S1), used for l-arabinose–induced expression of His6-ArcB78–778*, were constructed by cloning the NdeI-HindIII fragment from the respective pQE30ArcB78–778* between the same restriction sites of plasmid pMX517 (8). Plasmid pMAL-ArcB78–778, used for expression of ArcB78–778 fused to the maltose-binding protein (MBP-H1-D1-H2), was created by cloning the BamHI-HindIII fragment from pQE30ArcB78–778 between the same restriction sites of pMAL-c2x (New England Biolabs). Then the HpaI-HindIII restriction fragment from plasmid pMAL-ArcB78–778, containing the MBP-ArcB78–778-expressing gene and the tac promoter, was cloned between the SmaI and HindIII sites of plasmid pACT3 (41), resulting in pACT3MBP-ArcB78–778. Afterward, pACT3MBP-ArcB78–778* plasmids were constructed by replacing the BamHI-HindIII fragment of pACT3MBP-ArcB78–778 with the BamHI-HindIII fragment from the corresponding pQE30ArcB78–778* (where the asterisk refers to specific amino acid replacements; see Table S1). High-copy number plasmids carrying full-length arcB variants pMX546 (arcBH292Q), pMX547 (arcBH292Q,D576A), and pMX548 (arcBD576A,H717Q) were created by cloning the NruI-HindIII fragment from the corresponding pQE30ArcB78–778* between the NruI and HindIII sites of pMX712 (arcBwt) (8). The low-copy number plasmid pEXT22Cm, used for in vivo complementation assays, was obtained by cloning the blunt-ended XmnI-ScaI fragment from pACT3 (41), containing the chloramphenicol resistance cassette, into the SspI-restricted pEXT22 (41). Then the BamHI-HindIII fragments from pMX712, pMX546, and pMX547 were cloned between the BamHI and HindIII sites of pEXT22Cm to generate pEXT22CmArcBwt, pEXT22CmArcBH292Q, and pEXT22 CmArcBH292Q,D576A, respectively. Plasmids pBADArcBH292Q,D576Aand pBADArcBD576D,H717A, used to express full-length ArcB under the control of the l-arabinose–inducible promoter, were constructed by cloning the NdeI-HindIII restriction fragment from pMX547 and pMX548, respectively, between the NdeI and HindIII sites of pMX517 (8).
Bacteria were routinely cultured at 37 °C in LB. When necessary, media were supplemented with antibiotics, at the following concentrations: chloramphenicol, 20 μg/ml; kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; and tetracycline, 10 μg/ml. For β-galactosidase activity assays, the λΦ(cydA′-lacZ)-bearing strains were grown in LB containing 0.1 m MOPS (pH 7.4) and 20 mm d-xylose, whereas the λΦ(lldP′-lacZ)-bearing strains were grown in the above medium supplemented with 20 mm l-lactate as inducer.
Purification of His6- and MBP-tagged proteins and phosphorylation assays
Native His6-tagged ArcB78–778 variants and His6-ArcA, used in phosphorylation assays, were prepared as described previously (13, 47). MBP-tagged ArcB78–778 variants were purified by using amylose resin (New England Biolabs) as affinity matrix according to the instructions provided by the manufacturer. For the co-purification of the His6-/MBP-tagged ArcB versions, strain ECL5012 (arcB−) (40) carrying both pBADHis-ArcB78–778* and pACT3MBP-ArcB78–778* (where the asterisk refers to specific amino acid replacements) was grown in 1 liter of LB, supplemented with chloramphenicol and ampicillin, in a rotary shaker at 37 °C until an A600 of 0.6. Then the expression of the His6- and MBP-tagged ArcB78–778* was induced by the addition of 1 mm l-arabinose and 0.1 mm IPTG, respectively. Cells were harvested 5 h after induction, and the cell pellet was resuspended in 10 ml of lysis buffer (50 mm sodium phosphate, pH 8.0, 300 mm NaCl, 10 mm imidazole). Finally, the proteins were purified under native conditions by Ni-NTA–agarose affinity chromatography, as described previously (13, 47). Phosphorylation assays were carried out at room temperature in the presence of 40 μm [γ-32P]ATP (specific activity, 2 Ci mmol−1; New England Nuclear), 33 mm HEPES (pH 7.5), 50 mm KCl, 5 mm MgCl2, 1 mm DTT, 0.1 mm EDTA, and 10% glycerol. 5 pmol of purified ArcB78–778* (His6- or MBP-tagged) or 10 pmol of co-purified His6- and MBP-tagged ArcB78–778* and 50 pmol of His6-ArcA were used in each phosphorylation assay. The phosphorylation reactions were initiated by the addition of [γ-32P]ATP, and samples were withdrawn and mixed with SDS sample buffer after 15, 30, 60, 120, and 180 s and subject to analysis by SDS-PAGE on 12% polyacrylamide gels. The radioactivity of proteins resolved in the gels was analyzed by exposing to a Storage Phosphor Screen and scanning by a Typhoon FLA7000 biomolecular imager (GE Healthcare). The intensity of individual band signals was estimated using the ImageQuant version 5.2 software (Molecular Dynamics).
Isolation of His-ArcA1–136-P and dephosphorylation assays
Phosphorylation of ArcA1–136 and dephosphorylation assays were carried out as described previously (10, 47). Briefly, a 20-μl reaction mixture containing phosphorylation buffer (33 mm HEPES at pH 7.5, 50 mm KCl, 5 mm MgCl2, 1 mm DTT, 0.1 mm EDTA, and 10% glycerol), ArcA1–136 (20 mm, purified as a His6-tagged peptide as above), MBP-ArcB78–778 (2 mm), and 40 μm [γ-32P]ATP (specific activity 2 Ci/mmol) was incubated at room temperature. After 10 min, the reaction was stopped by the addition of 100 μl of buffer A, containing 50 mm Tris-HCl at pH 7.0, 150 mm KCl, 5 mm EDTA, and 3% Triton X-100. Separation of ArcA1–136-P from MBP-ArcB78–778-P was achieved by ultrafiltration using a Nanosep 30 K device (Pallfiltron), which retains MBP-ArcB78–778 but not ArcA1–136-P, ATP, and Pi. The eluate was then passed through a Nanosep 10K device (Pallfiltron), washed four times with 500 ml of buffer A to remove ATP and Pi, and once with 500 ml of dephosphorylation buffer (33 mm HEPES at pH 7.5, 50 mm KCl, 5 mm MgCl2, 0.1 mm EDTA, and 10% glycerol). The retained material containing ArcA1–136-32P (essentially free of MBP-ArcB78–778, ATP, and Pi) was recovered in 200 ml of dephosphorylation buffer, aliquoted, and used in dephosphorylating assays. Dephosphorylation reactions were carried out at room temperature in mixtures of 25 μl containing 50 pmol of His6-ArcA1–136-32P and 5 pmol of MBP-ArcB78–778 or 10 pmol of co-purified ArcB heterodimers in dephosphorylation buffer. At various time points, a 5-μl sample was withdrawn, mixed with 5 μl of SDS sample buffer, and analyzed by SDS-PAGE on 15% polyacrylamide gels. The radioactivity of proteins resolved in the gels was analyzed by using a PhosphorImager (Molecular Dynamics).
β-Galactosidase activity assay
In general, for aerobic growth, cells were cultured in 10–50 ml of medium in 250-ml baffled flasks at 37 °C with shaking (300 rpm), whereas for anaerobic growth, cells were cultured in a screw-capped tube filled with medium up to the rim at 37 °C and stirred by a magnet. For the aerobiosis to anaerobiosis shift, cells were aerobically grown and, at an A600 of 0.2, part of the culture was transferred to five prewarmed screw-capped tubes, filled up to the rim, and stirred by a magnet. The rest of the aerobic culture was further incubated with shaking at the same temperature. The time course of the experiment was followed by taking a sample from a filled screw-capped tube (anaerobiosis) and from the baffled flasks (aerobiosis) at each chosen time. For the anaerobiosis-to-aerobiosis shift, cells were cultured in seven screw-capped tubes filled with medium up to the rim at 37 °C and stirred by a magnet. At an A600 of 0.2, the content of a tube was passed to a 250-ml prewarmed baffled flask and incubated with shaking at the same temperature. Samples from aerobic and anaerobic cultures (the rest of the screw-capped tubes) were withdrawn at each chosen time after the shift. β-gal activity was assayed and expressed in Miller units as described previously (48).
Western blotting
Cells were harvested from cultures by centrifugation during mid-exponential growth. The cell pellet was resuspended in sample buffer and separated by SDS-PAGE (10% polyacrylamide gel), and the proteins were transferred to a Hybond-ECL filter (Amersham Biosciences). The filter was equilibrated in TTBS buffer (25 mm Tris, 150 mm NaCl, and 0.05% Tween 20) for 10 min and incubated in blocking buffer (1% milk in TTBS) for 1 h at room temperature. ArcB polyclonal antibodies, raised against His6-ArcB78–520 (12), were added at a dilution of 1:10,000 and incubated for 1 h at room temperature. The bound antibody was detected by using anti-rabbit IgG antibody conjugated to horseradish peroxidase and the ECL detection system (Amersham Biosciences).
Author contributions
J. L. T.-M., A. F. A., and D. G. conceptualization; J. L. T.-M., G. R. P.-S., H. S.-J., and C. R. investigation; J. L. T.-M., G. R. P.-S., H. S.-J., and C. R. methodology; A. F. A. and D. G. supervision; A. F. A. and D. G. funding acquisition; A. F. A. writing-original draft; D. G. writing-review and editing.
Supplementary Material
Acknowledgments
We thank Diego González-Halphen and Bertha Michel for critically reading the manuscript.
This work was supported by a doctoral fellowship from the Consejo Nacional de Ciencia y Tecnología (CONACyT) (to J. L. T. M.) as well as DGAPA-PAPIIT, UNAM, Grants IN208718 and IN209918 (to A. F. A. and D. G.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1 and Figs. S1–S3.
- ArcA-P
- phosphorylated ArcA
- ∼P
- phosphoryl group
- MBP
- maltose-binding protein
- β-gal
- β-galactosidase
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- LB
- lysogeny broth
- NTA
- nitrilotriacetic acid.
References
- 1. Georgellis D., Kwon O., Lin E. C., Wong S. M., and Akerley B. J. (2001) Redox signal transduction by the ArcB sensor kinase of Haemophilus influenzae lacking the PAS domain. J. Bacteriol. 183, 7206–7212 10.1128/JB.183.24.7206-7212.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jung W. S., Jung Y. R., Oh D. B., Kang H. A., Lee S. Y., Chavez-Canales M., Georgellis D., and Kwon O. (2008) Characterization of the Arc two-component signal transduction system of the capnophilic rumen bacterium Mannheimia succiniciproducens. FEMS Microbiol. Lett. 284, 109–119 10.1111/j.1574-6968.2008.01187.x [DOI] [PubMed] [Google Scholar]
- 3. Malpica R., Sandoval G. R., Rodríguez C., Franco B., and Georgellis D. (2006) Signaling by the Arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid. Redox Signal. 8, 781–795 10.1089/ars.2006.8.781 [DOI] [PubMed] [Google Scholar]
- 4. Lynch A. S., and Lin E. C. (1996) Regulation of gene expression in Escherichia coli. in Escherichia coli and Salmonella: Cellular and Molecular Biology (Neidhardt F. C., Curtis R., Ingraham A. L., Lin E. C. C., Low K. B., Magasanik B., Reznikoff W. S., Riley M., Schaechter M., and Umbarger H. E., eds) pp. 1526–1538, American Society for Microbiology, Washington, D. C. [Google Scholar]
- 5. Iuchi S., and Lin E. C. (1988) arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. U.S.A. 85, 1888–1892 10.1073/pnas.85.6.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iuchi S., Matsuda Z., Fujiwara T., and Lin E. C. (1990) The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol. Microbiol. 4, 715–727 10.1111/j.1365-2958.1990.tb00642.x [DOI] [PubMed] [Google Scholar]
- 7. Ishige K., Nagasawa S., Tokishita S., and Mizuno T. (1994) A novel device of bacterial signal transducers. EMBO J. 13, 5195–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nuñez Oreza L. A., Alvarez A. F., Arias-Olguín I. I., Torres Larios A., and Georgellis D. (2012) The ArcB leucine zipper domain is required for proper ArcB signaling. PLoS One 7, e38187 10.1371/journal.pone.0038187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhulin I. B., Taylor B. L., and Dixon R. (1997) PAS domain S-boxes in Archaea, Bacteria and sensors for oxygen and redox. Trends Biochem. Sci. 22, 331–333 10.1016/S0968-0004(97)01110-9 [DOI] [PubMed] [Google Scholar]
- 10. Georgellis D., Kwon O., and Lin E. C. (1999) Amplification of signaling activity of the Arc two-component system of Escherichia coli by anaerobic metabolites: an in vitro study with different protein modules. J. Biol. Chem. 274, 35950–35954 10.1074/jbc.274.50.35950 [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez C., Kwon O., and Georgellis D. (2004) Effect of d-lactate on the physiological activity of the ArcB sensor kinase in Escherichia coli. J. Bacteriol. 186, 2085–2090 10.1128/JB.186.7.2085-2090.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon O., Georgellis D., and Lin E. C. (2000) Phosphorelay as the sole physiological route of signal transmission by the Arc two-component system of Escherichia coli. J. Bacteriol. 182, 3858–3862 10.1128/JB.182.13.3858-3862.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Georgellis D., Lynch A. S., and Lin E. C. (1997) In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J. Bacteriol. 179, 5429–5435 10.1128/jb.179.17.5429-5435.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunsalus R. P., and Park S. J. (1994) Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and Fnr regulons. Res. Microbiol. 145, 437–450 10.1016/0923-2508(94)90092-2 [DOI] [PubMed] [Google Scholar]
- 15. Liu X., and De Wulf P. (2004) Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J. Biol. Chem. 279, 12588–12597 10.1074/jbc.M313454200 [DOI] [PubMed] [Google Scholar]
- 16. Lynch A. S., and Lin E. C. (1996) Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J. Bacteriol. 178, 6238–6249 10.1128/jb.178.21.6238-6249.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alvarez A. F., Malpica R., Contreras M., Escamilla E., and Georgellis D. (2010) Cytochrome d but not cytochrome o rescues the toluidine blue growth sensitivity of arc mutants of Escherichia coli. J. Bacteriol. 192, 391–399 10.1128/JB.00881-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Georgellis D., Kwon O., De Wulf P., and Lin E. C. (1998) Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem. 273, 32864–32869 10.1074/jbc.273.49.32864 [DOI] [PubMed] [Google Scholar]
- 19. Peña-Sandoval G. R., Kwon O., and Georgellis D. (2005) Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo. J. Bacteriol. 187, 3267–3272 10.1128/JB.187.9.3267-3272.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwon O., Georgellis D., and Lin E. C. (2003) Rotational on-off switching of a hybrid membrane sensor kinase Tar-ArcB in Escherichia coli. J. Biol. Chem. 278, 13192–13195 10.1074/jbc.M210647200 [DOI] [PubMed] [Google Scholar]
- 21. Georgellis D., Kwon O., and Lin E. C. (2001) Quinones as the redox signal for the Arc two-component system of bacteria. Science 292, 2314–2316 10.1126/science.1059361 [DOI] [PubMed] [Google Scholar]
- 22. Alvarez A. F., Rodriguez C., and Georgellis D. (2013) Ubiquinone and menaquinone electron carriers represent the yin and yang in the redox regulation of the ArcB sensor kinase. J. Bacteriol. 195, 3054–3061 10.1128/JB.00406-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malpica R., Franco B., Rodriguez C., Kwon O., and Georgellis D. (2004) Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc. Natl. Acad. Sci. U.S.A. 101, 13318–13323 10.1073/pnas.0403064101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bekker M., Alexeeva S., Laan W., Sawers G., Teixeira de Mattos J., and Hellingwerf K. (2010) The ArcBA two-component system of Escherichia coli is regulated by the redox state of both the ubiquinone and the menaquinone pool. J. Bacteriol. 192, 746–754 10.1128/JB.01156-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomomori C., Tanaka T., Dutta R., Park H., Saha S. K., Zhu Y., Ishima R., Liu D., Tong K. I., Kurokawa H., Qian H., Inouye M., and Ikura M. (1999) Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat. Struct. Biol. 6, 729–734 10.1038/11495 [DOI] [PubMed] [Google Scholar]
- 26. Tanaka T., Saha S. K., Tomomori C., Ishima R., Liu D., Tong K. I., Park H., Dutta R., Qin L., Swindells M. B., Yamazaki T., Ono A. M., Kainosho M., Inouye M., and Ikura M. (1998) NMR structure of the histidine kinase domain of the E. coli osmosensor EnvZ. Nature 396, 88–92 10.1038/23968 [DOI] [PubMed] [Google Scholar]
- 27. West A. H., and Stock A. M. (2001) Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26, 369–376 10.1016/S0968-0004(01)01852-7 [DOI] [PubMed] [Google Scholar]
- 28. Alvarez A. F., Barba-Ostria C., Silva-Jiménez H., and Georgellis D. (2016) Organization and mode of action of two component system signaling circuits from the various kingdoms of life. Environ. Microbiol. 18, 3210–3226 10.1111/1462-2920.13397 [DOI] [PubMed] [Google Scholar]
- 29. Stock A. M., Robinson V. L., and Goudreau P. N. (2000) Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 10.1146/annurev.biochem.69.1.183 [DOI] [PubMed] [Google Scholar]
- 30. Yang Y., and Inouye M. (1991) Intermolecular complementation between two defective mutant signal-transducing receptors of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 88, 11057–11061 10.1073/pnas.88.24.11057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ninfa E. G., Atkinson M. R., Kamberov E. S., and Ninfa A. J. (1993) Mechanism of autophosphorylation of Escherichia coli nitrogen regulator II (NRII or NtrB): trans-phosphorylation between subunits. J. Bacteriol. 175, 7024–7032 10.1128/jb.175.21.7024-7032.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Casino P., Rubio V., and Marina A. (2009) Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 139, 325–336 10.1016/j.cell.2009.08.032 [DOI] [PubMed] [Google Scholar]
- 33. Swanson R. V., Bourret R. B., and Simon M. I. (1993) Intermolecular complementation of the kinase activity of CheA. Mol. Microbiol. 8, 435–441 10.1111/j.1365-2958.1993.tb01588.x [DOI] [PubMed] [Google Scholar]
- 34. Cotter P. A., and Jones A. M. (2003) Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11, 367–373 10.1016/S0966-842X(03)00156-2 [DOI] [PubMed] [Google Scholar]
- 35. Peña-Sandoval G. R., and Georgellis D. (2010) The ArcB sensor kinase of Escherichia coli autophosphorylates by an intramolecular reaction. J. Bacteriol. 192, 1735–1739 10.1128/JB.01401-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jourlin C., Ansaldi M., and Méjean V. (1997) Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J. Mol. Biol. 267, 770–777 10.1006/jmbi.1997.0919 [DOI] [PubMed] [Google Scholar]
- 37. Uhl M. A., and Miller J. F. (1996) Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 15, 1028–1036 [PMC free article] [PubMed] [Google Scholar]
- 38. Kinoshita-Kikuta E., Kinoshita E., Eguchi Y., and Koike T. (2016) Validation of cis and trans modes in multistep phosphotransfer signaling of bacterial tripartite sensor kinases by using Phos-Tag SDS-PAGE. PLoS One 11, e0148294 10.1371/journal.pone.0148294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jovanovic G., Sheng X., Ale A., Feliu E., Harrington H. A., Kirk P., Wiuf C., Buck M., and Stumpf M. P. H. (2015) Phosphorelay of non-orthodox two component systems functions through a bi-molecular mechanism in vivo: the case of ArcB. Mol. BioSyst. 11, 1348–1359 10.1039/C4MB00720D [DOI] [PubMed] [Google Scholar]
- 40. Kwon O., Georgellis D., Lynch A. S., Boyd D., and Lin E. C. (2000) The ArcB sensor kinase of Escherichia coli: genetic exploration of the transmembrane region. J. Bacteriol. 182, 2960–2966 10.1128/JB.182.10.2960-2966.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dykxhoorn D. M., St Pierre R., and Linn T. (1996) A set of compatible tac promoter expression vectors. Gene 177, 133–136 10.1016/0378-1119(96)00289-2 [DOI] [PubMed] [Google Scholar]
- 42. Goulian M. (2010) Two-component signaling circuit structure and properties. Curr. Opin. Microbiol. 13, 184–189 10.1016/j.mib.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong J. M., Taylor J. S., Latour D. J., Iuchi S., and Lin E. C. (1993) Three overlapping lct genes involved in l-lactate utilization by Escherichia coli. J. Bacteriol. 175, 6671–6678 10.1128/jb.175.20.6671-6678.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soo V. W. C., Hanson-Manful P., and Patrick W. M. (2011) Artificial gene amplification reveals an abundance of promiscuous resistance determinants in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 108, 1484–1489 10.1073/pnas.1012108108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Batchelor E., and Goulian M. (2003) Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc. Natl. Acad. Sci. U.S.A. 100, 691–696 10.1073/pnas.0234782100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fu H.-A., Iuchi S., and Lin E. C. C. (1991) The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol. Gen. Genet. 226, 209–213 [DOI] [PubMed] [Google Scholar]
- 47. Alvarez A. F., and Georgellis D. (2010) In vitro and in vivo analysis of the ArcB/A redox signaling pathway. Methods Enzymol. 471, 205–228 10.1016/S0076-6879(10)71012-0 [DOI] [PubMed] [Google Scholar]
- 48. Miller J. H. (1972) β-Galactosidase assay. in Experiments in Molecular Genetics, pp. 352–355, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.