Abstract
Diet-induced obesity (DIO) is commonly associated with hyperleptinemia and leptin resistance. Leptin acts centrally to inhibit food intake and increase energy expenditure, thereby preventing body weight gain. Resistance to the biological effects of leptin represents a major obstacle in utilizing exogenously administered leptin as a treatment option for obesity. Of importance, recent studies demonstrate that naturally occurring compounds improve leptin sensitivity in DIO mice, as revealed by anorectic and body weight-lowering effects. To date, the role of sulforaphane (SFN, an isothiocyanate derived from cruciferous vegetables) on leptin responsiveness has not been examined, in spite of its known beneficial effects toward lowering body weight gain in DIO. In the present study, we determined the extent to which SFN regulates leptin responsiveness in high-fat high-sucrose (HFHS) diet-fed obese mice. SFN treatment (0.5 mg/kg/day, s.c.) for 23 days in HFHS-fed mice improved the responsiveness to intraperitoneally-injected leptin by promoting significant decreases in cumulative food intake and body weight gain. A single leptin injection (2 mg/kg; i.p.) resulted in significant decreases in food intake at 24 h and 38 h time points. In addition, a triple leptin injection (1 mg/kg/day, 3 days; i.p.) led to significant decreases in food intake at 14 h, 24 h, 38 h, 48 h, and 62 h time points. Furthermore, single and triple leptin injections prevented body weight gain at 38 h and 62 h time points, respectively. The present findings suggest that intervention with SFN, a naturally occurring isothiocyanate, has the potential to improve leptin responsiveness in DIO.
Keywords: Diet-induced obesity, Sulforaphane, Leptin responsiveness, Food intake, Weight gain
1. Introduction
Obesity in rodents and humans are associated with high circulating concentration of leptin and resistance to leptin action (Frederich et al., 1995; Considine et al., 1996). Leptin, an adipokine synthesized and released by the white adipose tissue, is a potent suppressant of appetite. It interacts with leptin-responsive neurons in the hypothalamus (e.g., arcuate nucleus) to decrease food intake Strategies to treat obesity using exogenously administered leptin have been hampered in a clinical setting due to resistance to leptin action.
Several lines of evidence suggest that naturally occurring compounds that improve leptin sensitivity may provide a realistic approach to target obesity. For instance, in a mouse model of diet-induced obesity (DIO), treatment with teasaponin (Yu et al., 2013), celastrol (Liu et al., 2015), or withaferin A (Lee et al., 2016) results in improved leptin responsiveness with anorectic and body weight-lowering effects. Sulforaphane (SFN) is an isothiocyanate derived from cruciferous vegetables such as broccoli and cabbage, and it plays a major role in energy metabolism (Vomhof-Dekrey & Picklo, 2012). To date, the role of SFN on leptin responsiveness has not been examined in DIO.
Previous studies, including our recent findings, demonstrate that in the mouse model of DIO, SFN or its precursor (glucoraphanin) treatment results in significant reduction in weight gain and adiposity (Choi et al., 2014; Shawky et al., 2016; Nagata et al., 2017). The protective effect of SFN against obesity is attributed in part to inhibition of adipogenesis through down-regulation of peroxisome proliferator activated receptor-γ (PPARγ) and suppression of lipogenesis through activation of AMP-activated protein kinase (AMPK) signaling (Choi et al., 2014). In addition, SFN has the potential to increase energy expenditure by enhancing uncoupling protein 1 (UCP1) expression in inguinal and epididymal adipose tissue depots (Nagata et al., 2017). Furthermore, SFN treatment diminishes leptin expression in adipose tissue and decreases serum/plasma leptin concentration (Choi et al., 2014; Shawky et al., 2016). Due to its beneficial effects toward reducing weight gain and lowering circulating leptin, it is critically important to determine whether SFN regulates leptin responsiveness in DIO.
In the present study, we used the high-fat high-sucrose diet (HFHS, western diet)-fed mice, an established mouse model of DIO that exhibits leptin resistance. To determine the effects of SFN treatment on leptin responsiveness, the mice were given leptin injections using two different protocols followed by measurements of changes in food intake and body weight.
2. Materials and methods
2.1. Materials
SFN (Cat# 574215) was purchased from EMD Millipore (Billerica, MA). It was supplied in the form of liquid and stored as a stock solution in dimethyl sulfoxide (DMSO) at a concentration of 4% and diluted in saline just before use to make the DMSO concentration in the injected solution equivalent to 0.12%. Recombinant mouse leptin (Cat# 498-OB) was purchased from R&D systems Inc. (Minneapolis, MN). It was supplied in the form of powder, which was reconstituted with HPLC grade water. DMSO was purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Animals
All animal experiments were performed in accordance with the Charlie Norwood Veterans Affairs Medical Center Institutional Animal Care and Use Committee guidelines and were approved by the committee. Male C57BL/6J mice (8 weeks, Jackson Laboratories, Bar Harbor, ME) were maintained in a room at a controlled temperature of 23 °C with a 12:12-hr dark-light cycle. Control diet (CON, D14022802) and HFHS diet (D12079B) were obtained from Research Diet (New Brunswick, NJ; see Table 1). In brief, CON diet consisted of ~10% fat-derived calories and ~72% carbohydrate-derived calories without sucrose. HFHS diet consisted of ~40% fat derived-calories (including 38.4% butter), ~42% carbohydrate-derived calories with sucrose (29.1% sucrose-derived calories), and cholesterol (0.15% w/w). The calories obtained by consumption of 1 g of CON or HFHS diets are indicated by the equations; 1 g CON diet = 3.91 Kcal and 1 g HFHS diet = 4.68 Kcal. The mice had free access to water and their assigned research diet.
Table 1.
Composition and energy content of control and high-fat high-sucrose diets.
| Control diet (CON) |
High-fat high-sucrose diet (HFHS) |
|||||
|---|---|---|---|---|---|---|
| Macronutrients | Calories (%) | Calories (%) | ||||
| Fat | 10 | 40 | ||||
| Carbohydrate | 72 | 42 | ||||
| Protein | 17 | 17 | ||||
| Ingredients | (g) | (kcal) | (kcal%) | (g) | (kcal) | (Kcal%) |
| Milk Fat (Butter) | 0 | 0 | 0 | 200 | 1800 | 38.4 |
| Corn Oil | 52.5 | 472.5 | 10.1 | 10 | 90 | 1.9 |
| Corn Starch | 745.4 | 2981.6 | 63.6 | 50 | 200 | 4.3 |
| Maltodextrin | 100 | 400 | 8.5 | 100 | 400 | 8.5 |
| Sucrose | 0 | 0 | 0 | 341 | 1364 | 29.1 |
| Casein | 195 | 780 | 16.6 | 195 | 780 | 16.6 |
| DL-Methionine | 3 | 12 | 0.3 | 3 | 12 | 0.3 |
| Cellulose | 50 | 0 | 0 | 50 | 0 | 0 |
| Mineral Mix | 35 | 0 | 0 | 35 | 0 | 0 |
| Calcium Carbonate | 4 | 0 | 0 | 4 | 0 | 0 |
| Vitamin Mix | 10 | 40 | 0.9 | 10 | 40 | 0.9 |
| Choline Bitartarate | 2 | 0 | 0 | 2 | 0 | 0 |
| Cholesterol | 0 | 0 | 0 | 1.5 | 0 | 0 |
2.3. HFHS diet feeding and SFN treatment
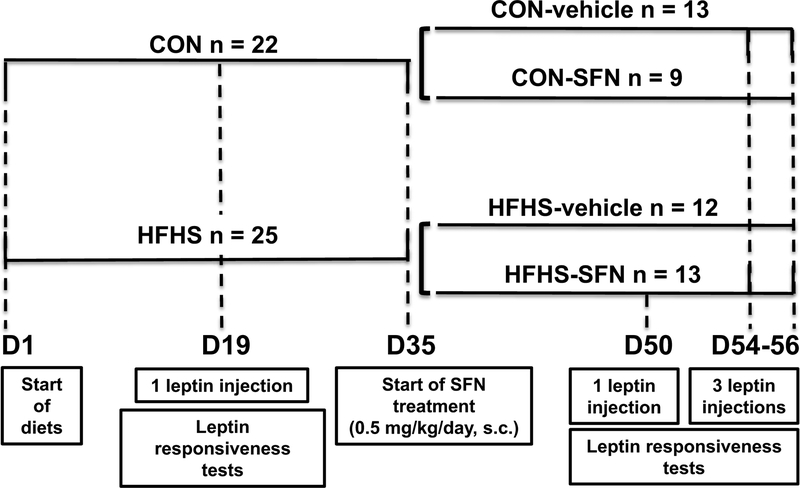
As shown in Fig. 1, mice were divided into 2 groups (22–25 mice per group). One group was fed a CON diet and the other group was fed a HFHS diet (western diet) for 35 days. Between day 35 and day 57, mice on CON diet were subdivided into: CON-vehicle (injected subcutaneously with 10 ml/kg/day of 0.12% DMSO) and CON-SFN (injected subcutaneously with 0.5 mg/kg/day SFN). Similarly, mice from the HFHS group were subdivided into: HFHS-vehicle and HFHS-SFN. The dosage and route of administration for SFN were based on our previous study with SFN (Shawky et al., 2016). It is noteworthy that in mice fed a high fat diet (with or without sucrose), there is a progressive increase in body weight for up to 50–60 days with significant increases in body weights being observed within 2 weeks (Van Heek et al., 1997; Shawky et al., 2016). This increase in body weight is accompanied by development of leptin resistance in 16 days with significant increases in leptin levels at 14–29 days (Roberts et al., 2002; Shawky et al., 2016). In the present study, we therefore chose days 19, 50, and 54 to perform leptin responsiveness tests.
Fig. 1.
Experimental protocol to determine leptin responsiveness to food intake and body weight in high-fat high-sucrose (HFHS) diet-fed obese mice before and after sulforaphane (SFN) treatment. Prior to SFN treatment, leptin responsiveness test was performed using a single-injection protocol (on day 19) in HFHS-fed mice versus control (CON) group. After the start of SFN treatment (on day 35), leptin responsiveness tests were carried out using a single-injection protocol (on day 50) and a triple-injection protocol (once daily for 3 consecutive days; on days 54–56).
2.4. Leptin responsiveness test
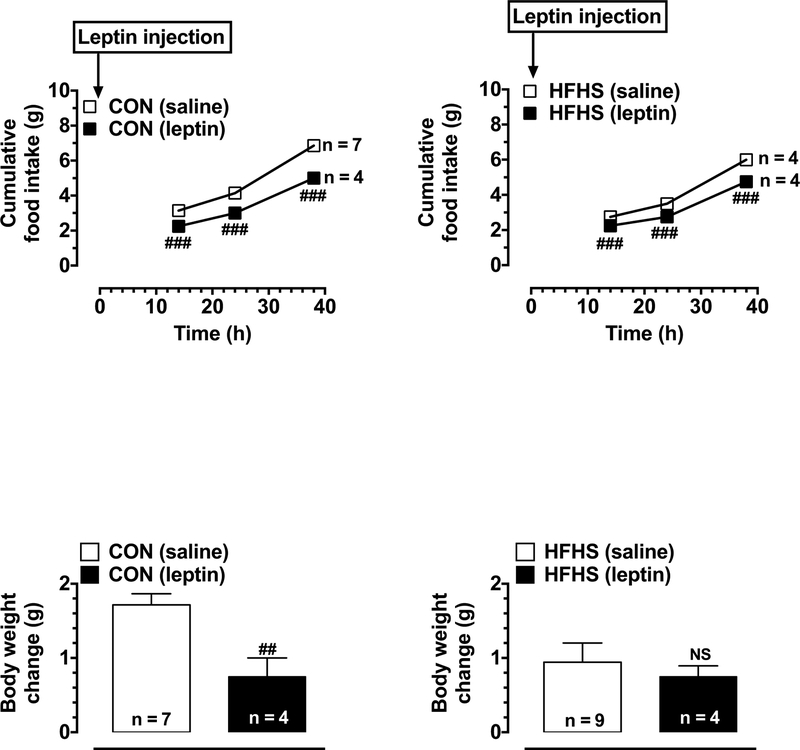
Previous studies have utilized different protocols to assess responsiveness to exogenously-administered leptin, including a single injection of leptin (Shapiro et al., 2008; Shapiro et al., 2011; Harris & Apolzan, 2012) and 3 injections of leptin separated by 24 h (Harris, 2010). To elucidate the effect of HFHS diet on leptin responsiveness to food intake and body weight, we initially performed leptin responsiveness test in mice (using a single injection protocol) on day 19 after a 9 h fast (Shapiro et al., 2008; Shapiro et al., 2011; Harris & Apolzan, 2012). In particular, food was removed from the cages at 8:00 am and mice were intraperitoneally (i.p.) injected with leptin [2 mg/kg] (Harris, 2010; Harris & Apolzan, 2012) or saline (10 ml/kg) at 5:00 pm. Food was returned to the cages immediately after injection. Cumulative food intake was recorded at 14, 24, and 38 h after injection (Shapiro et al., 2008). In addition, the changes in body weight were measured at the same time intervals. The measurements made at 38 h time point were shown in Fig. 2. The mice were deprived of food for 9 h prior to injection in order to optimize the conditions to examine inhibition of food intake by leptin (Harris, 2010).
Fig. 2.
Effects of HFHS diet on leptin responsiveness as assessed by a single leptin injection. After the start of respective diets, control group (CON) and HFHS diet-fed mice were fasted for 9 h during daytime (on day 19) followed by injection with leptin (2 mg/kg,i.p.). As additional controls, saline was injected (10 ml/kg,i.p.) in the respective treatment groups. Subsequently, cumulative food intake was determined at three different time points (14 h, 24 h, and 38 h; upper panels). In addition, body weight gain was determined at the 38 h time point (lower panels). The data shown are the means ± S.E.M (n values for different groups are indicated within the bar graphs and beside the linear graphs). Statistical significance between saline- and leptin-injected mice of the same group was determined using repeated measures two-way ANOVA followed by Bonferroni test (upper panel) or unpaired student t test (lower panel). # P < 0.05, ## P < 0.01, ### P < 0.001; and NS, non-significant compared with saline-injected mice from the same group.
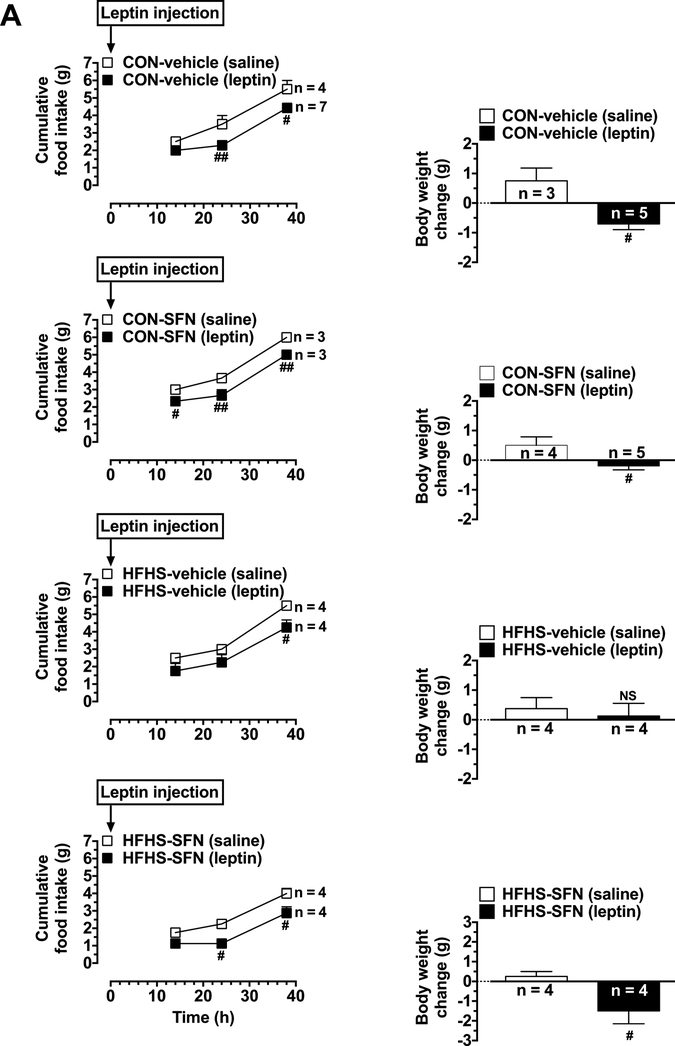
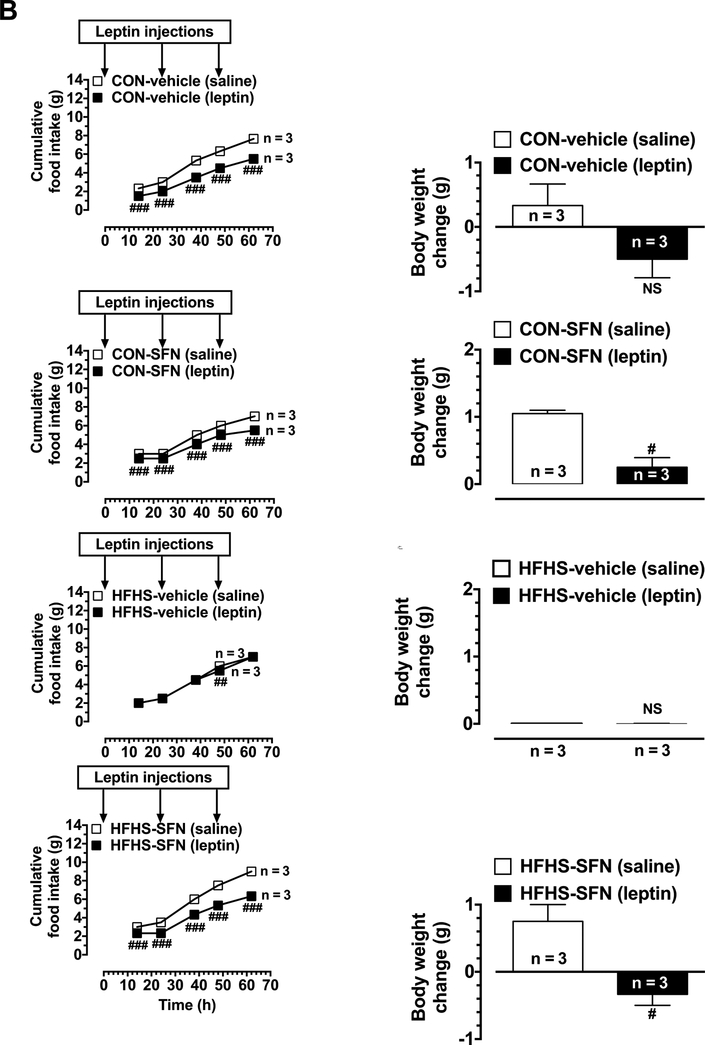
On day 50 (15 days after the start of SFN/vehicle treatment), leptin responsiveness test was carried out using a single injection protocol for the 4 groups of mice after a 9 h fast (as described for day 19). Furthermore, on day 54 (2 days after the completion of the single injection protocol), leptin responsiveness test was repeated using a slightly modified procedure where 3 leptin injections (1 mg/kg/day, i.p.) were given for 3 consecutive days (Harris, 2010). Mice were deprived of food during the day (at 8:00 am) and subjected to the first injection with leptin at 5:00 pm. This was followed by leptin injections for the second and third days. Cumulative food intake was measured as follows: i) in the morning after 14 h of each injection because mice are nocturnal feeders; and ii) at the time of 2nd and 3rd injections; 14 h, 24 h, 38 h, 48 h, and 62 h from the first leptin injection. The changes in body weight were recorded at the same time intervals. The measurements made at 62 h time point were shown in Fig. 3B.
Fig. 3.
Effects of HFHS diet on leptin responsiveness as assessed by single and triple leptin injection(s) under vehicle-treated or SFN-treated conditions. Mice were maintained on CON diet or HFHS diet without or with SFN treatment, as illustrated in Fig. 1. A) All four groups of mice were fasted for 9 h during daytime (on day 50) followed by a single injection with leptin (2 mg/kg,i.p.). As controls, saline was injected (10 ml/kg,i.p.) in the respective treatment groups. Subsequently, cumulative food intake was determined at three different time points (14 h, 24 h, and 38 h; left panels). Body weight gain was determined at the 38 h time point (right panels). B) On day 54, the test was repeated with triple leptin injections (1 mg/kg, i.p.; once a day for 3 days with a 24 h time interval between injections). Cumulative food intake was determined at five time points (14 h, 24 h, 38 h, 48 h, and 62 h from the first injection; left panels). Body weight gain was determined at the 62 h time point from the first injection (right panels). The data shown are the means ± S.E.M. (n values for the different groups are indicated at bases of bars and beside the linear graphs). Statistical significance between saline- and leptin-injected mice of the same group was determined using repeated measures two-way ANOVA followed by Bonferroni test (left panels) or unpaired student t test (right panels). # P < 0.05, ## P < 0.01, ### P < 0.001; and NS, non-significant compared with saline-injected mice from the same group.
2.5. Statistical analysis
Data are expressed as mean ± S.E.M. Statistical significance was determined between leptinand saline-injected mice of the same group for cumulative food intake (expressed in grams) in leptin responsiveness tests and weight gain at 38 h or 62 h after leptin/saline injection during leptin responsiveness test. Statistical analysis was carried out using repeated measures two-way ANOVA followed by Bonferroni multiple comparisons test for cumulative food intake. Weight gains were compared by unpaired student t-test. Statistical significance was also determined between the saline-injected mice of the different groups for total food intake (expressed in Kcal) at 38 h or 62 h following saline injection and initial body weights before saline injection; and between the 3 injections for the leptin-injected mice of different groups. Statistical analysis was carried out using unpaired student t-test (for total food intake and initial body weights of leptin responsiveness test performed on day 19) and regular two-way ANOVA followed by Bonferroni multiple comparisons test (for total food intake and initial body weights of leptin responsiveness test performed on days 50 and 54; and for comparing the response to each leptin injection in the different groups). Significance was calculated at P < 0.05. Statistical analyses were carried out using Graphpad Prism software (GraphPad Software Inc. V6.0f, San Diego, CA, USA).
3. Results
3.1. HFHS diet promotes resistance to leptin-mediated inhibition of weight gain in mice
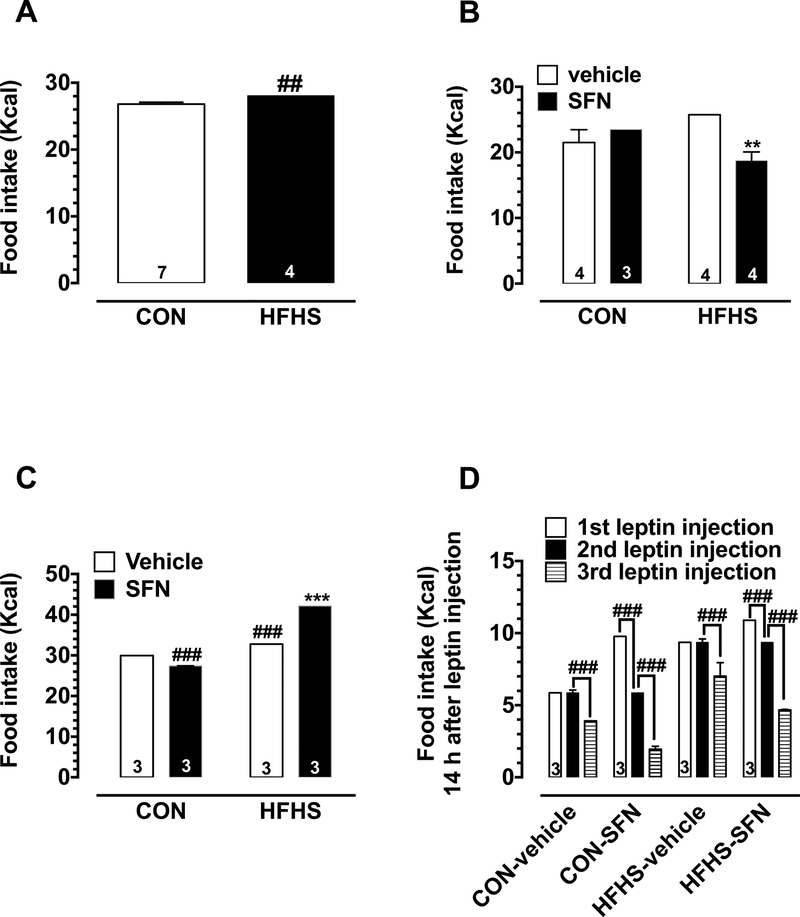
To determine whether the mice fed a HFHS diet for over 2 weeks exhibit changes in leptin response, we performed leptin responsiveness test on day 19 as described in the experimental protocol (Fig. 1). Following single leptin injection, both CON and HFHS diet-fed mice showed significant decreases in food intake at 14 h, 24 h, and 38 h time points (Fig. 2, upper panels). However, the decreases in food intake (28.4%, 27.6%, and 27.1%) in CON diet-fed mice were more pronounced, compared with the observed decreases in values (18.2%, 21.4%, and 20.8%)in HFHS diet-fed mice at the respective time points. For instance, upon comparing the food intake (expressed as Kcal) in saline-injected mice of CON and HFHS groups, it was noted that HFHS diet-fed mice consumed more calories (5% increase compared with CON group) at 38 h after saline injection (Fig. 4A). With regard to body weight, leptin injection in CON diet-fed mice led to a significant decrease in the weight gained by mice by 56.2% at the 38 h time point, compared with saline-injected mice of the same group (data not shown for the other time points). However, in HFHS diet-fed mice, leptin administration did not result in significant decreases in body weight gain at a similar time interval (Fig. 2, lower panels). Of note, HFHS mice showed a significantly higher initial absolute values of body weight before saline injection (17% increase) compared with CON group (Table 2).
Fig. 4.
Effects of HFHS diet on caloric intake during leptin responsiveness under vehicle-treated or SFN-treated conditions. Mice were maintained on CON diet or HFHS diet without or with SFN treatment, as illustrated in Fig. 1. A-B) All four groups of mice were fasted for 9 h during daytime (on days 19 and 50, respectively) followed by a single injection with saline (10 ml/kg, i.p.) in the four groups. Subsequently, total caloric intake after 38 h was determined using the following formulas: 1 g CON diet = 3.91 Kcal; and 1 g HFHS diet = 4.68 Kcal. C-D) On day 54, the test was repeated with triple saline or leptin injections injections (10 ml/kg and 1 mg/kg, respectively, i.p.; once a day for 3 days). Subsequently, total caloric intake after 62 h from the first injection for saline-injected mice of 4 groups (C) or caloric intake for the first 14 h after each injection for leptin-injected mice of 4 groups (D) were similarly calculated. The data shown are the means ± S.E.M. (n values for the different groups are indicated within the bar graphs). Statistical analysis was carried out using unpaired student t-test (for total caloric intake of leptin responsiveness test performed on day 19) and regular two-way ANOVA followed by Bonferroni multiple comparisons test (for total food intake of leptin responsiveness test performed on days 50 and 54). ## P < 0.01 and ### P < 0.001 compared with saline-injected mice from the CON or CON-vehicle groups (A and C). ** P < 0.01 compared with saline-injected mice from the HFHS-vehicle group (B). ### P < 0.001 compared with the food intake 14 h after the previous leptin injection (D).
Table 2.
Absolute values of body weight during leptin responsiveness test performed on day 19.
| CON | HFHS | |||
|---|---|---|---|---|
| Saline n = 7 |
Leptin n = 4 |
Saline n = 9 |
Leptin n = 4 |
|
| Initial body weight | 24.86 ± 0.4 | 23.75 ± 0.95 | 29.06 ± 0.72a | 26.75 ± 0.75 |
| Final body weight | 26.57 ±0.41 | 24.5 ± 0.87 | 30 ±0.65 | 27.5 ± 0.74 |
The data shown represent means ± S.E.M. Initial body weights represent the weights of mice before leptin/saline injection. Final body weights represent weights of mice 38 h after leptin/saline injection.
P < 0.001 compared with saline-injected mice of the CON group.
3.2. SFN improves leptin sensitivity in HFHS diet-fed mice
Previous studies have shown that in high fat diet- or HFHS diet-fed mice, SFN or SFN precursor (glucoraphanin) results in a marked decrease in body weight gain and plasma leptin concentration (Choi et al., 2014; Shawky et al., 2016; Nagata et al., 2017). To date, the likely regulatory effects of SFN on leptin regulation of food intake and body weight have not yet been examined in DIO. In the present study, we therefore treated CON and HFHS diet-fed mice with SFN to determine the extent to which SFN treatment impacts leptin responsiveness using two different leptin injection protocols (a single leptin injection and a triple injection protocol).
On day 50 (15 days after SFN treatment), leptin responsiveness test was performed using a single injection protocol in all four groups of mice. These four groups included CON-vehicle, CON-SFN, HFHS-vehicle, and HFHS-SFN. As shown in Fig. 3A (left panels), leptin injection in CON-vehicle group led to significant decreases in cumulative food intake by 34.7% and 19.5% at 24 h and 38 h time points, respectively, compared with saline-injected mice of the same group. Moreover, in CON-SFN group, leptin caused significant decreases in food intake by 22.2%, 27.3% and 16.7% at 14 h, 24 h and 38 h time points, respectively. On the other hand, leptin injection in HFHS-vehicle group showed a significant decrease in food intake by 22.7% only at 38 h time point. In HFHS-SFN group, leptin injection showed profound decreases in food intake by 50% and 28.1% at 24 h and 38 h time points, respectively. With regard to body weight (Fig. 3A, right panels), leptin injection in CON-vehicle and CON-SFN groups prevented the weight gain compared with respective saline-injected mice. However, in HFHS-vehicle group, leptin injection did not significantly affect weight gain compared with saline-injected group. Leptin injection in HFHS-SFN group prevented the weight gain seen in saline-injected mice.
On day 54 (19 days after starting SFN treatment), leptin responsiveness test was repeated using a triple injection protocol in all four groups of mice. As shown in Fig. 3B (left panels), leptin injection in CON-vehicle group resulted in significant decreases in cumulative food intake by 35.6%, 33.3%, 34.3%, 28.9%, and 28.2% at 14 h, 24 h, 38 h, 48 h, and 62 h time points, respectively, compared with saline injection. Moreover, in CON-SFN group, injection of leptin resulted in significant decreases in cumulative food intake at all measured time points (by 16.7%, 16.7%, 20%, 16.7% and 21.4% at 14 h, 24 h, 38 h, 48 h, and 62 h time points, respectively), compared with saline-injected mice of the same group. In HFHS-vehicle group, leptin injection did not show significant changes in food intake at the indicated time points, except for 8.3% decrease at the 48 h time point. In HFHS-SFN group, leptin injection resulted in marked decreases in food intake 22.3%, 33.4%, 27.8%, 28.9%, and 29.7% at 14 h, 24 h, 38 h, 48 h, and 62 h time points, respectively. With regard to body weight at the 62 h time point (Fig. 3B, right panels), leptin injection in CON-vehicle group promoted weight loss compared with saline-injected mice of the same group (data not shown for the other time points). In addition, in CONSFN group, leptin injection significantly attenuated body weight gain seen in saline-injected mice of the same group. In HFHS-vehicle group, no significant difference was noticed between leptin-injected and saline-injected mice; however, in HFHS-SFN group, leptin injection promoted weight loss thus showing significant weight change compared with saline-injected mice.
Upon comparing the food intake (expressed as Kcal) in saline-injected mice of different groups, HFHS diet-fed mice showed an increase in caloric intake (by 20% and 10% at 38 h and 62 h after saline injection on days 50 and 54, respectively) compared with CON-vehicle group. Moreover, in conformity with our previous study (Shawky et al., 2016), SFN did not show consistent changes in food intake compared with HFHS-vehicle group (Fig. 4B and C). Significantly higher initial body weights before saline injection in mice were noted in HFHS-vehicle group (34% and 21% increases on days 50 and 54, respectively) compared with CON-vehicle group. SFN treatment in HFHS group promoted a decrease in body weight, which is revealed by significantly lower initial body weight in saline-injected mice compared with saline-injected mice of HFHS-vehicle group (Tables 3 and 4).
Table 3.
Absolute values of body weight during leptin responsiveness test performed on day 50.
| CON-vehicle | CON-SFN | HFHS-vehicle | HFHS-SFN | |||||
|---|---|---|---|---|---|---|---|---|
| Saline n = 3 |
Leptin n = 5 |
Saline n = 4 |
Leptin n = 5 |
Saline n = 4 |
Leptin n = 4 |
Saline n = 4 |
Leptin n = 4 |
|
| Initial body weight | 26 ±0 | 27.6 ± 0.24 | 27.5 ± 0.87 | 25.8 ± 0.34 | 34.88 ± 0.52 a | 31.13 ± 0.52 | 30.5 ± 1.19bc | 29.75 ± 1.25 |
| Final body weight | 26.75 ± 0.43 | 26.9 ± 0.24 | 28 ± 0.58 | 25.6 ± 0.4 | 35.25 ± 0.75 | 31.25 ± 0.85 | 30.75 ± 1.03 | 28.25 ± 1.65 |
The data shown represent means ± S.E.M. Initial body weights represent the weights of mice before leptin/saline injection. Final body weights represent weights of mice 38 h after leptin/saline injection.
P < 0.05 and
P < 0.001 compared with saline-injected mice of the CON-vehicle.
P < 0.05 compared with saline-injected mice of the HFHS-vehicle group.
Table 4.
Absolute values of body weight during leptin responsiveness test performed on days 54–56.
| CON-vehicle | CON-SFN | HFHS-vehicle | HFHS-SFN | |||||
|---|---|---|---|---|---|---|---|---|
| Saline n = 3 |
Leptin n = 3 |
Saline n = 3 |
Leptin n = 3 |
Saline n = 3 |
Leptin n = 3 |
Saline n = 3 |
Leptin n = 3 |
|
| Initial body weight | 27 ± 1 | 25.17 + 0.17 | 24 ±0 | 24.42 ± 0.74 | 32.67 ± 0.33 a | 30.33 ± 1.2 | 27.25 ± 0.25 b | 29 ± 0.5 |
| Final body weight | 27.33 ± 1.33 | 24.67 ± 0.33 | 25.05 ± 0.05 | 24.67 ± 0.88 | 32.67 ±0.33 | 30.33 ± 1.2 | 28 ±0 | 28.67 ± 0.33 |
The data shown represent means ± S.E.M. Initial body weights represent the weights of mice before leptin/saline injection. Final body weights represent weights of mice 62 h after the first leptin/saline injection.
P < 0.01 compared with saline-injected mice of the CON-vehicle.
P < 0.01 compared with saline-injected mice of the HFHS-vehicle group.
Fig. 4D shows the changes in food intake during the first 14 h after each injection (1st, 2nd, and 3rd leptin injections) in four groups of mice on days 54–56. In vehicle-treated mice of both CON and HFHS groups, a significant decrease in food intake was observed only after 3rd leptin injection compared with 2nd leptin injection. However, in SFN-treated mice of both CON and HFHS groups, there were significant differences in food intake after the 2nd and 3rd leptin injections compared with 1st and 2nd leptin injections, respectively.
4. Discussion
Previous studies have shown that SFN, an Nrf2 activator derived from cruciferous vegetables, decreases body weight gain and food intake in mice fed a high fat diet (Choi et al., 2014) or HFHS diet (Shawky et al., 2016). This decrease in body weight in DIO is attributed in part to SFN-mediated diminutions in visceral adiposity and adipose tissue-derived leptin in the systemic circulation (Choi et al., 2014; Shawky et al., 2016). To date, the likely regulatory effects of SFN on leptin responsiveness to food intake and weight gain have not been reported. The present findings demonstrate that in HFHS diet-fed obese mice, SFN treatment (0.5 mg/kg/day, s.c.) for ~2–3 weeks improves the responsiveness to exogenously administered leptin as revealed by temporal decreases in food intake and weight gain.
Studies by several investigators, including our recent findings, demonstrate that rodents fed a HFHS diet for 2 to 22 weeks exhibit a marked increase in the circulating concentration of leptin (Harte et al., 1999; Roberts et al., 2002; Shapiro et al., 2011; Collino et al., 2014; Shawky et al., 2016). In addition, hyperleptinemia is associated with the induction of leptin resistance (Van Heek et al., 1997; Roberts et al., 2002; Shapiro et al., 2011). Of importance, mice fed a diet containing 45% fat for 16 days have been shown to develop resistance to peripherally-injected leptin (Van Heek et al., 1997). In mice fed a HFHS diet for 19–21 days (present study), a single leptin injection (2 mg/kg; i.p.) did not result in a significant reduction in body weight gain at 38 h time point, suggesting the development of leptin resistance. In parallel studies with control diet-fed mice, exogenously administered leptin led to ~56% decrease in weight gain, supporting the existence of leptin sensitivity under normal conditions. Furthermore, in mice fed a HFHS diet for 50–52 days or 54–56 days, the development of leptin resistance was further confirmed by the lack of significant inhibitory effects on food intake and body weight gain in response to single leptin injection (2 mg/kg; i.p.) or triple leptin injection (1 mg/kg/day for 3 days; i.p.).
Of importance, treatment of HFHS diet-fed mice with SFN (0.5 mg/kg/day, s.c.) for up to 22 days (days 35 to 56) resulted in improved sensitivity to exogenously administered leptin, as revealed by significant decreases in food intake and weight gain. First, a single leptin injection led to ~50% and ~28% decreases in food intake at 24 and 38 h time points, respectively. In addition, a triple leptin injection protocol showed significant decreases in food intake by ~22%, ~33%, ~28%, ~29%, and ~30% at 14 h, 24 h, 38 h, 48 h, and 62 h time points. Second, in HFHS diet-fed mice treated with SFN, exogenously administered leptin prevented body weight gain at 38 h and 62 h time points, as revealed by single leptin injection and triple leptin injection protocols, respectively. In conjunction with previously reported findings that show SFN-mediated decrease in circulating leptin concentration (Choi et al., 2014; Shawky et al., 2016), the present observations suggest the potential for SFN to improve leptin sensitivity in DIO.
Recent studies demonstrate leptin-sensitizing effects such as anorexia and a decrease in body weight upon treatment of DIO mice with naturally occurring compounds [e.g., teasaponin, celastrol, and withaferin A] (Yu et al., 2013; Liu et al., 2015; Lee et al., 2016). In addition, studies with metformin-treated obese mice demonstrate improved responsiveness to exogenously administered leptin, and this is revealed by a decrease in food intake, shedding of visceral fat, and lowering of body weight, compared with control group (Kim et al., 2006). From a mechanistic standpoint, naturally occurring compound (e.g., withaferin A) has been shown to reduce endoplasmic reticular (ER) stress in the hypothalamus, whereas metformin treatment results in enhanced hypothalamic phosphorylation/activation of signal transducer and activator of transcription-3 [STAT3] (Kim et al., 2006; Lee et al., 2016). Future studies should determine if SFN improves leptin sensitivity in DIO by targeting ER stress and/or STAT3 activity in the hypothalamus.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute/National Institutes of Health Grant (R01-HL-097090) and the University of Georgia Research Foundation Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, Lee MK, Lee YM, Hong JT, Yun YP & Yoo HS (2014) Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem, 25, 201–207. [DOI] [PubMed] [Google Scholar]
- Collino M, Benetti E, Rogazzo M, Chiazza F, Mastrocola R, Nigro D, Cutrin JC, Aragno M, Fantozzi R, Minetto MA & Thiemermann C (2014) A nonerythropoietic peptide derivative of erythropoietin decreases susceptibility to diet-induced insulin resistance in mice. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL & Caro JF (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med, 334, 292–295. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB & Flier JS (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature medicine, 1, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Friedman JM & Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature, 395, 763–770. [DOI] [PubMed] [Google Scholar]
- Harris RB (2010) Leptin responsiveness of mice deficient in corticotrophin-releasing hormone receptor type 2. Neuroendocrinology, 92, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB & Apolzan JW (2012) Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol, 302, R1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte RA, Kirk EA, Rosenfeld ME & LeBoeuf RC (1999) Initiation of hyperinsulinemia and hyperleptinemia is diet dependent in C57BL/6 mice. Horm Metab Res, 31, 570–575. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, Huh JY & Moon KH (2006) Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes, 55, 716–724. [DOI] [PubMed] [Google Scholar]
- Lee J, Liu J, Feng X, Salazar Hernandez MA, Mucka P, Ibi D, Choi JW & Ozcan U (2016) Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nature medicine, 22, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lee J, Salazar Hernandez MA, Mazitschek R & Ozcan U (2015) Treatment of obesity with celastrol. Cell, 161, 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y, Umeda R, Fuke N, Zhuge F, Ni Y, Nagashimada M, Takahashi C, Suganuma H, Kaneko S & Ota T (2017) Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes, 66, 1222–1236. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Berger JJ & Barnard RJ (2002) Long-term effects of diet on leptin, energy intake, and activity in a model of diet-induced obesity. Journal of applied physiology, 93, 887–893. [DOI] [PubMed] [Google Scholar]
- Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ & Scarpace PJ (2008) Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol, 295, R1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A, Tumer N, Gao Y, Cheng KY & Scarpace PJ (2011) Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr, 106, 390–397. [DOI] [PubMed] [Google Scholar]
- Shawky NM, Pichavaram P, Shehatou GS, Suddek GM, Gameil NM, Jun JY & Segar L (2016) Sulforaphane improves dysregulated metabolic profile and inhibits leptin-induced VSMC proliferation: Implications toward suppression of neointima formation after arterial injury in western diet-fed obese mice. J Nutr Biochem, 32, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM & Flier JS (1996) Adipogenesis and obesity: rounding out the big picture. Cell, 87, 377–389. [DOI] [PubMed] [Google Scholar]
- Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD & Davis HR Jr. (1997) Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest, 99, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomhof-Dekrey EE & Picklo MJ Sr. (2012) The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem, 23, 1201–1206. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wu Y, Szabo A, Wu Z, Wang H, Li D & Huang XF (2013) Teasaponin reduces inflammation and central leptin resistance in diet-induced obese male mice. Endocrinology, 154, 3130–3140. [DOI] [PubMed] [Google Scholar]