Abstract
Background: The emergence of psychoactive designer drugs has significantly increased over the last few years. Customs officials are responsible for the control of products entering the European Union (EU) market. This control applies to chemicals in general, pharmaceutical products and medicines. Numerous products imported from non-EU countries, often declared as ‘bath salts’ or ‘fertilizers’, contain new psychoactive substance (NPS).
Review: These are not necessarily controlled under international law, but may be subject to monitoring in agreement with EU legislation. This situation imposes substantial challenges, for example, for the maintenance of spectral libraries used for their detection by designated laboratories. The chemical identification of new substances, with the use of powerful instrumentation, and the time needed for detailed analysis and interpretation of the results, demands considerable commitment. The EU Joint Research Centre endeavors to provide scientific support to EU Customs laboratories to facilitate rapid identification and characterisation of seized samples. In addition to analysing known NPS, several new chemical entities have also been identified. Frequently, these belong to NPS classes already notified to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) by the European Early-Warning System (EWS).
Conclusion: The aim of this paper is to discuss the implementation of workflow mechanisms that are in place in order to facilitate the monitoring, communication and management of analytical data. The rapid dissemination of this information between control authorities strives to help protect EU citizens against the health risks posed by harmful substances.
Keywords: New psychoactive substances, chemical identification, cheminformatic, JRC, customs, cheminformatics
1. INTRODUCTION
New psychoactive substance (NPS), sometimes also referred to as designer drugs, are typically manufactured to mimic controlled psychoactive drugs that affect the central nervous system. These NPS are not necessarily controlled under the national or international law and their rapid appearance, as well as the lack of information regarding their biological effects, may seriously endanger the lives and health of users of these recreational drugs. The fact that they are not controlled by legislation and are often available from
online retailers, might contribute to the erroneous perception of their safety amongst naive users. Moreover, mislabelled products inconsistent with their contents frequently occur [1]. Due to their fast-changing chemical nature, adequate levels of information about psychoactive, toxicological and also physicochemical properties of NPS tend to emerge with considerable delay. Consequently, there is a need to promote understanding with regards to toxicology and metabolism studies [2-6]. NPS have also been reported as adulterants in controlled drugs and dietary/food supplements [7, 8]. The impact on public health has received a lot of media attention further to outbreaks of intoxications with new substances [9]. A particularly concerning development can be seen in the fentanyl crisis in Canada, where this potent opioid was detected in approximately 400 lethal overdose cases in 2016 [10], followed by an increasing number of cases in Europe [11].
The monitoring of these recreational drugs as well as fast identification of new substances are therefore important for protecting the health of EU citizens. The monitoring and exchange of information on NPS through the EWS (Early Warning System) were established by Council Decision 2005/387/JHA. The EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) collects information and provides the EU and its Member States (MS) with a factual overview of drug problems in Europe where currently over 620 NPS are being monitored [12]. The rapid identification and reporting of new compounds via the EWS enable the EU Member States to take timely decisions on the appropriate measures required to control the most harmful substances [1]. The European Union has recently adopted a new legislation to rapidly respond to public health and security threats caused by new psychoactive substances (NPS) which will significantly strengthen the existing processes by streamlining and accelerating data-collection and assessment procedures with shorter deadlines [13, 14].
Customs authorities are responsible for the control of goods and chemical products entering the European Community market. These include a broad range of substances such as pharmaceutical products, medicines and research chemicals. Moreover, they are responsible for collecting and safeguarding customs duties, and controlling the flow of goods into, and out of, the EU acting also as the first possible check-point for chemical products imported into Europe by its different borders (maritime, air and terrestrial). In the past decade, Customs authorities reported an increasing number of NPS being imported from non-European Union countries, often mislabelled as ‘bath salts’, ‘fertilizers’ or ‘research chemicals’ that represent analogues or precursors of known psychoactive substances, licit (medical) or illicit drugs [12, 15].
The routine control performed by the Customs laboratories generally includes the use of GC-MS (Gas Chromatography-Mass Spectrometry) and FT-IR (Fourier Transform Infrared Spectroscopy) followed by the search for a match with known chemicals in a number of spectral libraries. The Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) has established infrared and mass spectral libraries to help with the forensic examination of illicit and new drugs [16]. These libraries are available for download in several manufacturer proprietary formats and also in open format (NIST, JCAMP) on the SWGDRUG website. The company Cayman Chemical is also making available an electron ionisation mass spectral library originating from drug standards [17]. It is common practice in forensic and customs laboratories to install these spectral libraries on the computers of their analytical instruments to help with the recognition of a large number of illicit drugs and new drugs in seized samples. This procedure facilitates the efficient monitoring of substances found on the market. However, the time lag potentially arising from updating these libraries may hinder the fast detection of new substances recently identified elsewhere. Moreover, the validation, notification and possibly the publication of scientific data on the identification of a new molecule, may also cause delays. Consequently, the sharing and dissemination of useful analytical data within a broad forensic community can take up to several months.
The appearance of new substances on the EU market reached a peak of ca. hundred new compounds per year for 2014 and 2015 as presented in the EMCDDA report of 2017 [12]. The rapid sharing of validated spectral data is required to allow for both the efficient monitoring of NPS in Europe and to prevent the duplication of efforts in laboratories, which may inadvertently be “struggling” to identify the same new substance.
In addition to these spectral libraries, one can also check for information on systems available online where more frequent updates of analytical data on recently identified NPS can be found (e.g. the GC-MS Search Tool of Cayman Company [17], or the mass spectra database of “Designer Drugs Online”) [18].
The European Database on New Drugs (EDND) of the EMCDDA records the notifications of new substances and the detection of NPS reported by the EU MS. However, details about analytical data are not considered in the EU regulations for the monitoring of NPS. Without the establishment of a common standard for analytical reports, these data may be recorded in heterogeneous formats, thus, potentially making it impractical for use by analytical chemists.
The identification of these new substances is a challenge for both forensic and customs laboratories, predominantly due to the absence of scientific data and the lack of reference standards [19]. Analytical information on NPS related to NMR (Nuclear Magnetic Resonance), GC-MS, LC-MS (Liquid Chromatography-Mass Spectrometry) and Raman techniques, has been disseminated in recent years [20-33] and reviews on recent developments in this field, covering methods of detection, have also been published [34, 35].
The unambiguous identification and characterisation of unknown chemical substances require the use of advanced analytical techniques such as NMR and High-Resolution Mass Spectrometry (HR-MS). The Joint Research Centre (JRC) of the European Commission is equipped with such state of the art instrumentation and an agreement with DG TAXUD was established to provide scientific support for the characterisation and identification of the unknown substances that Customs laboratories may not be able to identify with routine analytical techniques. Besides analytical support for chemical identification, the JRC also identified a few key critical issues that have to be addressed to ensure the unambiguous reporting and sharing of analytical data on NPS. These issues are illustrated by a few cases that we present in results and discussion section.
2. MATERIALS AND METHODS
2.1. Samples
About 250 samples were analysed which were received from Customs laboratories from France (85), Belgium (42), Germany (35), Czech Republic (17), Italy (15), Norway (12), Sweden (6), Bulgaria (6), Austria (5), Poland (3), Netherlands (2) and Finland (3). Through collaboration with the project RESPONSE [36], a few other samples were sent by the Slovenian Forensic Institute and the French Police Scientific Laboratory of Lyon. Five other samples were received from the Indonesian Customs laboratory.
2.2. GC-MS and FT-IR
These analyses were performed by the customs and forensic laboratories which sent the samples. The raw electronic data of these analyses were sent to the JRC for further examination with the ACD/Labs (Advanced Chemistry Development, Inc., Toronto, Canada) software.
2.3. NMR and HR-MS Determinations
The analyses were performed with the NMR instruments of the JRC in Ispra (Bruker 600 MHz with a cryoprobe and/or 400 MHz with multinuclear probe). These spectrometers allow to perform mono and multi-dimension NMR experiments on 1H, 13C, 15N, 19F and 31P nuclei that may be required for the elucidation of the chemical structure of organic molecules. The HR-MS and LC-MS analyses were performed with the Orbitrap (Thermo) and/or Q-TOF (Time of Flight-Waters) instruments of the JRC which enabling the determination of the exact mass and MS-MS fragmentation, supporting the unambiguous structure elucidation. The procedures and experimental operating conditions can be found in our previous publication [37].
2.4. Cheminformatics
The analytical data (GC-MS, FT-IR, NMR, HR-MS) was processed using the ACD/Labs software. Following the structural identification, a variety of chemo-identifiers (SMILES (Simplified Molecular-Input Line-Entry System) [38], IUPAC (International Union of Pure and Applied Chemistry) names, InChi and InChiKey [39-42]) were generated. Their consistency was also checked with online tools such as OPSIN (Open Parser for Systematic IUPAC Nomenclature) [43, 44] and Chemicalize (Instant Chemo-informatics Solutions- ChemAxon Ltd.) [45]. The JRC portal ChemAgora was used to search through publicly available databases for chemical, physical and toxicological information of newly identified substance [46]. However, for newly discovered NPS the lack of information is not uncommon.
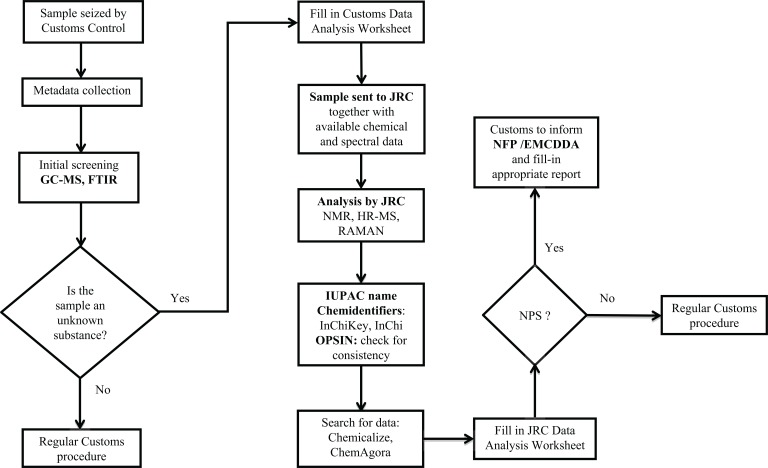
The above described analytical and data-handling process is illustrated in Fig. (1).
Fig. (1).
Workflow and decision process.
3. RESULTS AND DISCUSSION
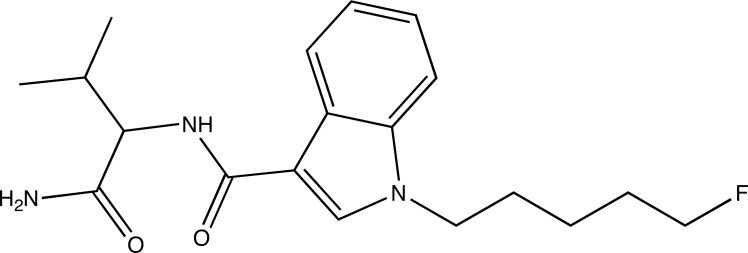
The European Customs laboratories first reflected on the emerging problems faced with regards to NPS at a dedicated workshop in Berlin in September 2012. The lack of reference materials [47] and the need for sharing of analytical data was highlighted in the conclusions of this meeting. The CLEN (Customs Laboratories European Network) Project Group on “designer drugs and other illicit products” was then established in the framework of the program Customs2020 with the involvement of the JRC for scientific support. In February 2014, the JRC hosted the first workshop of this CLEN Project Group with the presence of delegates from the CLEN and the EMCDDA. The chemical identification of the first substance in a sample received from the Bulgarian Customs in January 2014 (Fig. 2) was presented to the participants together with the analytical approach applied by the JRC. This substance, which has subsequently been registered in the EDND as 5F-AMBICA, which, incidentally, was also identified in another sample seized by Swedish Customs in March 2014 and reported to the EMCDDA as 5F-AB-144. Subsequently, its detection was also reported by Italian Customs in June 2014. The EDND record of that substance also indicates two other common names (5F-ABICA, 5F-ADBICA-144) as well as two different systematic chemical names: N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-1H-indol-3-carboxamide and N-(1-carbamoyl-2-methyl-propyl)-1-(5-fluoropentyl)indole-3-carboxamide. Both are compatible with IUPAC nomenclature but their equivalence is not obvious for non-chemists (Fig. 2 and caption).
Fig. (2).
5F-AMBICA. The systematic name of 5F-AMBICA in EDND is: N-(1-carbamoyl-2-methyl-propyl)-1-(5-fluoropentyl)indole-3-carboxamide. Another systematic name is also: N-(1-carbamoyl-2-methyl-propyl)-1-(5-fluoropentyl)indole-3-carboxamide. One can check that these two systematic names are compatible with the IUPAC nomenclature using the OPSIN Web tool which returns the same unique InChI and Standard InChIKey for both inputs:
InChI=1S/C19H26FN3O2/c1-13(2)17(18(21)24)22-19(25)15-12-23(11-7-3-6-10-20)16-9-5-4-8-14(15)16/h4-5,8-9,12-13,17H,3,6-7, 10-11H2,1-2H3,(H2,21,24)(H,22,25)
InChIKey: GQYZBLMAONPUNX-UHFFFAOYSA-N
Two different strings are respectively returned for their SMILES:
C(N)(=O)C(C(C)C)NC(=O)C1=CN(C2=CC=CC=C12)CCCCCF
NC(C(C(C)C)NC(=O)C1=CN(C2=CC=CC=C12)CCCCCF)=O
For synthetic cannabinoids the EMCDDA has proposed a model to apply to newly emerging substances, showing how the various components associated with molecular templates can be used to generate a unique short common name [48]. For unambiguous identification and communication of chemical information, one commonly used option is the InChiKey, (derived from IUPAC International Chemical Identifier InChi, Fig. 2).
Three more new substances were identified by NMR and HR-MS at the JRC in 2014 and were successively notified to the EMCDDA by the National Focal Points of the respective countries where the samples were seized. These were 4-Fluoropentedrone (1-(4-fluorophenyl)-2-(methylamino)pentan-1-one), 4-methyl-N,N-diethylcathinone (2-diethylamino-1-(4-methylphenyl)propan-1-one) and N-methyl-bk-MMDA-2 (1-(6-methoxy-1,3-benzodioxol-5-yl)-2-(methylamino) pro-pan-1-one) following nomenclature used in the EDND. Based on this experience, and after an exchange of views with the stakeholders, it was agreed that the traceability and availability of analytical data should be improved. One important consideration was to allow for its verification by peer experts in terms of consistency of the interpretation through the communication of comprehensive analytical reports. Moreover, the analytical methods should also be clearly mentioned in documents describing this process. The NMR and HR-MS methods were then edited according to an ISO template and presented in the JRC report discussed during the CLEN-EMCDDA meeting that followed, in February 2015 [49]. The description and harmonisation of the GC-MS and FT-IR methods used by the Customs laboratories were also edited according to the same template. These methods are now available in the Customs Laboratories ILIADe (Inter-Laboratory Inventory of Analytical Determination) database [50-54].
These approaches and methods were then systematically applied in collaboration with the European Customs laboratories according to the workflow presented in Fig. (1).
A data processing procedure was also set up using the ACD/Labs software in order to integrate multi-instrument, multi-techniques and format data from any Customs laboratory. ACD/Labs was also used to produce comprehensive analytical reports for the new substances that were encountered.
Of the ca. 250 samples analysed, several were already known NPS (mostly Synthetic Cannabinoids and Cathinones) but the data were not easily accessible to the Customs laboratory in question, which illustrated the need for fast access to data. In other cases, complex mixtures or other types of substances were received for confirmation (e.g. isomers).
Several new NPS which did not have data available elsewhere (at the time of their analysis and identification by the JRC) are listed in Table 1 with their common and systematic names as currently recorded in EDND.
Table 1.
List of the substances apprehended by the European Customs for which there was no data available at the time of seizure.
| MS | EDND Name | Systematic Chemical Name | InChiKey | Class |
|---|---|---|---|---|
| BG | 5F-AMBICA | N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-1H-indol-3-carboxamide | GQYZBLMAONPUNX-UHFFFAOYSA-N | Synthetic cannabinoid |
| FR | 4-Fluoropentedrone | 1-(4-Fluorophenyl)-2-(methylamino)pentan-1-one | QBFXBDUCRNGHSA-UHFFFAOYSA-N | Synthetic cathinone |
| FR | 4-methyl-N,N-diethylcathinone | 2-(Diethylamino)-1-(4-methylphenyl)-propan-1-one | SPARQPFHQXMJOR-UHFFFAOYSA-N | Synthetic cathinone |
| FR | N-methyl-bk-MMDA-2 | 1-(6-Methoxy-1,3-benzodioxol-5-yl)-2-(methylamino)propan-1-one | SONSUHOOEIVPFR-UHFFFAOYSA-N | Synthetic cathinone |
| FR | Despropionyl-2-fluoro fentanyl | N-(2-Fluorophenyl)-1-(2-phenylethyl)piperidin-4-amine | WUNLGTOLOUTCPE-UHFFFAOYSA-N | Opioid |
| BE | APP-CHMINACA | N-(2-Amino-1-benzyl-2-oxo-ethyl)-1-(cyclohexylmethyl)indazole-3-carboxamide | DMHWDSGURMXMGE-UHFFFAOYSA-N | Synthetic cannabinoid |
| FR | 5F-AB-FUPPYCA / 5F-5,3-AB-PFUPPYCA | N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-5-(4-fluorophenyl)-1H-pyrazole-3-carboxamide | GSXRDTDYPSATDE-UHFFFAOYSA-N | Synthetic cannabinoid |
| FR | 5F-PY-PICA | 1-(5-Fluoropentyl)-3-(pyrrolidine-1-carbonyl)-1-H-indole | AJOAHRJLOXOZKX-UHFFFAOYSA-N | Synthetic cannabinoid |
| FR | FUB-JWH-018 | [1-(4-Fluorobenzyl)-1H-indol-3-yl](naphthalen-1-yl)methanone | VREQTLWJHFQLEX-UHFFFAOYSA-N | Synthetic cannabinoid |
| FR | Nor-mephedrone | 2-Amino-1-(4-methylphenyl)-1-propanone | OHULHWHSUJEYIT-UHFFFAOYSA-N | Synthetic cathinone |
| BE | 4-Cl-alpha-PVP | 1-(4-Chlorophenyl)-2-(pyrrolidin-1-yl)pentan-1-one | NIGBFBTVONRYQN-UHFFFAOYSA-N | Synthetic cathinone |
| FR | tBuONE | 1-(1,3-Benzodioxol-5-yl)-2-(tert-butylamino)propan-1-one | BFOZYEAQPUMNIY-UHFFFAOYSA-N | Synthetic cathinone |
| BE | 3-CEC | 1-(3-Chlorophenyl)-2-(ethylamino)propan-1-one | ZMJIUWPWADQNLC-UHFFFAOYSA-N | Synthetic cathinone |
| BE | 4-CEC | 1-(4-Chlorophenyl)-2-(ethylamino)propan-1-one | RNBCGEIZCHBTGL-UHFFFAOYSA-N | Synthetic cathinone |
| BE | N-ethylhexedrone | 2-(Ethylamino)-1-phenylhexan-1-one | CWNKMHIETKEBCA-UHFFFAOYSA-N | Synthetic cathinone |
| FR | Valerylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)-4-piperidyl]pentanamide | VCCPXHWAJYWQMR-UHFFFAOYSA-N | Opioid |
| FR | 4-fluoromethylphenidate / 4F-MPH | Ethyl 2-(4-fluorophenyl)-2-(2-piperidyl)acetate | RKXQYWFDJDYSEN-UHFFFAOYSA-N | Piperidines & pyrrolidines |
| BE | Propylone | 1-(1,3-Benzodioxol-5-yl)-2-(propylamino)propan-1-one | YFVKHKCZBSGZPE-UHFFFAOYSA-N | Synthetic cathinone |
| DE | 4-chloropentedrone | 1-(4-Chlorophenyl)-2-(methylamino)pentan-1-one | OPCMVVKRCLOEDQ-UHFFFAOYSA-N | Synthetic cathinone |
| DE | alpha-PPP-MeO | 3-Methoxy-1-phenyl-2-(pyrrolidin-1-yl)propan-1-one | TVBNCCGCIPGJQO-UHFFFAOYSA-N | Synthetic cathinone |
MS: Member States (BE: Belgium, BG: Bulgaria, DE: Germany FR: France).
In several cases, the records of notification in the EDND show that several laboratories operating in different Member States were dealing with different seizures of the same new substances at more or less the same moment. For instance, N-ethylhexedrone (NEH) was identified in a sample from the Belgian Customs laboratory which was received at the JRC on 22/11/2015. In January 2016, it was identified at the JRC in a sample provided by French Customs. Subsequently, in February 2016, the EMCDDA received notifications of the identification of this substance from other countries, such as Sweden, The Netherlands, France, Belgium and Slovenia.
For most of the identified substances, the analytical reports produced by the JRC have also been included in the notification and stored in EDND. As per agreement by CLEN, the complete analytical data and reports of several NPS (propylone, valerylfentanyl, 4-fluoroethylphenidate, despropionyl-2-fluorofentanyl, 5F-MDMB-PICA, 5F-5,3-AB-FUPPYCA, and alpha-PPP-MeO) characterised in Customs seizures, were also integrated into the on-line drug monographs of the European project RESPONSE [55]. This helped with the sharing of analytical data and made it available to a broader community of law enforcement laboratories (as access to EDND is not open to all control laboratories). The RESPONSE project provides a systematic analytical characterisation of NPS purchased from reference materials producers, or from Internet vendors of research chemicals. Access to the data, through a simple Web interface, facilitates control laboratories with their identification work, when faced with new substances.
The EDND Names presented in table 1 are those used as key entry in this database. Often they are also known or found under other common names (e.g. 4-Cl-alpha-PVP as 4-Cl-α-PVP, alpha-PPP-MeO as α-PPP-MeO, N-ethylhexedrone as NEH). As discussed in the text the systematic chemical names or IUPAC names may not be unique.
The InChiKey provides a unique identifier for a chemical substance.
Several chemicals identified in Customs seizure were not reported to the EMCDDA as it is not always clear whether a substance would show psychoactive properties or not. This reflects the nature of subjective interpretation in the absence of data, especially in cases where compounds deviate from known NPS families.
However, in some cases, the circumstances related to a particular seizure can help Customs authorities and the NFP (National Focal Point) with the decision to include the communication of such substances in their notification to the EMCDDA.
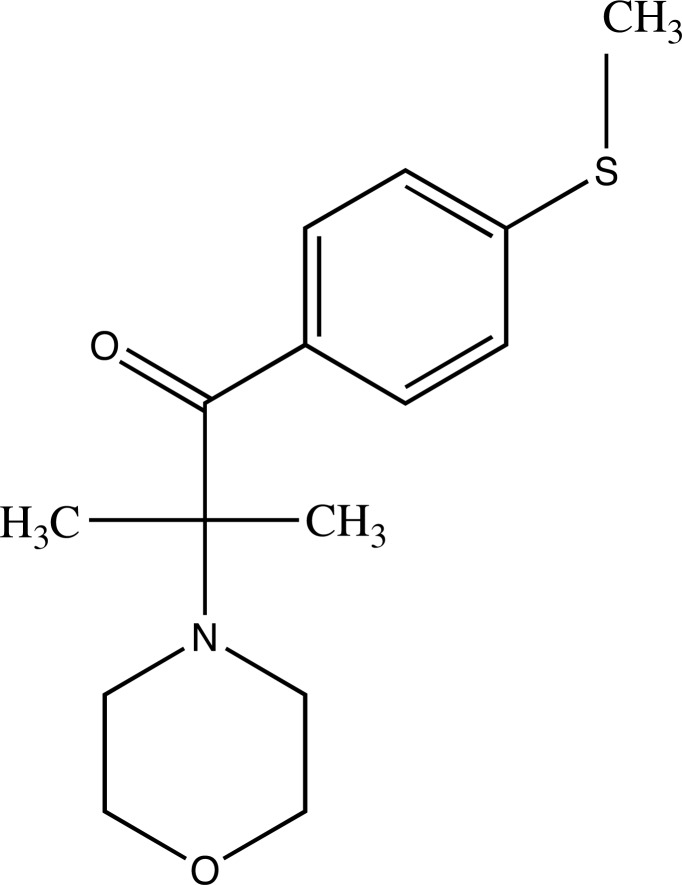
The substance Irgacure 907 (Fig. 3), was identified in two different samples received successively from the Belgian and the French Customs in March 2016. This substance is used by industry as an agent for paint and coating additives. It can also be found in consumer products such as ink, toner, and colorant products [56].
Fig. (3).
Irgacure 907. Irgacure 907 (2-methyl-1-[4-(methylthio) phenyl]-2-(4-morpholinyl)-1-propanone) is also known under many other common names such as CACCURE 907, Photocure 907, OMNIRAD 4817; ENVICURE 907, SPEEDCURE 907, MMTMP, MMMP/MTMP.
InChI=1S/C15H21NO2S/c1-15(2,16-8-10-18-11-9-16)14(17)12-4-6-13(19-3)7-5-12/h4-7H,8-11H2,1-3H3
InChIKey: LWRBVKNFOYUCNP-UHFFFAOYSA-N
However, it was unclear why it was found in seizures of parcels containing several cathinones. Six other Member States reported detections to the EMCDDA (Germany, Finland, The Netherlands, Portugal, Sweden and United Kingdom). It was also found outside Europe, in the US, Australia and Brazil, in powder form and in ecstasy-type tablet form as well as in seizures with internationally controlled substances, such as cocaine and methamphetamine.
In fact, apart from NPS, other types of substances have been identified in the samples sent by customs laboratories. In particular, they belong to the following families of compounds: SARMs (selective androgen receptor modulator), hair growth enhancers, sexual stimulants, nootropics, non-marketed or banned medicines, and also new drug precursors. It should be noted that such substances are also sold on the same internet sites as for where NPS can be purchased.
CONCLUSION
The integrated analytical approach presented in this article, combining analytical techniques and cheminformatics tools, has been shown to enable the rapid and unambiguous identification of substances apprehended by European Customs. The analytical reports produced by the JRC on newly identified substances provide the elements for a possible check of the chemical interpretation by peer experts in the field. The swift communication of data on NPS to the EMCDDA allows the rapid updating of EDND database. For an effective monitoring of NPS and to prevent duplication of efforts, access to analytical data such as those provided by the RESPONSE project should be facilitated. This practice could be considered as a base for strengthening the traceability and reliability of spectral libraries used in the routine control. Another goal of the JRC is to propose methods and approaches and support their adoption as guidelines (i.e. ILIADe-CLEN methods). Continuation of this work, in coordination with EMCDDA, ENFSI and CLEN, is planned within the action No. 53 of the EU Action plan 2017-2020 [57].
Apart from NPS, Customs laboratories are also facing the problem of identification of other type of chemicals sold on the “illicit market”. As the first rampart in the control of chemicals moving across European borders, the role of customs laboratories is of fundamental importance not only from an economic point of view but also in the interest of public health and the protection and safety of EU citizens. The principles presented in this paper may also be applied to the characterisation and development of spectral libraries used for a number of other chemicals. The ECICS (European Customs Inventory of Chemical Substances) database is widely used for checking the customs declaration of traded chemicals [58]. It is suggested that it can also be used as a repository of analytical data and spectra for substances characterised by Customs laboratories.
Consent for Publication
Not applicable.
Acknowledgements
The authors would like to thank the European Commission Directorate General for Taxation and Customs Unions (DG TAXUD) and the JRC for financial support (Administrative Arrangement JRC-Nr 33619-CLEN2SAND-DG TAXUD-Nr TAXUD/2014/DE/315).
The authors of the report would like to thank the members of the Customs Laboratories European Network (CLEN), the European Centre for Monitoring of Drugs and Drug Addictions (EMCDDA), the members of the Drug Working Group of the European Network of Forensic Sciences Institutes (ENFSI), the coordinators and the partners of the European project RESPONSE. Their active contribution through sending of samples and exchange of views and ideas have been very appreciated for the conception and execution of this work. Finally we are grateful to the reviewers whose comments and suggestions have allowed us to improve this article.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Brandt S.D., King L.A., Evans-Brown M. The new drug phenomenon: Editorial and perspective. Drug Test. Anal. 2014;6:587–597. doi: 10.1002/dta.1686. [DOI] [PubMed] [Google Scholar]
- 2.Dines A.M., Wood D.M., Yates C., Heyerdahl F., Hovda K.E., Giraudon I., Sedefov R., Dargan P.I., Euro-DEN Research Group Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months’ data collection from the european drug emergencies network (Euro-DEN). Clin. Toxicol. (Phila.) 2015;53:893–900. doi: 10.3109/15563650.2015.1088157. [DOI] [PubMed] [Google Scholar]
- 3.Menzies E.L., Hudson S.C., Dargan P.I., Parkin M.C., Wood D.M., Kicman A.T. Characterizing metabolites and potential metabolic pathways for the novel psychoactive substance methoxetamine: Identification of metabolites of methoxetamine using HRAM LCMS. Drug Test. Anal. 2014;6:506–515. doi: 10.1002/dta.1541. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet U., Mahler H. Synthetic cannabinoids: Spread, addiction biology & current perspective of personal health hazard. Fortschr. Neurol. Psychiatr. 2015;83:221–231. doi: 10.1055/s-0034-1399307. [DOI] [PubMed] [Google Scholar]
- 5.Erratico C., Negreira N., Norouzizadeh H., Covaci A., Neels H., Maudens K., van Nuijs A.L.N. In vitro and in vivo human metabolism of the synthetic cannabinoid AB-CHMINACA. Drug Test. Anal. 2015;7:866–876. doi: 10.1002/dta.1796. [DOI] [PubMed] [Google Scholar]
- 6.Negreira N., Erratico C., Kosjek T., van Nuijs A.L.N., Heath E., Neels H., Covaci A. In vitro phase I and phase II metabolism of α-pyrrolidinovalerophenone (α-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal. Bioanal. Chem. 2015;407:5803–5816. doi: 10.1007/s00216-015-8763-6. [DOI] [PubMed] [Google Scholar]
- 7.Giné C.V., Espinosa I.F., Vilamala M.V. New psychoactive substances as adulterants of controlled drugs. A worrying phenomenon? Drug Test. Anal. 2014;6:819–824. doi: 10.1002/dta.1610. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P.A., Travis J.C., Venhuis B.J. A methamphetamine analog (N,α-diethyl-phenylethylamine) identified in a mainstream dietary supplement. Drug Test. Anal. 2014;6:805–807. doi: 10.1002/dta.1578. [DOI] [PubMed] [Google Scholar]
- 9.Adams A.J., Banister S.D., Irizarry L., Trecki J., Schwartz M., Gerona R. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 2017;376:235–242. doi: 10.1056/NEJMoa1610300. [DOI] [PubMed] [Google Scholar]
- 10.Ireland N.N. http://www.cbc.ca/news/health/fentanyl-carfentanil-opioid-crisis-spreading-across-canada-1.3909986
- 11.Pichini S., Pacifici R., Marinelli E., Busardò F.P. European drug users at risk from illicit fentanyls mix. Front. Pharmacol. 2017;8:785. doi: 10.3389/fphar.2017.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EMCDDA . European Drug Report - Trends & Development 2017. Luxembourg: Publications Office of the European Union; 2017. [Google Scholar]
- 13.The European Parliament and The Council of the European Union 2017.
- 14.The European Parliament and The Council of the European Union 2017.
- 15.EMCDDA . European Drug Report - Trends & Developments 2013. Luxembourg: Publications Office of the European Union; 2013. [Google Scholar]
- 16.Scientific Working Group for the Analysis of Seized Drugs SWGDRUG Home Page Available on. http://swgdrug.org/
- 17.Chemicals C. Cayman Spectral Library Available on. https://www.caymanchem.com/forensic/csl
- 18. https://www.designer-drugs.de/
- 19.Smith J.P., Sutcliffe O.B., Banks C.E. An Overview of recent developments in the analytical detection of new psychoactive substances (NPSs). Analyst (Lond.) 2015;140:4932–4948. doi: 10.1039/c5an00797f. [DOI] [PubMed] [Google Scholar]
- 20.Shevyrin V., Melkozerov V., Nevero A., Eltsov O., Shafran Y., Morzherin Y., Lebedev A.T. Identification and analytical characteristics of synthetic cannabinoids with an indazole-3-carboxamide structure bearing a n-1-methoxycarbonylalkyl group. Anal. Bioanal. Chem. 2015;407:6301–6315. doi: 10.1007/s00216-015-8612-7. [DOI] [PubMed] [Google Scholar]
- 21.Adamowicz P., Tokarczyk B. Simple and rapid screening procedure for 143 new psychoactive substances by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2016;8:652–667. doi: 10.1002/dta.1815. [DOI] [PubMed] [Google Scholar]
- 22.Kanu A.B., Brandt S.D., Williams M.D., Zhang N., Hill H.H. Analysis of psychoactive cathinones and tryptamines by electrospray ionization atmospheric pressure ion mobility time-of-flight mass spectrometry. Anal. Chem. 2013;85:8535–8542. doi: 10.1021/ac401951a. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama N., Kawamura M., Kikura-Hanajiri R., Goda Y. URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci. Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama N., Asakawa K., Kikura-Hanajiri R., Tsutsumi T., Hakamatsuka T. A new pyrazole-carboxamide type synthetic cannabinoid ab-chfupyca [n-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1h-pyrazole-5-carboxamide] identified in illegal products. Forensic Toxicol. 2015;33:367–373. doi: 10.1007/s11419-015-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevyrin V., Kupriyanova O., Lebedev A.T., Melkozerov V., Eltsov O., Shafran Y., Morzherin Y., Sadykova R. Mass spectrometric properties of n-(2-methoxybenzyl)-2-(2,4,6-trimethoxy-phenyl)-ethanamine (2,4,6-tmpea-nbome), a new representative of designer drugs of NBOMe series and derivatives thereof. J. Mass Spectrom. 2016;51:969–979. doi: 10.1002/jms.3808. [DOI] [PubMed] [Google Scholar]
- 26.Concheiro M., Castaneto M., Kronstrand R., Huestis M.A. Simultaneous determination of 40 novel psychoactive stimulants in urine by liquid chromatography-high resolution mass spectrometry and library matching. J. Chromatogr. A. 2015;1397:32–42. doi: 10.1016/j.chroma.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Castro A., Lendoiro E., Fernández-Vega H., Steinmeyer S., López-Rivadulla M., Cruz A. Liquid chromatography tandem mass spectrometry determination of selected synthetic cathinones and two piperazines in oral fluid. Cross reactivity study with an on-site immunoassay device. J. Chromatogr. A. 2014;1374:93–101. doi: 10.1016/j.chroma.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Gaspar H., Bronze S., Ciríaco S., Queirós C.R., Matias A., Rodrigues J., Oliveira C., Cordeiro C., Santos S. 4F-PBP (4′-Fluoro-α-Pyrrolidinobutyrophenone), a new substance of abuse: Structural characterization and purity NMR profiling. Forensic Sci. Int. 2015;252:168–176. doi: 10.1016/j.forsciint.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Langer N., Lindigkeit R., Schiebel H-M., Ernst L., Beuerle T. Identification and quantification of synthetic cannabinoids in “spice-like” herbal mixtures: A snapshot of the German situation in the autumn of 2012. Drug Test. Anal. 2014;6:59–71. doi: 10.1002/dta.1499. [DOI] [PubMed] [Google Scholar]
- 30.Acton W.J., Lanza M., Agarwal B., Jürschik S., Sulzer P., Breiev K., Jordan A., Hartungen E., Hanel G., Märk L., Mayhew C.A., Märk T.D. Headspace analysis of new psychoactive substances using a selective reagent ionisation-time of flight-mass spectrometer. Int. J. Mass Spectrom. 2014;360:28–38. doi: 10.1016/j.ijms.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strano R.S., Odoardi S., Gregori A., Peluso G., Ripani L., Ortar G., Serpelloni G., Romolo F.S. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun. Mass Spectrom. RCM. 2014;28:1904–1916. doi: 10.1002/rcm.6969. [DOI] [PubMed] [Google Scholar]
- 32.Bell C., George C., Kicman A.T., Traynor A. Development of a rapid LC-MS/MS method for direct urinalysis of designer drugs. Drug Test. Anal. 2011;3:496–504. doi: 10.1002/dta.306. [DOI] [PubMed] [Google Scholar]
- 33.Guirguis A., Girotto S., Berti B., Stair J.L. Identification of new psychoactive substances (NPS) using handheld raman spectroscopy employing both 785 and 1064nm Laser Sources. Forensic Sci. Int. 2017;273:113–123. doi: 10.1016/j.forsciint.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Namera A., Kawamura M., Nakamoto A., Saito T., Nagao M. Comprehensive review of the detection methods for synthetic cannabinoids and cathinones. Forensic Toxicol. 2015;33:175–194. doi: 10.1007/s11419-015-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valente M.J. Guedes, de Pinho P.; de Lourdes, B.M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014;88:15–45. doi: 10.1007/s00204-013-1163-9. [DOI] [PubMed] [Google Scholar]
- 36.Slovenian Ministry of the Interior https://www. policija.si/eng/index.php/generalpolicedirectorate/1669-nfl-page-response
- 37.Lobo Vicente J., Chassaigne H., Holland M.V., Reniero F., Kolář K., Tirendi S., Vandecasteele I., Vinckier I., Guillou C. Systematic analytical characterization of new psychoactive substances: A case study. Forensic Sci. Int. 2016;265:107–115. doi: 10.1016/j.forsciint.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Weininger D. SMILES, a Chemical Language and Information System. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Model. 1988;28:31–36. [Google Scholar]
- 39.Frey J.G. The IUPAC International Chemical Identifier: Using InChi. Chem. Int. 2006:14–15. [Google Scholar]
- 40.McNaught A. The IUPAC International Chemical Identifier: InChi - a new standard for molecular informatics. Chem. Int. 2006;•••:12–14. [Google Scholar]
- 41.Heller S., McNaught A., Stein S., Tchekhovskoi D., Pletnev I. InChI - the Worldwide Chemical Structure Identifier Standard. J. Cheminform. 2013;5:7. doi: 10.1186/1758-2946-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heller S.R., McNaught A., Pletnev I., Stein S., Tchekhovskoi D. InChI, the IUPAC International Chemical Identifier. J. Cheminform. 2015;7:23. doi: 10.1186/s13321-015-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.OPSIN Open Parser for Systematic IUPAC Nomenclature Available on. http://opsin.ch.cam.ac.uk/
- 44.Lowe D.M., Corbett P.T., Murray-Rust P., Glen R.C. Chemical name to structure: Opsin, an open source solution. J. Chem. Inf. Model. 2011;51:739–753. doi: 10.1021/ci100384d. [DOI] [PubMed] [Google Scholar]
- 45. https://chemaxon.com/products/chemicalize
- 46.Zanzi A., Wittwehr C. Searching online chemical data repositories via the chemagora portal. J. Chem. Inf. Model. 2017;57:2905–2910. doi: 10.1021/acs.jcim.7b00086. [DOI] [PubMed] [Google Scholar]
- 47.Archer R.P., Treble R., Williams K. Reference materials for new psychoactive substances. Drug Test. Anal. 2011;3:505–514. doi: 10.1002/dta.317. [DOI] [PubMed] [Google Scholar]
- 48.EMCDDA http://www.emcdda.europa.eu/topics/pods/synthetic-cannabinoids
- 49.Reniero F., Lobo P.V.J., Chassaigne H., Holland M., Tirendi S., Kolar K., Guillou C. Report on Characterisation of New Psychoactive Substances (NPS); EUR - Scientific and Technical Research Reports. Publications Office of the European Union; 2014. [Google Scholar]
- 50.DG TAXUD-CLEN https://ec.europa.eu/taxation_ customs/business/customs-controls/eu-customs-laboratories/customs-laboratories-eu-network-overview/action-1-group-eu-customs-laboratories_en
- 51. https://ec.europa.eu/ taxation_customs/sites/taxation/files/nmr_iliade.pdf
- 52. https://ec.europa.eu/ taxation_customs/sites/taxation/files/hr_iliade.pdf
- 53. https://ec.europa.eu/taxation_customs/sites/taxation/files/gcms_iliade.pdf
- 54. https://ec.europa.eu/ taxation_customs/sites/taxation/files/ftir_iliade.pdf
- 55.Slovenian Ministry of the Interior https://www.policija.si/apps/nfl_response_ web/seznam.php
- 56.Buback M., Kuelpmann A. A suitable photoinitiator for pulsed laser-induced free-radical polymerization. Macromol. Chem. Phys. 2003;204:632–637. [Google Scholar]
- 57.2017.
- 58.Europan Commission - DG Taxation and Customs Union (TAXUD) European Customs Inventory of Chemical Substances Available on. https://ec.europa.eu/taxation_customs/online-services/eu-customs-inventory-chemical-substances_en