ABSTRACT
The neural crest, a progenitor population that drove vertebrate evolution, retains the broad developmental potential of the blastula cells it is derived from, even as neighboring cells undergo lineage restriction. The mechanisms that enable these cells to preserve their developmental potential remain poorly understood. Here, we explore the role of histone deacetylase (HDAC) activity in this process in Xenopus. We show that HDAC activity is essential for the formation of neural crest, as well as for proper patterning of the early ectoderm. The requirement for HDAC activity initiates in naïve blastula cells; HDAC inhibition causes loss of pluripotency gene expression and blocks the ability of blastula stem cells to contribute to lineages of the three embryonic germ layers. We find that pluripotent naïve blastula cells and neural crest cells are both characterized by low levels of histone acetylation, and show that increasing HDAC1 levels enhance the ability of blastula cells to be reprogrammed to a neural crest state. Together, these findings elucidate a previously uncharacterized role for HDAC activity in establishing the neural crest stem cell state.
KEY WORDS: HDAC, Histone acetylation, Pluripotency, Stem cell, Xenopus, Neural crest
Highlighted Article: HDAC activity is essential for the pluripotency of naïve blastula cells and formation of neural crest stem cells, and increasing HDAC activity enhances reprogramming to a neural crest state.
INTRODUCTION
First described by Wilhelm His 150 years ago, the neural crest is a vertebrate progenitor population distinguished by its ability to contribute cell types associated with multiple germ layers to the vertebrate body plan (Hall, 2000; Sauka-Spengler and Bronner-Fraser, 2008). Acquisition of these cells, referred to as the ‘Zwischenstrang’ by His, had a central role in the evolution of vertebrates, by layering myriad novel structures onto the simple chordate body plan (His, 1868; Le Douarin and Dupin, 2012). Understanding the mechanisms that control the genesis of these important cells is essential to understanding how vertebrates evolved, and is linked to understanding the establishment and maintenance of their developmental potential.
We recently demonstrated that much of the transcriptional circuitry that controls the potency of neural crest cells is shared with pluripotent blastula cells (the Xenopus equivalent of mammalian inner cell mass cells), and proposed that neural crest cells arise through retention of the characteristics of those earlier cells (Buitrago-Delgado et al., 2015). This revised model for neural crest formation raises fundamental questions regarding how these cells escape lineage restriction and retain their potency until after the basic chordate body plan has been laid down. These mechanisms are certain to involve signaling pathways and transcription factors previously linked to neural crest formation (Prasad et al., 2012; Taylor and LaBonne, 2007). For example, the transcription factors Snail and Sox5, which are both essential for the formation of the neural crest, are first required for the pluripotency of blastula cells, as are BMP and FGF signaling (Buitrago-Delgado et al., 2015; Geary and LaBonne, 2018; LaBonne and Bronner-Fraser, 2000; Nordin and LaBonne, 2014). Less understood is the role that chromatin remodelers and regulation of the epigenetic state might have in the retention of pluripotency leading to establishment of the neural crest (Hu et al., 2014).
The epigenetic landscape of a pluripotent cell determines its cellular competency and impacts its lineage choices (Atlasi and Stunnenberg, 2017; Li et al., 2012). The maternally defined chromatin state is crucial for controlling gene expression during embryonic development (Hontelez et al., 2015; Liang and Zhang, 2012). In cultured embryonic stem cells (ESCs), histone acetylation has an essential role in ensuring appropriate gene expression; acetylation of histone H3 at specific lysine residues (H3K9/14Ac and H3K27Ac) marks important developmentally regulated genes in murine and human ESCs as well as in early embryonic development (Bogdanovic et al., 2012; Creyghton et al., 2010; Gupta et al., 2014; Hezroni et al., 2011; Karmodiya et al., 2012; Rada-Iglesias et al., 2011). Histone deacetylases (HDACs), enzymes tasked with removing acetyl marks from histones, have also been shown to have a crucial role in maintaining pluripotency in cultured ESCs (Dovey et al., 2010; Jamaladdin et al., 2014). HDAC1 occupies the promoter regions of pluripotency genes in these cells, suggesting a positive regulatory role in maintaining the stem cell state, and HDAC-containing complexes, such as Sin3A-HDAC and nucleosome remodeling and deacetylase (NuRD), have been linked to the promotion of pluripotency in ESCs and during somatic cell reprogramming (Baltus et al., 2009; Saunders et al., 2017).
Whereas studies in cultured ESCs suggest that histone acetylation has a crucial, if complex, role in controlling pluripotency and lineage restriction, surprisingly little is known about the roles and regulation of HDAC activity and/or histone acetylation in the control of pluripotency in vivo during embryonic development. In contrast to ESC cultures, pluripotency is transient in early embryos, and lineage restriction events are dynamic and spatially controlled. We hypothesized that understanding how regulation of histone acetylation impacts the pluripotency of naïve blastula cells might shed light on the epigenetic mechanisms that contribute to a subset of cells escaping lineage restriction to form the neural crest. Xenopus embryos provide an ideal model for such studies, because explanted blastula cells retain the transient pluripotency characteristic of cells in vivo, and require no exogenous factors for survival or retention of potential. Remarkably, although decades of experiments utilizing these cells have shaped our understanding of the signals that direct pluripotent cells to adopt specific lineage states, less is known about how pluripotency is controlled and maintained in naïve blastula cells.
In this study, we examined the role of HDACs and histone acetylation in the establishment of the neural crest and the maintenance of pluripotency in Xenopus embryos. We show that HDAC activity is essential for formation of the neural crest stem cell population, and for the pluripotency of blastula stem cells. Inhibiting HDAC activity caused naïve blastula cells to lose pluripotency, and this was accompanied by precocious and aberrant expression of genes that direct multiple lineage states. We found that pluripotent blastula cells and neural crest cells are both characterized by low levels of histone acetylation, further emphasizing the similarities between these cell types. Finally, we show that increasing HDAC1 activity in blastula cells enhanced their reprogramming to a neural crest state. Together, these findings provide novel insights into the epigenetic mechanisms that control the maintenance of pluripotency in the developing embryo, and show that the regulation of HDAC activity is essential for establishing the neural crest state. These results shed important new mechanistic light on the genesis of a cell type central to the evolution of vertebrates.
RESULTS
HDAC activity is essential for neural crest and placode formation
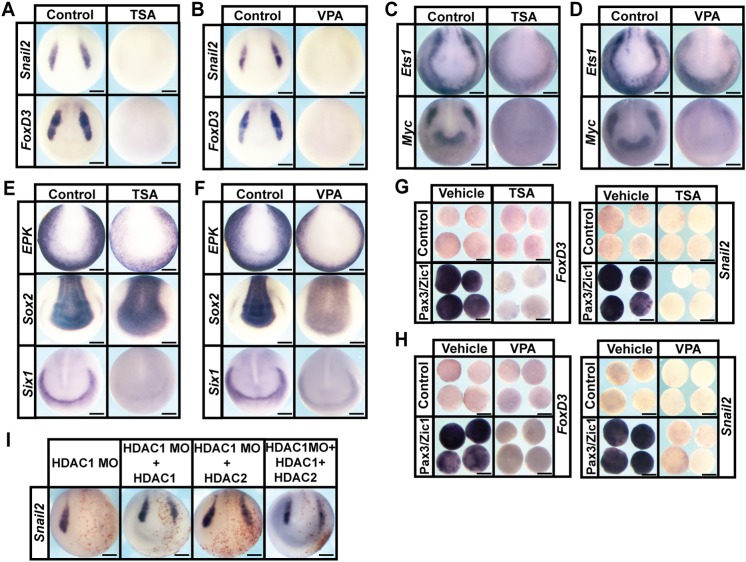
HDAC activity can be temporally controlled in developing embryos by using potent small-molecule inhibitors, such as Trichostatin A (TSA) and valproic acid (VPA) (Göttlicher et al., 2001; Yoshida et al., 1990). TSA inhibits Class I and IIB families of HDACs, whereas VPA specifically targets the Class I family. Treatment of Xenopus embryos with these inhibitors at early gastrula stages (Nieuwkoop and Faber stage 10.5-11) resulted in the complete absence of neural crest cells, as evidenced by loss of expression of snail2 (snai2 – Xenbase) (TSA: 100%, n=72; VPA: 100%, n=74) and foxD3 (TSA: 100%, n=65; VPA: 100%, n=70) compared with vehicle-treated control embryos [snail2 (DMSO: 0%, n=61; water: 0%, n=65); foxD3 (DMSO: 0%, n=66; water: 0%, n=68)] (Fig. 1A,B). This loss of neural crest cells was accompanied by developmental malformations in cranial regions and axial truncations (Fig. S1A,B) (Almouzni et al., 1994; Gurvich et al., 2005).
Fig. 1.
HDAC activity is required for neural crest formation. (A-D) In situ hybridization examining the expression of neural crest factors snail2, foxd3,ets1 and myc following treatment with vehicle or inhibitor [200 nM TSA (A,C) or 20 mM VPA (B,D)]. Embryos were treated at mid-gastrula stages (stage 11) and collected at mid-neurula stages (stage 15). (E,F) In situ hybridization examining expression of epk, sox2 and six1 following treatment with vehicle or inhibitor [200 nM TSA (E) or 20 mM VPA (F)]. (G,H) Explant assay examining snail2 and foxd3 expression in Pax3/Zic1-induced explants treated with vehicle or inhibitor [200 nM TSA (G) or 10 mM VPA (H)]. Explants were cultured alongside sibling embryos grown until late neurula stages (stage 18). (I) In situ hybridization examining the expression of neural crest factor snail2 in embryos after morpholino-mediated knockdown of HDAC1, and rescued with co-injection of HDAC1, HDAC2 or HDAC1+HDAC2 mRNA. Embryos were injected at the eight-cell stage and collected at mid-neurula stages (stage 15). Scale bars: 250 μm.
Based on these findings, we asked whether HDAC activity is essential for establishing the neural plate border (NPB) region, where neural crest cells and cranial placodes, another vertebrate novelty, reside (Groves and LaBonne, 2014). The NPB is characterized by expression of several key transcription factors, including ets1, myc, tfap2, msx1/2 and zic1 (Groves and LaBonne, 2014; Milet and Monsoro-Burq, 2012; Prasad et al., 2012). Treatment with TSA or VPA resulted in embryos with significantly reduced expression of several NPB factors, including myc (TSA: 100%, n=78; VPA: 100%, n=71) and ets1 (TSA: 100%, n=61; VPA: 100%, n=64) compared with vehicle-treated control embryos [myc (DMSO: 0%, n=64; water: 0%, n=76), ets1 (DMSO: 0%, n=61; water: 0%, n=57)] (Fig. 1C,D), as well as tfap2 and msx2 (Fig. S2B). Notable among these is myc, one of the four original factors that Yamanaka and colleagues showed could ‘reprogram’ somatic cells to form induced pluripotent stem cells (iPSCs), and before that had been shown to control stem cell potential in the neural crest (Bellmeyer et al., 2003; Takahashi et al., 2007). These results demonstrated that HDAC activity is essential for establishing the NPB region, and the vertebrate-specific progenitor cell populations that reside there. Consistent with this, the cranial placode region failed to form in TSA/VPA-treated embryos, as evidenced by loss of expression of six1 [TSA: 100%, n=77 (control: 0%, n=62); VPA: 100%, n=74 (control: 0%, n=76)] (Fig. 1E,F).
We noted that the expression of ets1 and myc outside of the NPB, in the prospective epidermis, was less affected by HDAC inhibition (Fig. 1C,D). Therefore, we examined the effects of TSA/VPA treatment on the establishment of other ectoderm-derived cell types. At neurula stages, we observed an expansion of the neural plate, as marked by sox2 expression, into the regions that would normally form neural crest, placodes and epidermis [TSA: 100%, n=62 (control: 0%, n=65); VPA: 100%, n= 58 (control: 0%, n=52)] (Fig. 1E,F). We also observed that expression of zic1, a gene that marks both neural and NPB cells, was expanded after HDAC inhibition [TSA: 100%, n=70 (control: 0%, n=68); VPA: 100%, n=66 (control: 0%, n=63)], consistent with an expansion of the neural plate (Fig. S2B). tfap2, which is expressed in the epidermis and the NPB, lost only its NPB expression upon HDAC inhibition [TSA: 100%, n=66 (control: 0%, n=66); VPA: 100%, n=64 (control: 0%, n=61)] (Fig. S2B). TSA and VPA treatment resulted in reduced expression of epk (xk81a1 – Xenbase), including ‘salt and pepper’-like expression at the NPB, but the overall domain of expression for this epidermal marker was largely unchanged [TSA: 100%, n=68 (control: 0%, n=60); VPA: 100%, n=56 (control: 0%, n=54)] (Fig. 1E,F). Together, these results indicated that HDAC activity has an essential role in patterning of the early ectoderm, including establishing sharp boundaries for the prospective epidermis and central nervous system (CNS). However, the most prominent effect of blocking HDAC activity was a failure to establish the neural crest and placode populations at the NPB.
HDAC activity is required for reprogramming to a neural crest state
To further investigate the striking loss of neural crest stem cells observed in embryos treated with HDAC inhibitors, we asked whether TSA/VPA treatment could also prevent explants of pluripotent blastula cells from being reprogrammed to a neural crest state. This reprogramming can be achieved following introduction of Pax3/Zic1 or Wnt/Chd, and leads to robust expression of neural crest regulatory factors, including snail2 and foxd3 (Hong and Saint-Jeannet, 2007; LaBonne and Bronner-Fraser, 1998; Plouhinec et al., 2014). We found that treatment with either TSA or VPA prevented reprogramming, as evidenced by a failure to induce the expression of markers of the neural crest cell state in response to either Pax3/Zic1 [TSA: Snail2: 100%, n=26 (control: 18%, n=28), FoxD3: 100%, n=23 (control: 18%, n=28); VPA: Snail2: 100%, n=28 (control: 18%, n=28), FoxD3: 100%, n=25 (control: 13%, n=31)] or Wnt/Chd (TSA: Snail2: 100%, n=26, FoxD3: 100%, n=25; VPA: Snail2: 100%, n=27, FoxD3: 100%, n=28) expression (Fig. 1G,H and Fig. S2C). These results indicated that the requirement for HDAC activity to establish the neural crest stem cell population is direct, and further suggested that it is linked to the retention of pluripotency in blastula stem cells.
HDAC1 is necessary for neural crest formation
Given that TSA and VPA are broad-spectrum HDAC inhibitors, we wished to identify which HDACs were specifically required for neural crest formation. Romidepsin (RMD) is an inhibitor that specifically targets HDAC1 and HDAC2 (Furumai et al., 2002). RMD treatment completely inhibited expression of neural crest markers in Wnt/Chd reprogrammed animal cap explants [Snail2: 100%, n=27 (control: 13%, n=30), FoxD3: 100%, n=30 (control: 15%, n=27)], indicating that HDAC1 and/or HDAC2 activity was required for neural crest formation (Fig. S2D).
hdac1 is more prominently expressed than hdac2 in both early embryos and naïve blastula cells [See ‘Expression' tab for hdac1 and hdac2 on Xenbase (www.xenbase.org); A.R. and C.L., unpublished data]. Therefore, we further investigated the requirement for HDAC1 by designing a translation-blocking morpholino that targets both alloalleles. We found that morpholino-mediated depletion of HDAC1 phenocopied the effects of pharmacological HDAC inhibition on neural crest formation, as evidenced by loss of snail2 expression (75% loss, n=52) (Fig. 1I). Interestingly, we found that these effects could be rescued by either HDAC1 (rescue 72%, n=54) or HDAC2 (rescue 60%, n=53) (Fig. 1I). This suggests that these two HDACs do not have distinct activities in this context. Furthermore, we found that introducing both HDAC1 and HDAC2 (rescue 62%, n=45) did not enhance the rescue (Fig. 1I). Consistent with a role in regulating neural crest genesis, hdac1 was broadly expressed at early neurula stages, and its expression was heightened in the neural crest by late neurula (Stage 18/19) stages (Fig. S2A) (Zhang et al., 2017). hdac1 is maternally provided, expressed from early cleavage stages and highly enriched in pluripotent cells at blastula stages (Fig. S2A), an expression profile consistent with a role in maintaining pluripotency and progenitor states (Carneiro et al., 2011).
HDAC activity is essential for proper gene expression in pluripotent blastula cells
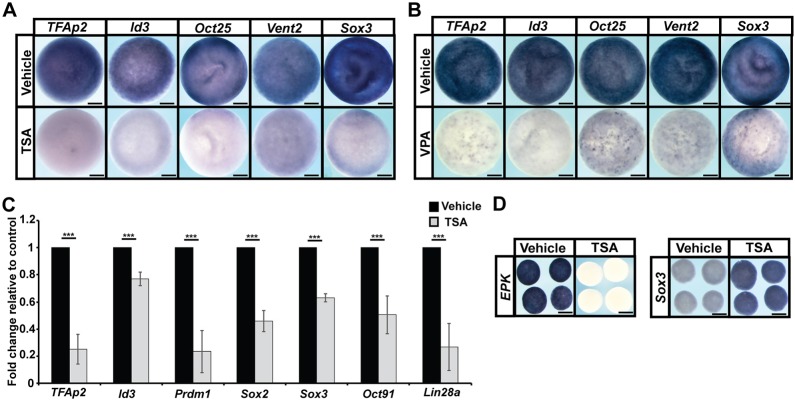
Given that HDAC activity is essential for pluripotent blastula cells to transit to a neural crest state, we wondered whether this might reflect an essential role in maintaining the pluripotency of those blastula stem cells. Accordingly, we examined the effects of TSA/VPA treatment on the expression of pluripotency genes in blastula embryos. Strikingly, inhibition of HDAC activity led to dramatically decreased expression of genes linked to pluripotency, including vent2 [ventx2.2 – Xenbase; TSA: 100%, n=71 (control: 0%, n=70); VPA: 100%, n=68 (control: 0%, n=63)], oct25 [pou5f3.2 – Xenbase; TSA: 98%, n=64 (control: 0%, n=63); VPA: 92%, n=71 (control: 0%, n=62)], sox3 [TSA: 100%, n=74 (control: 0%, n=79); VPA: 100%, n=70 (control: 0%, n=69)] as well as tfap2 [TSA: 100%, n=67 (control: 0%, n=63); VPA: 100%, n=71 (control: 0%, n=68)] and id3 [TSA: 100%, n=54 (control: 0%, n=68); VPA: 100%, n=61 (control: 0%, n=73)] in stage-9 embryos (Fig. 2A,B). Similar decreases were observed when gene expression changes were examined quantitatively in explants of pluripotent cells using qPCR (Fig. 2C). We observed a reduction in the expression of pluripotency genes previously examined by in situ hybridization, as well as in oct91 (pou5f3.1 – Xenbase), lin28a and prdm1, factors that also have been shown to be involved in the maintenance of pluripotency.
Fig. 2.
HDAC activity is essential for proper gene expression in pluripotent blastula cells. (A,B) In situ hybridization examining tfap2, id3, oct25, vent2 and sox3 expression in pluripotent blastula cells following treatment with vehicle or inhibitor [500 nM TSA (A) or 20 mM VPA (B)]. Embryos were treated at the two-cell stage and collected at the late blastula stage (stage 9). (C) qRT-PCR of animal pole explants examining the expression of pluripotency genes after treatment with vehicle or inhibitor (500 nM TSA) (***P<0.005). Explants were cultured alongside sibling embryos grown until late blastula stages (stage 9). (D) In situ hybridization examining epk and sox3 expression in animal pole explants treated with vehicle or inhibitor (500 nM TSA). Explants were cultured alongside sibling embryos until late gastrula stages (stage 13). Scale bars: 250 μm.
The pluripotency of explanted naïve blastula cells is transient; these cells will adopt an epidermal state in the absence of other inductive signals, expressing the epidermal marker EPK, while downregulating expression of pluripotency markers, such as sox3. Interestingly, we found that, following TSA treatment, explants failed to transit to an epidermal state and express EPK (100%, n=28), unlike control explants (control: 0%, n=29) (Fig. 2D). Conversely, we observed sustained expression of sox3 in TSA-treated explants [100%, n=29 (control: 0%, n=25)] (Fig. 2D). sox3 expression could be indicative of either a pluripotent state or a neural progenitor state, because sox3 marks both these cell populations. To distinguish between these possibilities, we examined the expression of another pluripotency marker, Oct60 (pou5f3.3 – Xenbase) and found that, unlike sox3, its expression was not maintained in TSA-treated explants (Fig. S3A). Thus, HDAC inhibition is neither blocking exit from pluripotency nor causing cells to prematurely transit to an epidermal state. Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assays also demonstrated that HDAC inhibition did not lead to a significant increase in cell death in these explants (Fig. S3B).
HDAC activity is essential for the pluripotency of blastula cells
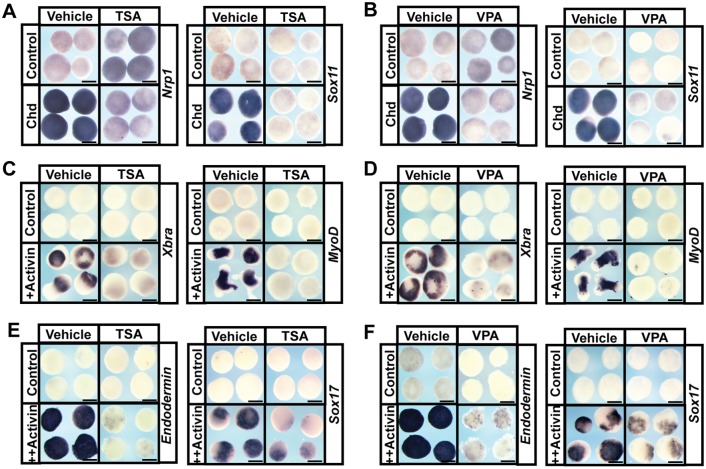
Given the striking changes in gene expression noted in pluripotent blastula cells following HDAC inhibition, we next asked whether these cells had lost their pluripotency by challenging them to adopt specific lineage fates. Isolated Xenopus animal poles cells (‘animal caps’) can be induced to form any cell type in the embryo given appropriate developmental cues (Ariizumi and Asashima, 2001). For example, these cells will transit to a neural state if treated with BMP inhibitors, such as noggin or chordin, and can be induced to form mesoderm or endoderm with low or high doses of TGFβ factors, such as activin (Asashima et al., 1990a,b; Lamb et al., 1993; Sasai et al., 1995). To determine whether pluripotent blastula cells can form neural tissue when HDAC activity is inhibited, animal pole regions explanted from embryos previously injected with mRNA encoding chordin were treated with vehicle or TSA/VPA. Explants treated with HDAC inhibitors were unable to adopt a neural fate, as evidenced by loss of expression of nrp1 [TSA: 100%, n=30 (control: 4%, n=27); VPA: 100%, n=26 (control: =4%, n=27)] and sox11 [TSA: 100%, n=23 (control: 0%, n=24); VPA: 96%, n=23 (control: 3%, n=30)] (Fig. 3A,B). When explants were challenged to form mesoderm in response to activin treatment, control explants displayed robust expression of mesodermal genes, such as xbra (tbxt – Xenbase; DMSO: 100%, n=29; water: 100%, n=30) and myod (DMSO: 100%, n=26; water: 100%, n=29). By contrast, explants treated with HDAC inhibitors failed to form mesoderm, as evidenced by failure to express these markers (TSA: xbra: 100%, n=28, myod: 100%, n=28; VPA: xbra: 100%, n=26, myod: 100%, n=29) (Fig. 3C,D). Similarly, inhibitor-treated explants failed to form endoderm in response to high doses of activin, as seen by failure to express genes such as endodermin [a2m – Xenbase; TSA: 96%, n=28 (control: 0%, n=25); VPA: 96%, n=27 (control: 0%, n=27)] and sox17 [TSA: 96%, n=25 (control: 0%, n=26); VPA: 96%, n=28 (control: 3%, n=30)] (Fig. 3E,F). Taken together, the inability of naïve blastula cells to adopt neural, mesodermal or endodermal states following HDAC inhibition, combined with the observed loss of pluripotency gene expression in these cells, suggests that HDAC activity is essential for the maintenance of pluripotency in blastula animal pole cells.
Fig. 3.
HDAC activity is required for the pluripotency of blastula stem cells. (A,B) In situ hybridization examining nrp1 and sox11 expression in animal pole explants induced with chordin mRNA and treated with vehicle or inhibitor [500 nM TSA (A) or 10 mM VPA (B)]. Explants were cultured alongside sibling embryos grown until late neurula stages (stage 18). (C-F) In situ hybridization examining expression of mesodermal markers xbra and myod and endodermal markers endodermin and Sox17 in animal pole explants induced with Activin and treated with vehicle (DMSO or water) or inhibitor [500 nM TSA (C,E) or 10 mM VPA (D,F)]. Explants were cultured alongside sibling embryos grown until mid-gastrula stages (stage 11.5) for xbra, endodermin and sox17 expression and late neurula stages (stage 18) for myod expression. Scale bars: 250 μm.
Inhibition of HDAC activity results in precocious expression of multi-lineage markers
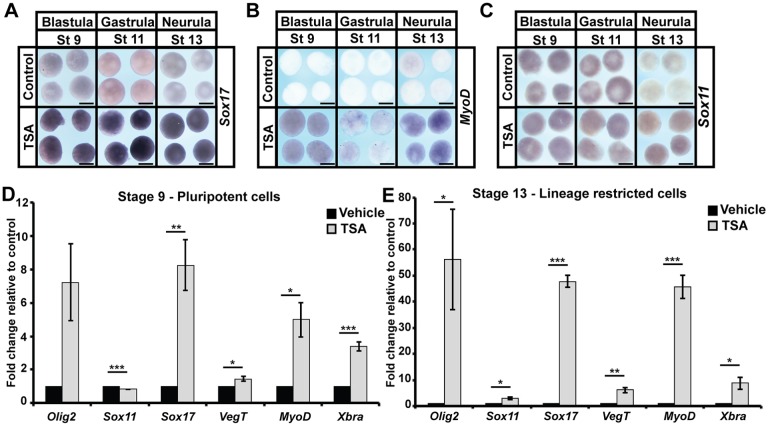
Given that blastula explants were no longer pluripotent following HDAC inhibition, yet were also not prematurely lineage restricted to an epidermal state, we wished to determine what the state of these cells was. We were intrigued by the expression of sox3 in TSA-treated explants at levels consistent with a weakly neuralized state (Fig. 2D), despite the apparent inability of explanted cells to adopt definitive neural fates (Fig. 3A,B). We wondered whether markers of other lineages might be similarly expressed following HDAC inhibition. To test this, we examined the expression of genes pertaining to the adoption of mesodermal (myod), endodermal (sox17) and neural (sox11) states in animal pole explants over developmental time via in situ hybridization. Control explants should not express any of these markers at stage 13, by which time cells should have lost pluripotency and become restricted to an epidermal state. However, in TSA-treated explants, we observed significant expression of myod [100%, n=26 (control: 0%, n=24)], sox17 [100%, n=28 (control: 0%, n=20)] and sox11 [96%, n=24 (control: 5%, n=20)] (Fig. 4A-C). As with sox3, these levels were lower than would be induced following activin- or chordin-mediated induction of specific lineage states. We used qPCR to quantify changes in the expression of genes linked to various lineage states at both stage 9, when control cells are pluripotent, and stage 13, when those cells should have become lineage restricted. Striking increases in lineage-linked gene expression were noted even in blastula stage explants treated with TSA (Fig. 4D), including olig2 and sox11 (neural markers), sox17 and vegt (endodermal markers) and myod and xbra (mesodermal markers), and this aberrant expression was maintained throughout the neurula stages (Fig. 4E). We compared the levels of sox17 and xbra expression in TSA-treated explants with the levels seen following activin treatment and found that they were significantly lower (Fig. S3C,D).
Fig. 4.
HDAC inhibition leads to aberrant expression of multiple lineage markers. (A-C) In situ hybridization examining the expression of lineage markers: endoderm (sox17; A), mesoderm (myod; B) and neural (sox11; C) in aging animal pole explants treated with vehicle or inhibitor (500 nM TSA). Explants were cultured alongside sibling embryos and grown until late blastula (stage 9), mid-gastrula (stage 11) and neural plate (stage 13) stages. (D,E) qRT-PCR examining markers of specific lineages [endoderm (sox17, vegt), mesoderm (myod, xbra) and neural (olig2, sox11)] in pluripotent and aged animal pole explants following treatment with vehicle or inhibitor (500 nM TSA) (*P<0.05, **P<0.01, ***P<0.005). Explants were cultured alongside sibling embryos until late blastula (stage 9) and neural plate (stage 13) stages. Scale bars: 250 μm.
Given the aberrant expression of multiple lineage markers observed in TSA-treated explants, we wished to examine makers of lineage determination in later-stage embryos. To determine effects on CNS development, we utilized a transgenic line in which GFP expression is driven by the N-β-tubulin promoter Xla.Tg(tubb2b:mapt-GFP) (Huang et al., 2007). To validate the line in our hands, Tubb2-GFP embryos were co-stained with an E7-N-Tubulin antibody (Fig. S4A). TSA-treated Tubb2-GFP embryos showed a pronounced increase in expression of the Tubb2-GFP reporter, particularly in the anterior CNS and in the eye (Fig. S4B), consistent with the expanded expression of sox3 noted in response to TSA treatment (Fig. 1E). To determine when this enhanced neural commitment commenced, we followed the expression of the Tubb2-GFP reporter in both TSA and vehicle-treated embryos as they developed. Strikingly, strong expression of Tubb2-GFP was noted in anterior regions of TSA-treated embryos as early as stage 16, a stage when no GFP was detected in control embryos, indicating that CNS development is not delayed as a consequence of HDAC inhibition (Fig. S4C). The precocious and expanded expression of this neural reporter in response to TSA treatment was not similarly accompanied by an increase in staining for muscle actin (Fig. S4B,D). Indeed, somatic muscle was shifted caudally in TSA-treated embryos compared with vehicle-treated controls (Fig. S4B,D), possibly as a consequence of enhanced neural development in anterior regions.
Histone acetylation increases as cells transit from pluripotency to lineage restriction
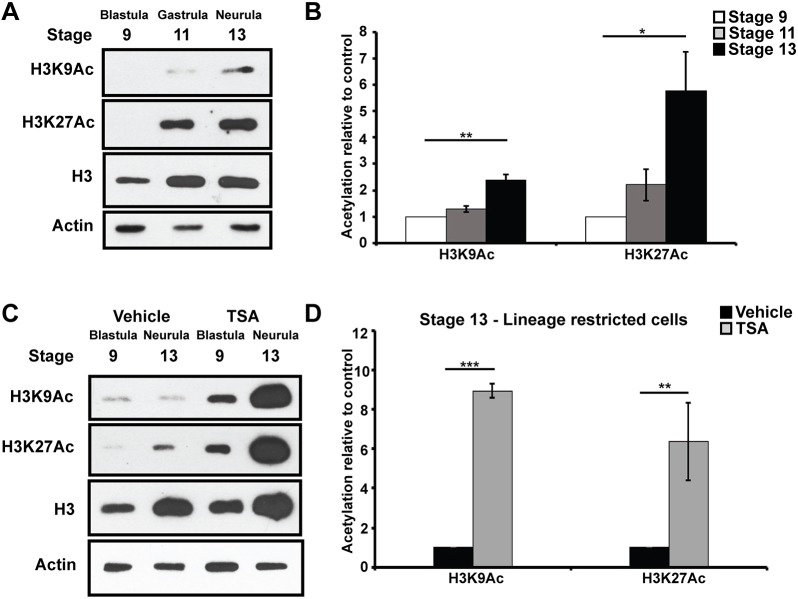
HDACs function by removing acetyl groups from lysine residues in the N-terminal tail of histones. Given that specific histone marks, including H3K9Ac and H3K27Ac, have previously been linked to determining the developmental state of a cell, we were interested in understanding when these marks accumulate as pluripotent cells progress through lineage restriction (Creyghton et al., 2010; Karmodiya et al., 2012). Western blot analysis of Xenopus animal pole explants showed that global levels of H3K9Ac and H3K27Ac were very low at blastula stages, when cells are pluripotent, and increased as cells progressed from gastrula to neurula stages and became lineage restricted (Fig. 5A). Although total H3 levels also increased in these explants, the observed changes in H3K9Ac and H3K27Ac were significant even when normalized to total H3 levels (Fig. 5B). Interestingly, TSA treatment dramatically increased the levels of H3K9Ac and H3K27Ac histone acetylation even in pluripotent cells (Stage 9) and more so in lineage-restricted explants (Stage 13) (Fig. 5C,D, Fig. S5A). This suggests that HDACs actively maintain low levels of H3K9Ac and H3K27Ac in pluripotent cells, and that increases in these marks underlie the loss of pluripotency observed following TSA/VPA treatment.
Fig. 5.
Histone acetylation increases as cells become lineage restricted. (A,B) Western blot analysis of lysates of aging animal caps examining H3K9Ac and H3K27Ac alongside total H3 levels via chemiluminescence (A) and quantified using Odyssey (B) (*P<0.05, **P<0.01). Explants were cultured alongside sibling embryos until late blastula (stage 9), mid-gastrula (stage 11) and neural plate (stage 13) stages. (C,D) Western blot analysis of lysates of aging animal caps treated with vehicle (DMSO) or inhibitor (500 nM TSA) examining H3K9Ac and H3K27Ac alongside total H3 and actin levels via chemiluminescence (C) and quantified using Odyssey (D) (**P<0.01, ***P<0.005).
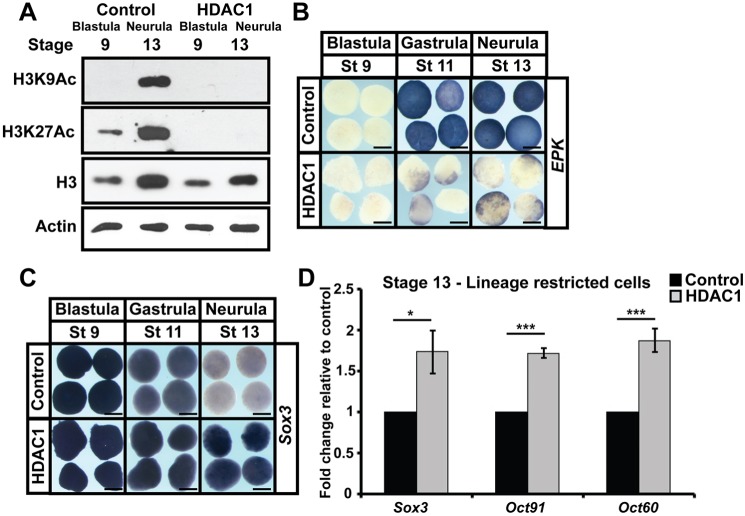
HDAC activity promotes retention of pluripotency
Given that low levels of H3K9Ac and H3K27Ac correlate with pluripotency, we wondered whether increasing HDAC activity might promote or sustain pluripotency gene expression animal pole cells. mRNA encoding HDAC1 was injected into two-cell Xenopus embryos targeting the animal pole, and explants were isolated at blastula stages. These explants displayed dramatically reduced levels of H3K9Ac and H3K27Ac relative to control explants (Fig. 6A). When we examined the changes in gene expression characteristic of progression from the pluripotent to lineage-restricted state in these explants, we found that HDAC1 activity promoted the sustained expression of sox3 [96%, n=28 (control: 0%, n=29)] and oct91 and oct60 (Fig. 6C,D), and a failure to initiate epk expression [90%, n=29 (control: 3%, n=30)] (Fig. 6B), consistent with a retention of pluripotency. Similar increases in sox3 expression were observed when HDAC1 (87%, n=31) or HDAC2 (81.5%, n=38) or HDAC1 and -2 in combination (86%, n=36; control: 5%, n=40) were expressed at high levels, suggesting that HDAC1/2 is functionally redundant in this context (Fig. S5B). No changes were observed in the expression of nrp1 or sox11 (neural markers) upon HDAC1 overexpression, confirming that these explants were not defaulting to a neural state [nrp1: Chd: 100%, n=28, HDAC: 0%, n=29), (sox11: Chd: 100%, n=28, HDAC1: 0%, n=28)] (Fig. S5C).
Fig. 6.
HDAC1 activity promotes pluripotency gene expression at the expense of lineage restriction. (A) Western blot analysis of lysates of aging animal pole explants from control embryos or embryos injected with HDAC1 mRNA examining H3K9Ac and H3K27Ac alongside total H3 and actin levels. Explants were grown alongside sibling embryos until late blastula (stage 9) and neural plate (stage 13) stages. (B,C) In situ hybridization examining epk and sox3 expression in aging animal pole explants from control embryos or embryos injected with HDAC1 mRNA. Explants were cultured alongside sibling embryos until late blastula (stage 9), mid-gastrula (stage 11) and neural plate (stage 13) stages. (D) qRT-PCR of explants from control embryos or embryos injected with HDAC1 mRNA examining expression of pluripotency genes (*P<0.05, ***P<0.005). Explants were cultured alongside sibling embryos until the neural plate stage (stage 13). Scale bars: 250 μm.
HDAC activity promotes the neural crest state
We have proposed that neural crest cells arise as a consequence of retaining characteristics of earlier, pluripotent blastula cells. Therefore, we would expect neural crest cells, like pluripotent blastula cells, to be characterized by low levels of histone acetylation, and we hypothesized that increased HDAC activity might help promote reprogramming to a neural crest state.
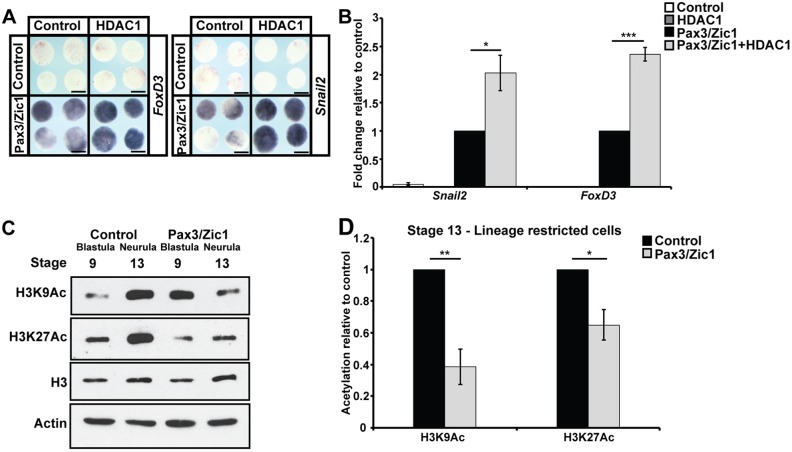
To test this, Pax3 and Zic1 were expressed in animal pole cells at levels that only weakly promoted the expression of neural crest markers. Strikingly, we found that co-expression of HDAC1 significantly enhanced expression of foxd3 (89%, n=36) and snail2 (81%, n=31) compared with Pax3/Zic1 alone (foxd3: 20%, n=44; snail2: 10%, n=30) (Fig. 7A,B). The ability of HDAC1 to enhance neural crest formation was consistent with the loss of neural crest observed when HDAC activity was inhibited, and suggests that proper levels of HDAC activity are crucial for the establishment of neural crest cells.
Fig. 7.
HDAC activity promotes neural crest formation. (A,B) In situ hybridization (A) and qRT-PCR (B) examining snail2 and foxd3 expression in animal cap explants induced with levels of Pax3/Zic1 titrated for weak neural crest establishment, with/without co-expression of HDAC1 (*P<0.05, ***P<0.005). Explants were cultured with sibling embryos until late neurula stages (stage 18). (C,D) Western blot analysis of lysates of control explants and explants induced with Pax3/Zic1 examining H3K9Ac and H3K27Ac alongside total H3 levels via chemiluminescence (C) and quantified using Odyssey (D) (*P<0.05, **P<0.01). Explants were cultured alongside sibling embryos until late blastula (stage 9) and neural plate (stage 13) stages. Scale bars: 250 μm.
A prediction of our revised model for neural crest origins is that Pax3/Zic1-mediated reprogramming to a neural crest state should at least partially preserve or restore the levels of H3K9Ac and H3K27Ac characteristic of pluripotent blastula cells. To test this, we examined H3K9Ac and H3K27Ac levels in the Pax3/Zic1 reprogrammed explants. We found that, by stage 13, the levels of these marks were significantly reduced in Pax3/Zic1 explants compared with age-matched control explants, and approximated the levels found at stage 9, when control explants are pluripotent (Fig. 7C,D). These data indicate that neural crest cells and pluripotent blastula cells share key aspects of their epigenetic state. Interestingly, although we observed an initial increase in H3K9Ac levels at stage 9, which likely accompany transcriptional changes occurring in response to Pax3/Zic1 activity, no similar increase in H3K27Ac was observed (Fig. 7C, Fig. S5E). Taken together, our findings demonstrate an essential role for HDAC activity in promoting both the pluripotent and neural crest states, and suggest that retaining the histone marks characteristic of pluripotent blastula cells is a key aspect of establishing the neural crest stem cell population.
DISCUSSION
The year 2018 marks the 150th anniversary of the discovery of the neural crest, the primary synapomorphy of vertebrates, by Wilhelm His in 1868 (His, 1868). The neural crest is distinguished by its retention of stem cell attributes long past the time when neighboring cells in the early embryo have undergone lineage restriction. Understanding the mechanisms underlying this maintenance of pluripotency is key to understanding the evolution of vertebrates, and is important for leveraging the power of these cells for regenerative medicine. In this study, we report a novel role for HDAC activity and histone acetylation in the maintenance of pluripotency and the genesis of neural crest cells in Xenopus. We showed that HDAC activity is required for the formation of the neural crest and for the pluripotency of the blastula stem cells it is derived from. Inhibition of HDAC activity using the chemical inhibitors TSA or VPA resulted in precocious expression of markers of multiple lineages, and an accompanying inability to commit to a specific lineage fate. Finally, we showed that pluripotent blastula cells and neural crest cells are both characterized by low levels of H3K9Ac and H3K27Ac acetylation, and that increased HDAC1 activity promotes reprogramming to a neural crest state. Together, these findings provide novel insights into the epigenetic mechanisms that control the maintenance of pluripotency in the early embryo, and show that regulation of HDAC activity is essential to establishing neural crest stem cells.
Our study is the first to functionally examine the epigenetic control of pluripotency in the naïve blastula cells of early Xenopus embryos, and its contribution to the retention of potential that underlies the genesis of the neural crest. By contrast, several studies have examined a role for epigenetic regulation in the later maintenance, migration and differentiation of neural crest cells in other systems. Epigenomic profiling of human neural crest cells derived from human (h)ESCs revealed several neural crest-specific enhancers that were marked by H3K27Ac and H3K4me1 and correlated with previously identified chick neural crest enhancer regions (Rada-Iglesias et al., 2012). This suggests that the neural crest state is under tight regulation by chromatin remodelers. The de novo methyltransferase DNMT3A has been shown to be expressed predominantly in the neural crest in avian embryos, and its loss results in downregulation of neural crest-specifier genes (Hu et al., 2012). Moreover, Jmjd2A, a histone demethylase, has been shown to be recruited to the promoter regions of Snail2 and Sox10 in chick embryos (Strobl-Mazzulla et al., 2010). Likewise, it has been shown that chromatin remodeler CHD7 associated with PBAF is essential for the activation of the neural crest transcriptional circuitry (Bajpai et al., 2010). Interestingly, during formation of the craniofacial skeleton in mouse, premigratory neural crest cells display prepatterned, poised (H3K27me3/H3K4me2) chromatin confirmation states that are maintained in postmigratory cells and might contribute to the plasticity that allows position-specific cues to direct neural crest differentiation post migration (Minoux et al., 2017).
HDACs have also been linked to the regulation of neural crest transcription, migration and differentiation. For example, Hdac1 has been shown to physically interact with Ets1 to control the expression of id3, a BMP target gene in Xenopus laevis (Wang et al., 2015). Similarly, Snail2 has been shown to recruit the HDAC-Sin3A complex to repress Cad6B expression, suggesting that HDAC activity is important for epithelial-mesenchymal transition (EMT) and migration (Strobl-Mazzulla and Bronner, 2012). Interestingly, injection of HDAC inhibitors into the closing neural tube of chick embryos resulted not only in defects in neural tube closure, but also in defects in dorsal neural tube patterning and increased expression of a subset of neural crest markers, including Pax3 and Sox10 (Murko et al., 2013). This suggests that HDAC activity has distinct consequences for neural crest cells at different developmental time points. Indeed, at later stages, HDAC activity has been shown to regulate the formation of several neural crest derivatives, including cartilage, melanocytes, cardiomyocytes and components of the peripheral nervous system (PNS), and has also been shown to be required for tail regeneration in Xenopus (Cunliffe and Casaccia-Bonnefil, 2006; Ignatius et al., 2008, 2013; Jacob et al., 2014; Montgomery et al., 2007; Pillai et al., 2004; Tseng et al., 2011).
Not surprisingly, HDAC inhibition has developmental consequences beyond the neural crest. In mouse, germ-line deletion of HDACs results in embryonic lethality at embryonic day (E) 10.5, indicating a requirement for HDAC function during key early developmental decisions in this system (Lagger et al., 2002; Montgomery et al., 2007). Similarly, treatment of gastrula or later-stage Xenopus embryos with VPA or other HDAC inhibitors results in severe developmental abnormalities, such as axial malformations and neural tube defects (Almouzni et al., 1994; Gurvich et al., 2005). Nevertheless, our finding that, in early embryos, neural crest cells and NPB cells are more sensitive to HDAC inhibition than are other cell types, including CNS and epidermal cells, suggests a crucial link to the regulation of developmental potential. Consistent with this, several studies have shown that HDACs are fundamental for the maintenance of pluripotency and/or appropriate differentiation in cultured ESCs (Dovey et al., 2010; Jamaladdin et al., 2014; Kidder and Palmer, 2012). In concordance with our findings here, HDAC inhibition in ESC cultures has been shown to result in both negative and positive changes in gene expression (Jamaladdin et al., 2014; Karantzali et al., 2008; Zupkovitz et al., 2006). Interestingly, genome-wide mapping of HDAC occupancy has found their enrichment on some active genes that might be regulated by a dynamic cycle of histone acetylation and deacetylation (Wang et al., 2009).
Although there is a wealth of literature on the role of HDACs in the maintenance of pluripotency in cultured ESCs, a single clear model has not emerged. HDAC-containing complexes have been shown to assemble on, and promote expression of, pluripotency genes in ESCs and pre-iPSCs, suggestive of a positive regulatory role for HDACs in pluripotency (Baltus et al., 2009; Saunders et al., 2017). Moreover, the reported effects of HDAC inhibition in cultured ESCs appear to vary significantly dependent on the inhibitor utilized, the concentration and duration of treatment and the source of the stem cells (murine versus human). Studies in cultured mouse and human ESCs revealed that levels of histone acetylation (H3K9/14Ac) increase and decrease dynamically when the cells are induced to differentiate (Hezroni et al., 2011; Liu et al., 2015; Markowetz et al., 2010; Melcer et al., 2012; Moussaieff et al., 2015; Qiao et al., 2015). Moreover, HDAC inhibition during differentiation of mouse epiblast stem cells (mEpiSCs) prevents neural differentiation, whereas, in hESCs, the effects are time dependent; early inhibition leads to maintenance of pluripotency, whereas later inhibition promotes neural fate commitment (Liu et al., 2015; Qiao et al., 2015; Yang et al., 2014). It has been suggested that HDAC inhibition promotes the progression of naïve murine (m)ESCs towards a primed mEpiSC state, and hESCs to an earlier state, but in both cases, it promotes self-renewal of these cells (Ware et al., 2009).
Our findings in early Xenopus embryos demonstrate a clear role for HDAC activity in the maintenance of pluripotency in naïve blastula stem cells. Importantly, we further showed that maintenance of this activity is linked to the events that preserve the potency in a subset of these cells, leading to formation of the neural crest. Interestingly, inhibition of HDAC activity in blastula explants led to enhanced expression of genes linked to multiple lineage states, in addition to a loss of pluripotency markers, yet these cells were unable to give rise to any lineage tested. However, in the context of whole embryos, HDAC inhibition promoted enhanced neural lineage commitment in at least some cells.
Significantly, we found that low levels of H3K9Ac and H3K27Ac acetylation are a shared feature of both pluripotent blastula cells and neural crest cells, building on our recent work showing that a requirement for high Map kinase activity and low Akt activity is similarly shared by these two cell types (Geary and LaBonne, 2018). Importantly, we also found that increased HDAC1 activity can enhance reprogramming to a neural crest state, which could have implications for regenerative medicine. Although future work will need to address the genome occupancy of HDAC1 at genes up- and downregulated following HDAC inhibition, the current study sheds important new light on the epigenetic mechanisms that control the maintenance of pluripotency and the establishment of neural crest stem cells.
MATERIALS AND METHODS
Embryological methods
Wild-type Xenopus laevis embryos were obtained using standard methods and staged according to Nieuwkoop and Faber (1994). For animal cap assays, ectodermal explants were manually dissected at early blastula (stage 8-9) from embryos microinjected with the indicated mRNA and/or treated with the specified inhibitor at the two-cell stage, and cultured in 1×Marc’s Modified Ringer's Solution (MMR) [0.1 M NaCl, 2 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM HEPES (pH 7.8), 0.1 mM EDTA] until sibling embryos reached the denoted stage. mRNA for microinjection was in vitro transcribed from a linearized DNA template using the SP6 mMessage mMachine Kit (Ambion). Pax3-GR and Zic1-GR expression explants were dissected from embryos treated at Stage 8 with 10 μM dexamethasone (Sigma-Aldrich) as previously described (Buitrago-Delgado et al., 2015). For morpholino experiments, a translation-blocking HDAC1 morpholino (Gene Tools, Sequence: 5′ GAGTCAGCGCCATTTTCCTTC 3′) was injected at the eight-cell stage in isolation or co-injected with HDAC1/2 mRNA. For activin experiments, animal cap explants from control or inhibitor-treated embryos were dissected at blastula stages and cultured with recombinant activin protein (R&D Systems) at a final concentration of 20-40 ng/ml for mesoderm, and 100 ng/ml for endoderm induction in 1×MMR supplemented with 0.1% bovine serum albumin (BSA) as a carrier. Manipulated embryos and/or explants were processed for in situ hybridization by fixing in 1×MEM [100mM MOPS (pH 7.4), 2mM EDTA, 1mM MgSO4] + 4% formaldehyde and dehydrating in 100% methanol. In situ hybridization was performed using digoxigenin-labeled RNA probes and developed using BM Purple substrate (Roche) (LaBonne and Bronner-Fraser, 1998). For TUNEL staining, dimethyl sulfoxide (DMSO)/TSA-treated explants were processed alongside DNMT3B mRNA-injected embryos (positive control) and processed as previously described (Bellmeyer et al., 2003). Results shown are representative of at least three independent experiments.
Western blot analysis
Animal cap explants (20-40 explants) were collected at the indicated stages and lysed in TNE lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5 mM EDTA, and 0.5% Triton X-100] supplemented with protease inhibitors [Aprotinin, Leupeptin and phenylmethylsulfonyl fluoride (PMSF)] and a complete Mini tablet (Roche). SDS-PAGE and western blot analyses were used to detect proteins and modifications using the following antibodies: H3K9Ac (#9649, Cell Signaling, 1:2000), H3K27Ac (ab4729, Abcam, 1:2000), H3 (#3638 and #4499, Cell Signaling, 1:1000) and Actin (A2066, Sigma-Aldrich, 1:4000). For enhanced chemiluminescence-based detection, horseradish peroxidase (HRP)-conjugated secondary antibodies were used. Results shown are representative of three independent experiments.
For the detection and quantification using the Odyssey platform (LI-COR Biosciences), blots were incubated simultaneously with primary antibodies for both H3K9Ac/H3K27Ac and H3. Histone acetylation was detected using IRDye (LI-COR Biosciences) secondary antibodies, and protein amounts were quantified using the Image Studio Lite software (LI-COR Biosciences). Relative histone acetylation (H3K29Ac or H3K27Ac) was calculated against total H3 levels. The results show the mean of three independent biological replicates, with error bars depicting the standard error of mean (s.e.m.). An unpaired, two-tailed t-test was utilized to determine significance.
RNA isolation, cDNA synthesis and qRT-PCR
RNA was isolated from control or manipulated animal cap explants (10-30 explants) using Trizol (Life Technologies) followed by LiCI precipitation. 1 µg of purified RNA was used as a template for synthesizing cDNA using a High Capacity Reverse Transcription Kit (Life Technologies). Quantitative (q)RT-PCR was performed using SYBR Premix ExTaq II (Takara Bio) and detected using the Bio-Rad CFX96 Connect system. The primer sequences used are available in the supplementary figures. Expression was normalized to ornithine decarboxylase (ODC) and the -fold change was calculated relative to control samples of the same stage. The results show the mean of at least three independent biological replicates, with error bars depicting the s.e.m. An unpaired, two-tailed t-test was utilized to determine significance.
Immunofluorescence and transgenic analysis
The Tubb2-GFP transgenic line was obtained from the National Xenopus Resource (www.mbl.edu/xenopus/) and generation of this line has been described elsewhere (Huang et al., 2007). Wild-type and Tubb2-GFP transgenic X. laevis embryos were blocked in whole-mount block solution (WMBS; 155 mM NaCl, 10 mM Tris-HCl, pH 7.5, 10% FBS, 5% DMSO) and incubated overnight with primary antibodies: 12/101-Actin (DSHB, 1:15), GFP (rabbit GFP, A-11122, Life Technologies, 1:250) and E7-Tubulin (DSHB, 1:100). After washing with Tris Buffer Saline with 0.1% Triton X-100, the embryos were re-blocked in WMBS and incubated overnight with secondary antibodies: goat anti-rabbit-Alexa Fluor 488 (A-11008, Life Technologies; 1:250) and goat anti-mouse-Cy3 (115-165-146, Jackson Laboratories; 1:500).
DNA constructs and inhibitors
Full-length X. laevis HDAC1 and HDAC2 clones were obtained from the Xenopus ORFeome (www.xenbase.org/reagents/static/orfeome.jsp) and subcloned into a pCS2 vector for microinjection, and into a pGEM-T vector for RNA probe synthesis. Pax3-GR and Zic1-GR constructs were a kind gift from Jean-Pierre Saint-Jeannet (New York University, NY, USA). For HDAC inhibition, embryos and/or explants were treated with TSA (Sigma-Aldrich) at a final concentration of 200-500 nM or VPA (Sigma-Aldrich) at a final concentration of 10-20 mM at the noted stage, or with Romidepsin (Sigma-Aldrich) at a final concentration of 5-15 µM.
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee, Northwestern University, and are in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Supplementary Material
Acknowledgements
We thank Joe Nguyen, Thomas Yeo and Fidak Khan for invaluable technical assistance, and members of the laboratory for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.L.; Formal analysis: C.L., A.R.; Investigation: A.R.; Resources: C.L.; Writing - original draft: A.R.; Writing - review & editing: C.L.; Supervision: C.L.; Funding acquisition: C.L.
Funding
This work was supported by the National Institutes of Health (R01GM116538 to C.L.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.163386.supplemental
References
- Almouzni G., Khochbin S., Dimitrov S. and Wolffe A. P. (1994). Histone acetylation influences both gene expression and development of Xenopus laevis. Dev. Biol. 165, 654-669. 10.1006/dbio.1994.1283 [DOI] [PubMed] [Google Scholar]
- Ariizumi T. and Asashima M. (2001). In vitro induction systems for analyses of amphibian organogenesis and body patterning. Int. J. Dev. Biol. 45, 273-279. [PubMed] [Google Scholar]
- Asashima M., Nakano H., Shimada K., Kinoshita K., Ishii K., Shibai H. and Ueno N. (1990a). Mesodermal induction in early amphibian embryos by activin A (erythroid differentiation factor). Roux's Arch. Dev. Biol. 198, 330-335. 10.1007/BF00383771 [DOI] [PubMed] [Google Scholar]
- Asashima M., Nakano H., Uchiyama H., Davids M., Plessow S., Loppnow-Blinde B., Hoppe P., Dau H. and Tiedemann H. (1990b). The vegetalizing factor belongs to a family of mesoderm-inducing proteins related to erythroid differentiation factor. Naturwissenschaften 77, 389-391. 10.1007/BF01135742 [DOI] [PubMed] [Google Scholar]
- Atlasi Y. and Stunnenberg H. G. (2017). The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 18, 643-658. 10.1038/nrg.2017.57 [DOI] [PubMed] [Google Scholar]
- Bajpai R., Chen D. A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C.-P., Zhao Y., Swigut T. and Wysocka J. (2010). CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463, 958-962. 10.1038/nature08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus G. A., Kowalski M. P., Tutter A. V. and Kadam S. (2009). A positive regulatory role for the mSin3A-HDAC complex in pluripotency through Nanog and Sox2. J. Biol. Chem. 284, 6998-7006. 10.1074/jbc.M807670200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmeyer A., Krase J., Lindgren J. and LaBonne C. (2003). The protooncogene c-myc is an essential regulator of neural crest formation in xenopus. Dev. Cell 4, 827-839. 10.1016/S1534-5807(03)00160-6 [DOI] [PubMed] [Google Scholar]
- Bogdanovic O., Fernandez-Miñán A., Tena J. J., de la Calle-Mustienes E., Hidalgo C., van Kruysbergen I., van Heeringen S. J., Veenstra G. J. C. and Gomez-Skarmeta J. L. (2012). Dynamics of enhancer chromatin signatures mark the transition from pluripotency to cell specification during embryogenesis. Genome Res. 22, 2043-2053. 10.1101/gr.134833.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Delgado E., Nordin K., Rao A., Geary L. and LaBonne C. (2015). Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 348, 1332-1335. 10.1126/science.aaa3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro K., Donnet C., Rejtar T., Karger B. L., Barisone G. A., Díaz E., Kortagere S., Lemire J. M. and Levin M. (2011). Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev. Biol. 11, 29 10.1186/1471-213X-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton M. P., Cheng A. W., Welstead G. G., Kooistra T., Carey B. W., Steine E. J., Hanna J., Lodato M. A., Frampton G. M., Sharp P. A. et al. (2010). Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 107, 21931-21936. 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe V. T. and Casaccia-Bonnefil P. (2006). Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech. Dev. 123, 24-30. 10.1016/j.mod.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Dovey O. M., Foster C. T. and Cowley S. M. (2010). Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA 107, 8242-8247. 10.1073/pnas.1000478107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumai R., Matsuyama A., Kobashi N., Lee K.-H., Nishiyama M., Nakajima H., Tanaka A., Komatsu Y., Nishino N., Yoshida M. et al. (2002). FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 62, 4916-4921. [PubMed] [Google Scholar]
- Geary L. and LaBonne C. (2018). FGF mediated MAPK and PI3K/Akt Signals make distinct contributions to pluripotency and the establishment of Neural Crest. eLife 7, 139 10.7554/eLife.33845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlicher M., Minucci S., Zhu P., Krämer O. H., Schimpf A., Giavara S., Sleeman J. P., Lo Coco F., Nervi C., Pelicci P. G. et al. (2001). Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20, 6969-6978. 10.1093/emboj/20.24.6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. K. and LaBonne C. (2014). Setting appropriate boundaries: Fate, patterning and competence at the neural plate border. Dev. Biol. 389, 2-12. 10.1016/j.ydbio.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Wills A., Ucar D. and Baker J. (2014). Developmental enhancers are marked independently of zygotic Nodal signals in Xenopus. Dev. Biol. 395, 38-49. 10.1016/j.ydbio.2014.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich N., Berman M. G., Wittner B. S., Gentleman R. C., Klein P. S. and Green J. B. A. (2005). Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 19, 1166-1168. 10.1096/fj.04-3425fje [DOI] [PubMed] [Google Scholar]
- Hall B. K. (2000). The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol. Dev. 2, 3-5. 10.1046/j.1525-142x.2000.00032.x [DOI] [PubMed] [Google Scholar]
- Hezroni H., Sailaja B. S. and Meshorer E. (2011). Pluripotency-related, valproic acid (VPA)-induced genome-wide histone H3 lysine 9 (H3K9) acetylation patterns in embryonic stem cells. J. Biol. Chem. 286, 35977-35988. 10.1074/jbc.M111.266254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- His W. (1868). Untersuchungen über die erste Anlage des Wirbeltierleibes: die erste Entwickelung des Hühnchens im Ei. Leipzig: Vogel FCW. [Google Scholar]
- Hong C.-S. and Saint-Jeannet J.-P. (2007). The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol. Biol. Cell. 18, 2192-2202. 10.1091/mbc.e06-11-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontelez S., van Kruijsbergen I., Georgiou G., van Heeringen S. J., Bogdanovic O., Lister R. and Veenstra G. J. C. (2015). Embryonic transcription is controlled by maternally defined chromatin state. Nat. Commun. 6, 10148 10.1038/ncomms10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Strobl-Mazzulla P., Sauka-Spengler T. and Bronner M. E. (2012). DNA methyltransferase3A as a molecular switch mediating the neural tube-to-neural crest fate transition. Genes Dev. 26, 2380-2385. 10.1101/gad.198747.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Strobl-Mazzulla P. H. and Bronner M. E. (2014). Epigenetic regulation in neural crest development. Dev. Biol. 396, 159-168. 10.1016/j.ydbio.2014.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. K., Dorey K., Ishibashi S. and Amaya E. (2007). BDNF promotes target innervation of Xenopus mandibular trigeminal axons in vivo. BMC Dev. Biol. 7, 59 10.1186/1471-213X-7-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. S., Moose H. E., El-Hodiri H. M. and Henion P. D. (2008). colgate/hdac1 Repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev. Biol. 313, 568-583. 10.1016/j.ydbio.2007.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. S., Unal Eroglu A., Malireddy S., Gallagher G., Nambiar R. M. and Henion P. D. (2013). Distinct functional and temporal requirements for zebrafish Hdac1 during neural crest-derived craniofacial and peripheral neuron development. PLoS ONE 8, e63218 10.1371/journal.pone.0063218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C., Lötscher P., Engler S., Baggiolini A., Varum Tavares S., Brügger V., John N., Buchmann-Moller S., Snider P. L., Conway S. J. et al. (2014). HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia. J. Neurosci. 34, 6112-6122. 10.1523/JNEUROSCI.5212-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaladdin S., Kelly R. D. W., O'Regan L., Dovey O. M., Hodson G. E., Millard C. J., Portolano N., Fry A. M., Schwabe J. W. R. and Cowley S. M. (2014). Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. USA 111, 9840-9845. 10.1073/pnas.1321330111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantzal E., Schulz H., Hummel O., Hubner N., Hatzopoulos A. K. and Kretsovali A. (2008). Histone deacetylase inhibition accelerates the early events of stem cell differentiation: transcriptomic and epigenetic analysis. Genome Biol. 9, R65 10.1186/gb-2008-9-4-r65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmodiya K., Krebs A. R., Oulad-Abdelghani M., Kimura H. and Tora L. (2012). H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics 13, 424 10.1186/1471-2164-13-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder B. L. and Palmer S. (2012). HDAC1 regulates pluripotency and lineage specific transcriptional networks in embryonic and trophoblast stem cells. Nucleic Acids Res. 40, 2925-2939. 10.1093/nar/gkr1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C. and Bronner-Fraser M. (1998). Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403-2414. [DOI] [PubMed] [Google Scholar]
- LaBonne C. and Bronner-Fraser M. (2000). Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev. Biol. 221, 195-205. 10.1006/dbio.2000.9609 [DOI] [PubMed] [Google Scholar]
- Lagger G., O'Carroll D., Rembold M., Khier H., Tischler J., Weitzer G., Schuettengruber B., Hauser C., Brunmeir R., Jenuwein T. et al. (2002). Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 21, 2672-2681. 10.1093/emboj/21.11.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. M., Knecht A. K., Smith W. C., Stachel S. E., Economides A. N., Stahl N., Yancopolous G. D. and Harland R. M. (1993). Neural induction by the secreted polypeptide noggin. Science 262, 713-718. 10.1126/science.8235591 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. and Dupin E. (2012). The neural crest in vertebrate evolution. Curr. Opin. Genet. Dev. 22, 381-389. 10.1016/j.gde.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Li M., Liu G.-H. and Belmonte J. C. I. (2012). Navigating the epigenetic landscape of pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 13, 524-535. 10.1038/nrm3393 [DOI] [PubMed] [Google Scholar]
- Liang G. and Zhang Y. (2012). Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 23, 49-69. 10.1038/cr.2012.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Dou X., Liu C., Wang L., Xing C., Peng G., Chen J., Yu F., Qiao Y., Song L. et al. (2015). Histone deacetylation promotes mouse neural induction by restricting Nodal-dependent mesendoderm fate. Nat. Commun. 6, 6830 10.1038/ncomms7830 [DOI] [PubMed] [Google Scholar]
- Markowetz F., Mulder K. W., Airoldi E. M., Lemischka I. R. and Troyanskaya O. G. (2010). Mapping dynamic histone acetylation patterns to gene expression in nanog-depleted murine embryonic stem cells. PLoS Comput. Biol. 6, e1001034 10.1371/journal.pcbi.1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcer S., Hezroni H., Rand E., Nissim-Rafinia M., Skoultchi A., Stewart C. L., Bustin M. and Meshorer E. (2012). Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nat. Commun. 3, 910 10.1038/ncomms1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milet C. and Monsoro-Burq A. H. (2012). Neural crest induction at the neural plate border in vertebrates. Dev. Biol. 366, 22-33. 10.1016/j.ydbio.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Minoux M., Holwerda S., Vitobello A., Kitazawa T., Kohler H., Stadler M. B. and Rijli F. M. (2017). Gene bivalency at Polycomb domains regulates cranial neural crest positional identity. Science 355, eaal2913 10.1126/science.aal2913 [DOI] [PubMed] [Google Scholar]
- Montgomery R. L., Davis C. A., Potthoff M. J., Haberland M., Fielitz J., Qi X., Hill J. A., Richardson J. A. and Olson E. N. (2007). Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21, 1790-1802. 10.1101/gad.1563807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A., Rouleau M., Kitsberg D., Cohen M., Levy G., Barasch D., Nemirovski A., Shen-Orr S., Laevsky I., Amit M. et al. (2015). Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 21, 392-402. 10.1016/j.cmet.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Murko C., Lagger S., Steiner M., Seiser C., Schoefer C. and Pusch O. (2013). Histone deacetylase inhibitor Trichostatin A induces neural tube defects and promotes neural crest specification in the chicken neural tube. Differentiation 85, 55-66. 10.1016/j.diff.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D. and Faber J. (ed.) (1994). Normal Table of Xenopus laevis (Daudin). New York: Garland Publishing. [Google Scholar]
- Nordin K. and LaBonne C. (2014). Sox5 is a DNA-binding cofactor for BMP R-Smads that directs target specificity during patterning of the early ectoderm. Dev. Cell 31, 374-382. 10.1016/j.devcel.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R., Coverdale L. E., Dubey G. and Martin C. C. (2004). Histone deacetylase 1 (HDAC-1) required for the normal formation of craniofacial cartilage and pectoral fins of the zebrafish. Dev. Dyn. 231, 647-654. 10.1002/dvdy.20168 [DOI] [PubMed] [Google Scholar]
- Plouhinec J.-L., Roche D. D., Pegoraro C., Figueiredo A. L., Maczkowiak F., Brunet L. J., Milet C., Vert J.-P., Pollet N., Harland R. M. et al. (2014). Pax3 and Zic1 trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Dev. Biol. 386, 461-472. 10.1016/j.ydbio.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M. S., Sauka-Spengler T. and LaBonne C. (2012). Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Dev. Biol. 366, 10-21. 10.1016/j.ydbio.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Wang R., Yang X., Tang K. and Jing N. (2015). Dual roles of histone H3 lysine 9 acetylation in human embryonic stem cell pluripotency and neural differentiation. J. Biol. Chem. 290, 2508-2520. 10.1074/jbc.M114.603761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S. A., Flynn R. A. and Wysocka J. (2011). A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279-283. 10.1038/nature09692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Prescott S., Brugmann S. A., Swigut T. and Wysocka J. (2012). Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11, 633-648. 10.1016/j.stem.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H. and De Robertis E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336. 10.1038/376333a0 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T. and Bronner-Fraser M. (2008). A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557-568. 10.1038/nrm2428 [DOI] [PubMed] [Google Scholar]
- Saunders A., Huang X., Fidalgo M., Reimer M. H., Faiola F., Ding J., Sánchez-Priego C., Guallar D., Saenz C., Li D. et al. (2017). The SIN3A/HDAC corepressor complex functionally cooperates with NANOG to promote pluripotency. Cell Reports 18, 1713-1726. 10.1016/j.celrep.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl-Mazzulla P. H. and Bronner M. E. (2012). A PHD12-Snail2 repressive complex epigenetically mediates neural crest epithelial-to-mesenchymal transition. J. Cell Biol. 198, 999-1010. 10.1083/jcb.201203098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl-Mazzulla P. H., Sauka-Spengler T. and Bronner-Fraser M. (2010). Histone demethylase JmjD2A regulates neural crest specification. Dev. Cell 19, 460-468. 10.1016/j.devcel.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861-872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Taylor K. M. and LaBonne C. (2007). Modulating the activity of neural crest regulatory factors. Curr. Opin. Genet. Dev. 17, 326-331. 10.1016/j.gde.2007.05.012 [DOI] [PubMed] [Google Scholar]
- Tseng A.-S., Carneiro K., Lemire J. M. and Levin M. (2011). HDAC activity is required during Xenopus tail regeneration. PLoS ONE 6, e26382 10.1371/journal.pone.0026382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zang C., Cui K., Schones D. E., Barski A., Peng W. and Zhao K. (2009). Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138, 1019-1031. 10.1016/j.cell.2009.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Kam R. K. T., Shi W., Xia Y., Chen X., Cao Y., Sun J., Du Y., Lu G., Chen Z. et al. (2015). The proto-oncogene transcription factor Ets1 regulates neural crest development through histone deacetylase 1 to mediate output of bone morphogenetic protein signaling. J. Biol. Chem. 290, 21925-21938. 10.1074/jbc.M115.644864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C. B., Wang L., Mecham B. H., Shen L., Nelson A. M., Bar M., Lamba D. A., Dauphin D. S., Buckingham B., Askari B. et al. (2009). Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell 4, 359-369. 10.1016/j.stem.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Tang Y., Liu H., Guo F., Ni J. and Le W. (2014). Suppression of histone deacetylation promotes the differentiation of human pluripotent stem cells towards neural progenitor cells. BMC Biol. 12, 95 10.1186/s12915-014-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Kijima M., Akita M. and Beppu T. (1990). Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265, 17174-17179. [PubMed] [Google Scholar]
- Zhang Z., Lei A., Xu L., Chen L., Chen Y., Zhang X., Gao Y., Yang X., Zhang M. and Cao Y. (2017). Similarity in gene-regulatory networks suggests that cancer cells share characteristics of embryonic neural cells. J. Biol. Chem. 292, 12842-12859. 10.1074/jbc.M117.785865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G., Tischler J., Posch M., Sadzak I., Ramsauer K., Egger G., Grausenburger R., Schweifer N., Chiocca S., Decker T. et al. (2006). Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol. Cell. Biol. 26, 7913-7928. 10.1128/MCB.01220-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.