Abstract
Decontamination of Bacillus spores adhered to common drinking water infrastructure surfaces was evaluated using a variety of disinfectants. Corroded iron and cement-mortar lined iron represented the infrastructure surfaces, and were conditioned in a 23 m long, 15 cm diameter (75 ft long, 6 in diameter) pilot-scale drinking water distribution pipe system. Decontamination was evaluated using increased water velocity (flushing) alone at 0.5 m sec−1 (1.7 ft sec−1), as well as free chlorine (5 and 25 mg L−1), monochloramine (25 mg L−1), chlorine dioxide (5 and 25 mg L−1), ozone (2.0 mg L−1), peracetic acid 25 mg L−1) and acidified nitrite (0.1 mol L−1 at pH 2 and 3), all followed by flushing at 0.3 m sec−1 (1 ft sec−1). Flushing alone reduced the adhered spores by 0.5 and 2.0 log10 from iron and cement-mortar, respectively. Log10 reduction on corroded iron pipe wall coupons ranged from 1.0 to 2.9 at respective chlorine dioxide concentrations of 5 and 25 mg L−1, although spores were undetectable on the iron surface during disinfection at 25 mg L-1. Acidified nitrite (pH 2, 0.1 mol L−1) yielded no detectable spores on the iron surface during the flushing phase after disinfection. Chlorine dioxide was the best performing disinfectant with >3.0 log10 removal from cement-mortar at 5 and 25 mg L-1.The data show that free chlorine, monochloramine, ozone and chlorine dioxide followed by flushing can reduce adhered spores by >3.0 log10 on cement-mortar.
Keywords: Drinking water, decontamination, disinfection, Bacillus, spores
1.0. Introduction
Bacillus spores are resistant to disinfection and other inactivation methods relative to vegetative bacteria (Young and Setlow 2003; Rose et al. 2005; Raber and Burklund 2010). Due to their resistance to disinfection, inactivation of spores can be used as a benchmark for decontamination of biological agents from drinking water infrastructure following intentional or unintentional contamination. Bacillus spores are persistent on common drinking water infrastructure surfaces like corroded iron (Szabo et al. 2007; Hosni et al. 2011) and cement-mortar (USEPA 2008; Shane et al. 2011). Spore germination followed by chlorine disinfection has been studied as a decontamination method for Bacillus spores on home plumbing materials (PVC and copper) using L-alanine and inosine (Morrow et al. 2008) and in a pilot-scale system using nutrient media (Szabo et al. 2012). Though effective, germination may not be practical over a wide area of a distribution system due to the large amount of germinant that would need to be added. Therefore, data is needed on the decontamination of spores from drinking water infrastructure using common disinfectant solutions, or disinfectants that can be formulated using widely available equipment and reagents.
Free chlorine and monochloramine are common distribution system disinfectants that are ideal biological decontamination agents as they are well known and widely applied in the drinking water industry. Ozone and chlorine dioxide have some niche applications at water treatment plants focused on disinfection, oxidation and taste and odor control (AWWA 1999). They are strong oxidants, but their reactivity may limit their application in a distribution system to small areas or lengths of pipe. Peracetic acid (PAA) is commonly used in the food and beverage industry for cleaning processing equipment and pipes, and has found use in the medical device industry (McDonnell and Russell 1999; USEPA 2012). The acceptance of PAA in the food and medical industry may make it a more palatable decontaminating agent in drinking water. Acidified nitrite is not a widely used disinfectant, but it can be effective against spores in water, and it uses common reagents (Szabo et al. 2014). Flushing is a common practice in drinking water distribution systems and is easily implemented (although large volumes of contaminated water could be generated).
This study examines the effectiveness of decontaminating Bacillus globigii spores attached to corroded iron and cement-mortar coupons with free chlorine at two pH levels, monochloramine, chlorine dioxide, ozone, peracetic acid (PAA) and acidified nitrite, followed by flushing. Data on flushing alone is also presented to show the persistence of spores on water infrastructure without high levels of disinfectants present. The goal of this study is to present a summary of data on spore decontamination from water infrastructure, which comes from a pilot-scale experimental system that simulates real word drinking water infrastructure surfaces.
2.0. Materials and Methods
2.1. Bacterial Strains and Cultures
Bacillus atrophaeus subsp. globigii (B. globigii) has been used as a surrogate for pathogenic B. anthracis in disinfection studies since it is more resistant to free chlorine (Sivaganesan et al. 2006). B. globigii spore preparation is presented in detail elsewhere (Nicholson and Setlow 1990). Briefly, tryptic soy broth (TSB) was inoculated with B. globigii cells and incubated for 5 days at 35°C with gentle shaking in a rotary shaker. Purified B. globigii endospores were produced using gradient separation. The presence of spores was confirmed using phase-contrast microscopy (<0.1% vegetative cells). Purified spores were stored in 40% ethanol at 4°C. Before an experiment, purified spores were reintroduced into TSB and cultured in their vegetative form for 5 days at 35 ± 0.5º C and allowed to sporulate. The resulting spore suspension was used in experiments.
2.2. Pilot Scale Drinking Water Distribution System
The drinking water distribution system simulator (DSS) is shown in Figure 1 and has been described previously (USEPA 2008; Szabo et al. 2012). Briefly, the DSS consists of 23 m (75 ft) of 15 cm (6 inch) diameter PVC pipe connected in a rectangular shape to an in-line recirculation tank. Total DSS volume is 832 L (220 gal). A 3780 L (1000 gal) feed tank supplied Cincinnati tap water to the DSS. This setup allows tap water to enter and exit the DSS while still having flow circulating around the rectangular pipe to impose shear on the inner pipe surfaces. Cincinnati tap water flowing into and out of the DSS was set at 3.78 L min−1, which maintained stable water quality. Free chlorine and pH ranged from 0.9 to 1.1 mg L−1 and 8.4 to 8.6, respectively, pressure ranged from 68,950 to 82,740 Pa (10 to12 psi), and temperature fluctuated between 25°C and 30°C. These flow and water quality values were the baseline operating conditions unless otherwise noted. Flow circulating around the loop that affects shear forces on the coupons and pipe inner surface was set at 314 L min−1 (83 gal min−1), which resulted in a flow velocity of 0.3 m sec−1 (1 ft sec−1). When flushing was used as a decontamination method, flow was increased to achieve a flow velocity of 0.5 m sec−1 (1.7 ft sec −1).
Figure 1:
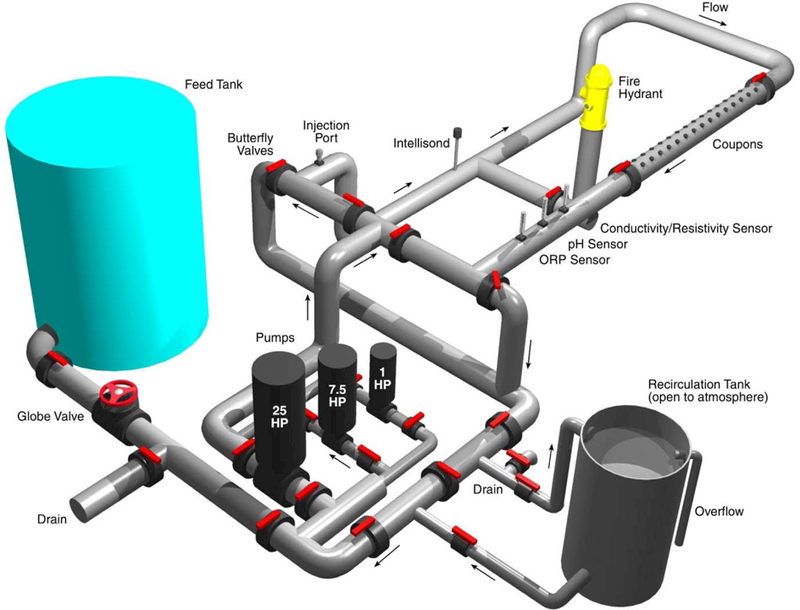
Schematic of the drinking water distribution system simulator used for decontamination studies
Thirty 6.5 cm2 coupons were inserted flush with the pipe inner surface. Half were unlined iron cut from a drinking water main and half were cement-mortar made using AWWA C104/A21.4–08 (AWWA 2008). Coupons were allowed to condition and form biofilm in the DSS for 1 month prior to contamination.
2.3. Experimental Procedure
Coupon surfaces were sampled before spores were injected to ensure that no detectable spores were present. Spore injection achieved 1×106 cfu/ml in the DSS bulk phase, which was allowed to circulate with flow present for two hours. Spores were flushed out of the DSS after the 2 hour contact period. Thereafter, a disinfectant was introduced into the DSS, mixed in the bulk phase and held for 22 hours (target concentrations of 5 and/or 25 mg L−1, or 0.1 M for acidified nitrite). During the decontamination period, no flow was present and the water was stagnant. The only exception to this protocol was ozone (2 mg/L), where decontamination occurred for 12 hours in the presence of flow at 314 L min−1 before flushing. After the decontamination period, any remaining disinfectant was flushed out of the DSS and water was subsequently recirculated around the DSS at a flow velocity of 0.3 m sec−1 (1 ft sec−1) for 22 hours to flush any remaining spores off of the coupon surfaces. Coupon and bulk water sampling times are listed in Tables 1 and 2.
Table 1:
For iron water infrastructure: Surface concentration of surviving Bacillus globigii spores in cfu cm−2 with decontaminant effectiveness (log reduction) in parenthesis for various decontamination and flushing durations (hr)
| Experimental Phase | Time | Disinfectant with its concentration (mg l−1) and water pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free Chlorine | Chlorine Dioxide | Monochlor-amine | Acidified Nitrite | Ozone | PAA | ||||||
| pH 8, 5 mg l−1 | pH 8, 25 mg l−1 | pH 7, 25 mg l−1 | 5 mg l−1 | 25 mg l−1 | 25 mg l−1 | pH 2, 0.1 mol l−1 | pH 3, 0.1 mol l−1 | 2 mg l−1 | 25 mg l−1 | ||
| Spore Injection | 0 min (initial) | 2.2×105 (0.0) | 2.5×105 (0.0) | 1.7×105 (0.0) | 2.2×105 (0.0) | 2.0×105 (0.0) | 1.9×105 (0.0) | 2.2×105 (0.0) | 7.1×105 (0.0) | 2.0×105 (0.0) | 1.5×105 (0.0) |
| Decontamin-ation with Disinfectant | 1 hr | 1.8×105 (0.1) | 4.2×104 (0.8) | 1.5×104 (1.1) | 6.3×104 (0.5) | 2.5×103 (1.9) | 2.0×105 (0.0) | 7.0×103 (1.5) | 9.7×104 (0.9) | 2.5×104 (0.9) | 8.6×104 (0.2) |
| 2 hr | 1.5×105 (0.2) | 3.8×104 (0.8) | 1.5×104 (1.1) | 5.6×104 (0.6) | 1.5×104 (1.1) | 2.0×105 (0.0) | 4.9×103 (1.6) | 1.5×105 (0.7) | 1.5×104 (1.1) | 7.0×104 (0.3) | |
| 3 hr | 1.3×105 (0.2) | 4.2×104 (0.8) | 1.7×104 (1.0) | 4.9×104 (0.7) | 2.8×103 (1.9) | 1.0×105 (0.3) | 4.0×103 (1.7) | 9.9×104 (0.9) | 2.0×104 (1.0) | 8.5×104 (0.2) | |
| 4 hr | 2.3×105 (0.0) | 4.1×104 (0.8) | 2.0×104 (0.9) | 3.5×104 (0.8) | 4.5×103 (1.6) | 1.1×105 (0.2) | 2.4×103 (2.0) | 1.8×105 (0.6) | 1.9×104 (1.0) | 4.7×104 (0.5) | |
| 6 hr | 1.4×105 (0.2) | 2.8×104 (1.0) | 1.7×104 (1.0) | 2.7×104 (0.9) | 0.0 (>3.6) | 9.8×104 (0.3) | 5.2×103 (1.6) | 1.1×105 (0.8) | 1.2×104 (1.2) | 4.5×104 (0.5) | |
| 18 hr | 1.6×105 (0.1) | 2.5×104 (1.0) | 1.8×104 (1.0) | 2.5×104 (0.9) | 0.0 (>3.6) | 2.5×104 (0.9) | 5.0×102 (2.6) | 2.7×104 (1.4) | 1.4×104 (1.2)* | 2.3×104 (0.8) | |
| 22 hr | 1.3×105 (0.2) | 1.6×104 (1.2) | 1.2×104 (1.2) | 2.7×104 (0.9) | 0.0 (>3.6) | 1.7×104 (1.0) | 1.0×103 (2.3) | 1.5×105 (0.7) | No Data | 1.9×104 (0.9) | |
| Flushing 0.3 m sec−1 (1 ft sec−1) | 24 hr | 9.8×104 (0.3) | 1.4×104 (1.3) | 9.8×103 (1.2) | 2.0×104 (1.0) | 2.5×102 (2.9) | 1.9×104 (1.0) | 0.0 (>3.6) | 1.6×104 (1.6) | 4.5×103 (1.6) | 1.6×104 (0.9) |
| 26 hr | 7.3×104 (0.5) | 1.2×104 (1.3) | 1.3×104 (1.1) | 1.6×104 (1.1) | 2.5×102 (2.9) | 1.7×104 (1.1) | 2.5×102 (2.9) | 1.9×104 (1.6) | 1.8×103 (2.1) | 2.1×104 (0.8) | |
| 44 hr | 8.1×104 (0.4) | 7.3×103 (1.5) | 7.3×103 (1.4) | 1.5×104 (1.2) | 5.0×102 (2.6) | 1.3×104 (1.2) | 0.0 (>3.6) | 6.6×103 (2.0) | 2.0×103 (2.0) | 1.6×104 (1.0) | |
Data is from 12 hours after the start of ozone treatment
Table 2:
For cement-mortar water infrastructure: Surface concentration of surviving Bacillus globigii spores in cfu cm−2 with decontaminant effectiveness (log reduction) in parenthesis for various decontamination and flushing durations (hr)
| Experimental Phase | Time | Disinfectant with its concentration (mg l−1) and water pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free Chlorine | Chlorine Dioxide | Monochlor-amine | Acidified Nitrite | Ozone | PAA | ||||||
| pH 8, 5 mg l−1 | pH 8, 25 mg l−1 | pH 7, 25 mg l−1 | 5 mg l−1 | 25 mg l−1 | 25 mg l−1 | pH 2, 0.1 mol l−1 | pH 3, 0.1 mol l−1 | 2 mg l−1 | 25 mg l−1 | ||
| Spore Injection | 0 min (initial) | 8.5×104 (0.0) | 4.1×104 (0.0) | 7.9×104 (0.0) | 5.5×104 (0.0) | 5.2×104 (0.0) | 4.4×104 (0.0) | 1.9×104 (0.0) | 1.2×105 (0.0) | 8.0×104 (0.0) | 2.6×104 (1.7) |
| Decontamin-ation with Disinfectant | 1 hr | 7.0×103 (1.1) | 4.6×103 (1.0) | 7.5×102 (2.0) | 5.0×102 (2.0) | 2.5×102 (2.3) | 3.5×104 (0.1) | 2.8×104 (0.0) | 1.1×105 (0.1) | 0.0 (>3.2) | 7.3×103 (0.6) |
| 2 hr | 1.3×104 (0.8) | 2.0×103 (1.3) | 1.5×103 (1.7) | 0.0 (>3.0) | 0.0 (>3.0) | 2.0×104 (0.3) | 2.5×104 (0.0) | 6.1×104 (0.3) | 0.0 (>3.2) | 4.8×103 (0.7) | |
| 3 hr | 7.8×103 (1.0) | 2.5×103 (1.2) | 2.5×102 (2.5) | 0.0 (>3.0) | 0.0 (>3.0) | 1.2×104 (0.6) | 1.8×104 (0.0) | 4.0×104 (0.5) | 0.0 (>3.2) | 3.3×103 (0.9) | |
| 4 hr | 7.0×103 (1.1) | 3.3×103 (1.1) | 1.5×103 (1.7) | 0.0 (>3.0) | 0.0 (>3.0) | 1.1×104 (0.6) | 1.2×104 (0.2) | 5.5×104 (0.3) | 0.0 (>3.2) | 3.3×103 (0.9) | |
| 6 hr | 6.8×103 (1.1) | 1.5×103 (1.4) | 3.3×103 (1.4) | 0.0 (>3.0) | 0.0 (>3.0) | 2.3×103 (1.3) | 1.5×104 (0.1) | 4.6×104 (0.4) | 0.0 (>3.2) | 1.5×103 (1.2) | |
| 18 hr | 2.0×103 (1.6) | 5.0×102 (1.9) | 0.0 (>3.2) | 0.0 (>3.0) | 0.0 (>3.0) | 0.0 (>2.9) | 2.0×103 (1.0) | 4.6×104 (0.4) | 0.0 (>3.2)* | 3.5×103 (0.9) | |
| 22 hr | 1.5×103 (1.8) | 2.5×102 (2.2) | 0.0 (>3.2) | 0.0 (>3.0) | 0.0 (>3.0) | 0.0 (>2.9) | 4.3×103 (0.6) | 7.9×104 (0.2) | No Data | 2.8×103 (1.0) | |
| Flushing 0.3 m sec−1 (1 ft sec−1) | 24 hr | 1.4×103 (1.8) | 5.0×102 (1.9) | 3.8×102 (2.3) | 0.0 (>3.0) | 0.0 (>3.0) | 5.0×101 (2.9) | 0.0 (>2.6) | 6.0×104 (0.3) | 0.0 (>3.2) | 1.9×103 (1.1) |
| 26 hr | 2.5×103 (1.5) | 0.0 (>2.9) | 0.0 (>3.2) | 0.0 (>3.0) | 0.0 (>3.0) | 0.0 (>2.9) | 5.0×102 (1.6) | 3.2×104 (0.6) | 0.0 (>3.2) | 5×102 (1.7) | |
| 44 hr | 5.0×102 (2.2) | 0.0 (>2.9) | 0.0 (>3.2) | 0.0 (>3.0) | 0.0 (>3.0) | 0.0 (>2.9) | 1.5×103 (1.1) | 5.4×103 (1.4) | 0.0 (>3.2) | 5×102 (1.7) | |
Data is from 12 hours after the start of ozone treatment
Infrastructure coupons were removed by unscrewing them from the pipe. Biofilm and corrosion particles were removed from the coupon using a sterile scalpel and suspended in 100 ml of 0.05 mol l−1 sterile KH2PO4 buffer (pH 7.2). The buffer was supplemented with 0.1 ml of sterile 10% (wt/vol) sodium thiosulfate when disinfectant was present. Suspended biofilm and corrosion particles were dispersed for 30 seconds using a tissue homogenizer. The homogenized suspension was vortexed and then serially diluted. B. globigii was enumerated by spread plating on tryptic soy agar (TSA) plates and incubating for 24 ± 2 hours at 35º C. All samples were plated in duplicate.
2.4. Decontaminating Agents
Free chlorine came from 10% Champion (Woodridge, IL) bleach/pool shock and was injected directly into the pipe system to achieve the desired free chlorine concentration. The pH was adjusted using concentrated (12.1 N) hydrochloric acid. Monochloramine was prepared by adding reagent grade ammonium hydroxide and free chlorine (10% bleach/pool shock) to the pipe system at a 5:1 Cl2:N ratio by weight to obtain a 25 mg L−1 monochloramine concentration in the loop water. Chlorine dioxide came from TwinOxide® (Best, Netherlands) pouches, the contents of which were diluted in the DSS to achieve the desired concentration. Ozone was made using a Clearwater Tech, Inc. (San Luis Obispo, CA) CD 2000 ozone generator with an air flow of 9 standard cubic feet per hour (SCFH) and vacuum of 5.4 inHg and injected into the DSS through the suction side of a pump. PAA was added directly to the pilot scale system using dilutions of Solvay Chemicals, LLC (Brussells, Belgium) Proxitane® WW-12 microbiocide (18.5% hydrogen peroxide, 12% peroxyacetic acid). Acidified nitrite was prepared by first injecting concentrated (12.1 N) hydrochloric acid into the pipe system and lowering the pH to 2 or 3. Reagent grade sodium nitrite dissolved in deionized water was then added to the pipe system and allowed to mix. If needed, disinfectants were diluted in deionized water before introduction into the pilot scale pipe system.
2.5. Measurement of Disinfectant Concentrations
Free chlorine and monochloramine residuals were measured using a Hach® (Loveland, CO) DR/2400 spectrophotometer using Hach methods 8021 (DPD) and 10171 (indophenol), respectively. AccuVac® Ampules containing N,N-Diethyl-p-phenylenediamine (DPD) were used for free chlorine analysis and powder pillows containing Monochlor F reagent were used for monochloramine. Chlorine dioxide was measured using Hach method 10126 (DPD) (equivalent to Standard Method 4500 ClO2 D) (Clesceri et al. 1999). Ozone was analyzed using Hach indigo method 8311 (High Range). PAA was measured using a Master’s Company (Wood Dale, IL) MP-9700 PAA colorimetric test kit (0–100 mg L−1 range). Nitrite was measured through ion chromatography using EPA Method 300.1. If necessary, samples were diluted in deionized water.
2.6. Data Treatment
Decontamination experiments were performed in duplicate. Data points in Figure 2 represent the average of two independent experiments and the bars represent the range between the two experiments. Spore concentration on coupons (CFU cm−2 ) and log10 data in Tables 1 and 2 are the mean of two independent experiments.
Figure 2:
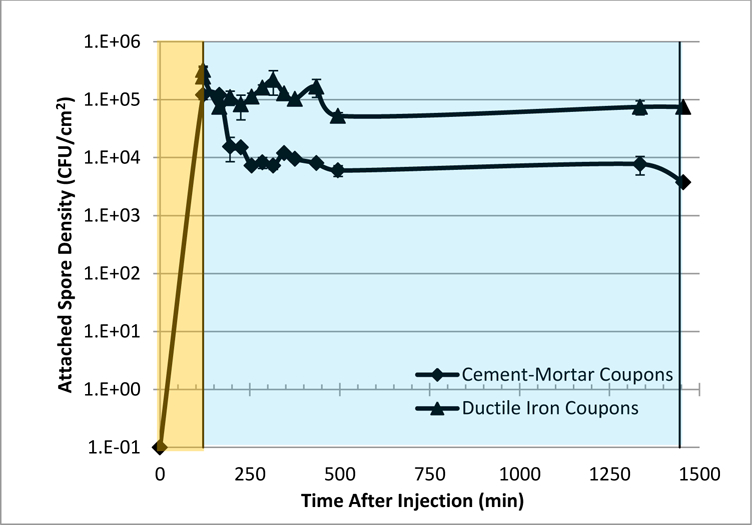
Spore density on iron and cement-mortar coupons after flushing at 0.5 m sec−1 (1.7 ft sec−1). The orange section represents the period when spores were injected. The blue section represents decontamination by flushing.
3.0. Results and Discussion
3.1. Flushing
Figure 2 shows the impact of flushing at 0.5 m sec−1 (1.7 ft sec−1) for one day on the number of adhered spores. Spores were more persistent on iron coupons compared to cement-mortar. The iron surface had a thick layer of corrosion, whereas the cement-mortar surface was comparatively smooth. The increased roughness of iron surface may have decreased the scouring effect of the flowing water on spores that were situated in a recessed area of the corrosion matrix. A decrease of 1.0 log10 from the cement mortar coupons occurred within two hours after flushing started. The change in the number of adhered spores after this point was minimal, which suggests that flushing at 0.5 m sec−1 (1.7 ft sec−1) for periods longer than two hours may not increase the effectiveness of flushing as a decontamination procedure.
Few studies have been conducted on the effect of flushing on the removal of spore forming bacteria from water infrastructure. One experiment examined spore persistence on corroded iron in biofilm annular reactors in the presence of flow at 0.3 m sec−1 (1 ft sec−1) and dechlorinated water. A 0.5 log10 decrease in adhered spores was observed over a period of 35 days (Szabo et al. 2007). A pilot scale study saw no spore removal from cement-mortar after flushing at 0.8 m sec−1 (2.5 ft sec−1) for two hours (USEPA 2008). This data suggests that spores are persistent on water infrastructure, and this suggestion is supported by the pilot scale data from this study. It is important to recognize that flushing is a viable mitigation tool following a drinking water contamination event. For instance, flushing water out of a fire hydrant can quickly remove large volumes of water and, with it, much of the contaminant. However, some of the contaminant adheres to the infrastructure surfaces. For removing residual spore forming bacteria from iron or cement-mortar water infrastructure, the data presented here suggest that flushing’s effectiveness is limited.
3.2. Free chlorine
Free chlorine is a common residual disinfectant in drinking water systems in the United States. Its widespread use and availability make it a candidate for decontamination of biological agents from drinking water infrastructure. At pH 8, 25 mg L−1 free chlorine was a more effective decontaminant than 5 mg L−1, but the difference between the two concentrations was more pronounced on spores adhered to iron compared to cement-mortar (Tables 1 and 2). A 1.0 log10 increase in spore inactivation on iron was seen at the higher free chlorine concentration after 18 to 22 hours of contact. On cement-mortar, the difference was 0.3 and 0.4 log10 at 18 and 22 hours of contact, respectively. The smoother and less reactive surface of cement-mortar (mostly calcium silicates from Portland cement and sand) relative to iron may have allowed free chlorine to penetrate the coupon surface at 5 mg L−1 to such a degree that increasing the concentration to 25 mg L−1 did not result in substantially increased inactivation. However, increased concentration may have resulted in more penetration of the free chlorine into the iron matrix, resulting in more inactivation.
One variable that was examined for its influence on decontamination efficacy was pH. With a pKa of 7.5, hypochlorous acid is more prevalent at pH 7, and hypochlorite ion is predominant at pH 8. Hypochlorous acid is a stronger disinfectant than the hypochlorite ion, so disinfection should be faster at pH 7. The data from this study show that at 18 and 22 hours of contact, 25 mg L−1 at pH 7 does appear to cause greater inactivation of spores adhered to cement-mortar compared to pH 8 (Tables 1 and 2). There was no obvious impact on inactivation of spores adhered to iron at pH 7 compared to pH 8. It could be that more hypochlorous acid at pH 7 reacted with the iron corrosion matrix before it could disinfect adhered spores, and decontamination efficacy was on par with less reactive hypochlorous acid at pH 8. The reactive iron species were not present on the cement-mortar surface, which gave the more predominant hypochlorous acid a better chance to disinfect at pH 7.
3.3. Monochloramine
At 25 mg L−1 monochloramine removed all detectable spores from cement-mortar after 18 hours of exposure (Table 2). Interestingly, the effectiveness of monochloramine on cement-mortar after 18 hours of exposure at 25 mg L−1 was similar to that of free chlorine at the same concentration and at pH 7 (Table 2). This similarity of decontamination effectiveness between monochloramine and free chlorine (pH 7, 25 mg L−1) at 18 hrs also holds for spores adhered to iron (Table 1). However, detectable spores remained on the iron coupons after decontamination with monochloramine and flushing.
Monochloramine was used in this study because it is a common residual drinking water disinfectant in the United States. It is an attractive residual disinfectant because it is not as reactive as free chlorine with organic matter, pipe material or biofilm (Chen and Stewart 1996). Because monochloramine is less reactive than free chlorine, the residual can remain detectable further out into the distribution system for longer periods of time. This same principle can make monochloramine an attractive infrastructure decontamination agent since it may penetrate into pipe material and/or biofilm deeper that free chlorine. Similar to another study on suppressing biofilm regrowth, the data in this study show that monochloramine was on par with free chlorine at 25 mg L−1 as a decontamination agent for spores (Zhang and DiGiano 2002). If monochloramine did penetrate deeper than free chlorine into the iron or cement-mortar matrix, this advantage was not enough to offset the superior disinfecting power of free chlorine.
3.4. Chlorine Dioxide
No spores were detected on cement-mortar at two hours after introduction of chlorine dioxide at 5 and 25 mg L−1 (Table 2). Spores were more persistent on iron coupons in the presence of chlorine dioxide (Table 1) compared to cement-mortar. Each experiment with iron coupons showed a similar decontamination trend characterized by a steep decrease within the first 60 minutes of chlorine dioxide introduction and slower disinfection or stable spore density thereafter. Spore density decreased by 0.5 and 1.9 log after 60 min of exposure to 5 and 25 mg L−1 chlorine dioxide, respectively. Spore inactivation increased to 0.9-log after 22 hours in the presence of 5 mg L−1 chlorine dioxide, and flushing removed little more. Continued exposure to chlorine dioxide at 25 mg L−1 decreased the number of spores to undetectable levels. During the flushing phase (after treatment at 25 mg L−1), spores reappeared on the coupons after chlorine dioxide had been flushed out of the loop. This may be due to spores becoming dislodged from a section of the DSS where disinfectant residual was low and spores persisted. No spores were detected in the bulk water phase during disinfection or flushing.
Spores adhered to cement-mortar were more susceptible to chlorine dioxide than those adhered to iron. This is likely due to cement mortar being smoother than corroded iron, which aids disinfectant transport to the surface. Cement-mortar can degrade commonly used disinfectants like free chlorine, which can lead to microbial persistence on cement-mortar as demonstrated in past similar studies using free chlorine as a decontaminating agent (Digiano and Zhang 2005; Al-Jasser 2007). Chlorine dioxide introduced at either concentration did not have these limitations, or the oxidative power of the disinfectant was enough to overcome them.
Chlorine dioxide may have been more effective than free chlorine since chlorine reacts through oxidation and electrophilic substitution, whereas chlorine dioxide acts through oxidation alone (AWWA 1999). Chlorine dioxide is a gas when dissolved in water, and is always present in its molecular form. In addition, estimated diffusivity coefficients of chlorine dioxide in a simulated dairy biofilm were found to be only slightly lower than in water (Jang et al. 2006). Chlorine’s action in biofilm is usually hindered by diffusion-reaction interactions (Chen and Stewart 1996). This is mainly due to its reaction with extracellular polymeric substances produced by the biofilms or reaction with corrosion matrices.
3.5. Ozone
No spores were detected on cement-mortar at 60 minutes after introduction of ozone (Table 2). Spores were persistent on iron coupons in the presence of ozone (Table 1). Adhered spores showed a 1.0 log10 drop 60 minutes after ozone was introduced, which increased to 1.2 log after 12 hours of exposure. Flushing increased log10 reduction to 2.0, but spores were still detected at the end of the experiment. Spore inactivation up to 5.0 log in water has been observed within minutes at ozone concentration ranging from 0.12 to 2.0 mg L−1 (Broadwater et al. 1973; Cho et al. 2003; Larson and Mariñas 2003; Young and Setlow 2004; Dow et al. 2006). In addition to the reactivity of the iron corrosion matrix, reduced ozone efficacy in this study may be due to the bubbling of ozone observed after its introduction into the DSS. If some ozone was trapped in bubbles, this would limit its ability to transport into the iron corrosion matrix. Increasing the ozone concentration exiting the generator may increase inactivation of adhered spores, but 2 mg L−1 was the highest concentration that could be sustained in these experiments.
3.6. Peracetic Acid
At 25 mg L−1, PAA was one of the least effective decontaminating agents used in this study (Tables 1 and 2). A 1.0 log10 removal from iron and 1.7 log10 removal from cement-mortar (only 0.9 log10 before flushing) is on par with monochloramine at 25 mg L−1 (for iron) and free chlorine at 5 mg L−1 for iron. The data in this study suggest that adding PAA to a water system to decontaminate spores would be no more effective than increasing the free chlorine or monochloramine levels.
Decontamination with PAA was attempted because it has been shown to be sporicidal in the food and medical industry, and can remain active in the presence of organics (McDonnell and Russell 1999). It has been reported that PAA is more potent when pH is maintained at 5 (Russell 1990). In this study, pH ranged between five and six during decontamination phase, which may have limited the effectiveness of PAA. It is possible that higher concentrations would be more effective, but adding large doses of disinfectant to a drinking water distribution system would be challenging. Thus, concentrations above 25 mg L−1 were not attempted. It should be noted that lowering pH to 5 could lead to increased corrosion of iron water pipes, and generation of discolored water. As PAA was no more effective than free chlorine or monochloramine, water utilities should consider whether applying PAA is worth the infrastructure damage that should occur from acidifying the water.
3.7. Acidified Nitrite
Little data on the use of acidified nitrite as a disinfectant in water systems was found beyond one study, but it was effective against spores in water given the right conditions (0.1 mol l−1, pH 2 or 3) (Szabo et al. 2014). Acidified nitrite’s disinfecting action is promising for water infrastructure decontamination since it relies on the production of nitrous acid, nitric oxide, peroxynitrite, dinitrogen trioxide, and nitrogen dioxide which can cause cell death through oxidation, formation of nitrosothiols and disruption of DNA replication (Nakaki et al. 1990; De Groote et al. 1995; Dykhuizen et al. 1998; Phillips et al. 2004). Furthermore, these compounds may not react with the iron corrosion or cement-mortar matrices present on pipe wall that can consume other oxidative disinfectants.
The decontamination data presented in Tables 1 and 2 show that acidified nitrite at either pH condition was less effective than any other disinfectant used in this study for cement-mortar. Interestingly, acidified nitrite was more effective against spores adhered to iron at pH 2 or 3 than it was against spores adhered to cement-mortar. Furthermore, at pH 2, it was second only to chlorine dioxide at 25 mg L−1 in its effectiveness as a decontaminating agent for spores. It is possible that the disinfecting compounds created by the acidified nitrite process were able to penetrate the iron corrosion matrix and cause disinfection to occur. However, it is also possible that the acidification of the water dissolved or degraded the iron corrosion matrix, which allowed disinfecting compounds to reach the spores or caused their removal through subsequent flushing. Acidification would not impact the cement-mortar matrix, which may explain why it was less effective against spores adhered to this surface. It should also be noted that past data on acidified nitrite disinfection in water shows that acidification at pH 2 or 3 does not cause a decrease in spore viability over the duration of the disinfection period used in this study (Szabo et al. 2014). However, like PAA, water utilities should consider whether the decontamination performance of acidified nitrite is worth the infrastructure damage that could occur from acidifying water in the distribution system to pH 2 or 3.
4.0. Conclusions
Flushing at 0.5 m sec−1 (1.7 ft sec−1) alone can be an effective means of removing contaminated water from a drinking water distribution system, which may contain most of the contaminant that was introduced. However, flushing at this rate leaves spores in place on iron and cement-mortar infrastructure surfaces, which could release into the water column in the future.
Chlorine dioxide (25 mg L−1) shows promise as a decontaminating agent for both surfaces used in this study. It was the only disinfectant studied that decreased adhered spores to undetectable levels on both iron and cement-mortar surfaces during the disinfection phase of decontamination.
Acidified nitrite (0.1 mol L−1) may have some application as a decontaminating agent for iron surfaces. Unlike the other disinfectants studied, it was more effective on iron surfaces compared to cement-mortar, which may be due to acidification dissolving or disrupting the iron surface enough that the disinfectants could reach the spores.
Ozone (2 mg L−1), free chlorine (25 mg L−1), and monochloramine (25 mg L−1) reduced the number of spores to undetectable levels on cement-mortar surfaces.
These data will help individuals such as incident commanders and drinking water utility personnel make informed decisions about how to decontaminate a drinking water distribution system after a biological contamination incident. Ultimately, decontamination performance along with the cost and effort of disseminating disinfectants over a sufficient area of the distribution system will dictate their use.
5.0. Acknowledgements
The authors thank Noreen Adcock (USEPA) for preparing the B. globigii spores. We also thank John Wright (US Army) for providing B. globigii spores.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The U.S. Environmental Protection Agency through its Office of Research and Development partially funded and collaborated in the research described here under contract EP-C-09–041 to CB&I (formerly Shaw Environmental). It has been subjected to the Agency’s review and has been approved for publication. Note that approval does not signify that the contents necessarily reflect the views of the Agency. Mention of trade names, products, or services does not convey official EPA approval, endorsement, or recommendation.
7.0 References
- Al-Jasser AO (2007) Chlorine decay in drinking-water transmission and distribution systems: Pipe service age effect. Water Res 41, 387–396. [DOI] [PubMed] [Google Scholar]
- AWWA (1999) Water Quality and Treatment: A Handbook of Community Water Supplies, 5th edition Denver: American Water Works Association. [Google Scholar]
- AWWA (2008) Standard for Cement–Mortar Lining for Ductile-Iron Pipe and Fittings In AWWA C104/A214–08. Denver: American Water Works Association. [Google Scholar]
- Broadwater WT, Hoehn RC and King PH (1973) Sensitivity of Three Selected Bacterial Species to Ozone. Applied Microbiology 26, 391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X and Stewart PS (1996) Chlorine penetration into artificial biofilm is limited by a reaction-diffusion interaction. Environ Sci Technol 30, 2078–2083. [Google Scholar]
- Cho M, Chung H and Yoon J (2003) Quantitative Evaluation of the Synergistic Sequential Inactivation of Bacillus subtilis Spores with Ozone Followed by Chlorine. Environ Sci Technol 37, 2134–2138. [DOI] [PubMed] [Google Scholar]
- Clesceri LS, Greenberg AE and Eaton AD eds. (1999) Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association, American Water Works Association, Water Environment Federation. [Google Scholar]
- De Groote MA, Granger D, Xu Y, Campbell G, Prince R and Fang FC (1995) Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. PNAS 92, 6399–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digiano FA and Zhang W (2005) Pipe section reactor to evaluate chlorine-wall reaction. J Am Water Works Assoc 97, 74–85. [Google Scholar]
- Dow SM, Barbeau B, von Gunten U, Chandrakanth M, Amy G and Hernandez M (2006) The impact of selected water quality parameters on the inactivation of Bacillus subtilis spores by monochloramine and ozone. Water Res 40, 373–382. [DOI] [PubMed] [Google Scholar]
- Dykhuizen RS, Fraser A, McKenzie H, Golden M, Leifert C and Benjamin N (1998) Helicobacter pylori is killed by nitrite under acidic conditions. Gut 42, 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosni AA, Szabo JG and Bishop PL (2011) The efficacy of chlorine dioxide as a disinfectant for Bacillus spores in drinking water biofilms. J Environ Eng 137, 569–574. [Google Scholar]
- Jang A, Szabo JG, Hosni AA, Coughlin M and Bishop PL (2006) Measurement of chlorine dioxide penetration in dairy process pipe biofilms during disinfection. Appl Microbiol Biotechnol 72, 368–376. [DOI] [PubMed] [Google Scholar]
- Larson MA and Mariñas BJ (2003) Inactivation of Bacillus subtilis spores with ozone and monochloramine. Water Res 37, 833–844. [DOI] [PubMed] [Google Scholar]
- McDonnell G and Russell AD (1999) Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin Microbiol Rev 12, 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JB, Almeida JL, Fitzgerald LA and Cole KD (2008) Association and decontamination of Bacillus spores in a simulated drinking water system. Water Res 42, 5011–5021. [DOI] [PubMed] [Google Scholar]
- Nakaki T, Nakayama M and Kato R (1990) Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol Mol Pharmacol 189, 347–353. [DOI] [PubMed] [Google Scholar]
- Nicholson WL and Setlow P (1990) Sporulation, germination and outgrowth In Molecular Biological Methods for Bacillus, eds. Harwood CR and Cutting SM pp.391–429. Chichester: John Wiley. [Google Scholar]
- Phillips R, Kuijper S, Benjamin N, Wansbrough-Jones M, Wilks M and Kolk AHJ (2004) In vitro killing of Mycobacterium ulcerans by acidified nitrite. Antimicrob Agents Chemother 48, 3130–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber E and Burklund A (2010) Decontamination options for Bacillus anthracis contaminated drinking water determined from spore surrogate studies. Appl Environ Microbiol 76, 6631–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose LJ, Rice EW, Jensen B, Murga R, Peterson A, Donlan RM and Arduino MJ (2005) Chlorine inactivation of bacterial bioterrorism agents. Appl Environ Microbiol 71, 566–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AD (1990) Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev 3, 99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane WT, Szabo JG and Bishop PL (2011) Persistence of non-native spore forming bacteria in drinking water biofilm and evaluation of decontamination methods. Environ Technol 32, 847–855. [DOI] [PubMed] [Google Scholar]
- Sivaganesan M, Adcock NJ and Rice EW (2006) Inactivation of Bacillus globigii by chlorination: a hierarchical Bayesian model. J Water Supply Res Technol AQUA 55, 33–43. [Google Scholar]
- Szabo JG, Adcock NJ and Rice EW (2014) Disinfection of Bacillus spores with acidified nitrite. Chemosphere 113, 171–174. [DOI] [PubMed] [Google Scholar]
- Szabo JG, Muhammad N, Heckman L, Rice EW and Hall JS (2012) Germinant enhanced decontamination of Bacillus spores adhered to iron and cement-mortar drinking water infrastructure. Appl Environ Microbiol 78, 2449–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo JG, Rice EW and Bishop PL (2007) Persistence and decontamination of Bacillus atrophaeus subsp. globigii spores on corroded iron in a model drinking water system. Appl Environ Microbiol 73, 2451–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (2008) Pilot-scale tests and systems evaluation for the containment, treatment, and decontamination of selected materials from T&E building pipe loop equipment (EPA/600/R08/016). Cincinnati, OH: US EPA. [Google Scholar]
- USEPA (2012) Anthrax spore decontamination using hydrogen peroxide and peroxyacetic acid. (Accessed May 7, 2015) http://www.epa.gov/pesticides/factsheets/chemicals/hydrogenperoxide_peroxyaceticacid_factsheet.htm
- Young SB and Setlow P (2003) Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J Appl Microbiol 95, 54–67. [DOI] [PubMed] [Google Scholar]
- Young SB and Setlow P (2004) Mechanisms of Bacillus subtilis spore resistance to and killing by aqueous ozone. J Appl Microbiol 96, 1133–1142. [DOI] [PubMed] [Google Scholar]
- Zhang W and DiGiano FA (2002) Comparison of bacterial regrowth in distribution systems using free chlorine and chloramine: a statistical study of causative factors. Water Res 36, 1469–1482. [DOI] [PubMed] [Google Scholar]


