Abstract
Objectives-
Recent evidence indicates that high-density lipoprotein (HDL) exerts vasculoprotective activities by promoting activating transcription factor 3 (ATF3), leading to down-regulation of TLR-induced inflammatory responses. Systemic lupus erythematosus (SLE) is associated with increased cardiovascular disease (CVD) risk not explained by the Framingham risk score. Recent studies have indicated oxidized HDL as a possible contributor. We investigated the potential mechanisms by which lupus HDL may lose its anti-inflammatory effects and promote immune dysregulation.
Methods-
Control macrophages were challenged with control and SLE HDL in vitro and examined for inflammatory markers by real time qRT-PCR, confocal microscopy, ELISA and flow cytometry. Lupus prone mice were treated with an HDL mimetic (ETC-642) in vivo and inflammatory cytokine levels measured by real time qRT-PCR and ELISA.
Results-
Compared to control HDL, SLE HDL activates NFκB, promotes inflammatory cytokine production, and fails to block TLR-induced inflammation in control macrophages. This failure of lupus HDL to block inflammatory responses is due to an impaired ability to promote ATF3 synthesis and nuclear translocation. This inflammation is dependent on lectin-like oxidized low-density lipoprotein receptor 1 (LOX1R) binding and ROCK1/2 kinase activity. HDL mimetic-treated lupus mice showed significant ATF3 induction and pro-inflammatory cytokine abrogation.
Conclusions-
Lupus HDL promotes pro-inflammatory responses through NFκB activation and decreased ATF3 synthesis and activity in a LOX1R- and ROCK1/2-dependent manner. HDL mimetics should be explored as potential therapies for inflammation and SLE cardiovascular risk.
Keywords: Systemic lupus erythematosus, inflammation, atherosclerosis, cytokines, lipids
INTRODUCTION
Systemic lupus erythematous (SLE) is an autoimmune syndrome of unclear etiology characterized by profound dysregulation of innate and adaptive immune responses. Inflammatory cytokines, particularly type I interferons (IFNs), interleukin (IL)-6, and tumor necrosis factor (TNF), may lead to altered cellular activation in SLE.1 Atherosclerotic cardiovascular disease (CVD) risk is significantly enhanced in SLE, independent of the Framingham risk score.2,3 Oxidized, dysfunctional high-density lipoprotein (HDL) has been linked to both “typical” and SLE-related CVD. When HDL becomes oxidized, it loses its cardioprotective effects and displays impaired cholesterol efflux capacity and decreased ability to block low-density lipoprotein (LDL) oxidation.4–10 We recently demonstrated that neutrophil extracellular traps in lupus promote pro-atherogenic modifications (3-nitrotyrosine and 3-chlorotyrosine oxidation) in HDL and that oxidized lupus HDL displays impaired cholesterol efflux capacity.7 What remains unclear is whether this modified SLE HDL promotes aberrant myeloid cell responses, leading to atherogenesis and systemic inflammation.
Monocytes and macrophages (Mϕ) within the vascular subendothelial space are key players in atherogenesis.11 These cells express scavenger receptors (SR), including lectin-like oxidized LDL receptor 1 (LOX1R), CD36, and SR-AI/II and SR-BI, which can internalize lipoproteins. This interaction can either promote repair or inflammation and plaque formation, depending on cardiovascular health status.11 As such, lipoprotein-mediated pathways that modify inflammatory responses in Mϕ can be crucial to mitigating CVD risk.
De Nardo et al. recently demonstrated that unmodified, healthy HDL blocks Toll-like receptor (TLR)-induced pro-inflammatory cytokine production through up-regulation of activating transcription factor 3 (ATF3).12 Upon activation, ATF3 translocates to the nucleus and recruits histone deacetylase-1 to the promoter regions of IL-6, TNF, and IL-12B to repress their expression.13 ATF3 may be particularly important for vascular health as, in vivo, HDL-induced arterial re-endothelialization requires ATF3.12 We therefore investigated if lupus oxidized HDL exerts similar anti-inflammatory pathways and affects ATF3 synthesis and function.
METHODS
See Supplementary Material online for details.
Subject recruitment
SLE subjects (diagnosed per American College of Rheumatology criteria) and healthy control (Ctrl, see Supplementary Table 1 for demographics) sample collection was approved by University of Michigan and National Institutes of Health IRBs. Disease activity was quantified by SLEDAI-2K.14,15 Pregnant or lactating women and individuals with recent or current infections or liver dysfunction were excluded.
HDL and ETC-642 preparation
Plasma HDL was purified (N=8 Ctrl, N=10 SLE) by sequential buoyant-density ultracentrifugation.16 ETC-642 HDL mimetic preparation is detailed online.17
Macrophage differentiation and culture
Adherent human PBMCs were cultured in X-Vivo-15 media (Lonza, Basel, Switzerland) with 10% fetal bovine serum (FBS) for 1 week. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, Grand Island, NY) with 2% lipoprotein deficient serum (LPDS, EMD Millipore, Billerica, MA), to minimize the effects of LDL in FBS. All HDL or ETC-642 in vitro treatments were at a concentration of 50 μg/ml. Individual Ctrl or SLE donor HDLs were used, not pooled samples. For receptor blocking experiments, Ctrl macrophages (CMϕ) were cultured with azide-free isotype control (1 μg/mL, Biolegend, San Diego, CA and SouthernBiotech, Birmingham, AL), anti-LOX1R blocking antibody (5 μg/mL, R&D Systems, Minneapolis, MN) or anti- SR-AI/II blocking antibody (1 μg/mL, R&D Systems) for 30 minutes (min), before adding HDL. To block ROCK1/2 activity, CMϕ were incubated with Y27632 (0.6 μM, Tocris, Bristol, UK) for 1 hr (for immunoblot, confocal microscopy, real time RT-qPCR) or 2hr (for ELISA) before HDL treatment.
Real Time qRT-PCR
To test the ability of HDL or ETC-642 to block TLR-induced inflammation, CMϕ were treated with media, Ctrl or SLE HDL for 4hr prior to challenge with LPS (100 ng/mL, Sigma-Aldrich, St. Louis, MO), Pam3CSK4 (300 ng/mL, InvivoGen, San Diego, CA), or R848 (1 μg/mL, InvivoGen) for 4hr. After treatment, RNA was isolated (Zymo Research, Irvine, CA), reverse transcribed, and real time RT-qPCR performed (BioRad, Hercules, CA). Primer sequences are listed in Supplementary Table 2.
ELISA
For LOX1R or SR-AI/II blockade experiments, CMϕ were treated with isotype control or blocking antibodies (see above) for 30min before addition of Ctrl or SLE HDL overnight (LOX1R blockade) or for 4hr before addition of Pam3CSK4 (300 ng/mL, InvivoGen) overnight (SR-AI/II blockade). Anti-human cytokine ELISAs (ALPCO, Salem, NH) were performed on supernatants. Anti-mouse IL-6 ELISA (eBioscience, San Diego, CA) was performed on mouse serum.
Confocal immunofluorescence microscopy
In the nuclear localization experiments, CMϕ were treated with isotype control/blocking antibody (see above), then HDL for 30min (ATF3) or 2hr (NFκB p65) before fixation. Cells were blocked, stained with Hoechst 33342 (1:1000, Life Technologies), anti-p65 (Abcam, Cambridge, MA), anti-LOX1R (Santa Cruz Biotechnology, Dallas, TX), or anti-ATF3 (Abcam) and fixed before imaging.
Immunoblot
Total lysate, nuclear fractions or immunoprecipitates were run on 4–12% Bis-Tris gels (Life Technologies), blocked with 10% BSA, stained, developed and densitometry calculated on an Odyssey CLx (Li-cor, Lincoln, NE).
Flow cytometry
Cells were blocked, stained with anti-CD14 (Biolegend, San Diego, CA), anti-LOX1R (Biolegend), or anti-ATF3 FITC (Bioss, Woburn, MA) and fixed before analysis.
In vivo administration of ETC-642
Lupus-prone NZM2328 (NZM) mice breeding pairs were obtained from Dr. Chaim Jacob (University of Southern California).7 NZM mice were treated (three times/week via tail vein injection) with ETC-642 (15 mg/kg, N=8) or equal volume phosphate-buffered saline (PBS, N=8) starting at 10 weeks of age until they reached 23 weeks of age. Balb/c mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Statistical analysis
Data are reported as mean ± SEM. Statistical analysis was performed using Student’s t-test or two-way ANOVA. A p value <0.05 (GraphPad Prism, La Jolla, CA) was considered significant.
RESULTS
SLE HDL induces inflammatory responses and fails to abrogate TLR-induced inflammatory cytokine synthesis.
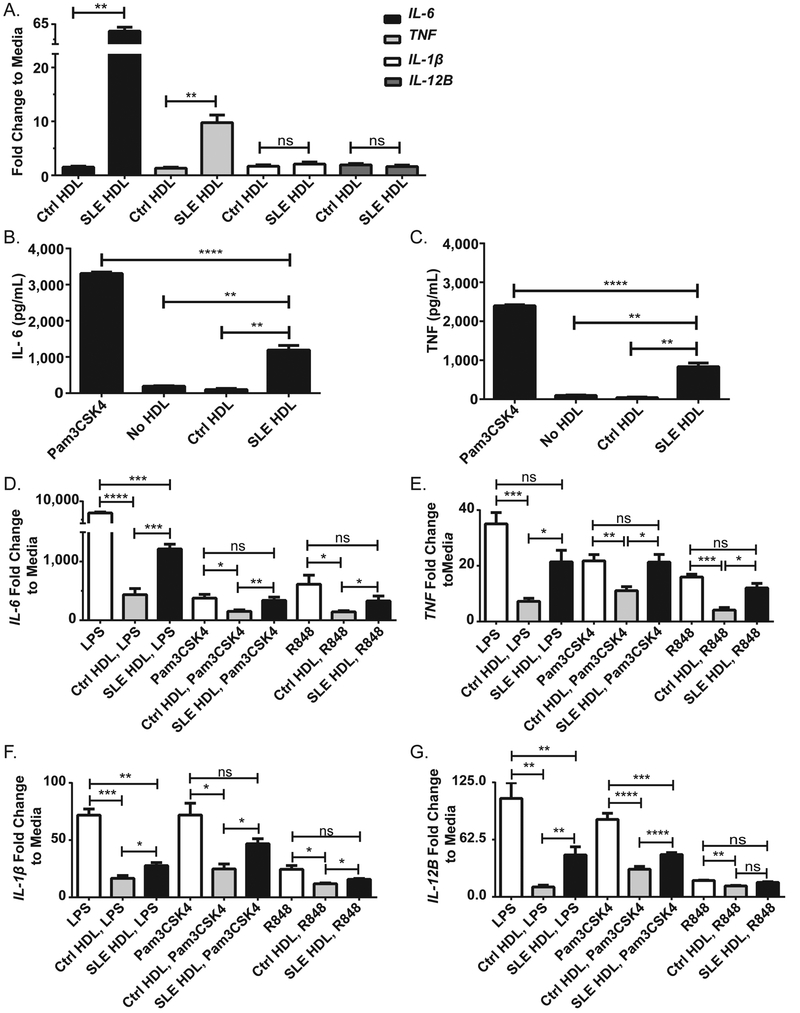
Compared to healthy control (Ctrl) HDL treatment, lupus HDL induced significant increases in IL-6 and TNF mRNA (Figure 1A) and protein (Figure 1B, IL-6, and 1C, TNF) in Ctrl Mϕ (CMϕ). Confirming recent reports, pre-incubation of CMϕ with Ctrl HDL prior to TLR-4, −1/2, or −7/8 stimulation decreased the synthesis of IL-6 (Figure 1D), TNF (Figure 1E), IL-1β (Figure 1F) and IL-12B mRNA (Figure 1G).4,12 In contrast, SLE HDL was impaired in its capacity to block TLR-induced inflammation (Figure 1D–G), independent of TLR internalization (Supplementary Figure 1A). Overall, these results indicate that SLE HDL promotes inflammatory responses in Mϕ and has an impaired ability to block TLR-induced inflammatory signals.
Figure 1. SLE HDL promotes pro-inflammatory cytokine production and fails to abrogate TLR-induced inflammation.
(A) Quantification of pro-inflammatory cytokine mRNA in Ctrl macrophages (CMϕ) incubated with Ctrl or SLE HDL for 4hr relative to media alone (no HDL). (B) IL-6 and (C) TNF levels in supernatants from CMϕ incubated with Pam3CSK4, no HDL, Ctrl HDL or SLE HDL overnight. (D-G) Measurement of pro-inflammatory genes in CMϕ pre-incubated with Ctrl or SLE HDL for 4hr before addition of TLR agonists for 4hr. Bar graphs are mean ± SEM, * p<0.05, ** p<0.01, *** p<0.0005, **** p<0.0001, ns =non-significant; fold change to media is relative to no HDL, no TLR agonist treatment.
Lupus HDL fails to promote ATF3 synthesis.
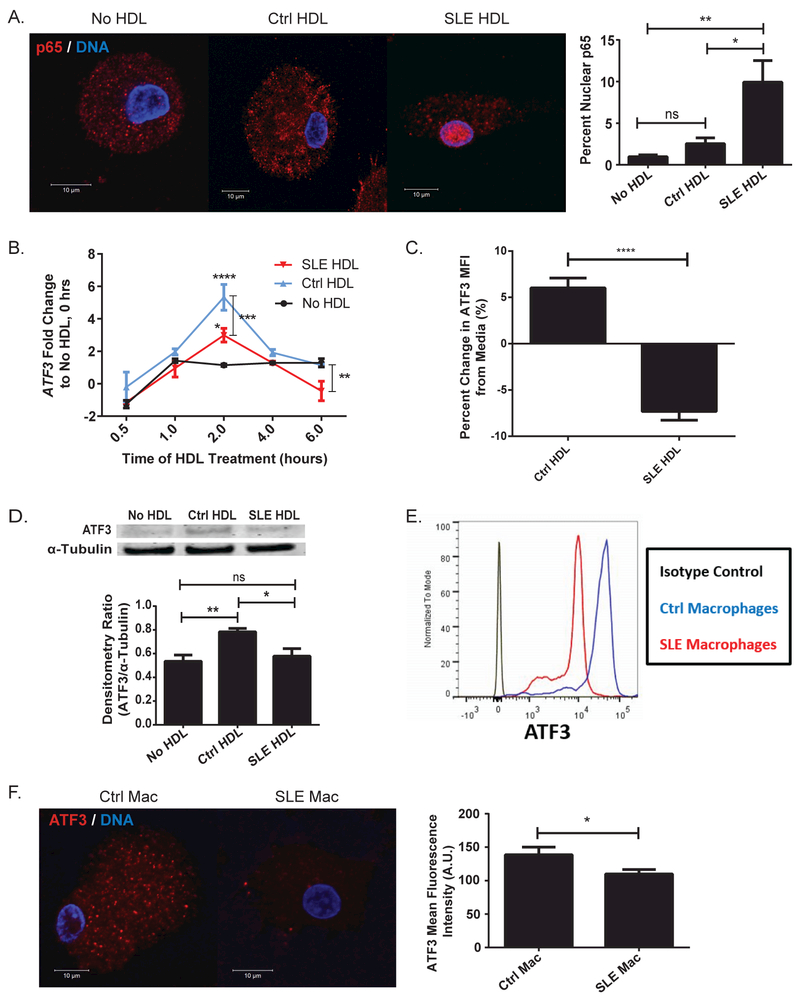
As lupus HDL promoted IL-6 and TNF production, we examined possible inflammatory signaling mechanisms. SLE HDL, but not Ctrl, promoted activation of the transcription factor NFκB in CMϕ, as quantified by p65 nuclear translocation (Figure 2A). ATF3 is an anti-inflammatory transcriptional repressor of IL-6, TNF, and IL-12B synthesis that is induced by Ctrl HDL.12,13 Two hours after exposing CMϕ to HDL, SLE HDL showed decreased capacity to induce ATF3 mRNA compared to Ctrl HDL (Figure 2B). By 6hr, lupus HDL induced significantly lower ATF3 mRNA than either Ctrl HDL or no HDL. This was confirmed at the protein level as Ctrl monocytes (Figure 2C) or CMϕ (Figure 2D) exposed to lupus HDL display significantly lower ATF3 compared to Ctrl HDL. Additionally, SLE macrophages (SMϕ) expressed lower levels of ATF3 than CMϕ by flow cytometry (Figure 2E) and microscopy (Figure 2F). These results suggest that, compared to Ctrl HDL, lupus HDL promotes NFκB activation and has decreased capacity to induce the expression of the inflammatory repressor ATF3.
Figure 2. SLE HDL induces NFκB activation and fails to induce ATF3 synthesis.
(A) NFκB p65 cellular localization in CMϕ (N=5) cultured with Ctrl or SLE HDL (N=5) for 2hr. Bar graph represents percent of p65 that co-localizes with DNA by weighted co-localization coefficient. (B) Kinetics of ATF3 induction response to HDL (N=6) or no HDL in CMϕ (N=9). Data are normalized to levels of ATF3 at time-0 in the absence of HDL stimulation. ATF3 levels in (C) Ctrl monocytes (N=5) by flow cytometry relative to media or (D) CMϕ (N=3) by immunoblot (α-tubulin loading control), following overnight incubation with Ctrl or SLE HDL (N=3). ATF3 levels in Ctrl and SLE macrophages (Mac) by (E) flow cytometry (N=3) or (F) confocal microscopy (Ctrl N=15, SLE N=12). Bar graphs are mean ± SEM, * p<0.05, ** p<0.01, *** p<0.0005, **** p<0.0001, ns =non-significant, A.U. = arbitrary units, percent change from media is relative to no HDL treatment.
Lupus HDL binds to LOX1R, prevents ATF3 nuclear translocation and promotes inflammatory responses in macrophages.
Previous work demonstrated that HDL purified from coronary artery disease patients or oxidized by myeloperoxidase acquires the ability to bind LOX1R and initiate inflammation.18,19 As lupus HDL contains enhanced levels of myeloperoxidase-catalyzed 3-chlorotyrosine, we investigated if the inflammatory responses induced by lupus HDL require LOX1R binding.7 We first verified LOX1R expression in Ctrl and SLE monocytes and Mϕ. While LOX1R was present in both Ctrl and SLE cells, a higher percentage of SLE monocytes were LOX1R+ compared to Ctrl (Supplementary Figure 2A), although LOX1R mean fluorescent intensity did not differ between Ctrl and SLE monocytes (Supplementary Figure 2B). In contrast, SMϕ displayed higher LOX1R expression at the protein (Supplementary Figure 2C and D) and mRNA level than CMϕ (Supplementary Figure 2E). LOX1R levels (quantified by microscopy, flow cytometry and real time RT-qPCR) did not correlate with SLEDAI. Furthermore, SLE HDL induced significantly higher LOX1R levels than Ctrl HDL (Supplementary Figure 2F) in CMϕ.
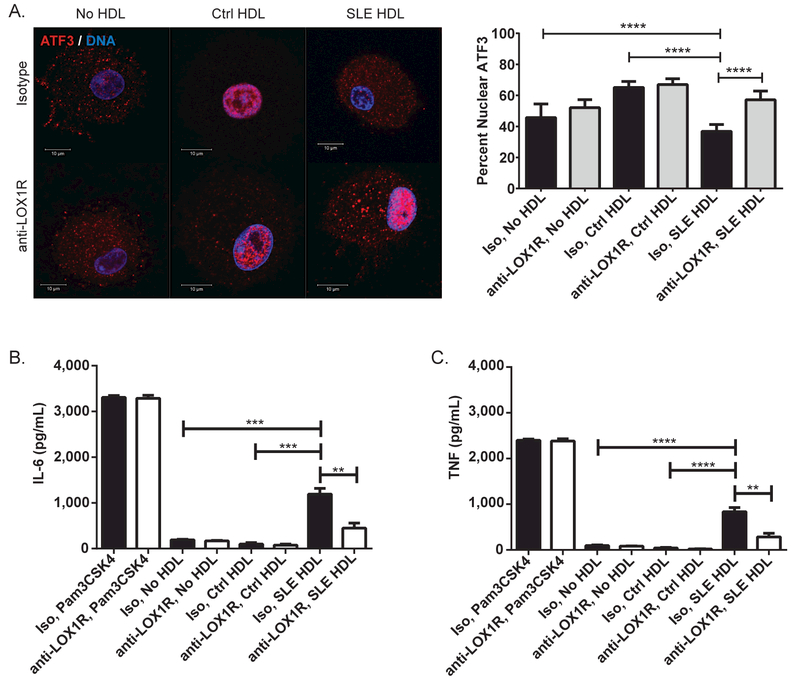
We then examined the effects of SLE HDL on ATF3 activity. Since ATF3 blocks inflammation via epigenetic promoter modifications, its activity was assessed by its ability to translocate to the nucleus. Compared to Ctrl HDL, SLE HDL-treated CMϕ displayed significantly less nuclear ATF3 (Figure 3A). To assess if this impairment was dependent on LOX1R binding, we incubated CMϕ with blocking anti-LOX1R or isotype control antibodies prior to addition of SLE or Ctrl HDL. By preventing the binding of SLE HDL to LOX1R, ATF3 nuclear translocation was restored (Figure 3A) while blockade prior to Ctrl HDL addition demonstrated no changes. This was confirmed by immunoblot (Supplementary Figure 3A). These results suggest that SLE HDL binds to LOX1R and initiates a pathway that suppresses ATF3’s anti-inflammatory activity. This was further examined by incubating CMϕ with anti-LOX1R blocking or isotype control antibodies, followed by SLE or Ctrl HDL overnight and quantifying IL-6 and TNF release by ELISA. LOX1R blockade significantly decreased SLE HDL-induced IL-6 (Figure 3B) and TNF (Figure 3C) release. Blocking LOX1R prior to addition of SLE HDL also significantly decreased NFκB activation by immunofluorescence (Supplementary Figure 3B) and immunoblot (Supplementary Figure 3C). These results indicate that lupus HDL engagement with LOX1R blocks the activity of ATF3 and enhances NFκB activation, thereby promoting inflammatory cytokine synthesis in Mϕ.
Figure 3. Lupus HDL binds LOX1R, blocks ATF3 nuclear translocation and promotes inflammatory cytokine synthesis.
(A) Pre-incubation with blocking anti-LOX1R antibody (anti-LOX1R) increases ATF3 nuclear translocation in CMϕ (N=12) exposed to SLE HDL (N=7) for 30min compared to isotype control (Iso). Images are representative of conditions performed in triplicate. Bar graph represents percent of ATF3 that co-localizes with DNA by weighted co-localization coefficient. Anti-LOX1R modifies (B) IL-6 and (C) TNF secretion by CMϕ (N=12) exposed overnight to SLE (N=10), but not Ctrl HDL (N=6). Bar graphs are mean ± SEM, ** p<0.01, *** p<0.0005, **** p<0.0001.
SLE HDL impairs ATF3 activity in a ROCK1/2-dependent manner
Stimulation of LOX1R has been linked to activation of Rho-associated, coiled-coil containing protein kinase 1 and 2 (ROCK1/2), which then activates NFκB.20 Given that ROCK1/2 are serine/threonine kinases and that ATF3 contains a threonine (Thr-162) phosphorylation site, we examined the role of ROCK1/2 on SLE HDL-induced inflammation and ATF3 activity.21
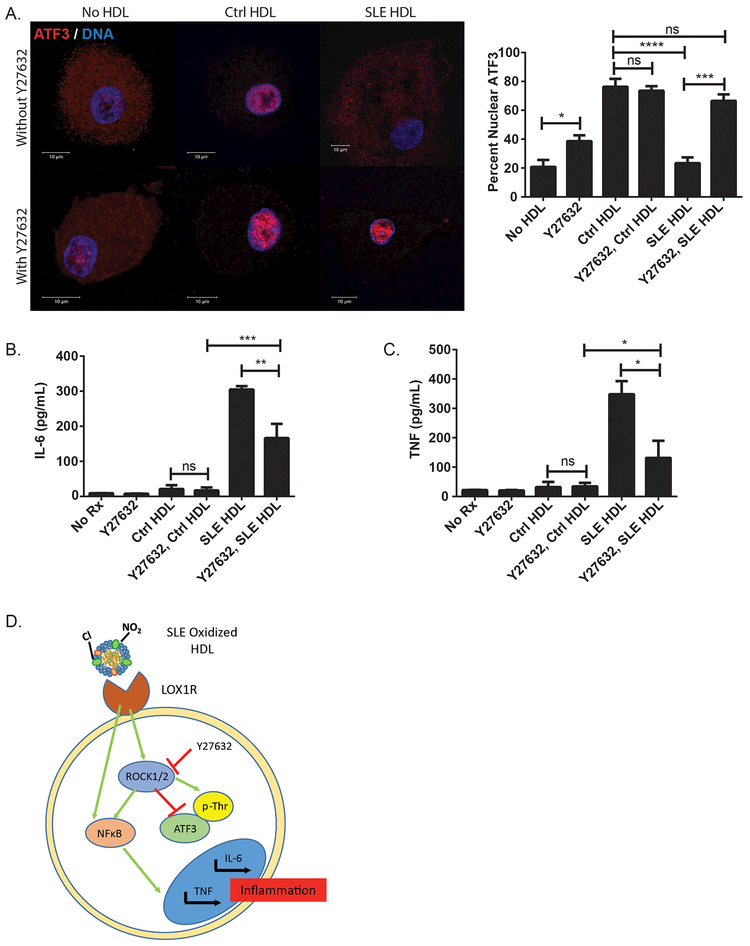
CMϕ were incubated with or without Y27632, at a concentration that specifically blocks ROCK1/2 activity, prior to adding Ctrl or SLE HDL.22 Immunoprecipitated ATF3 was then measured for phosphorylation. As Thr-162 on ATF3 is adjacent to a proline residue, we used a primary antibody that recognizes only phosphorylated serine or threonine residues adjacent to a proline residue (no phos-ATF3 antibodies are commercially available). SLE HDL enhanced ATF3 phosphorylation, but not when pre-incubated with Y27632 (Supplementary Figure 4A). This indicates that SLE HDL-activated ROCK1/2 targets ATF3 for phosphorylation.
We next examined the effect of ROCK1/2 on SLE HDL-induced inflammation and altered ATF3 localization. CMϕ pre-incubated with Y27632 before SLE HDL treatment showed restoration of ATF3 nuclear translocation (Figure 4A). In contrast, Y27632 did not modify Ctrl HDL-induced ATF3 nuclear localization. Y27632 by itself increased ATF3 nuclear translocation, suggesting a putative effect from FBS lipoproteins. Finally, pre-incubation with Y27632 also prevented SLE HDL-induced IL-6 (Figure 4B) and TNF protein (Figure 4C) and mRNA (Supplementary Figure 4B and C) synthesis. These results suggest that oxidized SLE HDL binds LOX1R, activates ROCK1/2, which phosphorylates ATF3 and blocks its nuclear translocation, resulting in increased inflammation (Figure 4D). As LOX1R expression is enhanced in SMϕ, these effects may be even more pronounced in lupus patients.
Figure 4. SLE HDL-induced inflammation and ATF3 inhibition are ROCK1/2-dependent.
(A) CMϕ (N=12) were pre-treated with the ROCK1/2 inhibitor Y27632 for 1hr followed by incubation with Ctrl (N=5) or SLE HDL (N=7) for 30min. Quantified DNA/ATF3 cellular localization in CMϕ. (B) IL-6 and (C) TNF supernatant protein levels in CMϕ pre-treated with Y27632 before overnight culture with HDL. (D) Proposed signaling pathways in SLE HDL-induced inflammation. Bar graphs mean ± SEM, * p<0.02, ** p<0.04, *** p<0.0004, **** p<0.0001, ns =non-significant.
SR-AI/II affects Ctrl HDL-induce anti-inflammatory activity and ATF3 mobility.
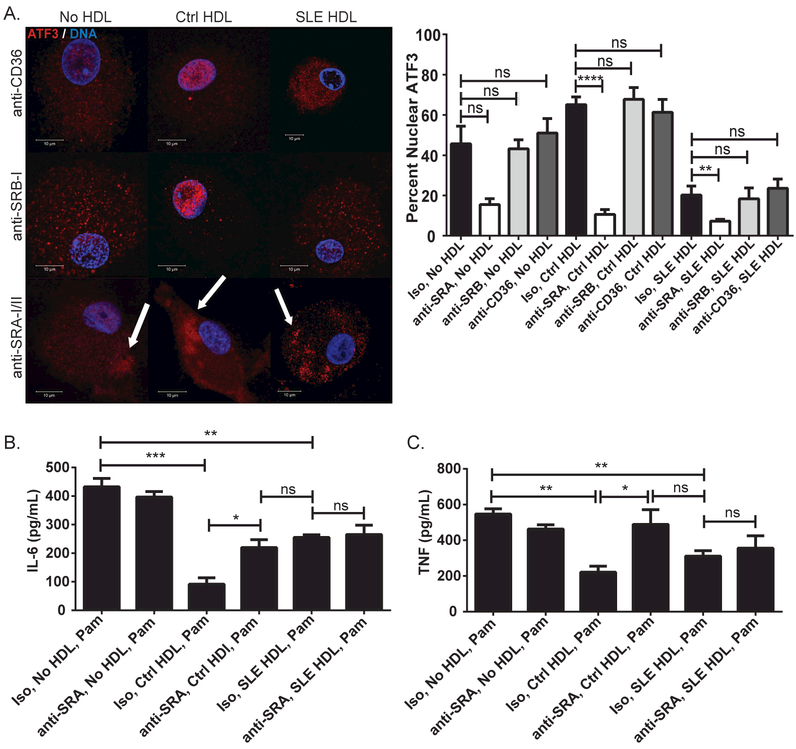
The signaling cascade leading to Ctrl HDL-induced ATF3 activity remains unclear. SRs possibly contribute to this pathway as they are abundant on Mϕ, have been linked to CVD, and interact with lipoproteins.10,11 We quantified mRNA levels of various SRs in CMϕ, compared to Ctrl monocytes, and found that SR-AI and –AII were the most significantly up-regulated during differentiation (Supplementary Figure 5A and B). We then tested whether Ctrl HDL interacts with SR-AI/II to modify ATF3 nuclear translocation. CMϕ pre-incubated with anti-SR-AI/II blocking antibody prior to addition of Ctrl HDL showed significantly lower nuclear ATF3 than isotype control or antibodies blocking other SRs (anti-CD36 or anti-SR-BI). Indeed, anti-SR-AI/II blockade prior to addition of Ctrl or SLE HDL promoted ATF3 aggregates in the cytoplasm (Figure 5A).
Figure 5. Binding of Ctrl HDL to SR-AI/II modulates ATF3 cellular localization and anti-inflammatory activity.
(A) Effect of SR blockade in ATF3 cellular localization in CMϕ (N=15) prior to exposure to Ctrl (N=7) or SLE (N=7) HDL treatment for 30min. White arrows indicated ATF3 cytoplasmic aggregates. Images are representative of conditions performed in triplicate. Co-localization bar graphs are as in Fig3A. Result of SR-AI/II blockade (or Iso control) on (B) IL-6 and (C) TNF synthesis induced in CMϕ (N=5) exposed to Ctrl (N=5) or SLE (N=5) HDL for 4hr, then Pam3CSK4 (Pam) overnight (protein in supernatants). Bar graphs are mean ± SEM, * p<0.04, ** p<0.008, *** p<0.0009, **** p<0.0001, ns =non-significant.
As Ctrl HDL-induced ATF3 blocks TLR-induced inflammatory cytokine production, we assessed whether blockade of SR-AI/II suppressed this effect. CMϕ treated first with anti-SR-AI/II blocking antibody, then Ctrl HDL, and finally TLR-2 agonist Pam3CSK4 showed significantly increased IL-6 (Figure 5B) and TNF (Figure 5C) protein and mRNA (Supplementary Figure 5C and D) levels compared to control conditions (Supplementary Figure 5E and F). This suggests that, in contrast to the observed interaction of SLE HDL with LOX1R, Ctrl HDL preferentially binds SR-AI/II to promote ATF3’s anti-inflammatory activity.
The HDL mimetic ETC-642 abrogates the inflammatory effects of lupus HDL.
ETC-642 is a HDL mimetic compound composed of 22 amino acids (22A) derived from apolipoprotein A-I (ApoA-I, the most abundant protein in HDL) and the phospholipids 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and sphingomyelin (SM). ETC-642 improves cardiovascular parameters in animal models and mobilizes cholesterol in plasma compartments in dyslipidemic patients.17,23 As this compound mimics some of the anti-inflammatory effects of healthy HDL, we assessed its role in down-regulating the deleterious effects of lupus HDL.
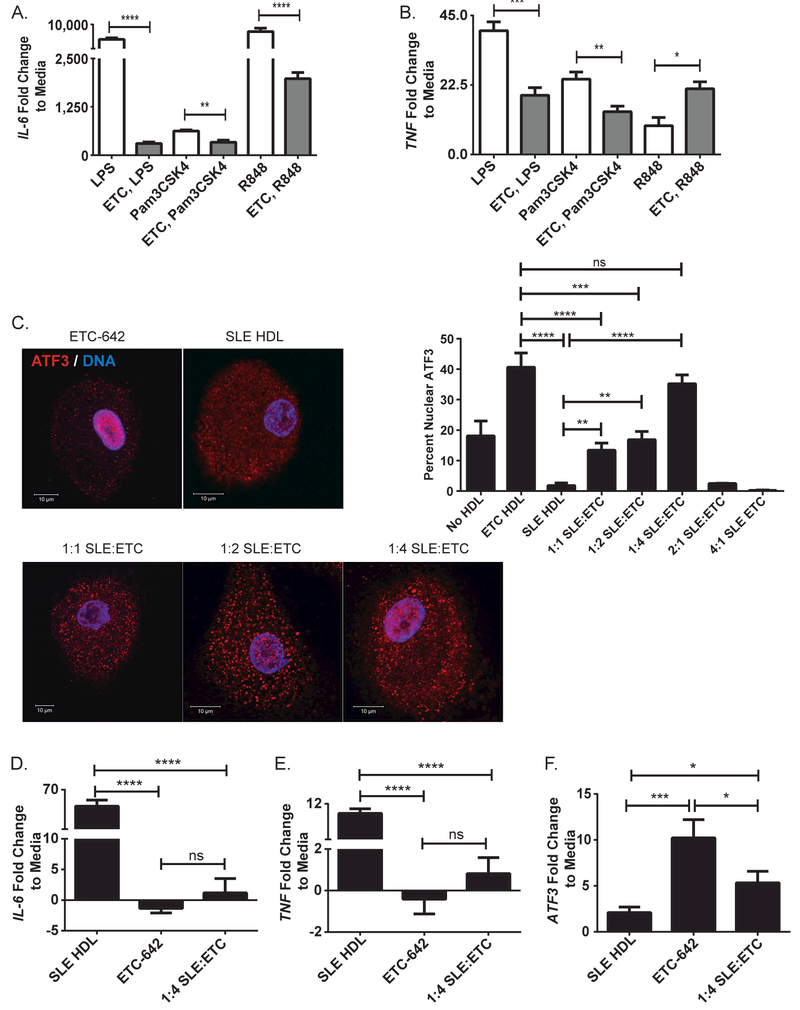
ETC-642 blocked TLR-induced IL-6 (Figure 6A) and TNF (Figure 6B) mRNA production in CMϕ (see Supplementary Figure 6A and B for IL1-β and IL-12B), while its individual protein or lipid components did not (Supplementary Figure 6C and D). Similarly, ATF3 nuclear translocation was induced by ETC-642 (Figure 6C), but not its individual components (Supplementary Figure 6E). To address if ETC-642 could reverse the pro-inflammatory effects of lupus HDL, CMϕ were exposed to various ratios (based on protein concentration) of lupus HDL:ETC-642. A 1:4 SLE HDL:ETC-642 ratio significantly enhanced ATF3 nuclear translocation (Figure 6C), blocked SLE HDL-induced IL-6 (Figure 6D) and TNF (Figure 6E) mRNA production and NFκB activation (Supplementary Figure 6F). ETC-642 also increased ATF3 levels in CMϕ (Figure 6F) and Ctrl monocytes (Supplementary Figure 6G–J). These results indicate that ETC-642 can mimic the effects of healthy HDL on ATF3 and NFκB activity in addition to hampering SLE HDL-induced Mϕ inflammation.
Figure 6. ETC-642 abrogates pro-inflammatory responses of TLR agonist and lupus HDL.
Pre-incubation of CMϕ (N=8) with ETC-642 (ETC) dampens TLR agonist-induced (A) IL-6 and (B) TNF mRNA synthesis. (C) ETC-642 promotes ATF3 nuclear translocation and blocks inhibitory effects of lupus HDL on ATF3 nuclear localization at a 1:4 ratio in CMϕ (N=6). Quantification is 30min post-stimulation. ETC-642 abrogates lupus HDL-induced (D) IL-6 and (E) TNF synthesis in CMϕ at a 1:4 ratio in CMϕ (N=6). (F) ETC-642 increases ATF3 mRNA at a 1:4 ratio in CMϕ (N=6). Bar graphs are mean ± SEM,* p<0.05, ** p<0.009, *** p<0.0008, **** p<0.0001, ns =non-significant, fold change to media is relative to no HDL treatment.
Similar to human SLE, we previously showed that lupus-prone NZM mice display CVD-like endothelial dysfunction and enhanced levels of oxidized HDL.24 We therefore assessed if in vivo systemic administration of ETC-642 could decrease inflammatory cytokine synthesis in female NZM mice (see Supplementary Figure 7A for pharmacokinetics). Splenocytes from ETC-642-treated NZM mice displayed significant increases in Atf3, significant decreases in Il6, Tnf, Il1β, Il12b and interferon-stimulated genes when normalized to Balb/c splenocytes and compared to PBS-treated NZM mice (Supplementary Figure 7B). Indeed, when normalized to PBS-treated NZM mice and compared to Balb/c mice (Supplementary Figure 7C), the splenocytes from ETC-treated lupus mice were not statistically different in Il6, Tnf, and Il12b mRNA levels than healthy Balb/c mouse splenocytes. ETC-treated NZM mice also showed significantly lower serum IL-6 levels (Supplementary Figure 6D). ETC-642 did not modify autoantibodies or proteinuria (data not shown). Overall, these results indicate that in vivo administration of the HDL mimetic ETC-642 can dampen cytokine synthesis in murine lupus.
DISCUSSION
Healthy (minimally oxidized) HDL shows an inverse correlation with CVD risk and promotes anti-inflammatory effects, including dampening of TLR-mediated responses in Mϕ.5–8,10,25 It has been unclear if oxidative modifications of HDL modify the capacity of this lipoprotein to modulate TLR-induced responses or affect ATF3 activity. We tested this hypothesis in SLE subjects because they exhibit enhanced inflammatory cytokine synthesis, aberrant innate immune responses, enhanced HDL oxidation and increased CVD risk.3,24,26 As Mϕ are crucial players in inflammation, understanding how oxidized HDL modulates Mϕ function is highly relevant in CVD and autoimmune disorders.4,11
In contrast to the anti-inflammatory effects of Ctrl HDL on Mϕ, exposure to SLE HDL enhanced inflammatory cytokine production (even in the absence of TLR agonists) and failed to block TLR-induced cytokine production. The inflammatory effects promoted by lupus HDL required its binding to LOX1R, leading to enhanced ROCK1/2 activity, and a failure to induce ATF3 synthesis and activity. In contrast, the anti-inflammatory effects of Ctrl HDL via ATF3 involved SR-AI/II binding. Importantly, an ApoA-I mimetic counteracted the deleterious effects of lupus HDL in vitro and also decreased levels of proinflammatory cytokines in murine SLE in vivo. While the mimetic did not affect autoantibody or proteinuria levels, future studies should explore if a beneficial effect may be observed using different doses of ETC-642, other lupus models or earlier assessments of disease activity. As this compound is devoid of tyrosine residues, it is not susceptible to CVD-associated regiospecific ApoA-I modifications. ETC-642 may therefore have therapeutic potential by counteracting pro-atherogenic and proinflammatory effects of lupus HDL and by engaging SRs associated with protective responses in macrophages. While to our knowledge clinical development of this compound has ceased, similar therapies could have potential benefit.
One conceivable explanation for how the SLE HDL:LOX1R interaction blocks ATF3 activity is by promoting degradation of ATF3 protein and/or mRNA. However, as differences in ATF3 nuclear translocation were detected as early as 30 minutes post-HDL treatment, this hypothesis is less likely. Instead, our results indicate that site-specific phosphorylation of ATF3 by ROCK1/2 promotes cytoplasmic ATF3 sequestration, thereby decreasing its anti-inflammatory effect. Future studies should address whether HDL from SLE and other chronic, inflammatory disorders (e.g. rheumatoid arthritis) have different affinities for LOX1R, SR-AI/II, CD36 or other SRs.16 The oxidation pattern alone may not fully explain lupus HDL’s inflammatory activity. Circulating HDL can bind and trap neighboring molecules that, in turn, could affect the lipoprotein’s immunogenicity. Changes in phospholipid composition can affect the anti-inflammatory capacity of HDL.27 Future analysis should explore differences in HDL lipid and protein cargo among different inflammatory conditions.
In conclusion, modified lupus HDL binds to LOX1R in Mϕ, leading to induction of NFκB activity and failure to induce ATF3 synthesis and activation through a ROCK1/2-dependent pathway, thereby promoting pro-inflammatory responses. As HDL mimetics modify pro-inflammatory effects of lupus HDL in vivo and in vitro, these therapies could potentially have cardioprotective and immunomodulatory roles in lupus and other autoimmune diseases associated to enhanced CVD risk.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Pragnesh Mistry, Carmelo Carmona-Rivera and Luz Blanco for helpful discussions.
Funding- This work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (project ZIA AR041199), the Lupus Research Institute and also by the NIH through grants: HL006193–01 (MP and NNM), AHA 13SDG17230049 and R01 GM113832 (AS and WY).
Footnotes
SUPPLEMENTARY MATERIAL: Refer to Web version on PubMed Central for supplementary material.
REFERENCES
- 1.Yap DY, Lai KN. The role of cytokines in the pathogenesis of systemic lupus erythematosus - from bench to bedside. Nephrology (Carlton) 2013;18:243–55. [DOI] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- 3.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med 2013;64:249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki M, Pritchard DK, Becker L, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation 2010;122:1919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon M, Grossman J, Skaggs B, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum 2009;60:2428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronda N, Favari E, Borghi MO, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis 2014;73:609–15. [DOI] [PubMed] [Google Scholar]
- 7.Smith CK, Vivekanandan-Giri A, Tang C, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol 2014;66:2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennathur S, Bergt C, Shao B, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem 2004;279:42977–83. [DOI] [PubMed] [Google Scholar]
- 9.Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707–14. [DOI] [PubMed] [Google Scholar]
- 10.Shao B, Bergt C, Fu X, et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem 2005;280:5983–93. [DOI] [PubMed] [Google Scholar]
- 11.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014;15:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrist M, Thorsson V, Li B, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 2006;441:173–8. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 15.Isenberg D, Bacon P, Bombardier C, et al. Criteria for assessing disease activity in systemic lupus erythematosus. J Rheumatol 1989;16:1395–6.2810268 [Google Scholar]
- 16.Vivekanandan-Giri A, Slocum JL, Byun J, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis 2013;72:1725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Gordon S, Schwendeman A, et al. Apolipoprotein mimetic peptides for stimulating cholesterol efflux. Apolipoprotein mimetics in management of human disease 2015:29–42. [Google Scholar]
- 18.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsche G, Levak-Frank S, Quehenberger O, et al. Identification of the human analog of SR-BI and LOX-1 as receptors for hypochlorite-modified high density lipoprotein on human umbilical venous endothelial cells. FASEB J 2001;15:1095–7. [DOI] [PubMed] [Google Scholar]
- 20.Mattaliano MD, Wooters J, Shih HH, et al. ROCK2 associates with lectin-like oxidized LDL receptor-1 and mediates oxidized LDL-induced IL-8 production. Am J Physiol Cell Physiol 2010;298:C1180–7. [DOI] [PubMed] [Google Scholar]
- 21.Berman HMWJ, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. www.rcsb.org. The Protein Data Bank Nucleic Acids Research 2000;28:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 2000;57:976–83. [PubMed] [Google Scholar]
- 23.Iwata A, Miura S, Zhang B, et al. Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis 2011;218:300–7. [DOI] [PubMed] [Google Scholar]
- 24.Thacker SG, Zhao W, Smith CK, et al. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum 2012;64:2975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem 2012;287:6375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwendeman A, Sviridov DO, Yuan W, et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.