Abstract
Long-term central venous access devices are increasingly prevalent and consequently often encountered by intensivists. This review introduces the different types of long-term central venous access devices, outlines their potential utility, examines potential complications associated with their use and outlines an approach to the management of these complications.
Keywords: Catheter, catheter complication, catheter infection, central venous catheter, long-term central venous access, thrombosis, vascular access
Introduction
Intensivists are familiar with standard short-term central venous access catheters; however, they increasingly encounter long-term central venous access devices (LCVADs). LCVADs are most commonly seen in patients receiving chemotherapy, home TPN, antimicrobial therapy or haemodialysis. Often dedicated multidisciplinary teams look after these devices, however in out-of-hours or emergency situations the intensivist may be required to use or manage problems with LCVADs. Different types of LCVADs exist and an awareness of how to use them appropriately and how to manage associated complications is therefore important.
Types of long-term central venous access catheter
LCVADs are usually defined as venous access devices intended to be in place greater than six weeks.1 LCVADs include external exiting catheters, which may or may not be tunnelled, have an anchoring cuff or be totally implanted devices (Ports).
Devices come in multiple variants but functionally can be broadly categorised as:
Single, double or multi-lumen
Small or large bore
Designed for antegrade or retrograde tunnelling
Preformed catheter tips, or tip cut to length
Implanted port or catheter which exits to Luer connector(s) via a skin incision
Rated as computed tomography (CT)/magnetic resonance imaging (MRI) pressure infusion compatible (e.g. 325 psi)
Rated for high volume flows suitable for dialysis
Presence of an anchoring cuff
Tunnelled cuffed externally exiting catheters
These catheters exit externally and are most commonly sited on the chest wall. They have single, double or triple lumens in variable sizes. The anchoring cuff provides internal fixation once tissue ingrowth occurs. It was previously believed that the cuffs reduced infection rates, but this has been challenged over recent years.2,3 The cuff generally prevents line removal by simple traction and should be surgically removed by an experienced operator.
Broviac and Hickman type catheters
The Broviac catheter was the prototype from which the Hickman catheter was developed. The Broviac catheter has a 1 mm internal diameter and allows flow rates of 25–65 ml/min.4 Although originally designed for children, it is frequently used in adults. Smaller neonatal versions are available. The Hickman catheter has an internal diameter of 1.6 mm allowing faster flow rates.5 Larger devices are also available with double or triple lumens.
Valved catheters
The Groshong catheter is similar in function to Broviac and Hickman catheters; however, it differs as it has a slit like orifice adjacent to the distal end which functions as a valve. The valve resists negative intrathoracic pressure and therefore potential air embolism. Equally the valve requires a positive pressure for opening. Closure of the valve as the positive pressure diminishes prevents back flow of venous blood into the catheter. The valve therefore requires that a pressurised system be used for the delivery of infusions and may alter the speed of continuous drug delivery. It also prevents the catheter being used for CVP monitoring. A Groshong catheter is recognisable by the labelling, blue colour and absence of an external clamp (an external clamp is found on both Broviac and Hickman catheters). This technology is being seen in other types of catheter as it obviates the requirement for external clamps and heparin locks.6 Some devices now also have a valve in their Luer hub working on the same principle. Despite theoretical attractions, valved catheters are more expensive and overall less widely used.
Long-term central venous vascular access for dialysis and apheresis (e.g. Tesio lines and Permcaths)
LCVADs may be used in haemodialysis patients without a functioning AV fistula or graft. They are also less commonly used in haematology patients having regular red cell exchange or apheresis. These may be two separate catheters, inserted side-by-side (e.g. Tesio) or a single dual lumen line (e.g. Permcath). Due to the diameter of the lumens, the catheters are often locked with high concentration anticoagulants (e.g. heparin 5000 units/ml). The volume used is variable (depending on catheter length and is stated on the hub end of the catheter typically around 1.6 ml). If this heparin is inadvertently flushed into the circulation, it can cause systemic anti-coagulation. Protocols for use therefore must involve aspiration of the locking volume before use. Some centres use thrombolytic agents or alternative anti-coagulant/anti-microbial solutions (e.g. Taurolock) to lock lines. A recent Cochrane review of anti-coagulants for preventing central venous catheter malfunction in haemodialysis patients reported that recombinant tissue plasminogen was the only locking solution shown to reduce catheter malfunction when compared to unfractionated heparin; however, this conclusion was based on the data from a single study.7,8 There is some evidence that alternative locking such as citarate solutions or antibiotic locks may reduce the risk of catheter-related blood stream infections although further high quality randomized trials are needed.7
Non-tunnelled externally exiting catheters
PICCs' (peripherally inserted central catheters)
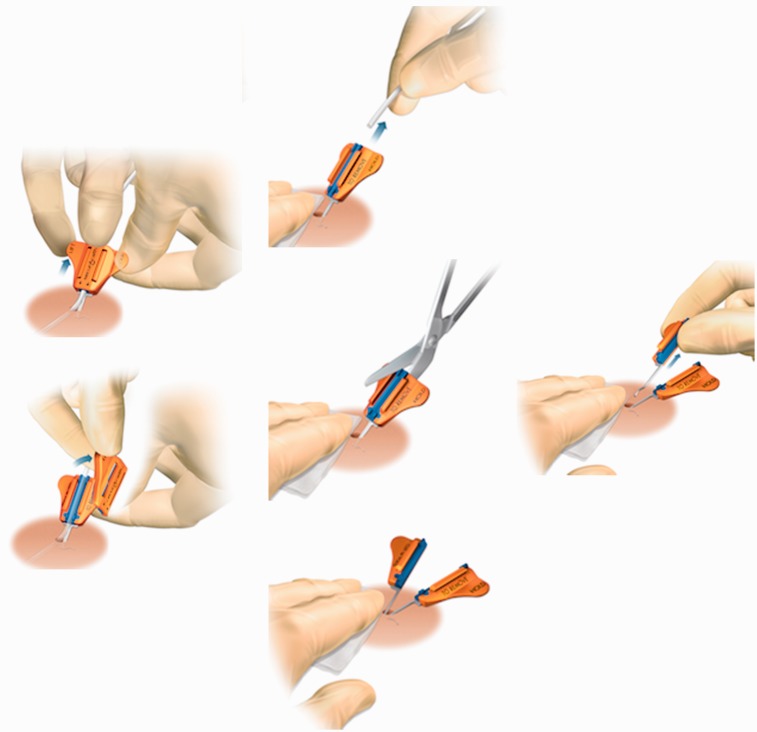
PICCs are usually inserted in the upper arm assisted by an external measuring technique and the aid of an ECG electrode or under fluoroscopy. They are used in increasing numbers for medium term access after insertion by non-medical staff in ward environments. Despite being relatively low cost and straightforward to insert, there is a higher thrombosis and occlusion rate due to their narrow lumens and reduced flow rates.9 With movement of the arm migration rates of up to 9 cm have been documented.10 This can cause endothelial damage and consequent vessel thrombosis or cardiac perforation,11 and arrhythmic episodes.12,13 PICCs do not possess a cuff and can be removed in a similar manner to standard central lines. They are traditionally anchored with a suture wing or adhesive device (e.g. Statlock), but a newer device (SecurAcath) (Figure 1) uses a blunt double Anchor (also referred to as legs and feet) inserted below the dermis into the subcutaneous tissue to secure devices. Removal of the legs and feet requires the base of the SecurAcath to be closed together by compressing the outside wings and the device can then be lifted out using one of two removal techniques (see website Interradmedical.com).
Figure 1.
The sequence of steps required when removing the SecurAcath device.
Port catheters
Ports are typically seen or felt on the chest wall or upper arm as a circular subcutaneous protuberance. They utilise the skin as a natural barrier to infection and patients can swim and bathe without issue. They have the lowest infection rates of all long-term central venous catheters, require little in the way of ongoing care, prolonged flush intervals (three to four weeks) and tend towards longevity.14 Each port membrane has a quoted survival of 1000–2000 punctures until it risks failure but this depends on needle size, operator skill and other factors. The system requires a non-coring Huber needle (Figure 2) for delivery through the skin, subcutaneous tissue and membrane into the chamber. The needle can be easily dislodged interrupting infusions and risking extravasation. Different sizes and lengths of needles (for different skin fat thicknesses over the port) are available. In an emergency situation, a standard (orange, blue or green) needle may be used. The needle is usually left in situ for a period after the port has been sited due to pain on repeated insertion (most centres cite up to seven days). The skin will denervate after a period of time, otherwise EMLA cream can be utilised. Correct needle placement is confirmed by the needle passing through a high resistance silicone membrane, with a loss or resistance, and then hitting the metal back wall of the port chamber. Blood should then be able to be aspirated and easy flushing occurs. Many adults and children have such devices in permanently or semipermanently, e.g. cancer chemotherapy, those with cystic fibrosis, life threatening asthma or allergies (for emergency use).
Figure 2.
Large double lumen port with 12 Fr catheter (Angiodynamics UK). There are two separate injection membranes, which are accessed with a non-coring Huber tip needle. The two lumens can be used at the same time or the injection site rotated to allow skin recovery.
Utilisation of LCVADs
LCVADs offer a lifeline for treatment or nutrition. Further venous access may be difficult and all central and peripheral venous sites may have been exhausted.15 Therefore, the parent team should be consulted regarding catheter use except in true emergency situations.
LCVADs' can be used in critical care for the induction of anaesthesia for intubation and ventilation and the delivery of drugs, fluids and blood products. Attention to sterility and line care is of paramount importance. The line should be tested to ensure it is working adequately with ease of aspiration of blood. A 10-ml syringe should be the smallest syringe used for drug delivery (other than line locks) to reduce the risk of catheter rupture. All lines should be adequately flushed after use, clamping the line as the last 0.5 ml of fluid is flushed, to prevent negative pressure from drawing blood into the tip of the catheter.6 Not using an existing line may reduce the likelihood of complications; however, the patients' wishes as well as the likelihood of successfully obtaining alternative access have to be considered. LCVADs' also allow central venous pressure measurement, with the exception of those with a Groshong valve or in lines which have developed a fibrin sleeve. The Groshong valve may also result in the pulsed delivery of infusing fluids, particularly undesirable when considering vasopressor use. Long-term dialysis lines can be used for haemofiltration on intensive care to avoid further line insertions.
As ports rely on the blunt needle staying in situ, it is usual practice to establish further access after emergency use. A dislodged needle runs the risk of extravasation and unsuccessful delivery of drugs.
A LCVAD may be the only existing venous access in patients requiring contrast enhanced CT imaging. Radiology traditionally has used peripheral access, as there are risks associated with contrast medium delivery through central catheters. Viscous contrast is delivered via an automated powered injector to ensure adequate high flow rates for imaging; consequently, there is a risk of catheter fracture leading to extravasation and possibly embolisation.16 Increasingly manufacturers are producing LCVADs, which tolerate this delivery.17 These are identifiable by external labelling which stipulates maximum acceptable pressure and flow rates, non-standard colour coding and manufacturers handbooks. In implantable devices, the patient's case notes will have to be referred to or alternatively there may be labelling evident on radiographs (an etched CT label may be seen with X-ray of a port). Most contrast delivery systems deliver pressures up to 325 Psi and flows up to 10 ml/s.17 In most instances, discussion with radiology explaining the catheter you have in situ will allow a risk assessment to be made and reduced pressures may provide satisfactory imaging.16 High flow devices like dialysis catheters, even if not CT rated, are unlikely to rupture due to their wide bores and stiff catheter walls.
Complications and their management
Understanding and recognising potential complications of LCVADs will allow a safe approach to their management. There are often risks and benefits that must be weighed up when considering removal of a LCVAD. Whilst line removal may be the only solution in certain instances, line insertions are not without risk and it is often prudent to consider whether it is possible, and in the patient's interest, to try and salvage an existing line. The immediate complication profile of an LCVAD is similar to that of short-term central venous lines and should be managed accordingly.18 Long-term complications can be divided into two broad categories: occlusion and infection.
Catheter occlusion
This may be due to mechanical causes, precipitation of drugs or parenteral nutrition, and thrombosis. Catheter occlusion is described as complete when unable to aspirate or flush, and partial when flushing is still possible (so-called persistent withdrawal occlusion).
Mechanical causes
Mechanical causes include simply resolved problems such as kinks in the external portion of a line, clamps left on, tightly placed sutures and dislodged Huber needles. Other mechanical causes include a suboptimal catheter tip position, kinking of the intra-luminal portion and pinching. Catheter tips can abut the vessel wall and this may be seen on a chest radiograph. Repositioning the patient may relieve the obstruction. If a tunnelled cuffed line is malpositioned, withdrawal may be difficult and require a trained expert. If an internal kink has occurred, this may be managed by re-insertion of a guide wire or repositioning of the line under fluoroscopic guidance.19 However, a new line is often required due to the risk of vessel or catheter damage when repositioning.
Precipitation of medicines and parenteral nutrition
Medicines that are alkaline or acidic in final solution may precipitate in the catheter and, therefore, it is important that protocols for preparation and delivery of medicines via a central venous catheter are referred to Lois et al.19 Parenteral nutrition may leave a lipid residue resulting in blockage of the lumen. Acidic preparations which precipitate in an alkaline environment have been treated with 0.1% hypochloric acid and alkaline preparations, which have precipitated in an acidic environment have been treated with sodium bicarbonate and sodium hydroxide.20–22 Ethanol 70% has also been used to clear obstructing lipid emulsion deposits from parenteral nutrition use; however, these patients may report side effects in keeping with excess alcohol intake.22
Pinch off syndrome
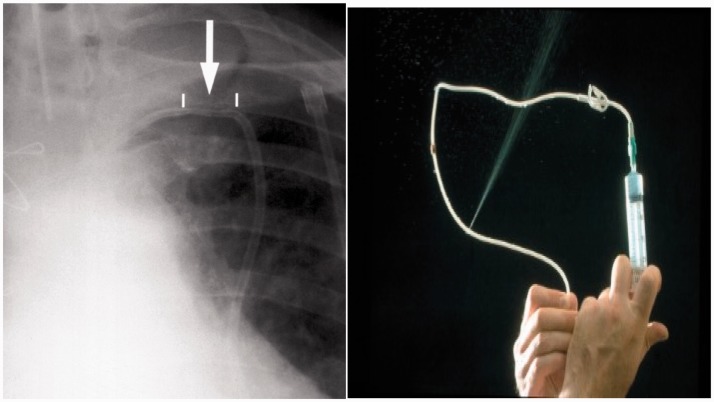
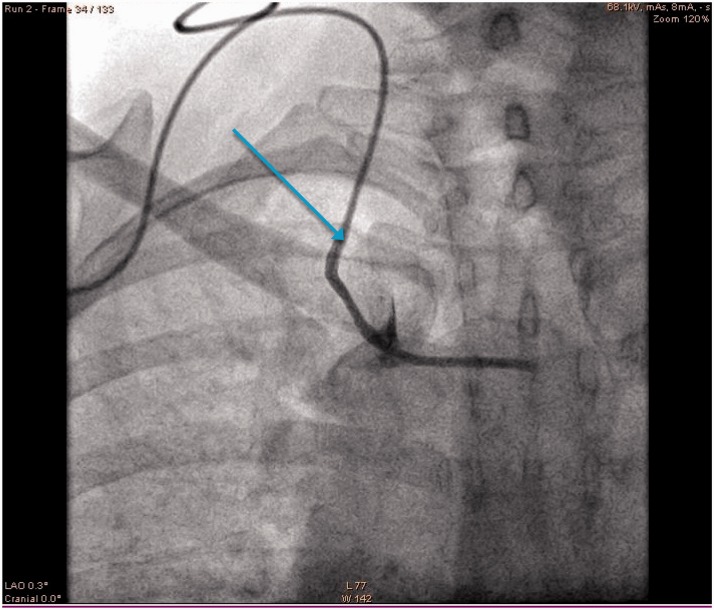
Sub-clavicular LCVADs are potentially exposed to shear forces between the first rib and clavicle. The risk is thought to be higher with more medial vein punctures (as per landmark techniques) as the catheter passes anteriorly between the clavicle and first rib before entering the subclavian vein.23,24 If repeatedly trapped it then fractures. More lateral punctures with ultrasound guidance into the axillary vein are thought to reduce this risk.25 The risk is greater in active patients where repeated intermittent compression of the catheter risks the complications of line fracture, extravasation, translocation and embolisation.21 The patient or nursing staff may report a postural effect on the ease of line use. Notably the catheter more easily aspirates and flushes in the supine position, with the ipsilateral arm raised than when the patient is upright. The patient may report infra-clavicular pain as a consequence of extravasation from a fractured line and inspection, may reveal skin changes and swelling in this area. A chest X-ray film may demonstrate scalloping of the catheter21 (Figures 3 and 4). If pinch off syndrome is suspected, then infusions will need to be stopped and the parent team involved with a view to replacing the line.
Figure 3.
Pinch off. Plain X-ray show scalloping in subclavian access. Hickman line within four days of insertion. Catheter started to leak and, on removal, a leak was evident with pressurized injection and catheter occlusion.
Figure 4.
Contrast leak from catheter damaged by shear forces between clavicle and first rib (pinch off).
Extravasation
Extravasation occurs when a drug enters the patients' soft tissue. The severity and presentation vary depending on the drug, concentration and volume extravasated. The typical presentation is pain at the site of extravasation and overlying skin changes. If untreated, tissue necrosis requiring amputation can result. Other consequences include infection, complex regional pain syndrome and loss of limb function. The management will vary depending on the responsible drug, volume involved and amount of resulting damage, however in all cases the infusion or injection should be stopped immediately and the site aspirated to remove as much drug as possible. If a port catheter is being used, the Huber needle should be removed immediately. Subsequently, the drug should be identified and guidance sought on specific management.26
External fracture
This is usually due to repeated clamping of a line. If an external line is fractured, it risks entraining air and therefore should immediately be clamped proximal to the fracture using artery forceps or similar apparatus. It is sometimes possible to repair an external fracture by replacing the damaged portion of the line with a manufacturer's repair kit. Ports or cuffs can also erode through the skin and usually require removal and replacement.
Thrombosis
Prevention and identification of this complication is important as it may lead to catheter-related infection, pulmonary embolus and post thrombotic syndrome. Catheter-related thrombosis (CRT) is broadly divided into extra-luminal and intra-luminal.
Extra luminal thrombosis
Fibrin sheath
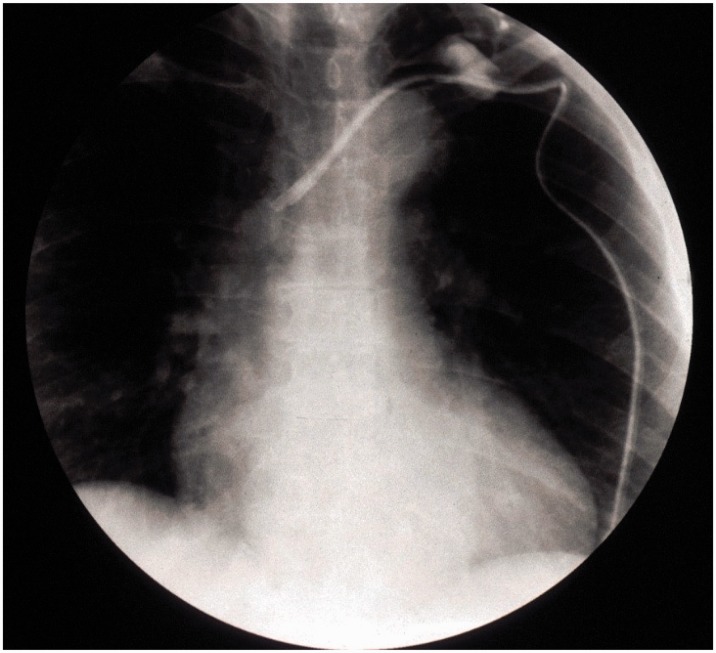
This is a commonly occurring phenomenon. The sheath may begin to form as early as 24 h after insertion.27 Sheaths usually initiate at the vessel entry site where there is endothelial damage and progress towards the tip. They may also initiate more distally as a result of the catheter rubbing on the endothelial lining of the vessel. A partial obstruction usually results and staff find difficulty aspirating as negative pressure sucks the sheath over the catheter tip. Drugs may collect and mix within the sheath and there is potential for drugs to backtrack to the skin entry point and consequently extravasate. Fibrin sheaths can be managed using thrombolytic locks or internal snare techniques and rarely necessitate catheter removal.28 These can sometimes be seen as a so-called “Ghost” in the vein after catheter removal (Figure 5).
Figure 5.
Flouroscopy image shows long-term catheter whose tip was misplaced in the left innominate vein and could not be resited due to a fibrin sleeve attached to vein wall. The catheter has been withdrawn so that its tip is in upper SVC (arrow) and injection of contrast shows a fibrin sleeve full of contrast (wider than catheter) with leak into the SVC shown by blush of contrast. The tip of the fibrin sleeve remains in the innominate vein and will remain in situ post catheter removal to hopefully be resorbed over time.
Venous thrombosis (CRT)
This may be mural (adhering to the vessel wall and potentially obstructing the catheter) or a deep venous thrombosis (completely obstructing flow within the vessel and therefore the catheter). Both typically present adjacent to the catheter and are collectively termed CRT.
CRT may be asymptomatic, however there are often reports of pain, swelling, erythema and occlusion of the catheter.29 Patients with malignancy are at particular risk.30 Diagnosis may be confirmed by ultrasonography or contrast imaging. The thrombotic process may progress to affect central veins such as the SVC and IVC. The occurrence of progressive central thrombosis, or stenosis, should be considered when prominent superficial collaterals are visible. Some local thrombosis around the catheter entry site is very common and does not warrant anticoagulation unless symptomatic. More extensive or symptomatic thrombosis usually requires anticoagulation.31,32 Catheter-directed thrombolysis (CDT) may be considered as heparin and coumarins have no thrombolytic properties.33 The catheter should usually remain in situ for the parent team to assess. Acute SVC obstruction can result from CRT or a catheter inserted into a stenosed vessel. This may rarely cause airway compromise and, in this instance, the patient may require intubation and catheter removal.34
Clinically significant pulmonary embolus and post-thrombotic syndrome are complications of deep vein thrombosis.35 In those with deep vein thrombosis, long-term anticoagulation may be required.31 Post-thrombotic syndrome is characterised by chronic oedema, pain and functional limitation of the affected limb. It is caused by persistent thrombosis and valvular dysfunction.36 The affected side should be avoided if future venous access is required.
Various strategies have been utilised to prevent CRT including heparin impregnated catheters, low dose warfarin37 and heparin administration.38 There is no evidence to support the routine use of these prophylactic measures in all patients with LCVADs39; however, treatment dose anticoagulation can be considered in high-risk cases.
Intra-luminal thrombosis
This refers to thrombus formation within the catheter itself. It can present as a partial or complete obstruction and accounts for 25% of all catheter obstructions.40 In order to prevent this, LCVADs are often locked with anticoagulants.41 The thrombus can be confirmed by ultrasound or venogram if this is felt necessary. A line blocked by thrombus may be salvaged and most centres have protocols for the use of thrombolytic agents for this purpose.42 If this fails, a guide wire or snare may be used to remove a clot at the tip of a catheter.
Thrombotic material provides an excellent medium for bacterial growth and many bacterial species produce thrombogenic proteins, consequently thrombosis and infection are risk factors for one another.43,44
Central venous stenoses
Central venous stenosis can become a significant problem for those requiring LCVADs. The risk of stenosis increases with the length of time a catheter is used and is consequently higher in those who have had previous LCVADs. Subclavian catheters pose a higher risk (42%) than internal jugular catheters (10%) and left-sided catheters carry an increased risk.45 Larger calibre lines (such as those used for haemodialysis) are also thought to increase the risk. Stenoses may be asymptomatic or symptomatic. Collaterals may be seen on physical examination of the face, arm and torso. Subclavian stenosis may also cause ipsilateral breast and upper limb swelling and innominate stenosis can also cause facial swelling. In addition to physical signs on examination of the patient, blockage or distention and a loss of variation in venous diameter with respiration on duplex ultrasound scanning should alert the operator to a potential central stenosis. Central venography is the diagnostic gold standard,46 however CTA and MRA studies may also be helpful in diagnosing central stenoses. Endovascular intervention including balloon angioplasty and stenting are the mainstay of treatment, however patients commonly require repeated interventions.46 Venous bypass procedures are rarely performed.
Catheter-related infection
(See EPIC47 or USA CDC guidelines for detail beyond the scope of this review.)
Catheter-related infections include exit site, tunnel and catheter-related blood stream infections (CRBSIs). Exit-site infections usually respond well to wound management and antibiotics, whereas tunnel infections usually require line removal and treatment with intravenous antibiotics. CRBSIs' occur from the skin puncture site, hub contamination or spread to the catheter from another sight of infection. A diagnosis of CRBSI can be made from blood cultures taken peripherally and from the catheter at the same time. Diagnosing a CRBSI does not require line removal,48 and it may be possible to salvage the catheter with antibiotic treatment. However, catheter salvage does carry the risk of serious complications from metastatic spread including septic arthritis, osteomyelitis, spinal epidural abscess and septic emboli. Catheter removal should always be considered in those with persistent CRBSIs not responding to treatment.49
Antibiotic delivery via alternate ports increases the likelihood of clearing a catheter infection.50 If an indwelling port reservoir becomes infected, antibiotics should be administered via alternative access (unless the Huber needle remains in situ) as needle introduction may introduce infection into the blood stream.
Prophylactic antibiotics
Locking LVCADs in paediatric and adult oncology patients with a combination of heparin and vancomycin and the use of prophylactic antibiotics prior to line insertion appear to reduce the rate of Gram positive infection of these lines.51 Antibiotic locks may also be considered in patients with repeated line infections.49 There is some evidence that antibiotic line locks and anti-microbial locking solutions may reduce the risk of CRBSIs; however, there is concern that their use may increase the risk of antibiotic resistance and that trials have not adequately assessed their potential harm.52 National guidelines for patients, such as those being treated with haemodialysis, have therefore not recommended their routine use in all patients.53
Removal of LCVAD
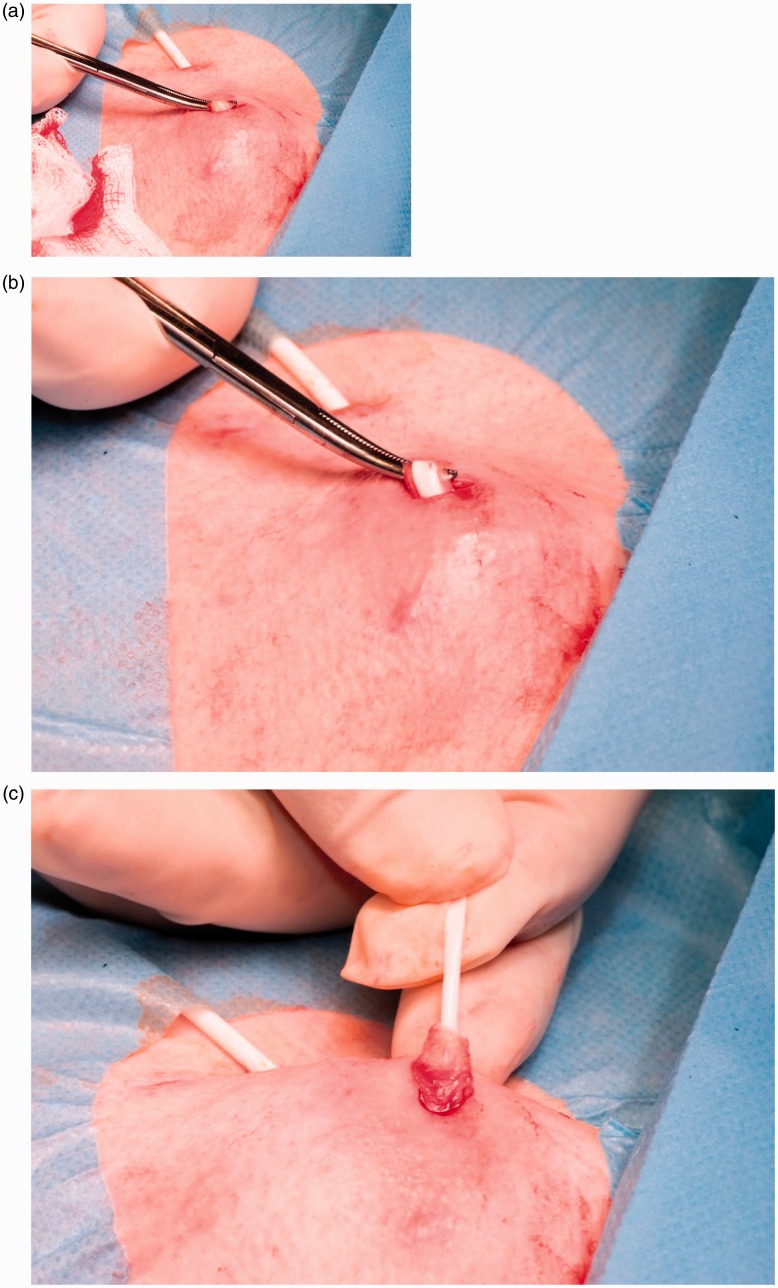
LCVADs may require urgent removal due to an unremitting infection and deteriorating clinical condition. Non-tunnelled LCVADs can be removed following the same general principles utilised in the removal of standard short-term central venous lines. Tunnelled catheters with a cuff sited less than three weeks ago can be removed using the same technique unless resistance is met when applying gentle traction. Some centres suggest traction alone can be used to remove the majority of cuffed catheters, but in our experience, this leads to patient discomfort, snapped catheters and retained cuffs. If the device had been in beyond three to four weeks or had additional internal anchoring sutures around the cuff, then removal requires infiltration of local anaesthetic and a cutdown to free the cuff. A superficial incision is made just above the cuff followed by blunt dissection to free the catheter from the surrounding soft tissue (Figure 6). Ideally the venous section of catheter is removed prior to any sharp dissection to avoid cutting the catheter and losing it internally as a catheter embolus (Figure 7). The cuff can then be sharp dissected free and removed. The external portion can then be pulled out from the exit site and the incision closed with appropriate sutures. Port catheter removal follows similar principles but requires a larger incision.54
Figure 6.
Cuffed catheter removal. (a) An incision has been made over the venous end of the anchoring cuff. Blunt dissection with artery forceps has allowed the catheter and its covering fibrous sheath to be brought to the skin surface. A very superficial longitudinal incision in this sheath reveals the white silicone catheter. (b) The catheter can be pulled from the sheath and out from the vein. Pressure is applied to allow clot to block the tract leading to the vein. (c) The cuff can then be freed with sharp dissection using small scissors. The concept is to minimise sharp dissection until the catheter is out of the vein to avoid catheter damage and loss centrally as a catheter embolus (see Figure 7).
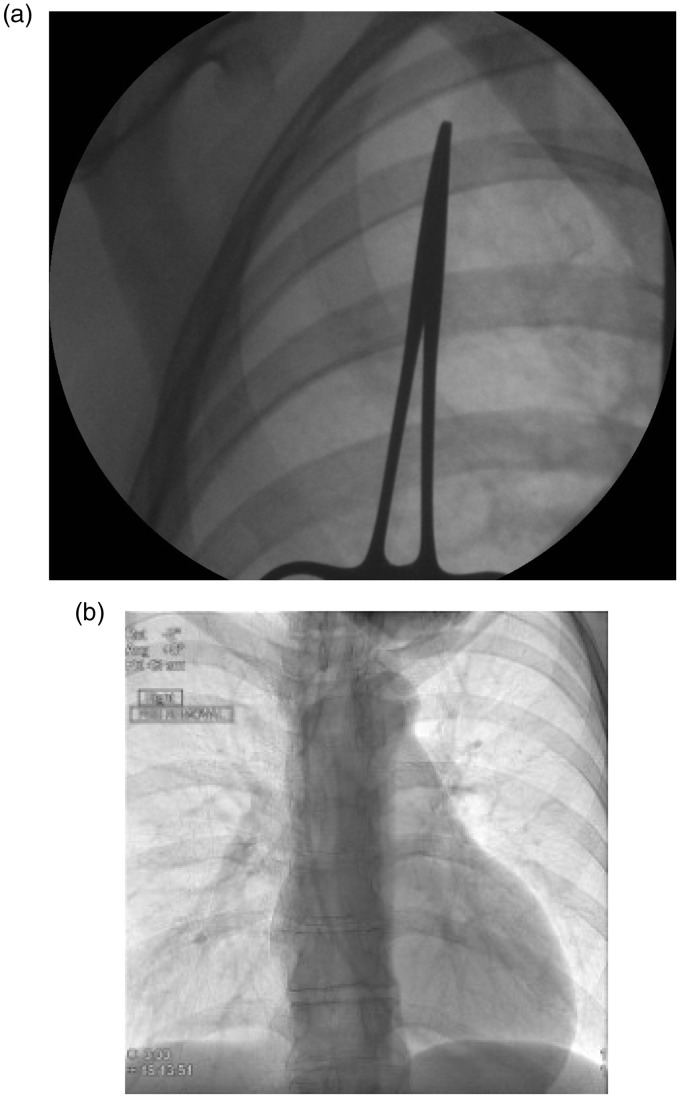
Figure 7.
An operator has inadvertently cut though a Hickman line whilst attempting to dissect out the cuff and has tried to retrieve the venous section but failed. (a) An image intensifier shows the proximal cut catheter lying in the subclavian vein. (b) This image shows the catheter has migrated centrally (embolised) crossing the tricuspid with its tip in the right ventricle. The catheter was snared from a femoral vein sheath and removed intact by interventional radiology. The patient developed arrhythmias when lying on her left side relieved by turning to the right.
Summary
LCVADs are increasingly used in a wide range of patients of all age groups and are therefore more likely to be encountered by intensivists. LCVADs provide critical access for patients and can enhance their quality of life. Those working in critical care should therefore be familiar with the different types of LCVADs and have a good working knowledge of potential complications and their management. This knowledge will encourage appropriate use, identification of complications and prevent unnecessary line removal.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DM declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. AB has been on the Editorial Board of JICS and has received consultancy payments from catheter manufacturers in relation to new devices.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Möller J, Reiss I, Schaible T. Vascular access in neonates and infants–indications, routes, techniques and devices, complications. Intensive Care World 1995; 12: 48–53. [PubMed] [Google Scholar]
- 2.de Cicco M, Chiaradia V, Veronesi A, et al. Source and route of microbial colonisation of parenteral nutrition catheters. Lancet 1989; 2: 1258–1261. [DOI] [PubMed] [Google Scholar]
- 3.Keohane PP, Jones BJ, Attrill H, et al. Effect of catheter tunnelling and a nutrition nurse on catheter sepsis during parenteral nutrition. A controlled trial. Lancet 1983; 2: 1388–1390. [DOI] [PubMed] [Google Scholar]
- 4.Leibundgut K, Muller C, Muller K, et al. Tunneled, double lumen Broviac catheters are useful, efficient and safe in children undergoing peripheral blood progenitor cell harvesting and transplantation. Bone Marrow Transplant 1996; 17: 663–667. [PubMed] [Google Scholar]
- 5.Bjeletich OJ, Hickman OR. The Hickman Indwelling Catheter. Am J Nurs 1980; 80: 62–65. [PubMed] [Google Scholar]
- 6.Goossens GA. Flushing and locking of venous catheters: Available evidence and evidence deficit. Nurs Res Pract 2015; 2015: 985686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Ivany JN, Perkovic V, et al. Anticoagulants and antiplatelet agents for preventing central venous haemodialysis catheter malfunction in patients with end-stage kidney disease. Cochrane Database Syst Rev 2016; 4: CD009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmelgarn BR, Moist LM, Lok CE, et al. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med 2011; 364: 303–312. [DOI] [PubMed]
- 9.Baskin JL, Pui C-H, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009; 374: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadroo A, Glass R, Lin J, et al. Changes in upper extremity position cause migration of peripherally inserted central catheters in neonates. Pediatrics 2002; 110: 131–136. [DOI] [PubMed] [Google Scholar]
- 11.Puel V, Caudry M, Le Métayer P, et al. Superior vena cava thrombosis related to catheter malposition in cancer chemotherapy given through implanted ports. Cancer 1993; 72: 2248–2252. [DOI] [PubMed] [Google Scholar]
- 12.Hacking MB, Brown J, Chisholm DG. Position dependent ventricular tachycardia in two children with peripherally inserted central catheters (PICCs). Pediatr Anesth 2003; 13: 527–529. [DOI] [PubMed] [Google Scholar]
- 13.Verdino RJ, Pacifico DS, Tracy CM. Supraventricular tachycardia precipitated by a peripherally inserted central catheter. J Electrocardiol 1996; 29: 69–72. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty L. Implanted ports: benefits, challenges and guidance for use. Br J Nurs 2011; 20: S12–S19. [Google Scholar]
- 15.Loveday H, Wilson J, Pratt R, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014; 86: S1–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plumb AAO, Murphy G. The use of central venous catheters for intravenous contrast injection for CT examinations. Br J Radiol 2011; 84: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LH. Implanted ports, computed tomography, power injectors, and catheter rupture. Clin J Oncol Nurs 2008; 12: 809–812. [DOI] [PubMed] [Google Scholar]
- 18.Bodenham A, Babu S, Bennett J, et al. Association of anaesthetists of Great Britain and Ireland: Safe vascular access. Anaesthesia 2016; 71: 573–585. [DOI] [PMC free article] [PubMed]
- 19.Lois JF, Gomes AS, Pusey E. Nonsurgical repositioning of central venous catheters. Radiology 1987; 165: 329–333. [DOI] [PubMed] [Google Scholar]
- 20.Werlin SL, Lausten T, Jessen S, et al. Treatment of central venous catheter occlusions with ethanol and hydrochloric acid. J Parenter Enteral Nutr 1995; 19: 416–418. [DOI] [PubMed] [Google Scholar]
- 21.Shulman RJ, Reed T, Pitre D, et al. Use of hydrochloric acid to clear obstructed central venous catheters. J Parenter Enteral Nutr 1988; 12: 509–510. [DOI] [PubMed] [Google Scholar]
- 22.Akinwande KI, Keehn DM. Dissolution of phenytoin precipitate with sodium bicarbonate in an occluded central venous access device. Ann Pharmacother 1995; 29: 707–709. [DOI] [PubMed] [Google Scholar]
- 23.Aitken DR, Minton JP. The “pinch-off sign”: a warning of impending problems with permanent subclavian catheters. Am J Surg 1984; 148: 633–636. [DOI] [PubMed] [Google Scholar]
- 24.Andris DA, Krzywda EA, Schulte W, et al. Pinch-off syndrome: a rare etiology for central venous catheter occlusion. J Parenter Enteral Nutr 1994; 18: 531–533. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Zhou Y, Yang P, et al. Optimized axillary vein technique versus subclavian vein technique in cardiovascular implantable electronic device implantation: A randomized controlled study. Chin Med J 2016; 129: 2647–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doellman D, Hadaway L, Bowe-Geddes LA, et al. Infiltration and extravasation: update on prevention and management. J Infus Nurs 2009; 32: 203–211. [DOI] [PubMed] [Google Scholar]
- 27.Balestreri L, De Cicco M, Matovic M, et al. Central venous catheter-related thrombosis in clinically asymptomatic oncologic patients: a phlebographic study. Eur J Radiol 1995; 20: 108–111. [DOI] [PubMed] [Google Scholar]
- 28.Reddy AS, Lang EV, Cutts J, et al. Fibrin sheath removal from central venous catheters: an internal snare manoeuvre. Nephrol Dial Transplant 2007; 22: 1762–1765. [DOI] [PubMed] [Google Scholar]
- 29.Blaivas M, Stefanidis K, Nanas S, et al. Sonographic and clinical features of upper extremity deep venous thrombosis in critical care patients. Crit Care Res Pract 2012; 2012: 489135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liem TK, Yanit KE, Moseley SE, et al. Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg 2012; 55: 761–767. [DOI] [PubMed] [Google Scholar]
- 31.Spiezia L, Simioni P. Upper extremity deep vein thrombosis. Intern Emerg Med 2010; 5: 103–109. [DOI] [PubMed] [Google Scholar]
- 32.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv 2013; 81: E269–E273. [DOI] [PubMed] [Google Scholar]
- 34.Woodyard TC, Mellinger JD, Vann KG, et al. Acute superior vena cava syndrome after central venous catheter placement. Cancer 1993; 71: 2621–2623. [DOI] [PubMed] [Google Scholar]
- 35.Van Rooden CJ, Tesselaar MET, Osanto S, et al. Deep vein thrombosis associated with central venous catheters – a review. J Thromb Haemost 2005; 3: 2409–2419. [DOI] [PubMed]
- 36.Kahn SR, Ginsberg JS. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood reviews 2002; 16: 155–165. [DOI] [PubMed]
- 37.Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med 1990; 112: 423–428. [DOI] [PubMed] [Google Scholar]
- 38.Randolph AG, Cook DJ, Gonzales CA, et al. Benefit of heparin in central venous and pulmonary artery catheters: a meta-analysis of randomized controlled trials. Chest 1998; 113: 165–171. [DOI] [PubMed] [Google Scholar]
- 39.Marnejon T, Angelo D, Abu Abdou A, et al. Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access 2012; 13: 231–238. [DOI] [PubMed] [Google Scholar]
- 40.Rosovsky RP, Kuter DJ. Catheter-related thrombosis in cancer patients: pathophysiology, diagnosis, and management. Hematol Oncol Clin North Am 2005; 19: 183–202. [DOI] [PubMed] [Google Scholar]
- 41.Hemmelgarn BR, Moist LM, Lok CE, et al. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. New Engl J Med 2011; 364: 303–312. [DOI] [PubMed] [Google Scholar]
- 42.Haire WD, Lieberman RP. Thrombosed central venous catheters: restoring function with 6-hour urokinase infusion after failure of bolus urokinase. J Parenter Enteral Nutr 1992; 16: 129–132. [DOI] [PubMed] [Google Scholar]
- 43.Mehall JR, Saltzman DA, Jackson RJ, et al. Fibrin sheath enhances central venous catheter infection. Crit Care Med 2002; 30: 908–912. [DOI] [PubMed] [Google Scholar]
- 44.Timsit JF, Farkas JC, Boyer JM, et al. Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest 1998; 114: 207–213. [DOI] [PubMed] [Google Scholar]
- 45.Schillinger F, Schillinger D, Montagnac R, et al. Post catheterisation vein stenosis in haemodialysis: Comparative angiographic study of 50 subdavian and 50 internal jugular accesses. Nephrol Dial Transpl 1991; 6: 722–724. [DOI] [PubMed] [Google Scholar]
- 46.Lumsden AB, MacDonald MJ, Isiklar H, et al. Central venous stenosis in the hemodialysis patient: incidence and efficacy of endovascular treatment. Cardiovasc Surg 1997; 5: 504–509. [DOI] [PubMed]
- 47.Loveday HP, Wilson J, Pratt RJ, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014; 86: S1–S70. [DOI] [PMC free article] [PubMed]
- 48.Seifert H, Cornely O, Seggewiss K, et al. Bloodstream infection in neutropenic cancer patients related to short-term nontunnelled catheters determined by quantitative blood cultures, differential time to positivity, and molecular epidemiological typing with pulsed-field gel electrophoresis. J Clin Microb 2003; 41: 118–123. [DOI] [PMC free article] [PubMed]
- 49.Kovalik EC, Raymond JR, Albers FJ, et al. A clustering of epidural abscesses in chronic hemodialysis patients: risks of salvaging access catheters in cases of infection. J Am Soc Nephrol 1996; 7: 2264–2267. [DOI] [PubMed] [Google Scholar]
- 50.Great Ormand Street Hospital for children. Central Venous Access Devices (long term). Available from: http://www.gosh.nhs.uk/health-professionals/clinical-guidelines/central-venous-access-devices-long-term#CVAD%20infections (accessed October 2017).
- 51.van de Wetering M, de Witte M, Kremer L, et al. Efficacy of oral prophylactic antibiotics in neutropenic afebrile oncology patients: a systematic review of randomised controlled trials. Eur J Cancer 2005; 41: 1372–1382. [DOI] [PubMed] [Google Scholar]
- 52.Zacharioudakis IM, Zervou FN, Arvanitis M, et al. Antimicrobial lock solutions as a method to prevent central line-associated bloodstream infections: A meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 59: 1741–1749. [DOI] [PubMed] [Google Scholar]
- 53.Kumwenda M, Mitra S, Reid C. Vascular access for haemodialysis, 6th ed. Bristol: UK Renal Association, 2015. Available from: https://renal.org/wp-content/uploads/2017/06/vascular-access.pdf (accessed October 2017), 2015. [Google Scholar]
- 54.Bishop L, Dougherty L, Bodenham A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol 2007; 29: 261–278. [DOI] [PubMed]