Abstract
Background
In the months after acute MI, risk for acute atherothrombotic events in non-culprit arteries increases several fold.
Objectives
We hypothesized that sustained pro-inflammatory and pro-thrombotic endothelial alterations occur in remote vessels after MI.
Methods
We studied wild type mice, atherosclerotic mice with double knockout (DKO) of the LDL receptor and Apobec-1, and DKO mice treated with the Nox-inhibitor apocynin were studied at baseline and 3 and 21 days after closed-chest MI. Ultrasound molecular imaging of P-selectin, VCAM-1, von Willebrand factor (VWF) A1-domain, and platelet GPIbα was performed. Intravital microscopy was used to characterize post-MI leukocyte and platelet recruitment in the remote microcirculation after MI.
Results
Aortic molecular imaging for P-selectin, VCAM-1, VWF-A1, and platelets was increased several fold (p<0.01) three days post-MI for both wild-type and DKO mice. At 21 days, these changes resolved in wild-type mice but persisted in DKO mice. Signal for platelet adhesion was abolished 1 hour after administration of ADAMTS13 which regulates VWF multimerization. In DKO and wild-type mice, apocynin significantly attenuated the post-MI increase for molecular targets, and platelet depletion significantly reduced P-selectin and VCAM-1 signal. On intravital microscopy, MI resulted in remote vessel leukocyte adhesion and platelet string or net complexes. On histology, high-risk inflammatory features in aortic plaque increased in DKO mice 21 days post-MI which were completely prevented by apocynin.
Conclusions
Acute MI stimulates a spectrum of changes in remote vessels including upregulation of endothelial inflammatory adhesion molecules, and platelet-endothelial adhesion from endothelial-associated VWF multimers. These remote arterial alterations persist longer in the presence of hyperlipidemia, are associated with accelerated plaque growth and inflammation, and are attenuated by Nox inhibition.
Keywords: Adhesion molecules, Myocardial infarction, Platelets, Von Willebrand Factor
Major acute atherothrombotic events such as myocardial infarction (MI), stroke, or limb ischemia lead to a markedly increased risk for recurrent events in separate vascular territories. After MI, the risk for stroke or recurrent MI from non-culprit lesions is increased by several-fold over the ensuing six to twelve months (1–3). These findings indicate that a focal ischemic event can lead to systemic adverse vascular responses. Murine studies have demonstrated that acute MI triggers the splenic production and mobilization of inflammatory Ly-6Chigh monocytes, and the accelerated entry of these cells and other CD11b+ myeloid cells into remote arterial plaque for weeks after the initial event (4). With regards to remote vascular endothelial responses, MI in mice leads to upregulation of mRNA for endothelial cell adhesion molecules within the non-infarct myocardial microcirculation (5). However, little is known about endothelial-specific alterations in remote arterial atherosclerotic lesions, including in non-coronary locations.
In this study, in vivo imaging methods unique in their ability to investigate events at the endothelial-blood pool interface were used to study post-MI endothelial alterations in remote arteries that can predispose to accelerated plaque growth and atherothrombotic events. Contrast-enhanced ultrasound (CEU) molecular imaging and direct microvascular observation with intravital microscopy were used to characterize the endothelial responses that occur in remote arteries after MI. CEU molecular imaging with targeted microbubble contrast agents that are confined to the vascular compartment was selected based on extensive experience in murine and non-human primate models to detect arterial events that occur at the endothelial-blood pool interface (6–8). Specifically, we hypothesized that acute MI leads to (i) remote arterial upregulation of endothelial cell adhesion molecules on the plaque surface, and (ii) platelet adhesion to the intact endothelial surface which contributes to pro-inflammatory activation and can occur, in part, from abnormal endothelial-associated ultra-large self-associated multimers of von Willbrand factor (VWF) which occur secondary to dysregulation of normal proteolytic regulation by ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats-13) (9–11).
Methods
Animal Model
The study was approved by the Animal Care and Use Committee of the Oregon Health & Science University. We studied wild-type C57Bl/6 mice and mice with susceptibility to age-related atherosclerosis through dual gene-targeted deletion or “double knockout” (DKO) of the LDL-receptor and apolipoprotein-B mRNA editing enzyme catalytic polypeptide 1 (Apobec-1) on a C57Bl/6 background. DKO mice develop reproducible, age-dependent development of atherosclerosis on a chow diet (6,12), and were studied at 20–25 weeks of age when plaque size is modest with early intraluminal encroachment (6). For all studies, mice were anesthetized with 1.0–2.0% inhaled isoflurane and a jugular cannula was placed for intravenous injection of contrast agents or drugs.
Imaging Study Design
The proximal thoracic aorta was selected as a remote arterial site to study endothelial activation after MI. CEU molecular imaging of the aorta was performed using microbubble contrast agents targeted to P-selectin (MBP), vascular cell adhesion molecule-1 (VCAM-1) (MBV), GPIbα as an indicator of platelet adhesion (MBPlt), and endothelial VWF (MBVWF). Studies were also performed with control non-targeted microbubbles (MB). Animals were studied in the following experimental conditions:
Molecular imaging performed at baseline and at either 3 or 21 days after MI in wild-type and DKO mice. Mice were also studied 3 days after sham procedure.
Molecular imaging was performed 3 days after MI in DKO mice treated 1 hour prior to imaging with recombinant human ADAMTS13 (5 μg I.V.), the key regulatory protease which reduces VWF multimer size through cleavage at the VWF A2 domain (10,11).
Molecular imaging performed 3 and 21 days after MI in DKO mice treated with apocynin (50 mg/kg/d) starting on the day of MI. Apocynin (acetovanillone) is a potent inhibitor of NADPH-oxidase (Nox) which has been implicated in transcriptional upregulation of adhesion molecule expression, and has been demonstrated to reduce platelet-endothelial adhesion in early and late atherosclerosis (13,14).
Molecular imaging was performed 21 days after MI in DKO mice undergoing platelet depletion by I.V. injection of 2 μg/g rat anti-mouse GPIbα mAb (7) at days 1, 4, 9 and 15 post-MI to determine the contribution of platelets to sustained endothelial adhesion molecule expression.
Myocardial Infarction
A closed-chest model of MI was used to allow for the resolution of the acute inflammatory responses after thoracotomy and cardiac exposure. At least five days prior to scheduled MI, mice were anesthetized, intubated, and placed on positive pressure mechanical ventilation with weight-adjusted tidal volumes and respiratory rates. A limited left lateral thoracotomy was performed to expose only the basal anterior wall. A 6-0 prolene suture was placed under the LAD but left unsecured. The free ends of the suture were exteriorized through the chest wall and left in a subcutaneous location after closure. After 5–7 days, mice were anesthetized, the suture was exteriorized through a limited skin incision and the tension was placed on the suture for 40 min to produce ST-elevation on ECG monitoring and wall motion abnormalities on high-frequency transthoracic 2-D echocardiography (Vevo 2100, Visualsonics Inc., Toronto, Canada). Sham-treated animals received a suture without tightening.
Targeted Microbubble Preparation
Biotinylated lipid-shelled decafluorobutane microbubbles were prepared by sonication of a gas-saturated aqueous suspension of distearoylphosphatidylcholine (2 mg/mL), polyoxyethylene-40-stearate (1 mg/mL), and distearoylphosphatidylethanolamine-PEG(2000)biotin (0.4 mg/mL). Conjugation of biotinylated ligand to the microbubble surface was performed with biotin-streptavidin bridging as previously described.(15) Ligands used for targeting were: dimeric murine recombinant A1 domain of VWF A1 (mature VWF amino acids 445 to 716) for targeting platelet GPIbα for MBPlt, a cell-derived peptide representing the N-terminal 300 amino acids of GPIbα for MBVWF,(7) and monoclonal antibodies against the extracellular domain of either P-selectin (RB40.34, BD Biosciences), or VCAM-1 (clone 429, BD Biosciences) for MBP and MBV, respectively. Control MB were prepared with isotype control antibody (R3-34, BD Biosciences). Microbubble concentrations and size distributions were measured by electrozone sensing (Multisizer III, Beckman Coulter).
Imaging Protocols
Contrast-enhanced ultrasound (CEU) molecular imaging of the ascending aorta and proximal aortic arch were imaged using a right parasternal window with a linear-array probe (Sequoia, Siemens Medical Systems). Multi-pulse phase-inversion and amplitude-modulation imaging at 7 MHz was performed with a dynamic range of 55 dB and a mechanical index of 1.0. Gain was set at a level that just eliminated pre-contrast background speckle and kept constant for all studies. Images were acquired 8 min after intravenous injection of targeted or control microbubbles (1×106), performed in random order, in order to allow almost all free microbubbles to clear from blood pool. Signal from retained microbubbles alone was determined as previously described by acquiring the first ultrasound frame and then digitally subtracting several averaged frames obtained after complete destruction of microbubbles at a mechanical index of 1.4 to eliminate signal from the low concentration of freely-circulating microbubbles in the blood pool (15). Signal intensity was measured from a region-of-interest encompassing the entire ascending aorta to just beyond the origin of the brachiocephalic artery. Region selection was facilitated by fundamental 2-D imaging at 14 MHz acquired after each CEU imaging sequence.
Echocardiography
High-frequency (40 MHz) transthoracic echocardiography was performed at each post-MI study interval in DKO mice to evaluate any differences in left ventricular (LV) diameter, infarct size, stroke volume, or peak aortic shear rate related to treatment with apocynin. A parasternal long-axis and parasternal short-axis at the mid-ventricular level imaging planes were used to assess infarct size quantified by calculating a wall motion score index. LV dimensions at end-systole and end-diastole were measured from the parasternal long-axis view using linear measurements of the LV at the level of the mitral leaflet tips during diastole. Stroke volume was calculated as the product of the left ventricular outflow tract cross-sectional area and time-velocity integral on pulsed-wave Doppler. Longitudinal strain was calculated using speckle-tracking echocardiography from a parasternal long-axis view and was quantified as the average of a standard single 6-segment model.
Histology
Histology of the aortic root and the mid-ascending aorta for DKO mice was performed 21 days or 3 months after MI (randomized evenly for each group). Perfusion-fixed transaxial sections were stained with Masson’s trichrome for assessment of plaque area and collagen content. Immunohistochemistry was performed with antibody against Mac-2 (M3/38, eBioscience) to assess macrophage content, against CD41 (sc20234, Santa Cruz Biotechnology, Santa Cruz CA) for platelets; and for VCAM-1 (BS-0369r, Bioss Inc Atlanta, GA). For each epitope, secondary staining was performed with species-appropriate secondary antibodies labeled with ALEXAFluor-488, -555, or -594 (Invitrogen Grand Island, NY). Plaque collagen content, necrotic core and MAC-2 area are expressed as percent of plaque area.
Intravital Microscopy
Direct observation of post-MI potentiation of platelet- and leukocyte-endothelial interactions was performed with intravital microscopy in wild-type and DKO mice, with or without MI 3 days prior to study. Mice were anesthetized with ketamine and xylazine (I.P.) and the cremaster muscle was exteriorized and prepared for intravital microscopy as previously described (16). Microscopy was performed with combined fluorescent epi-illumination and low-intensity transillumination (Axioskop2-FS, Carl Zeiss, Inc) and digital recordings were made with a high- resolution CCD camera (C2400, Hamamatsu Photonics). In vivo fluorescent labeling of platelets was performed with rhodamine-6G (1 mg/mL, 75 μL I.V.). The number of adherent leukocytes and platelet adhesive events (>5 s), and the formation of platelet “strings” indicative of ultra-large VWF multimers were quantified as fluorescent platelet area normalized to vessel area. Rolling velocity of leukocytes in 15–35 μm post-capillary venules was calculated by the distance traveled by video calipers divided by time.
Statistical Analysis
Data analysis was performed with Prism v7.0a (Graph Pad, La Jolla, California). Continuous variables that were normally distributed are displayed as mean ±SD unless stated otherwise; whereas those that were not normally distributed are displayed as box-whisker plots with a bar representing median, box representing 25–75% quartiles, and whiskers representing range. Student t test (paired or unpaired) were performed for comparisons of normally distributed data. For non-normally distributed data, either a Mann-Whitney U test or Wilcoxon signed-rank test was used as appropriate according to experimental conditions (group-wise comparisons versus paired data within a group). For multiple comparisons, a one-way ANOVA was performed for normally distributed data with post-hoc testing with Holm-Sidak’s multiple comparisons correction. A Kruskal-Wallis test followed by Dunn’s multiple comparison test was performed for non-normally distributed data.
Results
Post-MI Ventricular Function and Aortic Shear
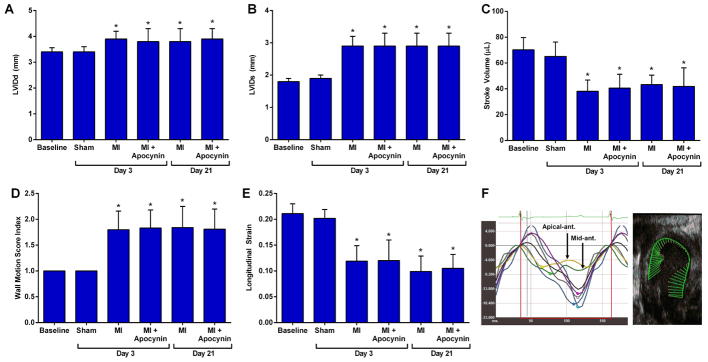
Electrocardiographic ST-segment elevation and regional hypokinesis or akinesis involving the mid to distal anterior, anterolateral, and anteroseptal walls on echocardiography was observed in all mice at the time of closed-chest LAD ischemia, but were not seen in any of the animals undergoing sham surgical procedure. Myocardial infarction resulted in LV enlargement, reduction of LV systolic function and stroke volume, and lower peak aortic shear rate at day 3 and 21 (Figure 1). Chronic therapy with apocynin starting on the day of MI did not affect the infarct-related changes in LV dimension or function on echocardiography, or the aortic peak shear rate.
Figure 1. Echocardiography and Vascular Ultrasound.
Mean (±SD) values are shown for LV internal diameter at (A) end-diastole and (B) end-systole, (C) stroke volume, (D) wall motion score index, and (E) longitudinal strain.. Data are combined for wild-type and DKO mice which were not significantly different from each other. *p<0.05 versus baseline. (F) Example of LV strain curves from a long-axis view illustrating severe hypokinesis in the LAD territory at day 3 post-MI and corresponding vector mapping of endocardial strain.
Remote Arterial Endothelial Activation and Platelet Adhesion After MI
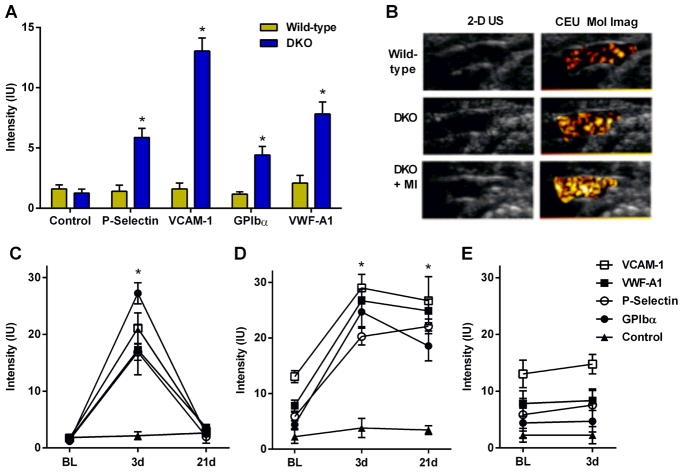
At baseline prior to any procedures, molecular imaging signal enhancement of the thoracic aorta for endothelial P-selectin, VCAM-1, Von Willebrand factor (VWF) A1-domain, and platelet GPIbα were all found to be higher in DKO than wild-type mice (Figure 2A), consistent with greater aortic endothelial adhesion molecule expression, endothelial-associated active form of VWF, and platelet adhesion in DKO mice. In DKO but not wild-type mice, signal for all four targeted microbubble agents was significantly greater than for control microbubbles. At day 3 post-MI, signal for endothelial P-selectin, VCAM-1, VWF and platelet GPIbα all increased significantly in both wild-type and DKO mice (Figure 2B to 2D). In wild-type mice, the post-MI increase for all four targeted agents completely resolved and returned to baseline levels at day 21; whereas in DKO mice signal enhancement remained elevated at 21 days. Signal from control MBs was low in all mice and did not change significantly after MI. Sham procedure did not produce any changes on CEU molecular imaging (Figure 2E).
Figure 2. Molecular Imaging of Remote Endothelial Activation.
(A) Background-subtracted signal intensity (mean±SEM) on CEU molecular imaging of the thoracic aorta from wild-type and DKO mice. *p<0.05 vs control non-targeted signal. (B) Examples of 2-D ultrasound and CEU molecular imaging for VCAM-1 of the thoracic aorta of wild-type, DKO, and post-MI (day 3) DKO mice. (C–E) Mean (±SEM) signal intensity on CEU molecular imaging of the aorta from: (C) post-MI wild-type mice; (D) post-MI DKO mice; and (E) DKO mice undergoing sham surgery. *p<0.05 for all actively-targeted agents vs baseline values and vs control non-targeted signal.
Effect of ADAMTS13 on Platelet Adhesion
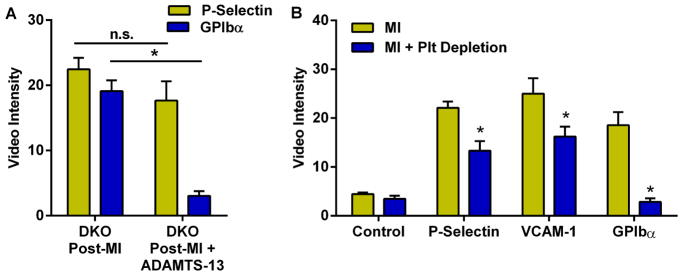
In DKO mice, aortic CEU molecular imaging 3 days post-MI was performed before and 1 hour after treatment with recombinant ADAMTS13, a protease that removes endothelial-associated VWF and controls VWF size (10,17). ADAMTS13 abolished platelet GPIbα signal (Figure 3A), indicating that platelet adhesion in remote vessels after MI occurs secondary to inducible abnormalities in the enzymatic regulation of endothelial-associated VWF. The lack of effect on P-selectin signal confirmed that this signal was primarily endothelial rather than platelet in origin.
Figure 3. Effect of ADAMTS13 Therapy and Platelet Depletion on Remote Arterial Activation.
(A) CEU molecular imaging intensity for P-selectin and platelet GPIbα in DKO mice at day 3 post-MI before and 1 hour after ADAMTS-13 (5 μg, I.V.). (B) Aortic molecular imaging signal in DKO mice with and without platelet depletion at 21 days post MI. *p<0.05 vs non-treated.
Effect of Platelet Depletion on Endothelial Activation
In DKO mice studied at day 21, platelet immune-depletion started on day 1 after MI completely prevented the post-MI increase in platelet adhesion on aortic molecular imaging, and also significantly but modestly reduced endothelial P-selectin and VCAM-1 signal enhancement (Figure 3B).
Nox Inhibition Reduced Endothelial Activation and Platelet Adhesion
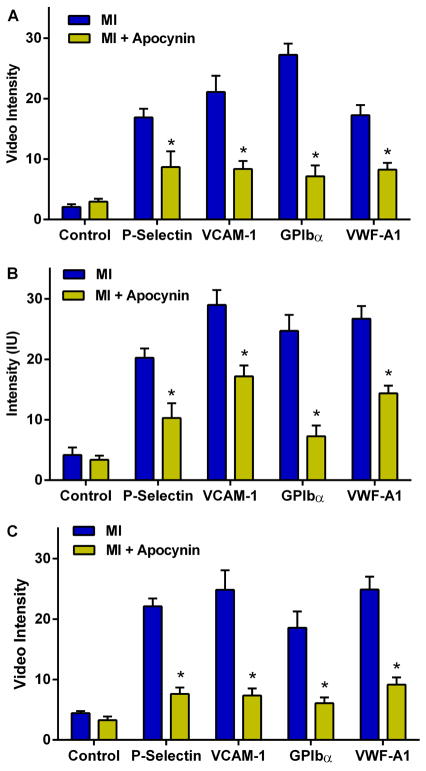
Apocynin started on the day of MI significantly attenuated the post-MI increase in remote aortic molecular imaging signal for P-selectin, VCAM-1, platelet GPIbα, and VWF A-1 in wild-type mice at day 3 and in DKO mice at day 3 and 21 (Figure 4A to 4C).
Figure 4. Effect of Apocynin on Remote Endothelial Activation.
Mean (±SEM) signal intensity on quantitative CEU molecular imaging depicting effect of apocynin in: (A) wild-type mice 3 days post-MI; (B) DKO mice 3 days post-MI; and (C) DKO mice 21 days post-MI. *p<0.05 vs non-treated.
Remote Endothelial Activation in the Microcirculation
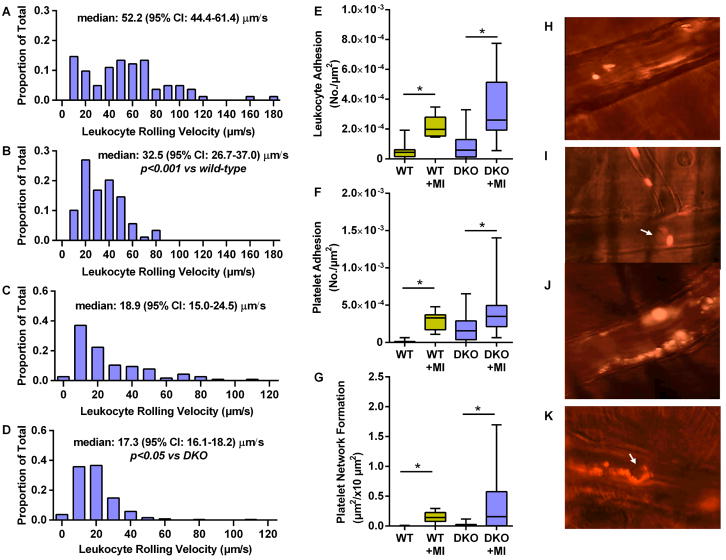
Intravital microscopy of the cremaster muscle was used to interrogate for similar post-MI remote endothelial-related events in the microcirculation. Venular leukocyte rolling velocity in both wild-type and DKO mice decreased after MI (Figure 5A to 5D, Online Videos). Leukocyte firm adhesion in venules and platelet adhesion were also significantly increased 3 days post-MI in both wild-type and DKO mice (Figure 5E to 5G). Almost all platelet adhesion events in non-ischemic mice were in the form of single platelets or small linear aggregates, whereas platelet adhesion post-MI was in the form of large string or net assemblies, consistent with ultra-large multimers of VWF on the endothelium or anchored to adherent leukocyte-platelet hetero-aggregates (Figure 5H to 5K, Online Videos).
Figure 5. Remote Microvascular Endothelial Activation.
Histograms and median leukocyte rolling velocities in cremasteric venules are shown for (A) wild-type mice, (B) wild-type mice 3 days post-MI, (C) DKO mice, and (D) DKO mice 3 days post-MI. Box-whisker plots depict (E) leukocyte adhesion (number per vessel area); (F) isolated platelet adhesion; and (G) platelet string or net complex formation rate on venules. *p<0.001; †p<0.0001. Pseudocolorized intravital microscopy images illustrate rhodamine-6G-labeled platelets adhering in post-capillary venules in the form of: (H), individual platelets; (I), single platelet-leukocyte complex; (J), large platelet net complexes on the endothelium; and (K), platelet strings attached (downstream) to adherent leukocytes. Arrow=leukocytes.
Post-MI Acceleration of Plaque Progression is Suppressed with Nox-2 Inhibition
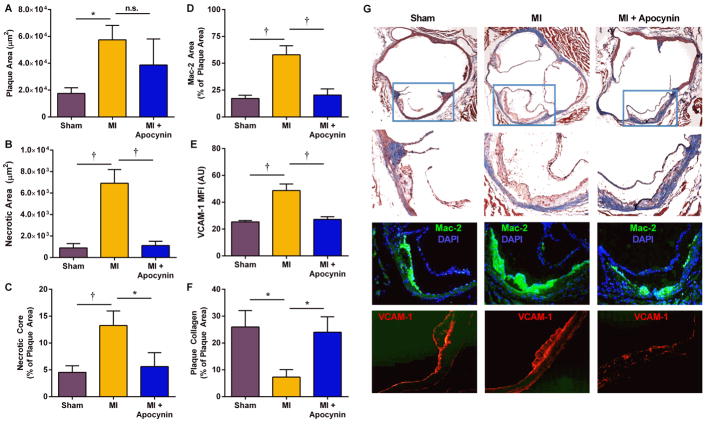
Aortic histology demonstrated that at 21 days, DKO mice undergoing MI compared to sham-treated mice had larger plaque area, larger necrotic core area, increased macrophage content, greater VCAM-1 staining, and lower collagen content (Figure 6, Online Figure 1). The increase in VCAM-1 staining was present in endothelium overlying atherosclerotic plaques, as well as non-plaque endothelium. Apocynin therapy in post-MI DKO mice attenuate all of the high-risk features in terms of macrophage content, VCAM-1 staining and collagen content; whereas the reduction in plaque size did not reach statistical significance. Histology obtained at 3 months demonstrated persistently larger plaque size and macrophage content, and lower collagen content in DKO mice undergoing MI compared with sham-treated controls (Online Figure 2).
Figure 6. Aortic histology from DKO mice.
Data are from DKO mice at 21 days after sham procedure or after MI, with or without apocynin treatment. (A) plaque cross-sectional area averaged for the aortic sinuses and distal ascending aorta; (B), necrotic core area; (C), necrotic core area as a percentage of total plaque area; (D), Mac-2 area averaged for plaques in the aortic sinuses and distal ascending aorta; (E), VCAM-1 staining area; and, (F) plaque collagen content as a percentage of the total plaque area. *p<0.05; †p<0.01 (J) Examples of histology with Masson’s trichrome, and immunohistochemistry for Mac-2 and VCAM-1 from DKO mice 21 days after either sham procedure or MI with or without apocynin treatment. Higher resolution images from Day 21 and 3 Month data are provided in the Online Figures.
Discussion
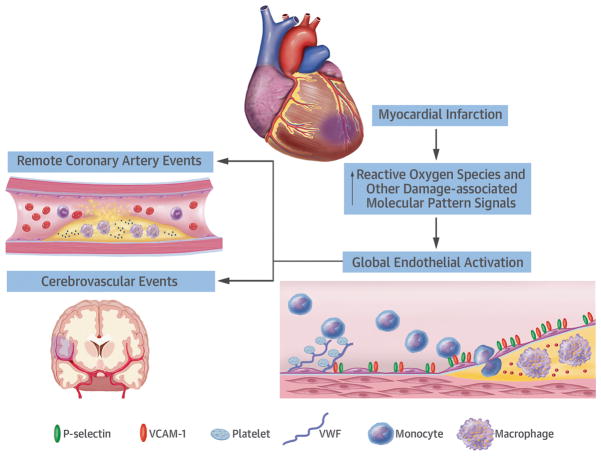
Our results indicate that acute MI stimulates a spectrum of adverse events in remote arterial and microvascular beds including endothelial upregulation of VCAM-1 and P-selectin, and platelet adhesion that occurs through primarily through VWF that is either endothelial-associated or from adherent leukocyte-platelet complexes (Central Illustration). These adverse changes at the vascular endothelial surface persist longer when there is pre-existing atherosclerosis and hyperlipidemia, and are associated with acceleration of plaque growth and inflammation in arteries spatially remote from the MI. Our data also suggest that reduction of oxidative stress through Nox inhibition attenuates adverse endothelial responses in remote arteries.
Central Illustration. Remote Endothelial Activation after Myocardial Infarction.
Myocardial infarction triggers systemic effects, including an increase in reactive oxygen species (ROS) and other damage-associated molecular patterns (DAMPs). These molecular intermediates result in endothelial activation including upregulation of adhesion molecules for inflammatory cells, increase in endothelial associated VWF, and secondary platelet adhesion. The pro-inflammatory and pro-thrombotic endothelial changes found in our experiments are changes could be associated with increased risk for atherothrombotic events in humans.
There is increasing evidence that global inflammatory responses after a major acute ischemic event contribute to the heightened risk for events in remote arterial beds. Leukocytosis and elevated plasma levels of cytokines and chemokines commonly occurs in the subacute phase after MI, the degree of which is associated with intermediate-term prognosis (18–20). In studies performed in atherosclerotic mice, acute MI has been shown to lead to an increase of pro-inflammatory Ly-6Chigh monocytes and other myeloid cells (4). Carotid activity with 18F-fluorodeoxyglucose positron emission tomography has been shown to be higher in patients with recent acute MI than those with chronic stable angina (21), although cross-sectional design precluded any comments on causation. Little is known regarding endothelial changes that promote the recruitment of innate immune cells into atherosclerotic lesions in distant vessels after MI.
We applied CEU molecular imaging with targeted tracers confined to the vascular compartment to reveal that reperfused MI triggers remote arterial upregulation of endothelial cell adhesion molecules that are critical for the recruitment of inflammatory cells (9,22). Although our primary aim was to study events in large arteries, corroborative evidence in other vascular territories was provided by intravital microscopy of a non-coronary microvascular bed where leukocyte rolling velocity and adhesion were used as indicators of endothelial expression of selectins and integrin counter-receptors. These studies confirmed that MI promotes global endothelial activation. We believe that endothelial activation was in part responsible for the acceleration of plaque growth, inflammation, and necrotic core at 21 days and 3 months post-MI; all consistent with a more “high risk” plaque appearance on histology. However, it is possible that increased plaque macrophage content contributed to the persistence of the endothelial activation at day 21 in DKO mice, consistent with the feed-forward nature of plaque inflammation.
Our investigation of endothelial-platelet adhesion after MI is based on the mounting evidence that these interactions play a multifactorial role in plaque inflammatory status (9,23). Local release of platelet-derived pro-inflammatory cytokines and CD40L leads to monocyte activation, upregulation of endothelial adhesion molecules, formation of neutrophil extracellular traps (NETs); and the generation of reactive oxygen species (ROS) (9,23–25). Inhibition of platelet activation or adhesion has been shown in animal models to reduce plaque size and inflammation (26,27).
Platelet adhesion in early to moderate-stage atherosclerosis is primarily mediated by interactions between the GPIbα component of the GPIb/IX/V complex on platelets and the A1 domain of “activated” VWF (27–29). This interaction appears to be facilitated by association of VWF with the intact endothelial surface, and ineffective cleavage at the A2 domain by ADAMTS13, resulting in ultra-large multimers of self-associated VWF (17,29,30). In this study, molecular imaging of a remote artery revealed a large increase in endothelial VWF-A1 domain and platelet adhesion after MI which paralleled endothelial inflammatory responses. Platelet signal in post-MI DKO mice was entirely eliminated by exogenous ADAMTS13 implicating abnormal regulation of VWF. Again, intravital microscopy provided corroborative evidence such as the formation of large linear platelet clusters that were dynamic in size and oscillated in flow, characteristic of the large VWF networks. To a lesser degree, adherent platelet-monocyte complexes were also observed. Many of these hetero-aggregates did not appear consistent with direct contact of single platelets to the leukocyte surface, but instead appeared as a dynamic clustering of platelets downstream from leukocytes possibly consistent with VWF net formation on leukocytes (31). The contribution of platelets to post-MI endothelial activation in this study was confirmed by a reduction, but not elimination, of P-selectin and VCAM-1 signal at 21 days in platelet-depleted DKO mice.
Oxidative stress measured by a variety of biomarkers is known to increase after recent MI (32), is known to be greater in the setting of dysplipidemia (33), and represents a pathway by which inflammation and platelet adhesion can self-propagate in a deleterious manner (9). We evaluated the contribution of oxidative stress by treating mice with apocynin which, among many of its effects, inhibits membrane translocation of the p47phox and gp91phox subunits of Nox (13), and in our experience has been more effective at reducing adhesion molecule expression than free radical scavenger approaches. Apocynin was found to reduce molecular imaging signal for P-selectin, VCAM-1, VWF-A1 and platelet GPIbα. These data suggest that increased oxidative stress plays an important role in remote endothelial activation after MI. These findings are consistent with known effects of reactive oxygen species to stimulate adhesion molecule expression through the transcription factor nuclear factor-κB (NF-κB), and to inhibit proteolytic regulation of VWF (34–36). The suppression of adhesion molecule expression and platelet adhesion with apocynin was associated with partial protection from post-MI accelerated plaque progression in terms of inflammation and necrotic core size.
There are several limitations of the study that should be acknowledged. Although the murine model was selected to reflect human disease based on reproducible plaque development without extreme diet, mice do not necessarily reproduce human condition based on both plaque location and lack of plaque rupture or erosion as the inciting event. It is also important to note that post-MI molecular imaging and plaque assessment were made only for the aorta based on the inability to resolve smaller branch non-culprit arteries and because of the unique distribution of disease in murine models. We did not make any direct measurements of oxidative stress in the different cohorts, largely because of the strength of evidence from previous studies demonstrating that oxidative biomarkers increase after MI and are reduced by apocynin (32,37). There are insufficient data to state that the primary mechanism by which apocynin reduced plaque progression after MI was directly attributable to adhesion molecule expression and platelet adhesion. Instead, we believe that lowering ROS would have pleiotropic effects that would include the endothelial abnormalities that were the focus of this study. Importantly, we do not yet have data revealing the primary inciting factor for remote plaque activation, nor do we have data on how infarct size or duration of ischemia influence the degree of remote plaque activation.
In summary, myocardial infarction leads to remote endothelial activation and abnormal regulation of endothelial-associated and possibly leukocyte-associated VWF (Central Illustration). These processes are associated with enhanced monocyte recruitment, platelet-endothelial adhesion, and acceleration of plaque progression. Interrupting the endogenous vascular production of ROS with the Nox inhibitor apocynin suppresses adverse remote endothelial changes. Our findings help understand mechanisms for heightened risk for recurrent events after MI, and reveal potentially modifiable processes. They could also contribute to the mechanistic understanding of the recently-described beneficial effects of pro-inflammatory cytokine inhibition in patients with MI (38).
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge
Acute atherothrombotic events such as myocardial infarction and stroke increase the risk of ischemic events in non-culprit arteries and other vascular territories for several months. These secondary events are mediated by upregulation of endothelial inflammatory adhesion molecules and platelet-endothelium interactions that are associated with accelerated plaque inflammation and persist longer in the presence of hyperlipidemia.
Translational Outlook
Future research should examine inhibition of systemic inflammatory processes that promote endothelial adhesiveness and platelet-endothelium interactions to reduce recurrent ischemic events after MI.
Acknowledgments
Dr. Lindner is supported by grants R01-HL078610 and R01-HL130046; Dr. López is supported by R01-HL091153 and R01-HL11763; and Dr. Ruggeri is supported by HL42846 annd HL78784 from the NIH. Dr. Lindner is also supported by a grant (14-14NSBRI1-0025) from the NASA National Space Biomedical Research Institute. Dr. Moccetti is supported by a grant from the Swiss National Science Foundation.
Abbreviations
- ADAMTS13
A disintegrin and metalloprotease with thrombospondin type I repeats-13
- CEU
Contrast-enhanced ultrasound
- DKO
Double knockout
- LAD
Left anterior descending coronary artery
- mAb
Monoclonal antibody
- VCAM-1
Vascular cell adhesion molecule-1
- VWF
Von Willebrand factor
Footnotes
Disclosures: There are no disclosures or relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 2.Witt BJ, Brown RD, Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Annals of internal medicine. 2005;143:785–92. doi: 10.7326/0003-4819-143-11-200512060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Milonas C, Jernberg T, Lindback J, et al. Effect of Angiotensin-converting enzyme inhibition on one-year mortality and frequency of repeat acute myocardial infarction in patients with acute myocardial infarction. Am J Cardiol. 2010;105:1229–34. doi: 10.1016/j.amjcard.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–63. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–9. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim CY, Liu YN, Atkinson T, et al. Molecular Imaging of Platelet-Endothelial Interactions and Endothelial von Willebrand Factor in Early and Mid-Stage Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002765. doi: 10.1161/CIRCIMAGING.114.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chadderdon SM, Belcik JT, Bader L, et al. Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation. 2014;129:471–8. doi: 10.1161/CIRCULATIONAHA.113.003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu MD, Atkinson TM, Lindner JR. Platelets and von Willebrand factor in atherogenesis. Blood. 2017;129:1415–1419. doi: 10.1182/blood-2016-07-692673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong JF. Cleavage of ultra-large von Willebrand factor by ADAMTS-13 under flow conditions. Journal of thrombosis and haemostasis: JTH. 2005;3:1710–6. doi: 10.1111/j.1538-7836.2005.01360.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. Journal of thrombosis and haemostasis: JTH. 2003;1:1335–42. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Powell-Braxton L, Veniant M, Latvala RD, et al. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat Med. 1998;4:934–8. doi: 10.1038/nm0898-934. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Weiwer M, Linhardt RJ, Dordick JS. The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr Vasc Pharmacol. 2008;6:204–17. doi: 10.2174/157016108784911984. [DOI] [PubMed] [Google Scholar]
- 14.Impellizzeri D, Esposito E, Mazzon E, et al. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol. 81:636–48. doi: 10.1016/j.bcp.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–84. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 16.Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000;101:668–75. doi: 10.1161/01.cir.101.6.668. [DOI] [PubMed] [Google Scholar]
- 17.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–9. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 18.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Moore XL, Dart AM, Wang LM. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol. 2015;12:305–12. doi: 10.11909/j.issn.1671-5411.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Quesada C, Frangogiannis NG. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Curr Atheroscler Rep. 2009;11:131–8. doi: 10.1007/s11883-009-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ Cardiovasc Imaging. 2014;7:454–60. doi: 10.1161/CIRCIMAGING.113.001093. [DOI] [PubMed] [Google Scholar]
- 22.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 23.Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Frontiers in immunology. 2015;6:98. doi: 10.3389/fimmu.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–6. doi: 10.1160/TH07-11-0685. [DOI] [PubMed] [Google Scholar]
- 25.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 26.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–7. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 27.Massberg S, Brand K, Gruner S, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–96. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theilmeier G, Michiels C, Spaepen E, et al. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–93. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Davidson BP, Yue Q, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan AK, Goerge T, Schneider SW, Wagner DD. Formation of platelet strings and microthrombi in the presence of ADAMTS-13 inhibitor does not require P-selectin or beta3 integrin. Journal of thrombosis and haemostasis: JTH. 2007;5:583–9. doi: 10.1111/j.1538-7836.2007.02361.x. [DOI] [PubMed] [Google Scholar]
- 31.Pendu R, Terraube V, Christophe OD, et al. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. 2006;108:3746–52. doi: 10.1182/blood-2006-03-010322. [DOI] [PubMed] [Google Scholar]
- 32.Neri M, Fineschi V, Di Paolo M, et al. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015;13:26–36. doi: 10.2174/15701611113119990003. [DOI] [PubMed] [Google Scholar]
- 33.Araujo FB, Barbosa DS, Hsin CY, Maranhao RC, Abdalla DS. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis. 1995;117:61–71. doi: 10.1016/0021-9150(94)05558-z. [DOI] [PubMed] [Google Scholar]
- 34.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–66. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Fu X, Wang Y, et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115:706–12. doi: 10.1182/blood-2009-03-213967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–71. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Tian J, Sun Y, et al. Activation of NADPH oxidase mediates increased endoplasmic reticulum stress and left ventricular remodeling after myocardial infarction in rabbits. Biochimica et biophysica acta. 2015;1852:805–15. doi: 10.1016/j.bbadis.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.