Abstract
Purpose
To evaluate BRAF, NRAS, and GNAQ mutations in surgical specimens of common and blue conjunctival melanocytic nevi.
Methods
Surgical specimens from 25 conjunctival melanocytic nevi (23 common and 2 blue) of 25 patients were evaluated. All common nevi were analyzed immunohistochemically for the expression of BRAF V600E or NRAS Q61R. One lesion with negative immunoreactivity and for all blue nevi, a hybridization capture-based next-generation sequencing method was employed for mutation analysis. For common nevi, genetic features were compared with clinical and histopathologic findings. Continuous variables (age at excision and largest basal diameter) were compared with a Students's t-test and all categoric variables were compared with Fisher's Exact Test.
Results
Of common melanocytic nevi, 9 (39.1%) were immunoreactive for NRASQ61R and 13 (56.5%) were immunoreactive for BRAFV600E. One common nevus, which was immunonegative for both BRAFV600E and NRASQ61R was found to harbor an NRASQ61K mutation by sequence analysis. Patients with NRAS-mutated nevi were more likely to report occurrence of the lesion prior to 18-years old and more likely to have intrinsic cysts. The mean largest basal diameter was 6.0 and 3.5 mm for NRAS- and BRAF-immunoreactive lesions, respectively (P = 0.003). GNAQ mutations were identified in each of the two blue nevi of this study.
Conclusions
These findings document that common conjunctival melanocytic nevi have mutually exclusive mutations in BRAF and NRAS. The two conjunctival blue nevi harbored GNAQ mutations. This suggests the driver mutations of conjunctival nevi are similar to those of nevi of the skin. At the molecular level, conjunctival nevi appear more like cutaneous nevi than choroidal nevi.
Keywords: conjunctiva, nevi, genetics, melanoma, eye
There are many parallels between melanocytic lesions of the skin and those of the conjunctiva. In both tissue types the originating melanocytes are derived from the neural crest and migrate toward the epithelium (or extraepithelial in the case of blue nevi) in their respective locations. There has been some evidence that they share the similar genetic underpinnings, however, this has not been fully expounded upon in the literature. For instance, cutaneous nevi have mutually exclusive mutations in NRAS and BRAF and these are shown to associate with particular clinic-pathological features.1–4 Even though conjunctival melanomas (for some of which conjunctival nevi are thought to be precursor lesions) have mutually exclusive mutations in NRAS and BRAF,5–9 only BRAF mutations have so far been documented in conjunctival nevi. Furthermore, the majority of cutaneous blue nevi have a unique genetic identity with mutations in GNAQ or GNA11, and the presence of these mutations have been confirmed in oral and central nervous system blue nevi,10–15 but not in the conjunctiva.
Thus, the purpose of this study was to evaluate the mutation spectrum of conjunctival melanocytic nevi. With the availability of mutant protein–specific antibodies we focused on documenting the distribution of BRAFV00E and NRASQ61R expression in common conjunctival melanocytic nevi and correlated the mutation status with clinicopathologic features. Using molecular studies we also document for the first time the presence of GNAQ mutations in blue nevi of the conjunctiva and the presence of an NRASQ61K mutation in a common nevus.
Methods
The institutional review board of Memorial Sloan Kettering Cancer Center and Emory University School of Medicine approved this retrospective study. It included 25 conjunctival nevi from 25 patients between September 2013 and May 2017. Lesions included in this study underwent consecutive surgical excision within the study timeframe and had histopathologic confirmation by a pathologist. Informed consent was obtained from each patient.
Patient data included sex, age, and ethnicity. Clinical data included initial occurrence of conjunctival nevus (juvenile [18 years or younger] or adulthood), age at time of excision, ocular site (bulbar, palpebral, caruncle), site of ultraviolet light exposure (intrapalpebral fissure or otherwise), iris pigmentation, largest basal diameter, degree of pigmentation (amelanotic, melanotic, deeply melanotic, or amelanotic/melanotic), thickness (flat or raised), and presence of intrinsic cysts, intrinsic vessels, or sentinel vessels. Location was defined histopathologically (compound, subepithelial, etc.). Clinical data was not available for the de-identified conjunctival blue nevus specimens. Representative clinical images are shown in Figures 1, 2, and 3.
Figure 1.
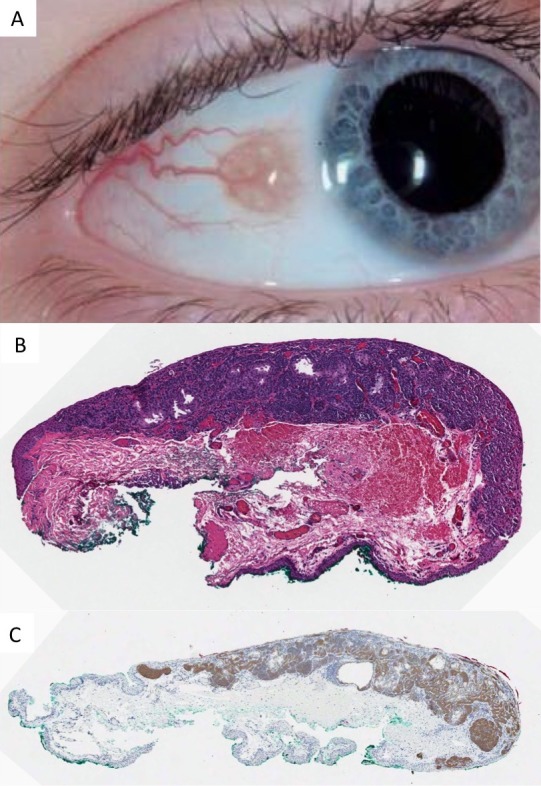
Conjunctival nevus occurring in childhood. (A) Clinical photo demonstrating intrinsic cysts, diameter of 3.5 mm and amelanotic appearance. (B) Histopathology of a compound melanocytic nevus with epithelial cysts and evidence of maturation (×10, hematoxylin and eosin). (C) The junctional and subepithelial melanocytes are immunohistochemically positive for NRASQ61R (×10, peroxidase antiperoxidase).
Figure 2.
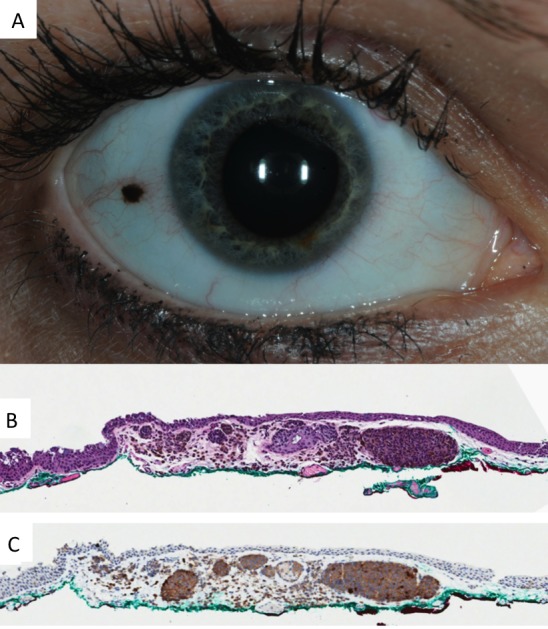
Conjunctival nevus occurring in adulthood. (A) Clinical photo demonstrating absence of intrinsic cysts, diameter of 1.5 mm and a deeply melanotic appearance. (B) Histopathology of a melanocytic nevus (×10, hematoxylin and eosin). (C) The melanocytes are immunoreactive for BRAFV600E (×10, peroxidase antiperoxidase).
Figure 3.
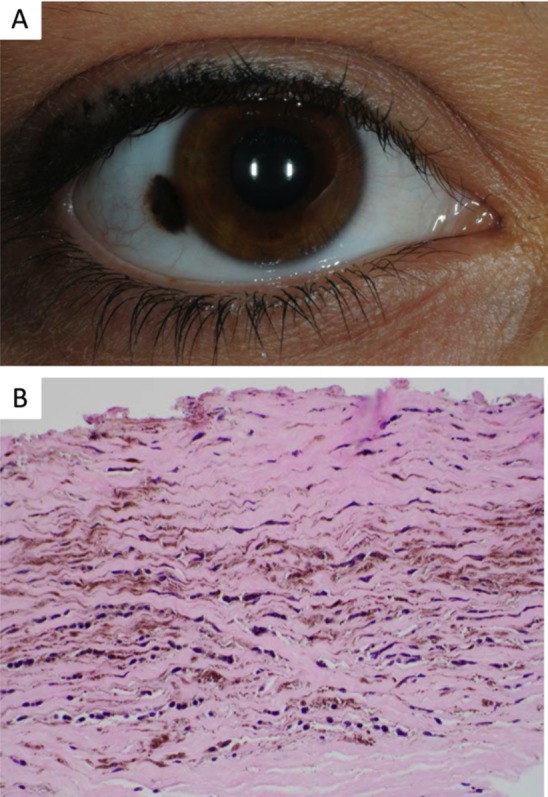
Conjunctival blue nevus. (A) Clinical photo demonstrating heterochromic bulbar lesion with areas of deep melanosis. (B) Histopathology of a blue nevus characterized by pigmented slender fusiform and dendritic melanocytes in the subepithelial stroma (×10, hematoxylin and eosin).
Tissue sections, 5-μm thick were cut from formalin-fixed and paraffin-embedded tissue blocks. For detection of BRAFV600E, an automated immunohistochemical system (Ventana BenchMark XT; Ventana Medical Systems, Inc., Tucson, AZ, USA) was used with the commercially available mouse monoclonal antibody VE1 (anti-BRAFV600E; Ventana). For NRASQ61R detection the commercially available rabbit monoclonal antibody SP174 (Spring Bioscience, Pleasanton, CA, USA) was used with the Leica Bond detection system (Leica, Wetzlar, Germany). The staining result was recorded as either positive or negative. In cases, all lesional cells were either homogeneous immunoreactive for the respective marker or completely negative.
For the single sample that did not reveal a mutation by the above method and the blue nevi, the MSKCC IMPACT assay was used as previously described,16 on formalin-fixed paraffin embedded tissue.
For statistical analysis, continuous variables (age, age at excision, and largest basal diameter) were compared with a Student's t-test and all categoric variables (clinicopathologic parameters) were compared with Fisher's Exact Test. Analysis was performed with Prism 7 (Graphpad Software, Inc., La Jolla, CA, USA) and a P value < 0.05 was considered statistically significant.
Results
Of nevi, nine (40.9%) were immunoreactive for NRAS Q61R and 13 (56.5%) were immunoreactive for BRAFV600E mutations. One specimen lacked immunoreactivity for all immunohistochemistry targets, for which the MSK-IMPACT assay identified an NRASQ61K mutation. Both blue conjunctival nevi had a mutation in GNAQ (Q209L and Q209H) detected by MSK-IMPACT.
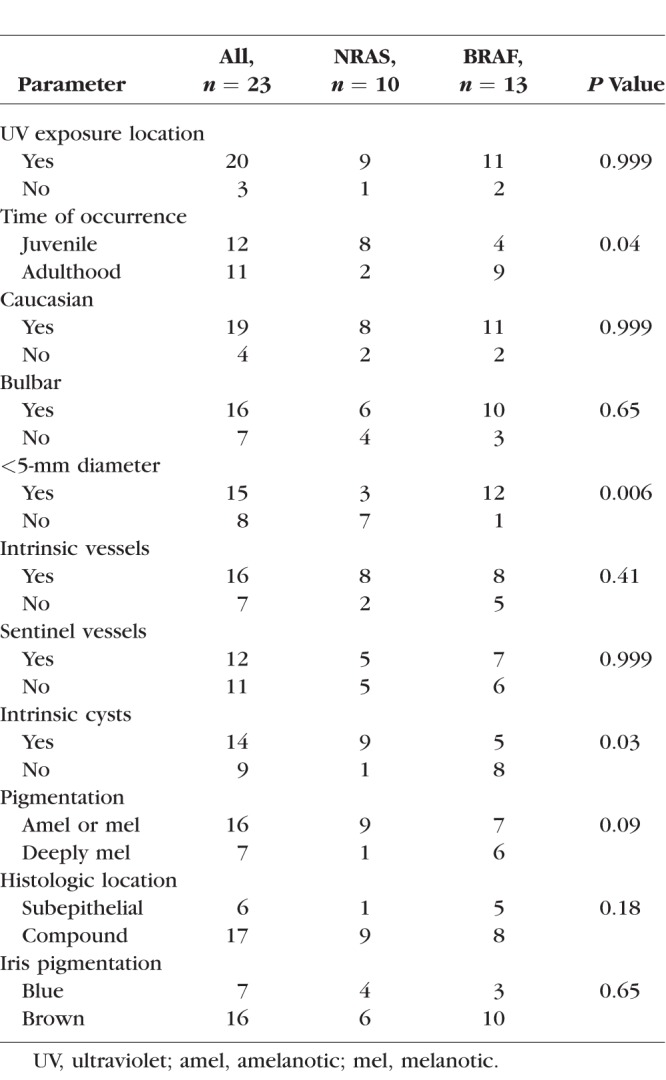
For the common nevi, the median age at the time of excision was 13.5 and 29 years for patients with an NRAS and BRAF mutated lesion, respectively (P = 0.09). The Table shows associations of NRAS and BRAF expression or mutational status with clinicopathologic features. There were three parameters that showed a statistical difference between patients with an NRAS- and BRAF-immunoreactive lesion: patients with NRAS-immunoreactive lesions were significantly more likely to report occurrence of the lesion prior to 18-years old. In addition, NRAS-immunoreactive lesions were significantly more likely to have intrinsic cysts and have a largest basal diameter more than 5 mm. The mean largest basal diameter was 6.0 and 3.5 mm for NRAS- and BRAF-immunoreactive lesions, respectively (P = 0.003).
Table.
Associations of NRAS and BRAF Expression or Mutational Status With Clinicopathologic Features in Common Conjunctival Nevi
At a mean follow-up of 13.1 months, there were not events of recurrence or malignant transformation.
Discussion
Ocular nevi differ in their genetic underpinnings depending on the anatomical derivation of their melanocytes. Nevi derived from uveal tract melanocytes harbor GNAQ/11 mutations.17 On the contrary, conjunctival nevi are more akin to their cutaneous counterpart and have been shown to have BRAF mutations.7 The present study confirms the similarity between cutaneous and conjunctival nevi by identifying further genetic aberrations and associations with clinical features that are found in cutaneous nevi.
Cutaneous nevi have NRAS and BRAF mutations.1,2 BRAF is a serine-threonine kinase and NRAS an isoform of the RAS family of GTPase proteins; and both activate the MAPK signaling cascade, which leads cell cycle progression and cell proliferation. Similarly, for all the conjunctival nevi in this study, immunoreactivity and mutational analysis allowed for identification of either an NRAS or BRAF aberration. For this cohort, NRAS and BRAF mutations occurred in a mutually exclusive manner, with just over half the lesions exhibiting immunoreactivity with the antibody VE1, thereby indicating the presence of a BRAFV600E mutation.
For cutaneous nevi, the presence of either a BRAF or NRAS mutation has been associated with specific clinical features.1–4 For instance, congenital cutaneous nevi are more likely to harbor NRAS mutations, while acquired nevi have a propensity toward BRAF mutations.1–3 One prior study on conjunctival nevi, that evaluated for BRAF mutations only, found no difference in the proportion of children and adults with mutant lesions.7 On the contrary, in our series, NRAS mutations were statistically more frequent in patients reporting the occurrence of their conjunctival nevus prior to the age of 18 years. However, the retrospective nature of this study limited our understanding of each lesion's occurrence to patient reporting. Furthermore, conjunctival nevi, particularly those that are congenital, are commonly amelanotic and difficult to fully appreciate overlying the white sclera; thereby, possibly influencing the recognition of their true occurrence date.
In cutaneous nevi, there is evidence to suggest that larger congenital lesions more commonly harbor NRAS mutations, while smaller lesions may have either BRAF or NRAS mutations.2 In line with this finding, in the present study, conjunctival nevi with a basal diameter greater than 5 mm were statistically more likely to have NRAS-immunoreactivity (P = 0.006). Evaluated another way, the mean largest basal diameter was significantly greater (almost double) in NRAS-immunoreactive lesions compared with BRAF-immunoreactive nevi. Finally, intrinsic cysts were statistically more likely in NRAS-immunoreactive lesions; which may be a clinical marker for juvenile/congenital nature of these lesions.
The risk of malignant transformation of conjunctival nevi into melanoma is predicted to be 1% (Gerner et al.18), and more common in adult-onset lesions compared with those that appear in childhood. Like conjunctival nevi, up to 14% to 50% of conjunctival melanomas have been found to harbor BRAF mutations and 18% have NRAS mutations (Griewank et al.,5 Lake et al.,6 Goldenberg-Cohen et al.,7 Gear et al.,9 Riechardt et al.8). In one study, BRAF mutant conjunctival melanomas were associated with a caruncle location and in another study with young age, male sex, sun-exposed location, mixed/nonpigmented color and nevus origin (Larsen et al.,19 Griewank et al.5). However, this latter study found no association between BRAF mutant status and prognosis. Along the same lines, Gear and colleagues9 found no correlation between BRAF mutations and location or other clinicopathologic characteristics (Gear et al.9). Therefore, the implications of BRAF mutations in conjunctival melanoma are controversial, and the potential of malignant transformation of nevi has an unknown association with BRAF or NRAS status.
It is proposed that lesions with GNAQ mutations possess the unique traits of being derived from extraepithelial melanocytes and particularly those that originate from cranial neural crest cells.11 Therefore, it would be conceivable that conjunctival blue nevi would be GNAQ mutant. This theory is strengthened by the discovery that cutaneous, central nervous system, and oral cavity blue nevi have GNAQ mutations.10–15 In keeping with this prediction, the present study demonstrates GNAQ mutations in both conjunctival blue nevi. Ultraviolet light exposure is not thought to play a role in GNAQ mutations, and remains debatable in BRAF and NRAS mutations. This shared genetic aberration in both cutaneous and conjunctival derived nevi is another example of the similarity between these two lesions from differing (skin and conjunctiva) anatomic origins.
Immunohistochemistry techniques for BRAF V600E and NRAS Q61R, are highly sensitive and specific.20–22 In our series, there was only one patient with negative immunohistochemistry for all targets. Instead, this specimen was evaluated with the MSK-IMPACT assay,16 which revealed a less common mutation in NRASQ61K, which is not recognized by the antibody SP174 The MSK-IMPACT assay is an invaluable tool with the advantage providing a combination approach for the detection of multiple categories of genetic alterations, and is particularly useful at identifying less common alterations that may not be easily detected through immunohistochemistry.
In summary, our findings confirm the parallels that exist between cutaneous and conjunctival melanocytic lesions. Our common conjunctival nevi cohort revealed data to support mutually exclusive genetic alterations of BRAFV600E and NRAS, with slightly higher presence of the former. Like cutaneous nevi, the NRAS-immunoreactivity was more common in larger lesions with an earlier occurrence in life. The similarities extend to blue nevi where we demonstrate a common genetic aberration in GNAQ, which were present in two blue nevi specimens. These findings would benefit from validation with a larger cohort study. Prior concerns of specialists in the field point to the semantic problems with classifying pigmented lesions of the conjunctiva.23 The association between genetics and histopathology of nevi was limited to blue versus nonblue nevi (and did not extend to distinguishing among common nevi); however, genetics did suggest an association with some clinical features of common nevi. These findings open up the possibility of organizing pigmented lesions molecularly or genetically, and this in turn may relate to pathogenesis and ultimately inform treatment approaches.
Acknowledgments
Supported by The Fund for Ophthalmic Knowledge, The New York Community Trust, Research to Prevent Blindness and Cancer Center Support Grant (P30 CA008748; New York, NY, USA).
Disclosure: J.H. Francis, None; H.E. Grossniklaus, P; L.A. Habib, None; B. Marr, Aura Biosciences (C); D.H. Abramson, None; K.J. Busam, None
References
- 1.Price HN. Congenital melanocytic nevi: update in genetics and management. Curr Opin Pediatr. 2016;28:476–482. doi: 10.1097/MOP.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 2.Roh MR, Eliades P, Gupta S, Tsao H. Genetics of melanocytic nevi. Pigment Cell Melanoma Res. 2015;28:661–672. doi: 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 4.Loewe R, Kittler H, Fischer G, Faé I, Wolff K, Petzelbauer P. BRAF kinase gene V599E mutation in growing melanocytic lesions. J Invest Dermatol. 2004;123:733–736. doi: 10.1111/j.0022-202X.2004.23402.x. [DOI] [PubMed] [Google Scholar]
- 5.Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143–3152. doi: 10.1158/1078-0432.CCR-13-0163. [DOI] [PubMed] [Google Scholar]
- 6.Lake SL, Kalirai H, Dopierala J, Damato BE, Coupland SE. Comparison of formalin-fixed and snap-frozen samples analyzed by multiplex ligation-dependent probe amplification for prognostic testing in uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53:2647–2652. doi: 10.1167/iovs.12-9584. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg-Cohen N, Cohen Y, Rosenbaum E, et al. T1799A BRAF mutations in conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2005;46:3027–3030. doi: 10.1167/iovs.04-1449. [DOI] [PubMed] [Google Scholar]
- 8.Riechardt AI, Maier A-KB, Nonnenmacher A, et al. B-Raf inhibition in conjunctival melanoma cell lines with PLX 4720. Br J Ophthalmol. 2015;99:1739–1745. doi: 10.1136/bjophthalmol-2015-306689. [DOI] [PubMed] [Google Scholar]
- 9.Gear H, Williams H, Kemp EG, Roberts F. BRAF mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci. 2004;45:2484–2488. doi: 10.1167/iovs.04-0093. [DOI] [PubMed] [Google Scholar]
- 10.Francis JH, Wiesner T, Milman T, et al. Investigation of somatic GNAQ, GNA11, BAP1 and SF3B1 mutations in ophthalmic melanocytomas. Ocul Oncol Pathol. 2016;2:171–177. doi: 10.1159/000442352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Raamsdonk CD, Fitch KR, Fuchs H, de Angelis MH, Barsh GS. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961–968. doi: 10.1038/ng1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamba S, Felicioni L, Buttitta F, et al. Mutational profile of GNAQQ209 in human tumors. PLoS One. 2009;4:e6833. doi: 10.1371/journal.pone.0006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Küsters-Vandevelde HVN, Klaasen A, Küsters B, et al. Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2010;119:317–323. doi: 10.1007/s00401-009-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen Y, Goldenberg-Cohen N, Akrish S, et al. BRAF and GNAQ mutations in melanocytic tumors of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:778–784. doi: 10.1016/j.oooo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerner N, Nørregaard JC, Jensen OA, Prause JU. Conjunctival naevi in Denmark 1960–1980. A 21-year follow-up study. Acta Ophthalmol Scand. 1996;74:334–337. doi: 10.1111/j.1600-0420.1996.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 19.Larsen A-C, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463–470. doi: 10.1111/aos.13007. [DOI] [PubMed] [Google Scholar]
- 20.Long GV, Wilmott JS, Capper D, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol. 2013;37:61–65. doi: 10.1097/PAS.0b013e31826485c0. [DOI] [PubMed] [Google Scholar]
- 21.Massi D, Simi L, Sensi E, et al. Immunohistochemistry is highly sensitive and specific for the detection of NRASQ61R mutation in melanoma. Mod Pathol. 2015;28:487–497. doi: 10.1038/modpathol.2014.137. [DOI] [PubMed] [Google Scholar]
- 22.Busam KJ, Hedvat C, Pulitzer M, Deimling von A, Jungbluth AA. Immunohistochemical analysis of BRAF(V600E) expression of primary and metastatic melanoma and comparison with mutation status and melanocyte differentiation antigens of metastatic lesions. Am J Surg Pathol. 2013;37:413–420. doi: 10.1097/PAS.0b013e318271249e. [DOI] [PubMed] [Google Scholar]
- 23.Jakobiec FA. Conjunctival primary acquired melanosis: is it time for a new terminology? Am J Ophthalmol. 2016;162:3–19.e1. doi: 10.1016/j.ajo.2015.11.003. [DOI] [PubMed] [Google Scholar]


