Abstract
U.S. breast cancer survivors (BCSs) are expected to increase to 4 million in the next 5–10 years. Cancer recurrence risk is highest among obese survivors. Inflammatory (Pro-I) biomarkers including C-reactive protein (CRP), Interleukins -3, -6, and -8 (IL-3, IL-6, IL-8), and Tumor Necrosis Factor (TNF)-α have been associated with cancer recurrence risk. Nutritional interventions aimed at reducing inflammation (INF) may contribute to reduced cancer recurrence risk, but studies have been limited to animal models. The goals of this one-year, culinary-based, pilot intervention were to: 1) decrease Pro-I biomarkers and increase anti-inflammatory (AI) cytokine, IL-10, by promoting AI food incorporation into BCS diets; and 2) examine intervention effects on cancer risk factors including body mass index (BMI) and circulating adipose stromal cells (ASCs). A total of 153 BCSs were recruited. Overweight and obese women aged 18 or older were randomized into Intervention (IG; n = 76) and Control (CG; n = 77) groups. CG received monthly nutritional brochures from the American Institute for Cancer Research. IG attended 6 monthly workshops (lectures on AI topics and chef-prepared food demonstrations), and received monthly newsletters and telephone calls incorporating Motivational Interviewing. At baseline, 6- and 12-month assessments, fasting serum was assayed for Pro-I/AI marker and ASC levels. Using R and Stata version 14 (Stata Corp, 2015), no significant differences were found between groups on baseline demographic variables. Correlations between serum cytokine levels, BMI, % body fat, ASCs, and self-reported variables are discussed.
Keywords: Inflammation, Obesity, Breast cancer survivors, Biomarker
1. Introduction
U.S. breast cancer survivors (BCSs) are expected to increase from 3 million in 2012 to 4 million by 2022 [1]. In 2015, there were 16,510 projected cases of breast cancer in Texas women; of these, 2710 were predicted to succumb to the disease [2]. The quality of life (QOL) of cancer survivors from diverse ethnic, cultural and socioeconomic backgrounds is an emerging priority area for studies on survivorship research and clinical care [3]. About 33% of cancer mortality in the U.S. is associated with obesity, physical inactivity, and/or poor nutrition, and is therefore preventable [4]. Several research studies have revealed that obese women diagnosed with breast cancer have higher recurrence and mortality risk than non-obese [5]. Circulating Adipose Stromal Cells (ASCs) have been linked to cancer recurrence risk [6,7]. Our previous work has shown higher levels of ASCs in obese BCSs [8]. Furthermore, excess body fat has been shown to promote cancer in postmenopausal women by increasing levels of inflammation, insulin, and other hormones [9].
Several cancers, including colorectal and prostate, are known to be influenced by inflammation [6,7]. Elevated C-reactive protein (CRP), a pro-inflammatory (Pro-I) biomarker, in serum is among many risk factors contributing to postmenopausal breast cancer [10]. CRP has also been positively correlated with a Pro-I diet [11]. Investigations of other Pro-I biomarkers: Interleukins (ILs)-6 and −8, and Tumor Necrosis Factor-alpha (TNFα), in overweight breast cancer survivors found weight loss via exercise training reduced high cytokine levels [12]. Furthermore, a Mediterranean-diet profile has been linked to lower breast cancer risk [13], compared to Western cuisine patterns which have a higher dietary glycemic load [12,14].
Relatively fewer studies have presented data on the linkage between diet and breast cancer risk. A meta-analysis of diet and mammary cancer experiments on mice showed a strong positive association between total energy intake and mammary tumor incidence, while fat intake was weakly associated [15]. Herbert and colleagues established a dietary “Inflammatory Index” (DII) that scores effects of various inflammatory foods on biomarkers IL-1β, IL-4, IL-6, IL-10, TNFα and CRP [16]. Several controlled studies have tested this index, with findings including increased incidence of esophageal squamous cell cancer in participants with high DII scores [17]; and positive correlations between high self-reported DII score and digestive cancer, and coronary heart disease mortality. Although no significant relationship was found between the DII and overall breast cancer, there were modest positive associations of pro-inflammatory diets as defined by DII with cancer mortality as well as tumor subtypes [18].
The goals of this one-year culinary-based pilot intervention were to: 1) decrease Pro-I biomarkers and increase the anti-inflammatory (AI) cytokine, IL-10, by promoting AI food incorporation into BCS diets; and 2) examine intervention effects on cancer risk factors including body mass index (BMI) and circulating ASCs. We report here on the study design and baseline characteristics of our participants, and discuss interactions between cytokine and ASC levels, BMI and dietary habits.
2. Methods
2.1. Study design and participants
Our trial recruited 153 overweight and obese (BMI ≥ 25kg/m2), early-stage (0-III), English-speaking breast cancer survivors who had completed their treatment 2 or more months prior to study enrollment to a two-arm randomized controlled trial (RCT) with a 2 (group) by 3 (time) repeated measures design. Computerized randomization was carried out via REDCap [19] using a 1:1 allocation ratio in blocks of size two. Intervention group (IG; n = 76) participants received individualized anti-inflammatory dietary prescriptions and behavior-change cues through six monthly workshops (culinary demonstrations, recipes and meal planning), reinforced by evidence- and theory-based patient navigation, motivational interviewing, and tailored newsletters personalized to individual readiness for change. The purpose of the intervention was to stimulate dietary behavior changes over the 1-year study duration resulting in corresponding changes in inflammatory biomarkers.
Control group (CG; n = 77) participants received minimal nutritional information at baseline, monthly American Institute for Cancer Research informational brochures, and two telephone calls prior to assessment appointments. They did not receive any navigational services. Groups were compared at baseline, 6-month, and 12-month follow-ups. Primary outcomes included dietary behaviors and levels of cancer associated biomarkers (Fig. 1).
Fig. 1.
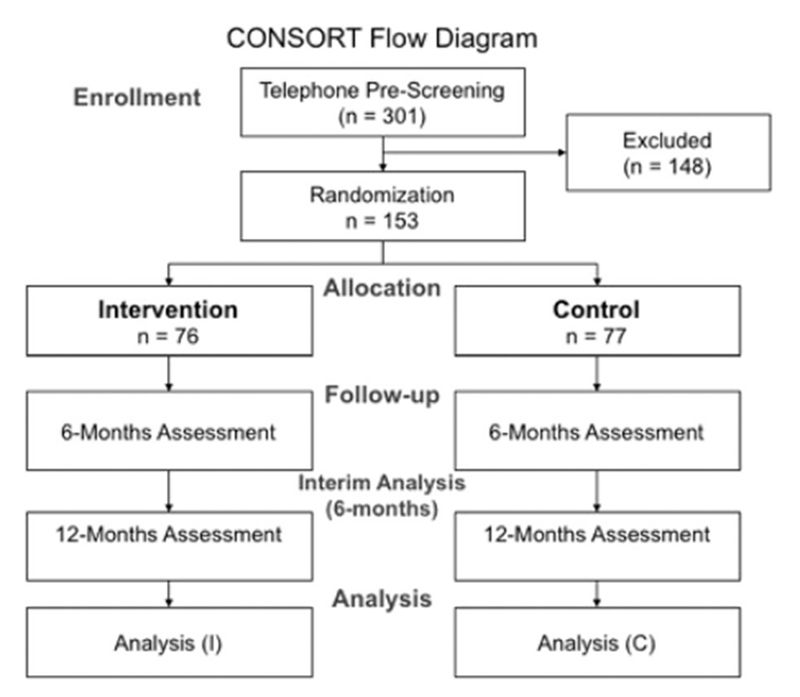
CONSORT study flow diagram.
2.2. Assessments
At the baseline visit and follow-up assessment periods (6 and 12 months), all participants underwent venipuncture to collect biomarker data, an anthropometric evaluation, and completed a questionnaire. At the 12-month assessment, additional blood was collected from consenting participants (n = 47) to obtain serum lipid panel (obesity marker) and hemoglobin A1C, a medium-term marker for diabetes risk. These results were correlated to other study measures.
Anthropometric data included height and weight (to calculate BMI). An initial estimate of BMI was taken over the phone during the formal eligibility screening and was confirmed at the first assessment visit. Participants were notified that if their measured BMI was below 25kg/m2, they would not be allowed to participate in the study. To calculate body fat percentage (%), three-site skinfold measurements (triceps, suprailium and quadriceps) were collected using American College of Sports Medicine (ACSM) guidelines [20]. Calipers (Lafayette Instruments, Lafayette IN) measured the skinfold tissue in mm with duplicate measurements taken at each site. Unless contraindicated by lymphedema, recent surgery, or participant preference, all measurements were taken on the right side of the body. Skin fold measurements were summed. Body density (Db) and % body fat were calculated using ACSM-recommended formulas:
Data were collected in private patient examination rooms at the Cancer Therapy and Research Center (CTRC).
At 6 months and 12 months, participants’ waist circumference (WC) was also measured. A flexible cloth tape measure (Seca) was placed against the skin at the anatomical location. WC was measured to the nearest 0.5 cm at the level of the iliac crest at the end of normal exhalation.
The questionnaire was administered in the same patient examination room at the CTRC as anthropometric data collection. Participants entered self-report responses into an iPad Air using REDCap [19], a web-based survey administration software hosted by the Department of Epidemiology & Biostatistics. Questionnaire included standard socio-demographic information; data specific to cancer diagnosis, stage, and treatment; and areas that the individual identified as potential or actual unmet needs (e.g., coping emotionally, learning more about their cancer and treatment options, community resources, etc.). This assessment also included questions to assess stage of change, depression, selfefficacy, self-esteem, locus of control, and cancer worry scales. Additionally, participants were also asked questions regarding their current diet history by completing an instrument based on the Mediterranean diet and an assessment of spice and herb consumption (see Table 1 for citations and descriptions of each instrument used). With the exception of demographics and cancer history, this battery of instruments was repeated at baseline, 6 and 12 months.
Table 1.
All assessments & scales descriptions and scoring methods.
| Scales | Description/scoring methods |
|---|---|
| Barriers to care [31] | Twenty “yes” (Y = 1) or “no” (N = 0) questions to measure existence of socio-behavioral procedures practices, which prevent effective assimilation in the health care system. Scoring: Responses were summed to a raw score between 0 and 20 points, where 0 indicated effective assimilation into the health care system and 20 indicated ineffective assimilation into the health care system. |
| Social support [32] | Twenty questions to evaluate awareness of family and friends supporting personal goals to modify patients’ diet. Reponses (response = x) for both family and friends were: “None” (x = 1), “rarely” (x = 2), “a few times” (x = 3), “often” (x = 4), “very often” (x = 5), and “does not apply” (x = 0). Scoring: Responses were summed to a raw score between 0 and 50 points, where 50 indicated a lot of family/friends support for diet change and 0 indicated no support for diet change. |
| Depression Scale CES-D [33] | Self-reported assessment consisting of five depression indicator questions with response ranges of “Rarely or none of the time (less than one day)” (x = 0), “Some or little of the time (1–2 days)” (x = 1), “Occasionally or moderate amount of time (3–4 days)” (x = 3), and “Most or all of the time (5–7 days)” (x = 3). Scoring: Responses were summed to a raw score between 0 and 15 points, where 0 indicated minor to no depression and 15 indicated severe depression. |
| Coping [34] | Two questions identifying patients’ means of solving issues and making important life decisions. |
| Self-esteem [35] | A ten-question survey evaluating overall measure of self-worth. Response (response = x) ranges were “Strongly Agree” (x = 1), “Agree” (x = 2), “Disagree” (x = 3), “Strongly Disagree” (x = 4), and “Refused/Don’t Know” (x = 0). Scoring: Responses were summed to a raw score between 10 and 40 points, where 10 indicated a high self-esteem and 40 indicated a low self-esteem. |
| Family Health History [36] | A two-question survey evaluating awareness of cancer related health problems in blood relatives. Response ranges were “yes” (points = 2), “no” (points = 1), and “don’t know” (points = 0). Scoring: Responses were summed to a raw score between 0 and 4, where 4 indicated knowledge of cancer in family history and 0 indicated no knowledge of family health history. |
| Health Behavior Change [37] | A fifteen-question assessment on stress coping & differences in health behavior among cancer survivors. Response (response = x) ranges were “less” (x = 1), “The Same Amount” (x = 2), “More” (x = 3), “Don’t Know” (x = 4), and “Refused” (x = 5). Scoring: Responses were summed to a raw score between 15 and 75 points. |
| Cancer Worry Scale [38] | An eight-question survey to assess patients’ personal worry about cancer diagnosis. Response (response = x) ranges were “Never or hardly ever” (x = 1), “Sometimes” (x = 2), “Often” (x = 3), and “Always or Almost Always” (x = 4). Scoring: Responses were summed to a raw score between 8 and 32 points, where 8 indicated no worry and 32 indicated high worry. |
| FACT-G [39] | FACT-G is an instrument with which respondent cancer patients are asked to respond to various health-related items as it applies to their experience within the past 7 days. The tool is composed of a total 27 questions and includes four subscales. Scoring: Subjects were asked to rate each item using a five point Likert-tvpe rating scale, where 1 = “Not at all,” 2 = “A little bit,” 3 = “Somewhat,” 4 = “Quite a bit,” and 5 = “Very much.” The total score for the FACT-G includes summation of the mean score of each of the four subscales; the mean score for each subscale is then calculated by adding each item response score, multiplying the total by seven and dividing by the number of questions answered. The score range for FACT-G is 0–108. |
| FACT-B (Breast Cancer Functional Assessment of Cancer Therapy Scale) [39] | FACT-B is a tool with which respondent breast cancer patients are asked to respond to various health-related items as it applies to their experience within the past 7 days. The tool is an additional subscale of F ACT-G and reflects experiences of specific importance to breast cancer patients. The FACT-B subscale is composed of ten total questions. Scoring: As in FACT-G, in FACT-B subjects are asked to rate each item using a five point Likert-type rating scale, where 1 = “Not at all,” 2 = “A little bit,” 3 = “Somewhat,” 4 = “Quite a bit,” and 5 = “Very much.” The subscale score for the FACT-B is its mean score; it is calculated by adding each item response score, multiplying the total by ten and dividing by the number of items answered. The Score range for FACT-B Subscale Score is 0–40. The Total FACT-B Score includes summation of its mean plus the Total FACT-G Score which is scored composed by adding the scores of each of the four subscales: Physical Well-Being, Social/Family Well-Being, Emotional Well-being, and Functional Well-Being. The score range for the FACT-B is 0–148. |
| Self-efficacy [40,41] | The Self Efficacy questionnaire explores how confident patients are; it is comprised of 24 questions which are organized in seven subscale categories. Scoring: For each of the questions, patients are asked to choose the number on the scale that corresponds to the level of confidence with which they can accomplish the tasks regularly at the present time. The scales are presented in a Likert-type approach ranging from “Not at all confident” to “Totally confident.” The total scale score includes summation of scores on all the questions, with lower scores indicating less satisfaction regarding personal confidence level, and higher scores indicating an observed greater confidence level. |
| IPAQ Short (Last 7 days) [42] | The International Physical Activity Questionnaire (IPAQ) was developed as an instrument for cross-national monitoring of physical activity and inactivity. Scoring: Both the short and long form data were used to estimate total weekly physical activity by weighting the reported minutes per week within each activity category by a MET energy expenditure estimate assigned to each category of activity. MET levels were obtained from the 2000 compendium of physical activities to include moderate-intensity activities between 3 and 6 METs and vigorous-intensity activities as 6 METs. The weighted MET-minutes per week were calculated as duration frequency per week. |
| PSS-14 (Perceived Stress Scale) [43] | A total of fourteen questions to evaluate self-reported amount of stress. Response (response = x) ranges were “Never” (x = 0), “Almost Never” (x = 1), “Sometimes” (x = 2), “Fairly Often” (x = 3), and “Very Often” (x = 4). Scoring: Reponses were summed to a raw score between 0 and 16 points, where 0 indicated a low amount of personal stress and 16 indicated a high amount of personal stress. |
| Brief Family Life Questionnaire [44] | A total of twenty questions designed to measure help and support between patient and family. Response (response = x) ranges were “Strongly Disagree” (x = 1), “Disagree” (x = 2), “Agree” (x = 3), and “Strongly Agree” (x = 4). Scoring: Responses were summed to a raw score between 20 and 80 points, where 80 indicated family support and 20 indicated no family support. |
| Apter Motivational Style Profile [45] | Survey of forty questions indicating level of motivation by personal experience recollection. Response (response = x) ranges were “Never” (x = 1), “Seldom” (x = 2), “Sometimes” (x = 3), “Often” (x = 4), “Very Often” (x = 5), and “Always” (x = 6). Scoring: Responses were summed to a raw score between 40 and 240 points. |
| Modeling [46,47] | A total of eight ‘yes’ (Y = 1) or ‘no’ (N = 0) questions of self-reported awareness of social support to improve personal diet. Scoring: Responses were summed to a raw score between 0 and 8 points, where 8 indicated strong patient-family role-model relationships and 0 indicated weak patient-family role model relationships. |
| Stages of Change [46,47] | A total of five ‘yes’ (Y = 1) or ‘no’ (N = 0) questions identify current change, intention to change, or no intention to change in diet. Scoring: Responses were summed to a raw score between 0 and 5 points, where 5 indicated current change of previous diet choices and 0 indicated no intention of changing current diet. |
| Medication List [48] | Participant’s self-reported medication list. Participants were asked for dose, frequency, duration, and route for each medicine the participant was taking at the time of the interview. Terms chosen for route are from the RxNorm developed at the NLM. |
| Diet Assessment Tool [49] | 1 Fourteen questions were coded to assign ‘1’ to responses in agreement with a Mediterranean Diet, and ‘0’ to other. Scoring: Items were summed up to get a raw score between 0 and 14 points. |
| Spices Assessment [49] | 2 Twelve questions constructed by the project’s team to evaluate consumption of Mediterranean style diet. Scoring: Each question ranges from 1 to 4 score points making for a raw score range of 12–48, where 48 indicated Mediterranean dietary choices and 12 indicated non-Mediterranean dietary choices. |
2.2.1. 3-Day food diary
Due to the fact that diet changes (e.g. caloric intake) unrelated to the intervention may influence biomarker levels, participants were instructed to maintain diet records for two weekdays and one weekend day prior to undergoing venipuncture. This was repeated at each time point (baseline, 6 and 12 months).
2.2.2. Biomarkers
Participants were instructed to abstain from physical activity, smoking, and alcohol; take all medications prescribed for regular daily use; and not take sporadic or “as needed” medications for 24 h before the blood draw.
Participants underwent venipuncture from a credentialed phlebotomist between 7:45 and 11:30 am the day after a 12-h fast, to minimize variability resulting from the circadian expression of most cytokines. Biomarker collection was done in the Breast Clinic at the CTRC. To assess pro- and anti-inflammatory biomarkers (Interleukins 3, 6, 8, and 10; CRP; TNFα), two blue “tiger top” EDTA (anti-coagulant) 10-mL Vacutainer tubes (BD Biosciences, USA) were collected, labeled with participant Study ID, and stored on ice for processing. Collected serum was aliquoted into 400 μL cryovials and stored at − 80 °C. Samples were analyzed in batches at the Bioanalytics and Single-Cell (BASiC) Core facility at UTHSCSA. The Luminex (Austin, TX) FlexMap 3D (FM3D) platform was used to analyze participant sera. Milliplex kits specific to each analyte — Human T-cell High-Sensitivity Panel (IL-6, IL-8, IL-10, TNFα), Human Cyto-Chemo Panel (IL-3) and Human Neurodegenerative Panel 2 (CRP) — were purchased from Millipore (Billerica, MA) to perform the assays. Serum samples were thawed, clarified, and 25 μL of each sample run in duplicate on a 96-well plate with blanks, standards, and assay controls. Depending on the targets for each kit, samples were diluted per manufacturer’s protocol. Assay plates were prepared as described by the manufacturer’s protocol and then read on the Flexmap 3D with xPONENT software, using the Luminex magnetic-bead detection technology. Quality controls were added to evaluate assay performance by determining high and low ranges of each individual cytokine level, and bead counts were performed to insure that sufficient data points are achieved to accurately assess cytokine levels. Analysis of cytokine concentrations was performed with the Millipore Analyst software. Analyte concentrations in sample wells were calculated from median fluorescent intensity using a standard curve based on concentrations of reference standards provided by the manufacturer. Raw results falling below detection limits were recorded as missing.
2.2.3. Isolation of circulating ASCs
ASCs were collected from the blood samples and analyzed as previously described [8]. Briefly, blood samples were centrifuged at room temperature for 25 min with no brake at 1600 × g. Fuzzy layers containing mononuclear cells (MNCs), located just above the gel barrier, were collected in 15 mL conical tubes. FcR-blocking reagent (Miltenyi Biotec, USA) was added to the MNCs according to the manufacturer’s recommendation (20 μL per 107 cells) and incubated on ice for 10 min. Each sample was aliquoted into 5 tubes and various antibodies added in each tube. After 30-min incubation on ice, cells were washed twice with ice-cold 1 × PBS and re-suspended in a final volume of 400 μL of ice-cold PBS. ASC analysis was conducted with an LSR-II flow cytometer and the FACSDiva Software (BD Bioscience). Cells were gated to exclude cell clumps, contaminating polymorphonuclear cells, red blood cells, platelets, endothelial microparticles, and cell debris. Viable MNCs (> 1000,000 per sample) used to enumerate individual populations. For FACS on MNCs, fluorescein anisothiocyanate-conjugated CD31 antibody (clone WM59) and eFluor–conjugated CD45 antibody (clone 2D1) purchased from e-Bioscience, and phycoerythrin-conjugated CD34 antibody (clone 563), along with appropriate isotype control immunoglobulin G purchased from BD Bioscience, were used. Cells with the immunophenotype of CD34+/CD31−/CD45− were identified as circulating ASCs.
2.2.4. Additional blood work at 12-month assessment
To measure lipid profile (total cholesterol, triglycerides, LDL, HDL) and Hemoglobin A1C, a 3-mL lavender-top and a 3-mL gold top tube were drawn during the regular draw for biomarkers. These were labeled with participant Study ID and transported on ice to the First Outpatient Research Unit for processing by a commercial laboratory (LabCorp). Lab reports were provided to Rx staff within 24–48 h, and made available to participants via mail. A cover letter accompanied each result form; if abnormal values were found, participants were encouraged to seek advice from their physician.
2.3. Intervention
2.3.1. Dietary workshops
The purpose of the workshops was to increase knowledge of the relationship between diet and cancer and promote behavior change to achieve dietary changes to increase AI foods in the diet and thus reduce cancer risk. The basic AI diet involves regular use of multiple spices and herbs, increased marine fish intake, cruciferous and colorful vegetables and fruit, olive oil, and green and black tea (Table 2). Intervention participants were grouped into 5 Cohorts (10–20/group). Each cohort was asked to attend six monthly intervention workshops which commenced immediately following randomization. Each workshop consisted of lectures on an AI theme, interactive activities with participants, and culinary cooking demonstrations with a chef skilled in AI food preparation. During each workshop, emphasis was placed on consumption of specific AI foods and personal goal-setting. Participants were asked to sign in to track workshop attendance. Participants who missed a session were contacted by a patient navigator and provided with electronic copies of all workshop materials.
Table 2.
Anti-inflammatory foods.
| AI food type | Examples |
|---|---|
| Spices | Cinnamon, ginger, turmeric, black cumin |
| Herbs | Chives, garlic, onion, cloves, rosemary, black pepper, chilies, oregano, thyme, lemongrass |
| Marine fish | Salmon, cod, mackerel, sardines, tuna (fresh or canned) |
| Cooking oils | Olive, canola, rapeseed |
| Drinks | Green and black tea, red wine |
| Vegetablesa | Crucifers like cauliflower, broccoli, cabbage, kale, collards, Brussels sprouts, watercress |
| Fruit | Colorful fruit like cherries, grapes, melons, plums, blackberries, raspberries, pomegranate; citrus |
| Desserts | Dark chocolate |
| Supplements | Vitamin D |
May be eaten fresh or frozen.
2.4. Tailored newsletters and motivational interviewing (MI) telephone calls
Participants received a tailored newsletter following each workshop to reinforce workshop messages by providing a summary of progress toward individual goals, provide tips, and additional recipes of AI foods discussed at the workshop. During the maintenance phase of the study, participants received additional monthly newsletters focused on continued progress toward their goals. Newsletters were tailored according to Prochaska’s stages of change [21] indicated on questions answered by participants on their questionnaires at baseline and in subsequent MI phone calls.
MI calls were conducted by research staff trained in MI by a certified member of the Motivational Interviewing Network of Trainers [22]. Calls occurred within 4 weeks after each workshop for the first 6 months of the study, then approximately monthly thereafter. Staff discussed progress toward individual goals, barriers, and facilitators, as well as importance and confidence scales for each goal [23].
2.5. Statistical analysis
This study evaluated multiple dependent variables associated variously with dietary behavior, carcinogenesis and/or cancer growth. Using sample/power calculation software provided by Dartmouth University [24], we calculated separate sample size requirements to detect a significant group-by-time interaction for each variable at (α) p ≤ 0.05, power (β) 0.90. Results suggest that conservative estimates of group sizes of 67–77 subjects would provide the desired results. We chose 75 as the maximum number of final group sample size repeatedly measured at 2 time points to detect significant moderate differences in targeted outcomes. Repeated measures in addition to likely correlation of outcomes are expected to increase power further.
Descriptive statistics were performed on all variables (mean, standard deviation). For categorical variables, count and percentage were calculated. Two-sample t-test or Wilcoxon rank-sum test -depending on normality- for continuous variables, and Fisher’s exact test for categorical variables were used to compare groups at baseline. Percent ASCs were determined by multiplying fraction of CD34+ cells by fraction of CD31−/CD45− cells. Spearman’s rank correlation coefficients were conducted to explore relationships between biomarkers, and between biomarkers and anthropometrics and dietary habits, all at baseline. For baseline, 6- and 12-month comparisons, a linear mixed model that included the experimental condition and time as fixed factors, cohort effect (if necessary), and other significant covariates as well as interactions (as appropriate) as random effects, was used. Two series of analyses were conducted for each dependent variable. We compared models with time-dependent direct effects at 6-month follow-up controlling for baseline and at 12-month follow-up controlling for baseline and 6-month follow-up using multiple logistic regression and/or general estimating equations as appropriate, and then used blended, mixed-method Structural Equation Modeling (SEM) and Latent Variable Growth Curve Modeling (LGM) to construct and compare hypothesized indirect paths to models with indirect paths only. All analyses were performed using Stata 14 (StataCorp, 2015) [25] and R [26].
3. Results
All individuals in this study were overweight or obese women aged 18 or older, diagnosed with Stage 0-III breast cancer, and were at least 2 months post-systemic therapy at the time of enrollment. Their baseline characteristics are shown in Table 3. Mean age was 56.6 years. Approximately 40% of participants self-identified as Anglo or Latino. The majority had monthly incomes > $2000 and were privately insured. There were no significant differences between groups on demographic variables, ASCs or % body fat. However, significantly more CG participants (39%) had BMI > 35 kg/m2 compared to IG (18%). IG participants also had higher levels of pro-I cytokines and IL-10 at baseline compared to CG.
Table 3.
Baseline participant characteristics.
| Variables | Intervention (n = 76) | Control (n = 77) | P |
|---|---|---|---|
| Age (years), mean (SD) | 55.28 (9.85) | 57.86 (8.81) | 0.091 |
| Ethnicity | 0.40 | ||
| Black or African American | 1 (1%) | 2 (3%) | |
| US Latino | 29 (38%) | 34 (44%) | |
| Other Latino (Mexican, Cuban, etc) | 10 (13%) | 6 (8%) | |
| Anglo | 33 (43%) | 33 (43%) | |
| Native American | 2 (3%) | 0 (0%) | |
| Asian | 1 (1%) | 0 (0%) | |
| Other | 0 (0%) | 2 (3%) | |
| Education level | 0.25 | ||
| Have received no formal education, did not receive GED | 1 (1%) | 0 (0%) | |
| Did not complete high school and did not receive a GED | 1 (1%) | 0 (0%) | |
| Completed high school or received GED | 9 (12%) | 8 (10%) | |
| Technical/vocation school | 0 (0%) | 2 (3%) | |
| Some college level credits or 2 year college degree | 29 (38%) | 21 (27%) | |
| Bachelors degree | 22 (29%) | 28 (36%) | |
| Masters degree | 10 (13%) | 17 (22%) | |
| MD, PhD or other Doctoral degree | 3 (4%) | 1 (1%) | |
| Other | 1 (1%) | 0 (0%) | |
| Insurance type | 0.38 | ||
| Private Insurance | 64 (84%) | 59 (77%) | |
| Medicaid | 9 (12%) | 15 (19%) | |
| No insurance | 2 (3%) | 3 (4%) | |
| Missing | 1 (1%) | 0 (0%) | |
| Income | 0.95 | ||
| Under $250 | 1 (1%) | 0 (0%) | |
| $500–$749 | 1 (1%) | 1 (1%) | |
| $750–$999 | 1 (1%) | 2 (3%) | |
| $1000–$1500 | 2 (3%) | 3 (4%) | |
| $1500–$2000 | 9 (12%) | 12 (16%) | |
| Over $2000 | 60 (79%) | 56 (73%) | |
| Refused | 2 (3%) | 2 (3%) | |
| Missing | 0 (0%) | 1 (1%) | |
| BMI (kg/m2), median (IQR) | 31.90 (28.18, 33.97) | 31.64 (29.18, 36.50) | 0.21 |
| % Body fat, median (IQR) | 0.43 (0.39, 0.47) | 0.43 (0.39, 0.47) | 0.79 |
| Adipose Stromal Cells (%), median (IQR) | 0.50 (0.26, 1.06) | 0.48 (0.23, 1.05) | 0.99 |
| C-Reactive Protein μg/mL × 10−4), median (IQR) | 1.77 (0.95, 3.64) | 2.44 (1.18, 4.59) | 0.17 |
| Interleukin-3 (pg/mL), median (IQR) | 0.22 (0.19, 0.62) | 0.22 (0.19, 0.62) | 0.70 |
| Interleukin-6 (pg/mL), median (IQR) | 0.71 (0.47, 1.51) | 0.70 (0.41, 1.25) | 0.43 |
| Interleukin-8 (pg/mL), median (IQR) | 3.23 (2.10, 4.84) | 3.11 (1.64, 5.12) | 0.53 |
| Interleukin-10 (pg/mL), median (IQR) | 3.62 (1.58, 9.73) | 4.57 (1.40, 7.41) | 0.64 |
| Tumor Necrosis Factor-α (pg/mL), median (IQR) | 1.93 (1.01, 3.87) | 1.86 (1.13, 3.42) | 0.85 |
Table 4 shows correlations between selected variables at baseline. Moderate positive relationships (ρ ~ 0.4–0.7; p < 0.05) were seen between % body fat and BMI, BMI and serum CRP levels, and between the cytokines IL-6, IL-8 and TNF-α. Weaker relationships (ρ ≥ 0.2) were observed between % body fat and ASCs, IL-3, IL-10, IL-6 and CRP; ASCs and IL-3; IL-10, IL-8 and TNF-α; and IL-6 and CRP. Weak negative relationships were observed between age and IL-10 levels, and ASCs and IL-8. There were no correlations between dietary habits (based on responses to the Diet Assessment Tool, see Table 1) and any other variables at baseline.
Table 4.
Correlations between selected variables.
| Age | % body fat | BMI | % ASCs | IL-3 | IL-10 | IL-6 | IL-8 | TNFα | |
|---|---|---|---|---|---|---|---|---|---|
| % Body fat | 0.008 | ||||||||
| BMI | 0.002 | 0.545* | |||||||
| % ASCs | −0.018 | 0.203* | 0.040 | ||||||
| IL-3 | −0.120 | 0.204* | −0.048 | 0.198* | |||||
| IL-10 | −0.216* | 0.225* | 0.110 | −0.018 | 0.135 | ||||
| IL-6 | −0.041 | 0.184* | 0.108 | 0.000 | 0.102 | 0.465* | |||
| IL-8 | 0.032 | −0.062 | −0.024 | −0.193* | −0.037 | 0.334* | 0.660* | ||
| TNFα | 0.061 | 0.034 | 0.058 | −0.034 | −0.006 | 0.336* | 0.693* | 0.665* | |
| CRP | 0.027 | 0.292* | 0.427* | 0.106 | −0.008 | 0.122 | 0.234* | −0.037 | 0.107 |
P < 0.05.
4. Discussion
This paper presents the study design and baseline characteristics of an anti-inflammatory nutritional study of breast cancer survivors. Comparative analysis results at 6 and 12 months are published separately. Notable aspects of this study design include the tailored approach to the IG participants, including newsletters and telephone calls. The latter is innovative in the Motivational Interviewing (MI) field; MI interventions have historically been conducted face-to-face [27], although this is changing [28]. Analyses of the call logs will determine effectiveness of this method of using MI.
Study participant randomization resulted in equivalent distribution of characteristics, with the exception of BMI and baseline cytokine levels. Since ASCs and % body fat were equivalent between groups, the differences in BMI may be insignificant for analysis purposes.
As expected, positive relationships were seen between % body fat and BMI; BMI and a major pro-I cytokine (CRP); and between pro-I cytokines IL-6, IL-8 and TNF-α. Interestingly, age was negatively correlated with the anti-inflammatory cytokine IL-10, albeit weakly. This is consistent with the notion that aging results in an increased inflammatory state, and/or a decreased ability to combat inflammation [29,30]. Also of interest was the lack of correlation between dietary habits and any other variables prior to intervention. Pre-post intervention comparisons will be interesting to examine. Future analyses will focus on relationships between QOL variables, dietary responses and inflammatory markers, and changes that occur as a result of the intervention. Future studies will expand this intervention to other populations, including other cancer survivors, active duty military and the general population.
Acknowledgments
This research was supported by Susan G. Komen for the Cure (SAB08-0005); the National Cancer Institute (P20 CA165589 to UTHSCSA&P20 CA165583 to UTSA); and the Cancer Therapy and Research Center (CTRC) at The UT Health Science Center at San Antonio, through the NCI Cancer Center Support Grant (P30 CA054174).
References
- [1].American Cancer Society, New Report Tracks Growing Population of Cancer Survivors in the US, 2012. [cited 2015 December 22]; Available from: http://www.cancer.org/cancer/news/new-report-tracks-growing-population-of-cancer-survivors-in-the-us, (2012 June 14).
- [2].Texas Department of State Health Services, Expected New Cancer Cases and Deaths by Primary Site, Texas, Texas.xls, Editor Texas Cancer Registry, 2015. [Google Scholar]
- [3].Ashing-Giwa KT, et al. , Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors, Psycho-Oncology 13 (6) (2004) 408–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].American Cancer Society, Does body weight affect cancer risk? 2015. [cited 2015 December 22]; Available from: http://www.cancer.org/cancer/cancercauses/dietandphysicalactivity/bodyweightandcancerrisk/body-weight-and-cancer-risk-effects, (2015 April 24).
- [5].Ligibel JA, et al. , American Society of Clinical Oncology position statement on obesity and cancer, J. Clin. Oncol. 32 (31) (2014) 3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bellows CF, et al. , Circulation of progenitor cells in obese and lean colorectal cancer patients, Cancer Epidemiol. Biomark. Prev. 20 (11) (2011) 2461–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prantl L, et al. , Adipose tissue-derived stem cells promote prostate tumor growth, Prostate 70 (15) (2010) 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ghosh S, et al. , Association of obesity and circulating adipose stromal cells among breast cancer survivors, Mol. Biol. Rep. 41 (5) (2014) 2907–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anderson AS, et al. , European code against cancer 4th edition: obesity, body fatness and cancer, Cancer Epidemiol. 39 (Suppl. 1) (2015) S34–S45. [DOI] [PubMed] [Google Scholar]
- [10].Gunter MJ, et al. , Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk, J. Natl. Cancer Inst. 107 (9) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wirth MD, et al. , Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers, J. Occup. Environ. Med. 56 (9) (2014) 986–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pakiz B, et al. , Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors, Int. J. Behav. Med. 18 (4) (2011) 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Castello A, et al. , Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study, Br. J. Cancer 111 (7) (2014) 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Finocchiaro C, et al. , Effect of specific educational program on dietary change and weight loss in breast-cancer survivors, Clin. Nutr. 35 (4) (2016) 864–870. [DOI] [PubMed] [Google Scholar]
- [15].Hunter DJ, Willett WC, Diet, body size, and breast cancer, Epidemiol. Rev. 15 (1) (1993) 110–132. [DOI] [PubMed] [Google Scholar]
- [16].Hebert JR, A dietary inflammatory index to predict changes in inflammatory markers, in: Gussler J, Graham MA (Eds.), The 111th Abbott Nutrition Research Conference, Abbott Nutrition Health Institute, Columbus, Ohio, 2011. [Google Scholar]
- [17].Shivappa N, et al. , Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy, Cancer Causes Control 26 (10) (2015) 1439–1447. [DOI] [PubMed] [Google Scholar]
- [18].Shivappa N, et al. , Association between inflammatory potential of diet and mortality in the Iowa Women’s Health study, Eur. J. Nutr. 55 (4) (2016) 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harris PA, et al. , Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform. 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].American College of Sports Medicine, ACSM’s Guidelines for Exercise Testing and Prescription, Ninth ed, Lippincott Williams&Wilkins, Philadelphia. PA, 2013. [DOI] [PubMed] [Google Scholar]
- [21].Prochaska JO, DiClemente CC, Stages and processes of self-change of smoking: Toward an integrative model of change, J. Consult. Clin. Psychol. 51 (3) (1983) 390–395. [DOI] [PubMed] [Google Scholar]
- [22].Motivational Interviewing Network of Trainers, Motivational Interviewing Network of Trainers, 03/23/2016]; Available from: http://www.motivationalinterviewing.org/, (2016).
- [23].Rollnick S, Miller WR, Butler CC, Motivational Interviewing in Health Care: Helping Patients Change Behavior, first ed, Guilford Press, New York, NY, 2008. [Google Scholar]
- [24].Dartmouth-Hitchcock Norris Cotton Cancer Center, Power Calculations, 08/08/2013]; Available from: http://biostat.hitchcock.org, (2003).
- [25].StataCorp, Stata Statistical Software: Release 14, StataCorp LP, College Station, TX, 2015. [Google Scholar]
- [26].R Core Team R: A Language and Environment for Statistical Computing, R Foundation fo Statistical Computing, Vienna, Austria, 2014. [Google Scholar]
- [27].Tuccero D, et al. , Behavioral health in prevention and chronic illness management: motivational interviewing, Prim. Care 43 (2) (2016) 191–202. [DOI] [PubMed] [Google Scholar]
- [28].Spencer JC, Wheeler SB, A systematic review of motivational interviewing interventions in cancer patients and survivors, Patient Educ. Couns. 8 (16) (2016) 30064–30067. [DOI] [PubMed] [Google Scholar]
- [29].Zhang J, et al. , Ageing and the telomere connection: an intimate relationship with inflammation, Ageing Res. Rev. 25 (2016) 55–69. [DOI] [PubMed] [Google Scholar]
- [30].Goldberg EL, Dixit VD, Drivers of age-related inflammation and strategies for healthspan extension, Immunol. Rev. 265 (1) (2015) 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maly RC, et al. , Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons, J. Am. Geriatr. Soc. 46 (7) (1998) 889–894. [DOI] [PubMed] [Google Scholar]
- [32].Bonevski B, et al. , Evaluation of an instrument to assess the needs of patients with cancer, Cancer 88 (1) (2000) 217–225. [DOI] [PubMed] [Google Scholar]
- [33].Bohannon RW, Maljanian R, Goethe J, Screening for depression in clinical practice: reliability and validity of a five-item subset of the CES-depression, Percept. Mot. Skills 97 (3 Pt 1) (2003) 855–861. [DOI] [PubMed] [Google Scholar]
- [34].Park CL, et al. , Positive and negative health behavior changes in cancer survivors: a stress and coping perspective, J. Health Psychol. 13 (8) (2008) 1198–1206. [DOI] [PubMed] [Google Scholar]
- [35].Rosenberg M, Society and the Adolescent Self-image, Princeton University Press, Princeton, N.J., 1965. [Google Scholar]
- [36].United States Census Bureau, [cited 2015 July 15]; Available from: http://www. census.gov/, (2015).
- [37].Stein K, et al. , The American Cancer Society’s Studies of Cancer Survivors: the largest, most diverse investigation of long-term cancer survivors so far, Am. J. Nurs. 106 (3 Suppl) (2006) 83–85. [DOI] [PubMed] [Google Scholar]
- [38].Custers JA, et al. , The Cancer Worry Scale: detecting fear of recurrence in breast cancer survivors, Cancer Nurs. 37 (1) (2014) E44–E50. [DOI] [PubMed] [Google Scholar]
- [39].Cella DF, et al. , The Functional Assessment of Cancer Therapy scale: development and validation of the general measure, J. Clin. Oncol. 11 (3) (1993) 570–579. [DOI] [PubMed] [Google Scholar]
- [40].Stanford Patient Education Research Center, Chronic Disease Efficacy Scales, Chronic Disease Self-Efficacy Scales - Research Instruments Developed, Adapted or Used by the Stanford Patient Education Research Center - Patient Education - Department of Medicine - Stanford University School of Medicine, 2015[cited 2015 December 22]; Available from: http://patienteducation.stanford.edu/research/secd32.html. [Google Scholar]
- [41].Lorig K, et al. , Outcome Measures for Health Education and Other Health Care Interventions, SAGE Publications, 1996. [Google Scholar]
- [42].Craig CL, et al. , International physical activity questionnaire: 12-country reliability and validity, Med. Sci. Sports Exerc. 35 (8) (2003) 1381–1395. [DOI] [PubMed] [Google Scholar]
- [43].Cohen S, Kamarck T, Mermelstein R, A global measure of perceived stress, J. Health Soc. Behav. 24 (4) (1983) 385–396. [PubMed] [Google Scholar]
- [44].Foxcroft DR, Lowe G, Adolescent drinking, smoking and other substance use involvement - links with perceived family-life, J. Adolesc 18 (2) (1995) 159–177. [Google Scholar]
- [45].Apter M, Mallows R, Williams S, The development of the motivational style profile, Personal. Individ. Differ. 24 (1998) 7–18. [Google Scholar]
- [46].Bandura A, Social Foundations of Thought and Action: A Social Cognitive Theory, Prentice Hall: Englewood Cliffs, NJ, 1986. [Google Scholar]
- [47].Bandura A, Self-efficacy: The Exercise of Control, New York, W H Freeman, NY, 1997. [Google Scholar]
- [48].U.S. National Library of Medicine, Appendix 2 - RxNorm Dose Forms (TTY = DF) with Definitions, [cited 2015 July 15]; Available from: https://www.nlm.nih.gov/research/umls/rxnorm/docs/2012/appendix2.html, (2015).
- [49].Martinez-Gonzalez MA, et al. , Cohort profile: design and methods of the PREDIMED study, Int. J. Epidemiol. 41 (2) (2012) 377–385. [DOI] [PubMed] [Google Scholar]


