Abstract
Objective
Adverse childhood experiences may be associated with cardiometabolic morbidity and mortality in adulthood. There is heterogeneity in this literature regarding the type of items in cumulative adversity indices, sample sizes and demographics, and covariates. The present review used quantitative meta-analysis to examine this association and potential moderators.
Methods
Included studies had a measure of cumulative adversity (an index of at least 2 adverse childhood experiences from age 0 to 18) and a measure of cardiometabolic disease: cardiovascular (CVD) clinical outcomes (hypertension, coronary heart disease, ischemic heart disease, myocardial infarction, stroke, cerebrovascular disease) and metabolic outcomes (diabetes, metabolic syndrome) at age 18 or older. Given different interpretations of odds ratios (OR) versus hazard ratios (HR), effects were pooled separately. Overall, 9 HR studies (15 effects) based on 179,612 participants and 29 OR studies (62 effects) based on 247,393 participants were included.
Results
Based largely on retrospectively-assessed adversity, combined studies showed a significant estimated effect of cumulative childhood adversity on adult cardiometabolic disease (HR=1.42, 95% CI [1.20, 1.67]; OR=1.36 [1.27, 1.46]). Results varied somewhat by type of cardiometabolic disease, analytic strategy, and number and type of covariates.
Conclusions
The literature suggests that cumulative childhood adversity is modestly related to adult cardiometabolic disease, with effects somewhat stronger for CVD clinical outcomes. The absence of a consistent operational and conceptual definition of adversity and paucity of prospective designs temper the conclusions. It is time for further evaluation of the types and timing of childhood events that have maximal impact on adult cardiometabolic disease.
Keywords: childhood adversity, abuse, cardiovascular, diabetes, development
A growing body of evidence points to stressful experiences early in life, more recently called “adverse childhood experiences”, setting the trajectory of poor mental and physical health across the life course. The Centers for Disease Control and Prevention (CDC) define adverse childhood experiences as family environments that lack safety, stability, or nurturing relationships (2013). However, the concept of childhood adversity is applied more broadly in the literature, and generally indicates exposure to a combination of poverty, abuse, neglect, and household dysfunction (defined as domestic violence, substance use, mental illness, or incarceration of family members). Furthermore, exposure to adverse childhood experiences is prevalent in the United States, and types of adversities are inter-related and often overlap (M. Dong, Anda, et al., 2004). For example, in a sample of 29,229 adult men and women, over 50% of the sample reported at least one form of childhood adversity and 17% reported four or more adverse experiences (Font & Maguire-Jack, 2015).
The overarching rationale for research on childhood adversities has been quite simple: the more bad things that occur in childhood, the worse the long-term mental and physical health, although some investigators have questioned whether there may be a cutoff or threshold effect (e.g., Evans, Li, & Whipple, 2013). Review papers have reported associations between various types of adversity and poor outcomes in adulthood that span mental health, physical health, and psychosocial domains, including: increased substance abuse and risky sexual behavior; depression, anxiety, suicidality, and eating disorders; sleep disorders; inflammation and obesity; increased health care utilization; and low adult education and income (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2015; Danese & Tan, 2014; Font & Maguire-Jack, 2015; Kajeepeta, Gelaye, Jackson, & Williams, 2015; Kalmakis & Chandler, 2015; Midei & Matthews, 2011; Norman et al., 2012).
Globally, cardiovascular disease (CVD) and diabetes are two of the leading causes of death, accounting for 31% and 5.2% of deaths in 2011, as well as a combined economic burden exceeding $550 billion in the United States alone (Go et al., 2014). Evidence also suggests the metabolic syndrome (a composite of risk factors including elevated waist circumference, triglycerides, blood pressure, and fasting glucose, as well as reduced HDL cholesterol) predicts additional risk for diabetes, CVD events, and CVD-related mortality (Go et al., 2014). Risk factors for CVD and diabetes, including pre-diabetes and hyperinsulinemia (C. Li, Ford, Zhao, & Mokdad, 2009), obesity (Ogden, Carroll, Kit, & Flegal, 2014), and blood pressure (Muntner, He, Cutler, Wildman, & Whelton, 2004) are prevalent, including in American youth. Moreover, these risk factors track into adulthood to predict CVD (Hartiala et al., 2012; S. Li et al., 2003; Rademacher et al., 2009; Raitakari et al., 2003).
Several meta-analyses have summarized the associations between some form of childhood adversity (assessed almost exclusively using retrospective measures), and CVD and/or diabetes-related outcomes. Wegman and Stetler (2009) found moderate effects of physical, sexual, and emotional abuse and neglect in childhood on CVD and metabolic outcomes, the latter defined as a combination of obesity and diabetes but did not include metabolic syndrome. However, their analysis included a small number of studies for each type of outcome, and studies were included whether they assessed cumulative abuse/neglect or single types of abuse/neglect. In another analysis of the individual contributions of physical abuse, emotional abuse, and neglect on mental and physical health outcomes, Norman and colleagues (2012) found weak and inconsistent results for diabetes and any CVD outcomes (though they had a relatively small number of studies for each outcome). A more recent meta-analysis (Huang et al., 2015), which included only seven studies, found a small relationship between cumulative abuse and neglect and diabetes. Collectively, these meta-analyses yield different conclusions, are based on relatively small numbers of studies that typically utilized retrospective report of adversity, and have focused exclusively on abuse and neglect to the exclusion of other important and commonly assessed early childhood adversities. Thus, the field would benefit from a more inclusive review and analysis of cumulative adverse childhood experiences, consistent with the CDC’s definition, and cardiometabolic disease.
Furthermore, there has not been a thorough assessment of potential moderators of the relationship between childhood adversity and cardiometabolic outcomes, though multiple moderators have been suggested in the literature. For example, earlier studies suggest that both females (e.g., Danese & Tan, 2014; Wegman & Stetler, 2009) and blacks (e.g., Slopen et al., 2016; Umberson et al. 2016) may experience more childhood adversity and/or worse health outcomes related to adversity, and that childhood adversity is associated with worse outcomes through lower adult SES (e.g., Font & Maguire-Jack, 2015). Additionally, recent reviews (e.g., Appleton, Holdsworth, Ryan, & Tracy, 2017) have noted that the extant literature includes many different indices of adversity, e.g. some include household dysfunction, some do not; some include childhood socioeconomic status (SES), which is known to predict CVD (Cohen, Janicki-Deverts, Chen, & Matthews, 2010; Galobardes, Lynch, & Smith, 2008).
In summary, the primary aim of the present review is to estimate the cumulative association between an inclusive definition of childhood adversity and cardiometabolic disease in adulthood (CVD clinical outcomes and metabolic outcomes). A secondary aim is to evaluate factors that may modify this overall association, including childhood SES, participant demographics, study design and methods, and type of cardiometabolic disease.
Method
Literature Search Strategy
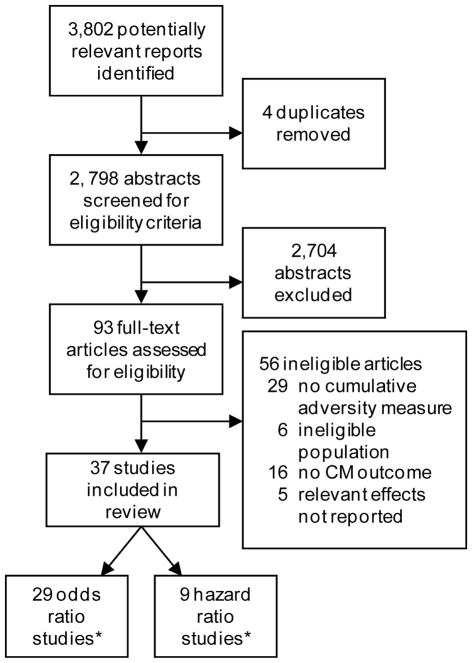
A literature search was conducted in the PubMed database for articles appearing through September 2017 using the following search terms: (“adverse childhood experiences” or “cumulative adversity” or “childhood adversity” or “childhood maltreatment” or “childhood trauma” or “risky families” or “early life adversity” or “stressful life events” or “psychosocial stress”) AND (“cardiovascular” or “hypertension” or “diabetes” or “CHD” or “MI” or “metabolic syndrome” or “stroke”). Reference lists of relevant articles were used to identify additional studies. The search returned 3,802 possible abstracts (see Figure 1 for the number of articles identified, screened, and excluded). Abstracts were reviewed to assess for the presence of a measure of cumulative adversity (i.e., an index that included at least two adverse childhood experiences) and at least one measure of cardiometabolic disease obtained at age 18 or beyond in adult populations without severe psychiatric illness (i.e., schizophrenia and psychosis).
Figure 1.
Flow chart showing inclusion/exclusion of studies identified from initial search.
Included studies a) had a measure of cumulative adversity (i.e., an index that included at least two adverse childhood experiences) and b) had at least one measure of cardiometabolic (CM) disease assessed at age 18 or beyond. Excluded from review were studies that measured single items of adversity (e.g., physical abuse) or studies that exclusively sampled individuals with severe psychiatric illness or children (age 17 or younger).
*One study (McCrory et al., 2015) included outcomes relevant to both hazard ratio and odds ratio analyses and was included in both sets of pooled effects.
Cardiometabolic disease included the following: 1) CVD clinical outcomes: clinically-diagnosed hypertension (but not continuous measures of blood pressure, as clinical cutoffs could not be applied without obtaining and grouping individual-level data from the parent study), coronary heart disease (CHD), ischemic heart disease (IHD), myocardial infarction (MI), stroke or cerebrovascular disease, or combinations of the aforementioned endpoints; and 2) metabolic outcomes: type 2 diabetes and metabolic syndrome. Measurement of outcomes typically involved diagnosis by a nurse, physician, or other healthcare provider (as indexed in national registers, hospital registers, or medical records); assessment by trained research staff; self-report of a previous diagnosis made by a physician or other healthcare provider; or a composite of items assessed by physician, research staff, and/or self-report.
The present meta-analysis does not include pre-clinical CVD risk factors per se, such as weight-related outcomes (e.g., body mass index, waist circumference, obesity), sub-clinical indicators of CVD (e.g., carotid intima-media thickness), or inflammation, as these outcomes have previously been reviewed in association with early life adversity in adults (Appleton et al., 2017; Basu, McLaughlin, Misra, & Koenen, 2017; Baumeister et al., 2015; Danese & Tan, 2014; Norman et al., 2012; Su, Jimenez, Roberts, & Loucks, 2015), and are predictors of later CVD, rather than CVD, which is the focus of this study.
Our search resulted in 93 articles that were assessed in detail for eligibility. Of these full-text articles, 29 were excluded because they did not have a cumulative measure of adversity; 6 involved ineligible populations (e.g., adults with severe psychiatric illness; children); 16 studies did not have an appropriate measure of cardiometabolic disease; and 5 studies did not report relevant effects. Thus, 37 studies met the stated inclusion criteria. Surprisingly, there were no articles evaluating CVD mortality.
Data Extraction
The first author (KJ) performed the data searches and coding in consultation with KM. The following information was extracted from each study: 1) sample size; 2) sample demographics (age, sex [i.e., 80% or more of one sex], and geographic region [U.S. vs. non-U.S. samples]); 3) study design (prospective vs. retrospective report of adversity); 4) type of cardiometabolic disease (i.e., CVD clinical outcomes vs. metabolic outcomes); 5) type of cumulative adversity measure and whether it included childhood SES, household dysfunction (defined as domestic violence, substance use, mental illness, and incarceration), and abuse (defined as physical, sexual, or emotional/psychological/verbal abuse or neglect); 6) measurement of cardiometabolic disease (independently-assessed disease [i.e., outcomes assessed in a laboratory and/or medical setting or diagnosis made by a healthcare provider and indexed in national registers, hospital registers, or medical records]) vs. self-report of a previous diagnosis made by a physician or other healthcare provider); 7) analytic strategy (i.e., comparing individuals who reported the greatest number or severity of adversities to a reference group of those who reported the fewest or least severe adversities or none at all vs. using a continuous total/average score on an adversity-related scale); and 8) total number and type of covariates included in analytic models, including adult SES, adult psychosocial risk factors for CVD (e.g., depression or anxiety symptoms, marital status, social support), and adult health behaviors (e.g., BMI, smoking, alcohol use, diet, physical activity, sleep); these variables were counted as covariates if they were tested as mediators or part of the pathway between adversity and cardiometabolic disease.
In order to calculate reliability statistics, a random sub-sample of eight studies (n=3 HR studies; n=5 OR studies), or 13% of all HR and OR studies, were double-coded for reliability by KJ and KM. Overall reliability across extracted study data was 95%, with individual category reliabilities ranging from 75% to 100%.
The reported effect size was extracted for each health outcome examined. Effect sizes were reported as regression weights, odds ratios (OR), relative risks (RR), or hazard ratios (HR), and were drawn from fully adjusted models. Effects involving regression weights or RR were pooled with OR effects. An OR reflects the ratio of the odds of an outcome in exposed persons (i.e., those exposed to cumulative childhood adversity) relative to the odds in non-exposed persons, thus, it can be interpreted as the risk of developing a disease given a certain exposure. In contrast, a HR reflects time to event for a particular outcome, or a survival analysis. Thus, one might hypothesize that persons exposed to greater cumulative adversity would demonstrate greater risk of cardiometabolic disease (as indicated by OR analyses) and earlier/faster disease onset (as indicated by HR analyses). Given differences in the interpretation of OR versus HR and the inability to harmonize these effect sizes statistically, these effects were pooled separately, as has been the case in other meta-analyses (Bhattacharjee, Bhattacharya, Kelley, & Sambamoorthi, 2013; Kronish et al., 2011). The direction of effects was consistently coded such that values indicate a relationship between increased exposure to cumulative adversity and greater risk of cardiometabolic disease or faster time to disease event.
It was common for studies to analyze multiple cumulative adversity-health relationships (i.e., multiple health outcomes were tested) or the same adversity-health relationship within multiple subgroups (men vs. women) so each of these effects was coded. Non-overlapping groups were treated as independent effects. If multiple comparisons were tested within the same group for the same outcome (e.g., 0 adversities vs. 1–2 adversities vs. 3–4 adversities), the most extreme comparison was retained. The majority of studies used an extreme groups approach with a reference group of 0 adversities (HR studies: K = 7; OR studies: K = 17), although three studies used a reference group other than 0 adversities (Halonen et al., 2015; Rich-Edwards et al., 2010; Wilson et al., 2012). All remaining studies (HR studies: K = 1; OR studies: K = 10) did not use an extreme groups approach, and instead analyzed continuous associations between a total/average score on a trauma/adversity-related scale with no reference group and cardiometabolic outcomes.
Overall, 37 studies were included: 9 HR studies (15 effects; total N = 203,017) and 29 OR studies (62 effects; total N = 555,532). One study (McCrory, Dooley, Layte, & Kenny, 2015) was included in both HR and OR pooled effects because it repeated both types of analyses in the health outcomes tested. A description of samples, measures, and analytic strategies used in HR and OR studies are listed in Supplemental Tables 1 and 2, respectively, by author and year of publication.
Treating multiple effects from the same study sample as independent can artificially reduce the SE, making it more likely that results will be accompanied by a lower p value. However, aggregating across effects reduces power, could artificially deflate estimates, and makes it more difficult to adequately test whether the strength of the association may vary depending on the type of outcome assessed (Borenstein, Hedges, Higgins, & Rothstein, 2009). Thus, meta-analytic results are presented in two ways: (a) using a more conservative approach, which aggregated effects within studies across dependent measures (HR studies: K = 9; OR studies: K = 29), and (b) using a less conservative approach which treated each cumulative adversity-cardiometabolic disease effect as independent (HR studies: k = 15; OR studies: k = 62). By necessity, moderators were tested using the less conservative approach. To be thorough, we also performed analyses assuming a correlation of .4 among outcomes within the same study, for HR and OR studies, respectively. This correlation was chosen because it represents an analytic strategy in between the extreme strategies presented above (treating outcomes as independent is akin to correlating outcomes within studies at 0, and treating them as redundant is akin to correlating outcomes within studies at 1).
Analyses were conducted with Comprehensive Meta-Analysis analytic software version 3 (BiostatTM, USA) to employ random effects modeling of associations between adversity and cardiometabolic disease. Random effects models assume that samples are drawn from populations with different effect sizes, thus allowing for both random variance and variance due to true differences between populations. Accordingly, the sample size in a study contributes less weight in random effects models compared to fixed effects models (Borenstein, Hedges, & Rothstein, 2007). Furthermore, random effects models yield more accurate confidence intervals in meta-analysis (Schmidt, Oh, & Hayes, 2009). The additional analyses assuming a correlation of .4 were also conducted in the CMA program; however, within study effects were first aggregated in excel using a template provided by the CMA software’s creator, who is an expert in meta-analysis (M. Borenstein).
The heterogeneity statistic (QT) provides an estimate of variability of effect sizes. If there was significant heterogeneity of effects (i.e., significant QT coefficient), then moderators were examined as a potential explanation for variability of effect size (see “Data Extraction” section for specific moderators). Because of different numbers of total effects in HR (k=15) and OR (k=62) analyses, arbitrary cutoffs of “3” and “12,” respectively, were chosen for the minimum number of effects needed in each category to test moderators. This corresponded to cutoffs of at least 20% and 19% of available effects for HR and OR analyses, respectively. These cutoffs were not applied to moderation by sex analyses, which involved fewer total HR (k = 5) and OR (k = 25) effects.
Finally, because null and negative findings are less likely to be submitted for publication by authors and/or less likely to be accepted for publication, there may be biased effects in the available literature. Proposed guidelines (Rothstein, Sutton, & Borenstein, 2006) were used to examine the presence and impact of publication bias in both HR and OR pooled effects, including examination of (1) forest plots for individual effects that appear to be outliers; (2) funnel plots of analyzed HR and OR effects (Light & Pillemer, 1984); (3) Kendall’s tau, which provides a statistic of the relationship between standard errors and standardized effect sizes (Begg & Mazumdar, 1994); and (4) Egger’s regression, where the intercept reflects the slope of the association (Egger, Smith, Schneider, & Minder, 1997).
Results
Descriptive Data Regarding HR and OR Pooled Effects
Table 1 provides a summary of the frequencies of demographic, moderator, and outcome variables for both HR and OR pooled effects. The analysis for HR effects were based on 203,017 participants across all effects (i.e., counted for each outcome and males and females separately for sex-stratified analyses) and 179,612 participants across studies, whereas the analysis for OR effects were based on 555,532 across effects and 247,393 participants across studies. Overall, HR and OR effects were based on predominantly female samples and retrospectively-reported adversity data. The majority of HR studies were conducted in European samples, while the majority of OR studies were conducted in U.S. samples, with non-U.S. samples spread amongst several European countries, Saudi Arabia, New Zealand, Canada, and the Philippines. The Philippines (Ramiro, Madrid, & Brown, 2015) is the only country that is grouped with low and middle-income countries (LMIC) for the current 2018 fiscal year by the World Bank Atlas method (World Bank Group, 2017). The majority of both HR and OR effects reflected CVD clinical outcomes, compared to metabolic outcomes. Roughly half of HR effects reflected independently-assessed disease, compared to 36% of OR effects. The majority of both HR and OR effects utilized an analytic strategy in which they compared extreme categories of adversities, as opposed to studies that used a total count or average. Five OR studies utilized prospectively-collected data on cumulative adversity, primarily reported by parents and/or teachers, and longitudinal associations with cardiometabolic disease; follow-up length for these studies ranged from 27 to 38 years (M = 31.8 years, SD = 4.02).
Table 1.
Frequencies of demographic, outcome, and moderator variables in hazard ratio and odds ratio studies.
| Number (%) of effects unless otherwise noted | Hazard Ratio Effects (k=15) | Odds Ratio Effects (k=62) |
|---|---|---|
| Participants | ||
| Total number across all effects | 203,017 | 555,532 |
| Mdn, [range] | 6,407 [3,032–37,699] | 2,209 [192–53,998] |
| Total female participants across all effects | 153,952 (75.8%) | 323,920 (58.3%) |
| Non-U.S. samples | 13 (87.0%) | 28 (45.2%) |
| % participants who report 2+ adversities, M (SD) | 25.0% (18.0%)a | 30.0% (21.4%)b |
| Study Design | ||
| Retrospective assessment of adversity | 15 (100%) | 56 (90.3%) |
| Years of follow-up, M (SD) | 10.0 (4.4)c | 19.9 (13.0)d |
| Outcomes | ||
| CVD clinical outcomes | 12 (80.0%) | 36 (58.1%) |
| Metabolic outcomes | 3 (20.0%) | 26 (41.9%) |
| Independently-assessed disease | 8 (53.3%) | 22 (35.5%) |
| Cumulative Adversity Index | ||
| Total number of items, M (SD) | 7.4 (3.2) | 9.81 (6.0) |
| ACE Questionnaire vs. Others | 3 (20.0%) | 36 (58.1%) |
| Index included childhood SES | 11 (73.3%) | 13 (21.0%) |
| Index included household dysfunction | 10 (66.7%) | 48 (77.4%) |
| Index included abuse/neglect | 8 (53.3%) | 58 (93.5%) |
| Analytic Strategy | ||
| Comparison of most vs. least adversities | 14 (93.3%) | 48 (77.4%) |
| Number of covariates in analytic models, M (SD) | 8.3 (4.0) | 5.2 (4.4) |
| Covariate type: Adult SES | 13 (86.7%) | 46 (74.2%) |
| Covariate type: Psychosocial | 11 (73.3%) | 22 (35.5%) |
| Covariate type: Health behaviors | 10 (66.7%) | 22 (35.5%) |
Reflects data from 7 out of 15 effects that provided sufficient information.
Reflects data from 47 out of 62 effects that provided sufficient information.
Reflects data from 6 out of 9 studies that provided sufficient information.
Reflects data from 10 out of 29 studies, including both retrospective and prospective designs, that provided sufficient information.
Association between Cumulative Adversity and Cardiometabolic Disease
HR results
Results for HR studies are presented in Table 2. A forest plot examining all effects individually is presented in Figure 2 and a forest plot of the individual effects aggregated within study is pictured in Supplemental Figure 1. Results treating all effects as independent (k = 15) and results aggregating effects within studies (K = 9) both revealed that exposure to cumulative adversity in childhood was associated with decreased time to event for all outcomes combined. The cumulative effect size was similar in magnitude across these two analytic strategies (Table 2, first two rows). Results assuming a correlation of .4 among outcomes tested in the same sample (HR=1.35, 95% CI [1.14, 1.60]) were very similar to the estimated effect sizes from analyses treating all outcomes as independent or redundant (Table 2, first two rows), likely due to the number of large samples in our analyses.
Table 2.
Cumulative hazard ratios of cumulative childhood adversity on cardiometabolic disease.
| Cumulative Hazard Ratio | 95%CI | QT (heterogeneity) | |
|---|---|---|---|
| All effects (k=15) | 1.42* | 1.20 – 1.67 | 61.61* |
| Using study as the unit of analysis (K=9) | 1.38* | 1.15 – 1.66 | 52.11* |
| Participants | |||
| Sexa | |||
| Women (k=3) | 1.62* | 1.08 – 2.43 | 6.30* |
| Men (k=2) | 1.01 | .59 – 1.73 | .002 |
| QM (1, 3) | 1.92 | ||
| Outcomec | |||
| Type | |||
| Metabolic outcomes (k=3) | 1.63* | 1.16 – 2.29 | 10.89* |
| CVD clinical outcomes (k=12) | 1.36* | 1.13 – 1.63 | 41.11* |
| QM (1, 13) | .84 | ||
| Measurement | |||
| Self-report (k=7) | 1.59* | 1.23 – 2.05 | 42.91* |
| Independently-assessed disease (k=8) | 1.30* | 1.02 – 1.66 | 15.51* |
| QM (1, 13) | 1.26 | ||
| Cumulative Adversity Index | |||
| Type of Adversity Measure | |||
| ACE Study Questionnaire (k=3) | 3.41* | 2.31 – 5.03 | 1.81 |
| Other Scale/Ad-hoc Composite (k=12) | 1.23* | 1.09 – 1.38 | 24.44* |
| QM (1, 13) | 24.29* | ||
| Index Included Childhood SES | |||
| No (k=4) | 2.26* | 1.66 – 3.07 | 19.11* |
| Yes (k=11) | 1.22* | 1.04 – 1.43 | 19.46* |
| QM (1, 13) | 12.13* | ||
| Index Included Household Dysfunction | |||
| No (k=5) | 1.16 | 0.86 – 1.56 | 5.63 |
| Yes (k=10) | 1.61* | 1.29 – 2.01 | 55.74* |
| QM (1, 13) | 3.04† | ||
| Index Included Abuse/Neglect | |||
| No (k=7) | 1.27* | .98 – 1.66 | 14.21* |
| Yes (k=8) | 1.56* | 1.24 – 1.96 | 46.99* |
| QM (1, 13) | 1.28 | ||
| Analytic Strategy | |||
| Number of Covariates in Analytic Models | |||
| 5 or fewer (k=5) | 1.99* | 1.48 – 2.62 | 27.48* |
| 6 or more (k=10) | 1.22* | 1.01 – 1.47 | 19.49* |
| QM (1, 13) | 7.48* | ||
| Psychosocial Covariate | |||
| No (k=4) | 2.26* | 1.66 – 3.07 | 19.11* |
| Yes (k=11) | 1.22* | 1.04 – 1.43 | 19.46* |
| QM (1, 13) | 12.13* | ||
| Health Behavior Covariate | |||
| No (k=5) | 1.97* | 1.48 – 2.62 | 27.48* |
| Yes (k=10) | 1.22* | 1.01 – 1.47 | 19.49* |
| QM (1, 13) | 7.48* | ||
Note. QM = test of between group difference. K denotes studies and k denotes effects (i.e., cumulative adversity on one outcome). Cumulative effect sizes are represented using hazard ratios. Results reflect estimated effects of cumulative adversity on combined cardiometabolic outcomes, with the exception of results for moderation by “Outcome Type.” Bolded values highlight significant moderator variables.
Greater than 80% of the sample had to be of the same sex or results presented separately by sex to code the sample as predominantly composed of men or women.
p<.05.
p<.10.
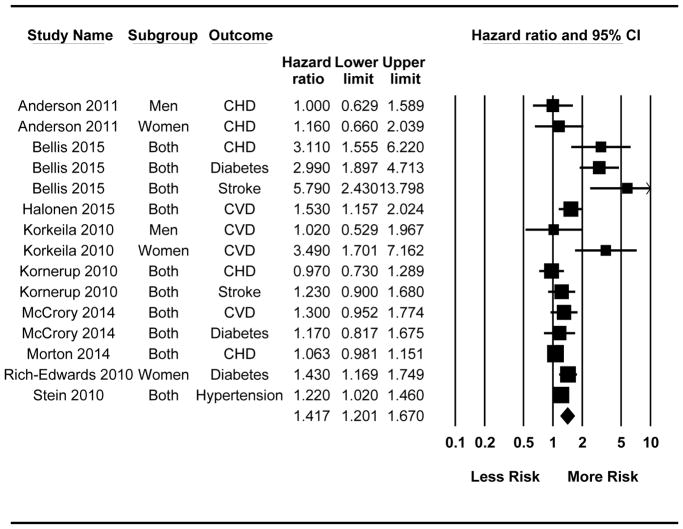
Figure 2.
Forest plot for all Hazard Ratio (HR) effects (k = 15). When all effects were treated as independent, results from random-effects meta-analysis revealed that exposure to cumulative adversity in childhood was associated with faster time to event to cardiometabolic disease for all outcomes combined (k = 15; HR = 1.42 [1.20, 1.67]). Following the exclusion of Bellis et al. (2015), the effect size was reduced and still significant (k = 12; HR = 1.23, [1.09, 1.39]).
Examining the forest plot raised the possibility that the Bellis et al. (2015) study may be an outlier, given relatively large effects across multiple outcomes. Thus, analyses were re-run excluding Bellis et al. (2015). Results indicated a reduced, but still significant, effect size whether treating all effects as independent or aggregated within study (see Figure 2 and Supplemental Figure 1 captions).
OR results
Results for OR studies are presented in Table 3. A forest plot examining all effects individually is presented in Figure 3 and a forest plot of the individual effects aggregated within study is pictured in Supplemental Figure 2. Results treating all effects as independent (k = 62) and results aggregating effects within studies (k = 29) both revealed that exposure to cumulative adversity in childhood was associated with increased risk for all outcomes combined, with almost identical cumulative effect sizes (Table 3, first two rows). Results assuming a correlation of .4 among outcomes tested in the same sample (OR=1.34, 95% CI [1.24, 1.45]) were very similar to the estimated effect sizes from analyses treating all outcomes as independent or redundant (Table 3, first two rows), likely due to the number of large samples in our analyses.
Table 3.
Cumulative odds ratios of cumulative childhood adversity on cardiometabolic disease.
| Cumulative Odds Ratio | 95%CI | QT (heterogeneity) | |
|---|---|---|---|
| All effects (k=62) | 1.36* | 1.27 – 1.46 | 396.71* |
| Using study as the unit of analysis (K=29) | 1.36* | 1.24 – 1.50 | 339.11* |
| Participants | |||
| Sexa | |||
| Women (k=14) | 1.42* | 1.24 – 1.62 | 141.34* |
| Men (k=11) | 1.52* | 1.28 – 1.80 | 52.92* |
| QM (1, 23) | .35 | ||
| Geography/Culture | |||
| Non-US (k=28) | 1.56* | 1.41 – 1.72 | 130.53* |
| US (k=34) | 1.22* | 1.13 – 1.33 | 175.20* |
| QM (1, 60) | 13.52* | ||
| Outcomec | |||
| Type | |||
| Metabolic outcomes (k=26) | 1.26* | 1.14 – 1.40 | 137.25* |
| CVD clinical outcomes (k=36) | 1.46* | 1.33 – 1.61 | 231.04* |
| QM (1, 60) | 4.12* | ||
| Measurement | |||
| Self-report (k=40) | 1.60* | 1.46 – 1.75 | 288.19* |
| Independently-assessed disease (k=22) | 1.08 | 0.96 – 1.21 | 84.65* |
| QM (1, 60) | 27.96* | ||
| Cumulative Adversity Index | |||
| Type of Adversity Measure | |||
| ACE Study Questionnaire (k=36) | 1.37* | 1.26 – 1.50 | 306.99* |
| Other Scale/Ad-hoc Composite (k=26) | 1.35* | 1.20 – 1.52 | 85.15* |
| QM (1, 60) | .85 | ||
| Index Included Childhood SES | |||
| No (k=49) | 1.34* | 1.24 – 1.44 | 342.49* |
| Yes (k=13) | 1.50* | 1.26 – 1.80 | 33.73* |
| QM (1, 60) | .24 | ||
| Index Included Household Dysfunction | |||
| No (k=14) | 1.29* | 1.11 – 1.51 | 45.64* |
| Yes (k=48) | 1.39* | 1.28 – 1.50 | 350.92* |
| QM (1, 60) | .43 | ||
| Analytic Strategy | |||
| Comparison of Extreme Groups vs. Count/Average | |||
| Comparison of most vs. least adversities (k=48) | 1.49* | 1.37 – 1.61 | 256.14* |
| Continuous total/average adversity score (k=14) | 1.15* | 1.04 – 1.27 | 43.38* |
| QM (1, 60) | 15.39* | ||
| Number of Covariates in Analytic Models | |||
| 5 or fewer (k=41) | 1.52* | 1.38 – 1.67 | 325.55* |
| 6 or more (k=21) | 1.20* | 1.07 – 1.34 | 65.62* |
| QM (1, 60) | 9.39* | ||
| Adult SES Covariate | |||
| No (k=16) | 1.09* | 0.94 – 1.27 | 72.64* |
| Yes (k=46) | 1.45* | 1.34 – 1.57 | 323.29* |
| QM (1, 60) | 10.25* | ||
| Psychosocial Covariate | |||
| No (k=40) | 1.30* | 1.17 – 1.43 | 198.31* |
| Yes (k=22) | 1.46* | 1.31 – 1.62 | 188.69* |
| QM (1, 60) | 2.60 | ||
| Health Behavior Covariate | |||
| No (k=40) | 1.47* | 1.34 – 1.61 | 311.43* |
| Yes (k=22) | 1.23* | 1.10 – 1.38 | 72.93* |
| QM (1, 60) | 5.52* | ||
Note. QM = test of between group difference. K denotes studies and k denotes effects (i.e., cumulative adversity on one outcome). Cumulative effect sizes are represented using odds ratios. Results reflect estimated effects of cumulative adversity on combined cardiometabolic outcomes, with the exception of results for moderation by “Outcome Type.” Bolded values highlight significant moderator variables.
Greater than 80% of the sample had to be of the same sex or results presented separately by sex to code the sample as predominantly composed of men or women.
Study Design refers to retrospective vs. prospective assessment of adversity.
p<.05.
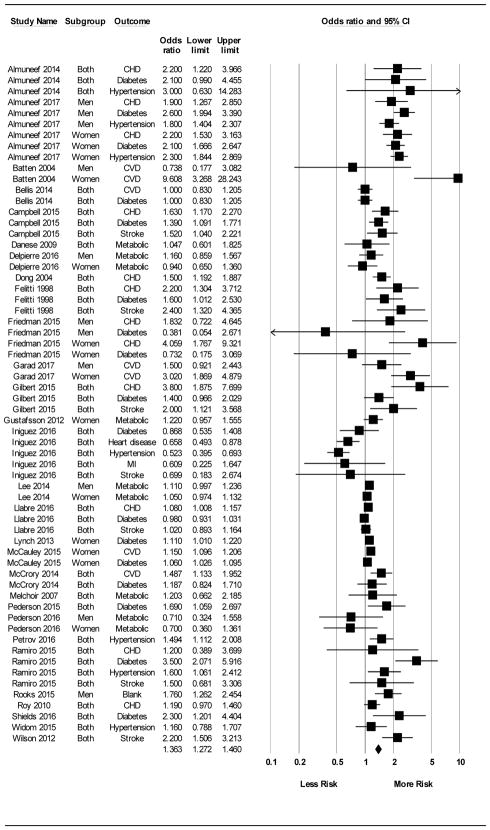
Figure 3.
Forest plot for all Odds Ratio (OR) effects (k=62). When all effects were treated as independent, results from random-effects meta-analysis revealed that exposure to cumulative adversity in childhood was associated with increased cumulative risk for cardiometabolic disease for all outcomes combined (k = 62; OR = 1.36 [1.27, 1.46]).
Moderators
HR results
Results of moderator analyses for HR studies are presented in Table 2 and revealed significant between-group differences for 5 of the 10 moderators tested: larger effects were observed for the ACE questionnaire compared to other adversity questionnaires or ad-hoc composites; for studies that did not include an indicator(s) of child SES in the composite index of cumulative adversity compared to those that did; for studies that did not adjust for adult psychosocial factors or health behaviors, respectively, in analytic models, compared to models that did; and for studies that had 5 or fewer covariates included in analytic models, compared to 6 or more covariates.
OR results
Results of moderator analyses for OR studies are presented in Table 3 and revealed significant between group differences for 7 of the 12 moderators tested: effects were larger for non-U.S. vs. U.S. samples; for CVD clinical outcomes compared to metabolic outcomes; for self-reported compared to independently-assessed disease; for studies that used an analytic strategy comparing individuals who reported the most adversities to a reference group of those who reported the fewest adversities (usually categorized as 0 adversities) vs. using a continuous total/average score on a trauma/adversity-related scale with no reference group; for studies that did not adjust for adult health behaviors in analytic models, compared to models that did; for studies that did adjust for adult SES in analytic models, compared to those that did not; and for studies that had 5 or fewer covariates, compared to 6 or more covariates.
Other Relevant Data
Results suggested no evidence of moderation by sex in HR or OR effects, but there were relatively few studies that had at least 80% males or females. Given limited variability in effects (see Table 1), for HR effects it was not possible to test moderation by adult SES, study design, analytic approach, and U.S. vs. non-U.S. samples, while for OR effects it was not possible test study design and whether the cumulative index included abuse/neglect.
Publication Bias
Evaluation of the funnel plot for HR effects (Supplemental Figure 3) indicated evidence of bias, such that smaller studies (i.e., those closer to the bottom of the plot) showed larger effects (because effects would need to be larger in order to reach statistical significance) and effects were less symmetrically distributed about the mean. Additionally, the funnel plot for OR effects (Supplemental Figure 4) suggests there may be some bias. Notably, results for Kendall’s Tau did not suggest significant publication bias in HR effects (Tau = .24, p = .23) or in OR effects (Tau = .05, p = .53), however, results for Egger’s regression did suggest bias in both HR (Intercept = 2.14, SE = .67, p<.05) and OR effects (Intercept = 1.62, SE = .36, p<.05).
Given evidence that results are influenced by publication bias, Duval and Tweedie’s (2000) trim-and-fill procedure was used to provide a bias-corrected estimate of the cumulative effect size; see Supplemental Figures 3 and 4. Overall, for both HR and OR studies, results suggest there may be bias in publication, depending on the approach used. However, statistically correcting for this bias did not nullify associations found here, but reduced the estimated effect size in OR analyses to 1.31, 95% CI [1.22, 1.40].
Discussion
The present review provides a quantitative analysis of the relationship of cumulative childhood adversity with cardiometabolic disease in adulthood. Based on 179,612 participants in 9 HR studies and on 247,393 participants in 29 OR studies that met eligibility criteria, results show overall effect sizes of 1.38 for HR studies and 1.36 for OR studies (Tables 2 and 3). The estimate for OR studies was reduced to 1.31 after correcting for publication bias. These results are based on retrospectively-reported adversity for the majority of HR (100%) and OR (90%) studies. Overall, results are surprisingly robust considering that most of the studies adjusted for multiple covariates (some of which are likely mediators of the association of interest) as well as the diversity of samples, study designs, and outcomes. Furthermore, the effect sizes are comparable to the aggregated effects of other psychosocial risk factors on cardiovascular morbidity and mortality. For example, in healthy populations (i.e., individuals without pre-existing CVD), meta-analytic results demonstrate a significant effect of anger and hostility (HR = 1.19, 95% CI [1.05, 1.35]; Chida & Steptoe, 2009), depression (RR = 1.81 [1.53, 2.15]; Nicholson, Kuper, & Hemingway, 2006), and anxiety (HR = 1.26 [1.15, 1.38]; Roest, Martens, de Jonge, & Denollet, 2010) on CVD events, as well as an effect of depression on risk of stroke (RR = 1.34 [1.17, 1.54]; J. Y. Dong, Zhang, Tong, & Qin, 2012) and anxiety on cardiac death (HR = 1.48 [1.14, 1.92]; Roest et al., 2010). Additionally, similar to effect sizes found here, recent meta-analytic results suggest a moderate combined estimated effect of loneliness, social isolation, and living alone on risk of incident CVD (RR = 1.29 [1.04, 1.59]) and stroke (RR = 1.32 [1.04, 1.68]; Valtorta, Kanaan, Gilbody, Ronzi, & Hanratty, 2016), as well as mortality (OR = 1.30 [1.16, 1.46]; Holt-Lunstad, Smith, Baker, Harris, & Stephenson, 2015).
The present meta-analysis also examined moderators. Of particular interest was whether childhood adversity was more closely associated with CVD clinical outcomes or metabolic outcomes. Findings suggest that cumulative adversity may be more closely associated with CVD clinical outcomes than metabolic outcomes (based on OR effects), although associations were significant for both types of outcome. Notably, there was no evidence of moderation by type of outcome for HR effects.
This weaker effect for metabolic results is consistent with the small cumulative association between abuse and neglect and diabetes reported in Huang et al. (2015). It is also consistent with the results of five studies (k = 6 effects) in the present review that involved “exemplary” measurement of both predictor and outcome variables (i.e., prospectively-reported childhood adversity and objective metabolic outcomes). Null relationships with metabolic disorders were found in four of these five studies (Danese et al., 2009; Delpierre et al., 2016; Gustafsson & Hammarstrom, 2012; Melchior, Moffitt, Milne, Poulton, & Caspi, 2007). Further, although not included in this meta-analysis, several recent studies using exemplary measurement have reported positive associations between prospective measures of childhood adversity and subclinicial CVD in adulthood (Hakulinen et al., 2016; Juonala et al., 2016), providing strong support for increased risk for later clinical events among those who experienced childhood adversity.
Regarding vulnerable or resilient subgroups, the meta-analysis found no evidence that cumulative childhood adversity has a more negative effect on men or women, although this is based on a limited number of HR and OR effects that provided sex-specific estimates. In contrast, previous meta-analytic evidence suggests stronger effects of abuse and neglect in females relative to males for obesity (Danese & Tan, 2014) and CVD problems (Wegman & Stetler, 2009). Ultimately, it is important that future studies examine sex-specific effects and not simply statistically adjust for sex, as males and females may be vulnerable to different types of adversity, such as child maltreatment versus household dysfunction. Importantly, it was not possible to test for moderation by race in HR and OR analyses because most studies (both prospective and retrospective) are conducted in predominantly non-Hispanic White samples. Consequently, little is known about the impact of cumulative childhood adversity on cardiometabolic disease in ethnically and racially diverse samples.
Regarding method issues, although overall effects were similar for HR and OR studies, effects for some moderators varied by HR versus OR analyses. The total number of covariates included in analytic models moderated associations with cardiometabolic disease for both types of analyses, suggesting indirectly that some of the covariates (particularly those related to health behaviors) may be part of the pathways connecting cumulative adversity with health outcomes. However, smaller effects were seen in HR studies that adjusted for psychosocial covariates. As much as possible, future studies should adjust for known correlates of both childhood adversity and long-term disease risk, in order to better understand the direct association of childhood adversity on cardiometabolic disease and identify potential mediators that may serve as intervention targets (see also Su et al., 2015, for a review of these issues).
Interestingly, the type of analytic strategy emerged as a significant moderator, such that OR effects were larger when analytic models involved comparison of extreme groups of adversities relative to a total score or average of a trauma/adversity scale, although it was not possible to test this moderator in HR effects (93% used an extreme groups approach). Given that all studies rely on the distribution of total number of adversities reported within their own sample in order to categorize individuals, there are stark differences among studies in how many adversities are included in the “extreme” category; for example, using 2+ adversities (e.g., Danese et al., 2009) vs. 4+ (Felitti et al., 1998) vs. 7–9 (e.g., Gilbert et al., 2015). As the field develops, it would be helpful to develop standard ways to measure and describe exposures to childhood adversity to allow comparisons across studies and populations, including using both continuous measures and discrete categories of exposure.
Considering the broader literature, there are significant methodological limitations that should be addressed and also limit the strength of the findings of this meta-analysis (see also Appleton et al., 2017 and Basu et al., 2017 for excellent discussions of these issues). First, there is wide variability regarding the number of adversity items included in cumulative indices, with some studies assessing as many as 27 items. Thus, there are many unique combinations of adversities that could be endorsed, and it is unclear whether it is actually the combination of certain types of adversities, a particular number of adversities, or the duration (c.f. Alastalo et al., 2009; Pesonen et al., 2007), chronicity (c.f. Slopen, Koenen, & Kubzansky, 2014), severity (c.f. Schilling, Aseltine, & Gore, 2008), or timing of exposure (c.f. Friedman, Montez, Sheehan, Guenewald, & Seeman, 2015; McLaughlin et al., 2015; Pesonen et al., 2010; Slopen et al., 2014; Slopen, Kubzansky, McLaughlin, & Koenen, 2013) that is most health-damaging. Future research should take a more exhaustive approach to measurement and analysis to overcome these limitations, as these details may provide a window into how and when to best intervene.
Second, not all studies include a measure of childhood SES in cumulative indices of adversity, despite established literatures from the areas of developmental and health psychology that indicate associations between childhood SES and poor long-term physical health and psychosocial outcomes (Cohen et al., 2010). Moreover, some researchers include childhood poverty as an adverse exposure. Thus, it is important for researchers to come to consensus about whether poverty or low childhood SES should be consistently included in cumulative measures of adversity. Given that the inclusion of childhood SES in measures of adversity may influence estimated cumulative effects (e.g., Table 2), it seems wise to investigate SES and adversity as independent variables in the same analysis, in order to inform our understanding of their overlap and their independent and aggregate predictive utility (see Appleton et al., 2017).
Third, the majority of studies analyzed here (over 90%) used retrospective self-reports of childhood adversity. Some evidence suggests that this may lead to inflated results, both in terms of the report of adversity and relationships with self-reported health outcomes, and that retrospectively-reported adversity may be less related to objectively-measured outcomes than prospectively-reported adversity (see Reuben et al., 2016; Widom, Raphael, & DuMont, 2004). Prospective adversity data have their own set of limitations, such as the possibility of under-reporting by parents or children due to fear of legal or social consequences. However, at face value, one may hypothesize that prospective data are “closer to the truth” and less subject to poor memory, biased post-hoc interpretation of past events, or the influence of concurrent negative mood or psychosocial adversity. Thus, it is important in future research, when feasible, to measure exposure to adversity both prospectively and retrospectively to elucidate the role of childhood adversity in adult cardiometabolic disease.
Ultimately, although exposure to cumulative childhood adversity appears to have long-term negative consequences for adult cardiometabolic disease, there are several promising avenues with regard to the mitigation of cardiometabolic risk in youth and adults. First, several studies in racially diverse samples indicate that interventions designed to improve parenting may have indirect but salubrious relationships on health outcomes, including decreased BMI (Smith, Montano, Dishion, Shaw, & Wilson, 2015), blood pressure (Brotman et al., 2012), and inflammation (Miller, Brody, Yu, & Chen, 2014) in youth samples, as well as decreased CVD risk factors and clinical events in adults, decades after the intervention (F. Campbell et al., 2016). Second, extant data suggests that adults who report a history of early adversity but also report greater resilience may demonstrate relatively better mental and physical health (e.g., Surtees & Wainwright, 2007; Wingo et al., 2010). Indeed, a recent review describes the scientific and clinical utility of investigating domains of development that may be shaped by exposure to stressful life experiences (e.g., learning, memory, attention) to potentially enhance long-term social, emotional, and functional outcomes (Ellis, Bianchi, Griskevicius, Frankenhuis, 2017).
In conclusion, the present analysis adds to the existing literature in a number of ways. First, it is the most thorough and recent review of the childhood adversity literature that also includes relevant papers from the child stress literature, and it provides the first quantitative analysis focused on clinical cardiometabolic disease. Second, it goes beyond the definition of adversity in terms of individual types of maltreatment to include other forms of adversity, which resulted in a large number of studies and effects to include. Finally, it examined a broad range of potential moderators of the effects. Based on the reviewed literature, this meta-analysis revealed a moderate cumulative effect of childhood adversity on risk for cardiometabolic diseases, with the evidence stronger for CVD clinical outcomes than for metabolic outcomes.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Health (T32 #HL07560).
References
References marked with an asterisk indicate studies included in the meta-analysis.
- Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, … Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Annals of Medicine. 2009;41(1):66–72. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- *.Almuneef M, ElChoueiry N, Saleheen HN, Al-Eissa M. Gender-based disparities in the impact of adverse childhood experiences on adult health: findings from a national study in the Kingdom of Saudi Arabia. Int J Equity Health. 2017;16(1):90. doi: 10.1186/s12939-017-0588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Almuneef M, Qayad M, Aleissa M, Albuhairan F. Adverse childhood experiences, chronic diseases, and risky health behaviors in Saudi Arabian adults: a pilot study. Child Abuse and Neglect. 2014;38(11):1787–1793. doi: 10.1016/j.chiabu.2014.06.003. [DOI] [PubMed] [Google Scholar]
- *.Andersen I, Diderichsen F, Kornerup H, Prescott E, Rod NH. Major life events and the risk of ischaemic heart disease: does accumulation increase the risk? International Journal of Epidemiology. 2011;40(4):904–913. doi: 10.1093/ije/dyr052. [DOI] [PubMed] [Google Scholar]
- Appleton AA, Holdsworth E, Ryan M, Tracy M. Measuring childhood adversity in life course cardiovascular research: A systematic review. Psychosomatic Medicine. 2016;79(4):434–440. doi: 10.1097/PSY.0000000000000430. [DOI] [PubMed] [Google Scholar]
- Basu A, McLaughlin KA, Misra S, Koenen KC. Childhood Maltreatment and Health Impact: The Examples of Cardiovascular Disease and Type 2 Diabetes Mellitus in Adults. Clinical Psychology. 2017;24(2):125–139. doi: 10.1111/cpsp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. Journal of Clinical Psychology. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Molecular Psychiatry. 2015 doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- *.Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. Journal of Public Health. 2015;37(3):445–454. doi: 10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bellis MA, Lowey H, Leckenby N, Hughes K, Harrison D. Adverse childhood experiences: retrospective study to determine their impact on adult health behaviours and health outcomes in a UK population. Journal of Public Health. 2014;36(1):81–91. doi: 10.1093/pubmed/fdt038. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: a retrospective self-report manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Bhattacharjee S, Bhattacharya R, Kelley GA, Sambamoorthi U. Antidepressant use and new-onset diabetes: a systematic review and meta-analysis. Diabetes/Metabolism Research and Reviews. 2013;29(4):273–284. doi: 10.1002/dmrr.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester, UK: Wiley; 2009. [Google Scholar]
- Borenstein M, Hedges L, Rothstein H. Meta-analysis: Fixed effect vs. random effects. 2007 doi: 10.1002/jrsm.12. Retrieved from https://www.meta-analysis.com/downloads/Meta-analysis fixed effect vs random effects 072607.pdf. [DOI] [PubMed]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. Journal of Nervous and Mental Disease. 2007;195(3):211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman LM, Dawson-McClure S, Huang KY, Theise R, Kamboukos D, Wang J, … Ogedegbe G. Early childhood family intervention and long-term obesity prevention among high-risk minority youth. Pediatrics. 2012;129(3):e621–628. doi: 10.1542/peds.2011-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science. 2014;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Campbell JA, Walker RJ, Egede LE. Associations Between Adverse Childhood Experiences, High-Risk Behaviors, and Morbidity in Adulthood. American Journal of Preventive Medicine. 2015 doi: 10.1016/j.amepre.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Violence Prevention. Essentials for childhood: steps to creating safe, stable and nurturing relationships. 2013 Retrieved from http://www.cdc.gov/violenceprevention/pdf/essentials_for_childhood_framework.pdf.
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. Journal of the American College of Cardiology. 2009;53(11):936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- *.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, … Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Molecular Psychiatry. 2014;19(5):544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- *.Delpierre C, Fantin R, Barboza-Solis C, Lepage B, Darnaudery M, Kelly-Irving M. The early life nutritional environment and early life stress as potential pathways towards the metabolic syndrome in mid-life? A lifecourse analysis using the 1958 British Birth cohort. BMC Public Health. 2016;16(1):815. doi: 10.1186/s12889-016-3484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43(1):32–37. doi: 10.1161/STROKEAHA.111.630871. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, … Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse and Neglect. 2004;28(7):771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- *.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997:315–369. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed]
- Ellis BJ, Bianchi J, Griskevicius V, Frankenhuis WE. Beyond Risk and Protective Factors: An Adaptation-Based Approach to Resilience. Perspectives on Psychological Science. 2017;12(4):561–587. doi: 10.1177/1745691617693054. [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, Whipple SS. Cumulative Risk and Child Development. Psychological Bulletin. 2013;139(6):1342–1396. doi: 10.1037/a0031808.supp. [DOI] [PubMed] [Google Scholar]
- *.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Font SA, Maguire-Jack K. Pathways from childhood abuse and other adversities to adult health risks: The role of adult socioeconomic conditions. Child Abuse and Neglect. 2015 doi: 10.1016/j.chiabu.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Friedman EM, Montez JK, Sheehan CM, Guenewald TL, Seeman TE. Childhood Adversities and Adult Cardiometabolic Health: Does the Quantity, Timing, and Type of Adversity Matter? Journal of Aging and Health. 2015;27(8):1311–1338. doi: 10.1177/0898264315580122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- *.Garad Y, Maximova K, MacKinnon N, McGrath JJ, Kozyrskyj AL, Colman I. Sex-Specific Differences in the Association Between Childhood Adversity and Cardiovascular Disease in Adulthood: Evidence From a National Cohort Study. Canadian Journal of Cardiology. 2017;33(8):1013–1019. doi: 10.1016/j.cjca.2017.05.008. [DOI] [PubMed] [Google Scholar]
- *.Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, Parks SE. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. American Journal of Preventive Medicine. 2015;48(3):345–349. doi: 10.1016/j.amepre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ … Stroke Statistics, S. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gustafsson PE, Hammarstrom A. Socioeconomic disadvantage in adolescent women and metabolic syndrome in mid-adulthood: an examination of pathways of embodiment in the Northern Swedish Cohort. Social Science and Medicine. 2012;74(10):1630–1638. doi: 10.1016/j.socscimed.2012.01.044. [DOI] [PubMed] [Google Scholar]
- *.Halonen JI, Stenholm S, Pentti J, Kawachi I, Subramanian SV, Kivimaki M, Vahtera J. Childhood Psychosocial Adversity and Adult Neighborhood Disadvantage as Predictors of Cardiovascular Disease: A Cohort Study. Circulation. 2015;132(5):371–379. doi: 10.1161/CIRCULATIONAHA.115.015392. [DOI] [PubMed] [Google Scholar]
- Hartiala O, Magnussen CG, Kajander S, Knuuti J, Ukkonen H, Saraste A, … Raitakari OT. Adolescence risk factors are predictive of coronary artery calcification at middle age: the cardiovascular risk in young Finns study. Journal of the American College of Cardiology. 2012;60(15):1364–1370. doi: 10.1016/j.jacc.2012.05.045. [DOI] [PubMed] [Google Scholar]
- Hakulinen C, Pulkki-Raback L, Elovainio M, Kubzansky LD, Jokela M, Hintsanen M, … Raitakari OT. Childhood Psychosocial Cumulative Risks and Carotid Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. Psychosomatic Medicine. 2016;78(2):171–181. doi: 10.1097/PSY.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspectives on Psychological Science. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, … Liu L. Adverse childhood experiences and risk of type 2 diabetes: A systematic review and meta-analysis. Metabolism: Clinical and Experimental. 2015;64(11):1408–1418. doi: 10.1016/j.metabol.2015.08.019. [DOI] [PubMed] [Google Scholar]
- *.Iniguez KC, Stankowski RV. Adverse Childhood Experiences and Health in Adulthood in a Rural Population-Based Sample. Clinical Medicine & Research. 2016;14(3–4):126–137. doi: 10.3121/cmr.2016.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juonala M, Pulkki-Raback L, Elovainio M, Hakulinen C, Magnussen CG, Sabin MA, … Raitakari OT. Childhood Psychosocial Factors and Coronary Artery Calcification in Adulthood: The Cardiovascular Risk in Young Finns Study. JAMA Pediatr. 2016;170(5):466–472. doi: 10.1001/jamapediatrics.2015.4121. [DOI] [PubMed] [Google Scholar]
- Kajeepeta S, Gelaye B, Jackson CL, Williams MA. Adverse childhood experiences are associated with adult sleep disorders: a systematic review. Sleep Medicine. 2015;16(3):320–330. doi: 10.1016/j.sleep.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: a systematic review. Journal of the American Association of Nurse Practitioners. 2015;27(8):457–465. doi: 10.1002/2327-6924.12215. [DOI] [PubMed] [Google Scholar]
- *.Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96:298–303. doi: 10.1136/heart.2009.188250. [DOI] [PubMed] [Google Scholar]
- *.Kornerup H, Osler M, Boysen G, Barefoot J, Schnohr P, Prescott E. Major life events increase the risk of stroke but not of myocardial infarction: results from the Copenhagen City Heart Study. European Journal of Cardiovascular Prevention and Rehabilitation. 2010;17(1):113–118. doi: 10.1097/HJR.0b013e3283359c18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123(15):1611–1621. doi: 10.1161/CIRCULATIONAHA.110.983874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lee C, Tsenkova V, Carr D. Childhood trauma and metabolic syndrome in men and women. Social Science and Medicine. 2014;105:122–130. doi: 10.1016/j.socscimed.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32(2):342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. Journal of the American Medical Association. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- Light R, Pillemer D. Summing Up: The Science of Reviewing Research. Cambridge: Harvard University Press; 1984. [Google Scholar]
- *.Llabre MM, Scneiderman N, Gallo LC, Arguelles W, Daviglus ML, Gonzalez F, 2nd, … Penedo FJ. Childhood Trauma and Adult Risk Factors and Disease in Hispanics/Latinos in the US: Results From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Sociocultural Ancillary Study. Psychosomatic Medicine. 2016 doi: 10.1097/PSY.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lynch L, Waite R, Davey MP. Adverse Childhood Experiences and Diabetes in Adulthood: Support for a Collaborative Approach to Primary Care. Contemporary Family Therapy. 2013;35(4):639–655. doi: 10.1007/s10591-013-9262-6. [DOI] [Google Scholar]
- *.McCauley HL, Blosnich JR, Dichter ME. Adverse Childhood Experiences and Adult Health Outcomes Among Veteran and Non-Veteran Women. Journal of Women’s Health. 2015;24(9):723–729. doi: 10.1089/jwh.2014.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McCrory C, Dooley C, Layte R, Kenny RA. The lasting legacy of childhood adversity for disease risk in later life. Health Psychology. 2015;34(7):687–696. doi: 10.1037/hea0000147. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA., 3rd Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(18):5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- *.Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. American Journal of Epidemiology. 2007;166(8):966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midei AJ, Matthews KA. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obesity Reviews. 2011;12(5):e159–172. doi: 10.1111/j.1467-789X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(31):11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Morton PM, Mustillo SA, Ferraro KF. Does childhood misfortune raise the risk of acute myocardial infarction in adulthood? Social Science and Medicine. 2014;104:133–141. doi: 10.1016/j.socscimed.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. Journal of the American Medical Association. 2004;291(17):2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pedersen JM, Lund R, Andersen I, Clark AJ, Prescott E, Rod NH. Psychosocial risk factors for the metabolic syndrome: A prospective cohort study. International Journal of Cardiology. 2016;215:41–46. doi: 10.1016/j.ijcard.2016.04.076. [DOI] [PubMed] [Google Scholar]
- *.Pedersen JM, Rod NH, Andersen I, Lange T, Poulsen G, Prescott E, Lund R. Accumulation of Major Life Events in Childhood and Adult Life and Risk of Type 2 Diabetes Mellitus. PloS One. 2015;10(9):e0138654. doi: 10.1371/journal.pone.0138654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Feldt K, Heinonen K, Osmond C, Phillips DI, … Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35(5):758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Heinonen K, Kajantie E, Forsen T, Eriksson JG. Depressive symptoms in adults separated from their parents as children: a natural experiment during World War II. American Journal of Epidemiology. 2007;166(10):1126–1133. doi: 10.1093/aje/kwm254. [DOI] [PubMed] [Google Scholar]
- *.Petrov ME, Davis MC, Belyea MJ, Zautra AJ. Linking childhood abuse and hypertension: sleep disturbance and inflammation as mediators. Journal of Behavioral Medicine. 2016;39(4):716–726. doi: 10.1007/s10865-016-9742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher ER, Jacobs DR, Jr, Moran A, Steinberger J, Prineas RJ, Sinaiko A. Relation of blood pressure and body mass index during childhood to cardiovascular risk factor levels in young adults. Journal of Hypertension. 2009;27(9):1766–1774. doi: 10.1097/HJH.0b013e32832e8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, … Viikari J. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- *.Ramiro LS, Madrid BJ, Brown DW. Adverse childhood experiences (ACE) and health-risk behaviors among adults in a developing country setting. Child Abuse and Neglect. 2010;34(11):842–855. doi: 10.1016/j.chiabu.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, … Danese A. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2016;57(10):1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, Wright RJ. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. American Journal of Preventive Medicine. 2010;39(6):529–536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. Journal of the American College of Cardiology. 2010;56(1):38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- *.Rooks C, Veledar E, Goldberg J, Votaw J, Shah A, Bremner JD, Vaccarino V. Long-Term Consequences of Early Trauma on Coronary Heart Disease: Role of Familial Factors. Journal of Traumatic Stress. 2015;28(5):456–459. doi: 10.1002/jts.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein H, Sutton A, Borenstein M. Publication bias in meta-analysis: Prevention, assessment and adjustments 2006 [Google Scholar]
- *.Roy A, Janal MN, Roy M. Childhood trauma and prevalence of cardiovascular disease in patients with type 1 diabetes. Psychosomatic Medicine. 2010;72(8):833–838. doi: 10.1097/PSY.0b013e3181eafc2d. [DOI] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Gore S. The impact of cumulative childhood adversity on young adult mental health: measures, models, and interpretations. Social Science and Medicine. 2008;66(5):1140–1151. doi: 10.1016/j.socscimed.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F, Oh I, Hayes T. Fixed-versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. British Journal of Mathematical and Statistical Psychology. 2009;62:97–128. doi: 10.1348/000711007X255327. [DOI] [PubMed] [Google Scholar]
- *.Shields ME, Hovdestad WE, Pelletier C, Dykxhoorn JL, O’Donnell SC, Tonmyr L. Childhood maltreatment as a risk factor for diabetes: findings from a population-based survey of Canadian adults. BMC Public Health. 2016;16(1):879. doi: 10.1186/s12889-016-3491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Cumulative adversity in childhood and emergent risk factors for long-term health. Journal of Pediatrics. 2014;164(3):631–638. e631–632. doi: 10.1016/j.jpeds.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38(2):188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, Williams DR. Racial Disparities in Child Adversity in the U.S.: Interactions With Family Immigration History and Income. American Journal of Preventive Medicine. 2016;50(1):47–56. doi: 10.1016/j.amepre.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Smith JD, Montano Z, Dishion TJ, Shaw DS, Wilson MN. Preventing weight gain and obesity: indirect effects of the family check-up in early childhood. Prev Sci. 2015;16(3):408–419. doi: 10.1007/s11121-014-0505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stein DJ, Scott K, Haro Abad JM, Aguilar-Gaxiola S, Alonso J, Angermeyer M, … Von Korff M. Early childhood adversity and later hypertension: data from the World Mental Health Survey. Annals of Clinical Psychiatry. 2010;22(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada. [Accessed 18 Dec 2016];General Social Survey – Victimization. http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=4504.
- Straus MA, Gelles RJ. Physical violence in American families: risk factors and adaptations to violence in 8, 145 families. New Brunswick: Transaction Publishers; 1990. [Google Scholar]
- Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Current Cardiology Reports. 2015;17(10):88. doi: 10.1007/s11886-015-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW. The shackles of misfortune: social adversity assessment and representation in a chronic-disease epidemiological setting. Social Science and Medicine. 2007;64(1):95–111. doi: 10.1016/j.socscimed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Umberson D, Williams K, Thomas PA, Liu H, Thomeer MB. Race, gender, and chains of disadvantage: childhood adversity, social relationships, and health. Journal of Health and Social Behavior. 2014;55(1):20–38. doi: 10.1177/0022146514521426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102(13):1009–1016. doi: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CA, MacMillan HL, Trocme N, Jamieson E, Boyle MH. Measurement of victimization in adolescence: development and validation of the Childhood Experiences of Violence Questionnaire. Child Abuse and Neglect. 2008;32(11):1037–1057. doi: 10.1016/j.chiabu.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71(8):805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- *.Widom CS, Czaja SJ, Bentley T, Johnson MS. A prospective investigation of physical health outcomes in abused and neglected children: new findings from a 30-year follow-up. American Journal of Public Health. 2012;102(6):1135–1144. doi: 10.2105/AJPH.2011.300636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, Raphael KG, DuMont KA. The case for prospective longitudinal studies in child maltreatment research: commentary on Dube, Williamson, Thompson, Felitti, and Anda (2004) Child Abuse and Neglect. 2004;28(7):715–722. doi: 10.1016/j.chiabu.2004.03.009. [DOI] [PubMed] [Google Scholar]
- *.Wilson RS, Boyle PA, Levine SR, Yu L, Anagnos SE, Buchman AS, … Bennett DA. Emotional neglect in childhood and cerebral infarction in older age. Neurology. 2012;79:1534–1539. doi: 10.1212/WNL.0b013e31826e25bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo AP, Wrenn G, Pelletier T, Gutman AR, Bradley B, Ressler KJ. Moderating effects of resilience on depression in individuals with a history of childhood abuse or trauma exposure. Journal of Affective Disorders. 2010;126(3):411–414. doi: 10.1016/j.jad.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank Group. World Bank Country and Lending Groups. 2017 Retrieved October 25, 2017, from https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ) 2012 Retrieved from http://www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.