Key Points
Question
Does early postoperative acetaminophen exposure reduce the incidence of acute kidney injury in children who undergo cardiac surgery?
Findings
In this cohort study, acetaminophen exposure in the first postoperative 48 hours was associated with a lower rate of acute kidney injury in pediatric patients who underwent cardiac surgery in primary and validation cohorts from 2 tertiary referral children’s hospitals.
Meaning
Postoperative use of acetaminophen may protect pediatric cardiac surgery patients from acute kidney injury.
Abstract
Importance
Acute kidney injury (AKI) is a common and serious complication for pediatric cardiac surgery patients associated with increased morbidity, mortality, and length of stay. Current strategies focus on risk reduction and early identification because there are no known preventive or therapeutic agents. Cardiac surgery and cardiopulmonary bypass lyse erythrocytes, releasing free hemoglobin and contributing to oxidative injury. Acetaminophen may prevent AKI by reducing the oxidation state of free hemoglobin.
Objective
To test the hypothesis that early postoperative acetaminophen exposure is associated with reduced risk of AKI in pediatric patients undergoing cardiac surgery.
Design, Setting, and Participants
In this retrospective cohort study, the setting was 2 tertiary referral children’s hospitals. The primary and validation cohorts included children older than 28 days admitted for cardiac surgery between July 1, 2008, and June 1, 2016. Exclusion criteria were postoperative extracorporeal membrane oxygenation and inadequate serum creatinine measurements to determine AKI status.
Exposures
Acetaminophen exposure in the first 48 postoperative hours.
Main Outcomes and Measures
Acute kidney injury based on Kidney Disease: Improving Global Outcomes serum creatinine criteria (increase by ≥0.3 mg/dL from baseline or at least 1.5-fold more than the baseline [to convert to micromoles per liter, multiply by 88.4]) in the first postoperative week.
Results
The primary cohort (n = 666) had a median age of 6.5 (interquartile range [IQR], 3.9-44.7) months, and 341 (51.2%) had AKI. In unadjusted analyses, those with AKI had lower median acetaminophen doses than those without AKI (47 [IQR, 16-88] vs 78 [IQR, 43-104] mg/kg, P < .001). In logistic regression analysis adjusting for age, cardiopulmonary bypass time, red blood cell distribution width, postoperative hypotension, nephrotoxin exposure, and Risk Adjustment for Congenital Heart Surgery score, acetaminophen exposure was protective against postoperative AKI (odds ratio, 0.86 [95% CI, 0.82-0.90] per each additional 10 mg/kg). Findings were replicated in the validation cohort (n = 333), who had a median age of 14.1 (IQR, 3.9-158.2) months, and 162 (48.6%) had AKI. Acetaminophen doses were 60 (95% CI, 40-87) mg/kg in those with AKI vs 70 (95% CI, 45-94) mg/kg in those without AKI (P = .03), with an adjusted odds ratio of 0.91 (95% CI, 0.84-0.99) for each additional 10 mg/kg.
Conclusions and Relevance
These results indicate that early postoperative acetaminophen exposure may be associated with a lower rate of AKI in pediatric patients who undergo cardiac surgery. Further analysis to validate these findings, potentially through a prospective, randomized trial, may establish acetaminophen as a preventive agent for AKI.
This study tests the hypothesis that early postoperative acetaminophen exposure is associated with reduced risk of acute kidney injury in pediatric patients undergoing cardiac surgery.
Introduction
Acute kidney injury (AKI) occurs in approximately 25% of children admitted to intensive care units (ICUs).1,2,3,4,5,6,7,8,9 Among pediatric ICU patients, AKI is associated with increased length of stay and consistently demonstrates odds ratios (ORs) exceeding 3 for mortality.1,2,8,9,10,11,12 Among children who have cardiac surgery, rates of AKI are even higher, and AKI is associated with increased morbidity, mortality, and length of stay.13,14,15,16,17,18,19,20,21,22,23,24,25,26 To date, efforts to reduce the effect of AKI in pediatric patients are focused on early detection and risk reduction because there are no known preventive or therapeutic agents for AKI.
For children who undergo cardiac surgery, the necessary cardiopulmonary bypass (CPB) causes hemolysis, which together with the tissue damage from surgery and administration of packed red blood cells leads to high circulating levels of free hemoglobin in these patients.27,28 Cell-free plasma hemoglobin has been associated with AKI after cardiac bypass in children.29 Based on data from animal models and adults, it is hypothesized that free hemoglobin contributes to AKI through the oxidation of the central iron of the heme moiety to the highly reactive and nephrotoxic ferryl (Fe4+) form.30,31,32 This pathogenic mechanism for AKI illuminates a potential therapeutic agent. Acetaminophen, a well-established drug used for analgesia and fever reduction, is known to reduce the oxidation of free hemoglobin by preventing the oxidation of iron from Fe3+ to Fe4+.32,33 The hemoreductant activity of acetaminophen derives from the structural similarity between the heme moiety of hemoglobin and the peroxidase moiety of cyclooxygenase. In recent years, acetaminophen has been increasingly used after surgery in children to reduce opioid exposure.
The primary objective of this study was to test the hypothesis that acetaminophen exposure in the first 48 hours after cardiac surgery is associated with reduced risk of AKI in children. To test this hypothesis, we performed a retrospective observational study of children who had cardiac surgery in a primary cohort from the Monroe Carell Jr Children’s Hospital at Vanderbilt, and we validated our findings in a second independent cohort from Duke Children’s Hospital.
Methods
Population and Electronic Health Record Data Extraction
This study was reviewed and approved by the institutional review boards of Vanderbilt University Medical Center, Nashville, Tennessee, and Duke University, Durham, North Carolina. Written informed consent was obtained from parents or guardian, as well as written assent from study participants as appropriate by age. Inclusion criteria for the primary cohort were cardiac surgery at the children’s hospital between July 1, 2008, and June 1, 2016, and age older than 28 days. Exclusion criteria were postoperative extracorporeal membrane oxygenation (because this may include renal replacement therapy and thus affect serum creatinine values) and absence of either the baseline or postoperative serum creatinine measurements to determine AKI status. If individuals had multiple cardiac surgical procedures, data from only the first surgery were included. After the primary cohort was analyzed, a second cohort was assembled to determine if these findings were unique to the institution or if they could be replicated in an independent validation cohort. Inclusion criteria for the validation cohort were the same as for the primary cohort, with the exception that the study inclusion dates were June 25, 2013, to March 29, 2016. All study data, including demographic, laboratory, medication administration, and administrative data, were extracted from electronic health record data sources. Medications administered in the first 48 postoperative hours were categorized as high-risk nephrotoxins (eg, aminoglycosides, ketorolac tromethamine, and tacrolimus), moderate-risk nephrotoxins (eg, loop diuretics, enalapril maleate, and trimethoprim-sulfamethoxazole [eTable 1 in the Supplement]), or nonnephrotoxins. Postoperative hypotension was defined using the systolic blood pressure measurements documented in the first 48 postoperative hours, irrespective of any vasopressor support. Hypotension was defined as one or more systolic blood pressures lower than 70 mm Hg plus 2 times the age in years if 10 years or younger and lower than 90 mm Hg if older than 10 years.34
Exposure and Outcome Definitions
Exposure was defined as dichotomous exposure to acetaminophen for the initial analysis and then the cumulative weight-adjusted intravenous, enteral, and/or rectal acetaminophen dose (in milligrams per kilogram), alone or in combination with other medications, received in the first 48 hours after surgery. Individuals were also categorized as those receiving no acetaminophen or the lowest (<40 mg/kg), middle (40-80 mg/kg), or highest (>80 mg/kg) doses of acetaminophen. The AKI outcome was defined by change in serum creatinine values. Baseline serum creatinine measurements were defined as the lowest obtained in the 28 days before surgery. Postoperative serum creatinine measurements were defined as those obtained in the 7 days after surgery. Acute kidney injury was defined solely using the creatinine criteria because reliable urine output data are not available for all pediatric inpatients35 and accurate height measurements were not consistently available among pediatric inpatients for estimated glomerular filtration rate. Individuals were classified as having AKI using Kidney Disease: Improving Global Outcomes (KDIGO)7 serum creatinine criteria if any postoperative serum creatinine measurement increased by 0.3 mg/dL or more from baseline (to convert creatinine level to micromoles per liter, multiply by 88.4) or was at least 1.5-fold more than the baseline in the first postoperative week. Those not meeting these criteria were classified as having no AKI. Acute kidney injury stage was determined for each AKI case using serum creatinine–based KDIGO definitions for stage 1 (≥0.3 mg/dL or 1.5-fold increase), stage 2 (2-fold increase), and stage 3 (≥4 mg/dL or 3-fold increase) based on the highest serum creatinine measurement in the 7 days after surgery.
Statistical Analysis
Descriptive statistics were presented as the median (interquartile range [IQR]) or number (percentage) as appropriate. Continuous variables were compared using the Wilcoxon rank sum test and categorical variables using the Pearson χ2 test. The Kruskal-Wallis test was used to test the weight-adjusted acetaminophen dose across AKI stages. We analyzed the association between AKI and dichotomous acetaminophen exposure using the Pearson χ2 test. We also investigated the association of acetaminophen dose (continuous variable, in milligrams per kilogram) with AKI using logistic regression, after adjusting for prespecified risk factors, such as age, CPB time, red blood cell distribution width (RDW) given prior associations with AKI,36,37 postoperative hypotension, nephrotoxin exposure, and Risk Adjustment for Congenital Heart Surgery (RACHS) score.38 The nonlinear association of age with AKI was modeled using the restricted cubic spline with 3 knots chosen at the 10th, 50th, and 90th percentiles. The same logistic regression model was used for both the primary and validation cohorts. Subgroup analyses were also done. First, logistic regression was performed for each cohort using the same methods and covariates as in the full cohort, excluding those individuals who had no acetaminophen exposure. In addition, a subgroup analysis was done by pooling individuals from both cohorts with a RACHS score of 3 or higher as an alternative method to account for variability in clinical status after surgery and likely degree of cell-free hemoglobin. For this analysis, the same logistic regression was performed as above, with the inclusion of clinical site as an additional covariate. Sensitivity analyses of unmeasured confounding were also performed (see the eAppendix in the Supplement). Multiple imputation was performed based on 10 completed data sets by applying predictive mean matching to handle missing data for the variables of CPB time, RDW, and RACHS score.39 All analyses were performed with a statistical programming language (R, version 3.3.0; R Development Core Team). The statistical significance was determined at the level of .05, and all P values were based on 2-sided tests.
Results
Study Cohorts
The primary cohort included 666 individuals with baseline and postoperative serum creatinine measurements. Half of them were female, and half met criteria for AKI (Table 1). In all, 159 individuals (23.9%) had stage 1 AKI, 131 individuals (19.7%) had stage 2 AKI, and 51 individuals (7.7%) had stage 3 AKI. Those with AKI had more postoperative serum creatinine measurements than those without AKI (8 [IQR, 4-7] vs 5 [IQR, 5-10]). Primary cardiac diagnoses are listed in eTable 2 in the Supplement. The validation cohort comprised 333 individuals, with similar incidence of AKI (Table 1). In the validation cohort, 51 patients (15.3%) had stage 1 AKI, 91 patients (27.3%) had stage 2 AKI, and 20 (6.0%) had stage 3 AKI. Primary cardiac diagnoses for the validation cohort are listed in eTable 3 in the Supplement.
Table 1. Characteristics of the Primary and Validation Cohorts.
| Variable | Primary Cohort (n = 666) | Validation Cohort (n = 333) |
|---|---|---|
| Age, median (IQR), mo | 6.5 (3.9-44.7) | 14.1 (3.9-158.2) |
| Female sex, No. (%) | 328 (49.2) | 172 (51.7) |
| Weight, median (IQR), kg | 7.1 (5.0-14.6) | 9.2 (4.7-49.0) |
| Serum creatinine, median (IQR), mg/dL | ||
| Preoperative baseline | 0.35 (0.25-0.45) | 0.30 (0.20-0.60) |
| Postoperative peak | 0.53 (0.45-0.66) | 0.50 (0.40-0.90) |
| RACHS score, No./total No. (%)a | ||
| 1 | 143/636 (22.5) | 88/273 (32.2) |
| 2 | 256/636 (40.3) | 112/273 (41.0) |
| ≥3 | 237/636 (37.3) | 73/273 (26.7) |
| CPB time, median (IQR), minb | 87 (59-122) | 95 (39-149) |
| AKI outcome, No. (%) | ||
| No AKI | 325 (48.8) | 171 (51.4) |
| Stage 1 | 159 (23.9) | 51 (15.3) |
| Stage 2 | 131 (19.7) | 91 (27.3) |
| Stage 3 | 51 (7.7) | 20 (6.0) |
Abbreviations: AKI, acute kidney injury; CPB, cardiopulmonary bypass; IQR, interquartile range; RACHS, Risk Adjustment for Congenital Heart Surgery.
SI conversion factor: To convert creatinine level to micromoles per liter, multiply by 88.4.
Score available for 636 in the primary cohort and 273 in the validation cohort.
Time available for 665 in the primary cohort and all 333 in the validation cohort.
AKI and Acetaminophen Exposure
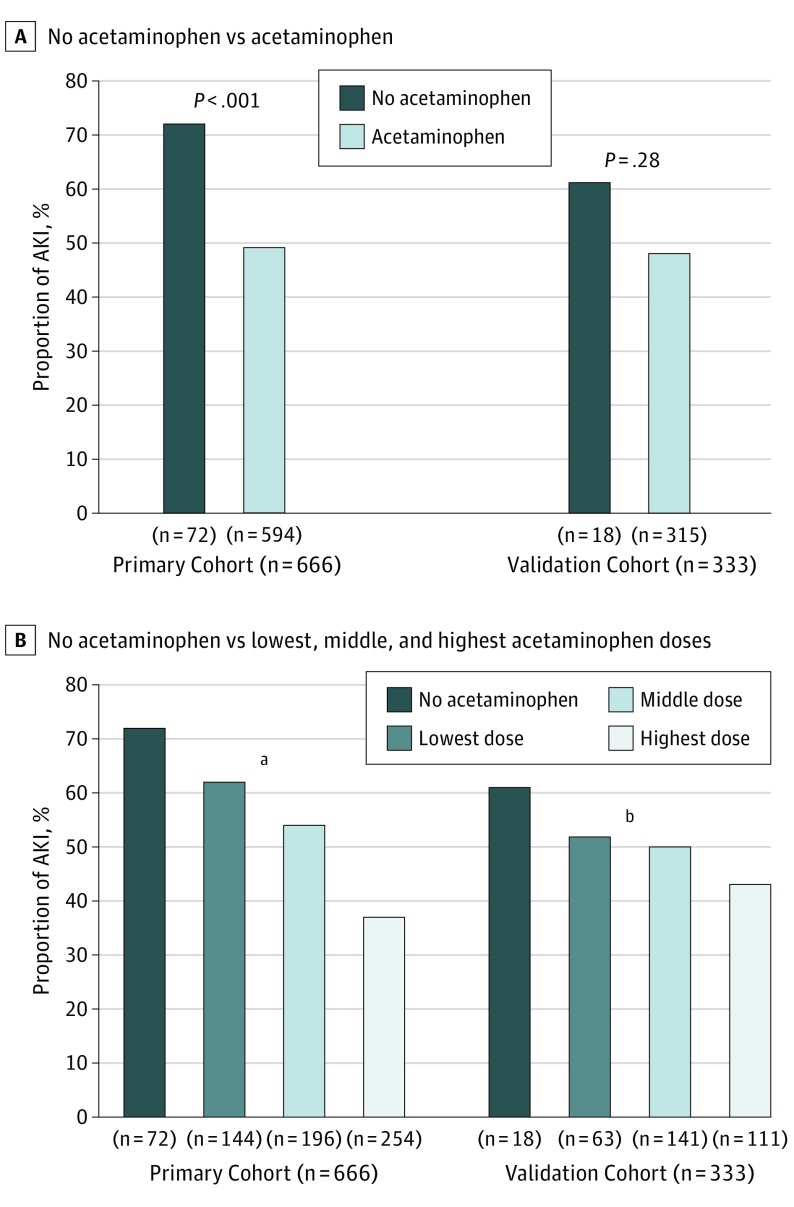
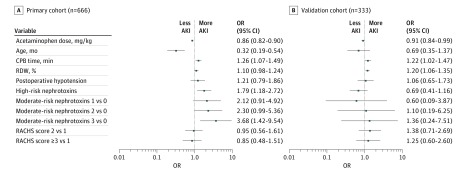
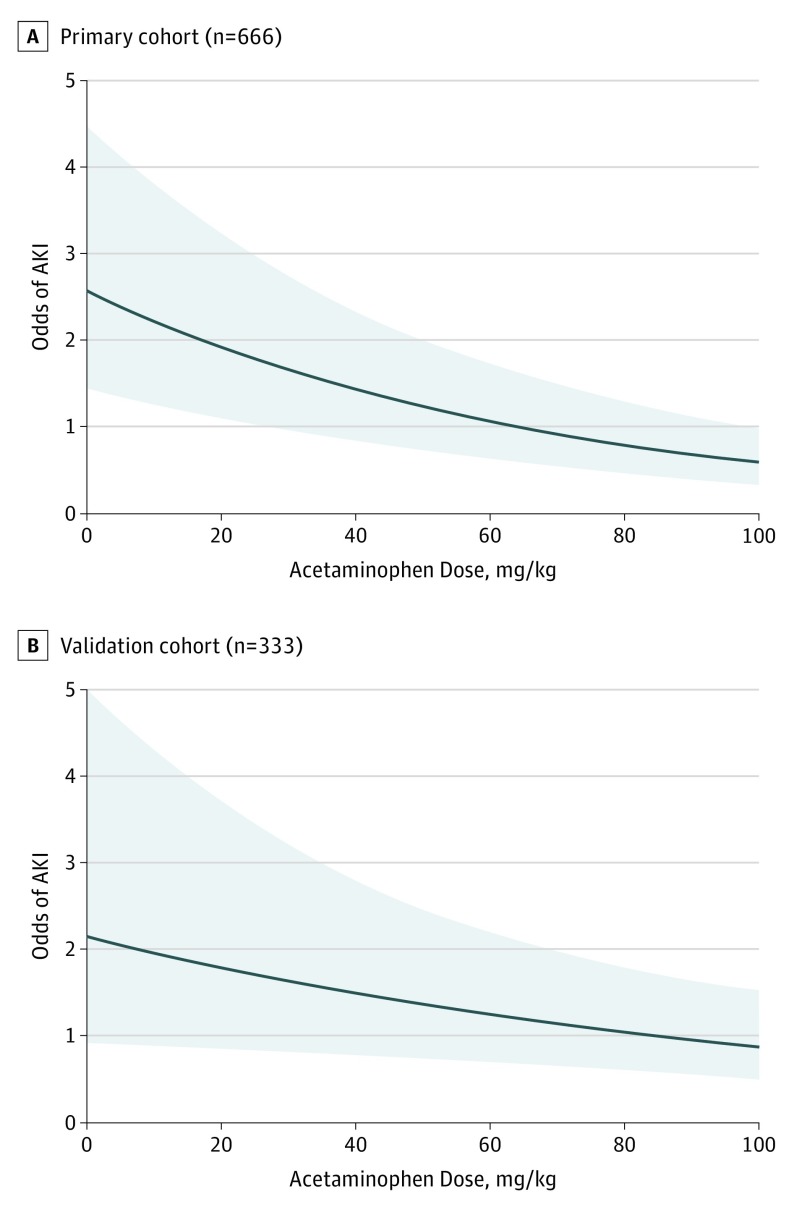
In the primary cohort, 72 (10.8%) had no acetaminophen exposure in the first 48 hours after surgery. The incidence of AKI was higher among those with no acetaminophen exposure than among those with acetaminophen exposure (Figure 1A). Comparing those with and without AKI, those without AKI had a significantly higher weight-adjusted acetaminophen dose in the first 48 hours after surgery compared with those with AKI (Table 2). Other variables significantly associated with AKI included younger age, longer CPB time, higher RDW, presence of postoperative hypotension, fewer high-risk nephrotoxins, and higher RACHS score. When stratified by AKI stage, those with no AKI had the highest median acetaminophen dose (78 [IQR, 43-104] mg/kg), followed by those with stage 1 AKI (57 [IQR, 17-100] mg/kg), stage 2 AKI (48 [IQR, 28-78] mg/kg), and stage 3 AKI (31 [IQR, 0-70] mg/kg (P < .001 for difference in distribution across groups). Those with no acetaminophen exposure had the highest rates of AKI, with decreasing incidence in the lowest, middle, and highest acetaminophen dose groups (Figure 1B). In the logistic regression analysis adjusting for age, CPB time, RDW, postoperative hypotension, nephrotoxin exposure, and RACHS score, acetaminophen exposure was protective against postoperative AKI, with an odds ratio (OR) of 0.86 (95% CI, 0.82-0.90) per each additional 10 mg/kg (Figure 2). The association between continuous acetaminophen dose and adjusted odds for AKI is shown in Figure 3. The patients who did not receive acetaminophen were younger and had longer CPB times, higher RDW, and more postoperative hypotension than those who received acetaminophen (eTable 4 in the Supplement). When subset analysis of the primary cohort was performed that excluded those with no acetaminophen exposure, acetaminophen remained protective against AKI (OR, 0.85 [95% CI, 0.80-0.90] per each additional 10 mg/kg; P < .001). We performed analyses to determine the sensitivity of our findings to unmeasured confounding. To make our findings statistically insignificant, an unmeasured confounder must have a minimum strength of association greater than 3.07 for both acetaminophen exposure and AKI (eTable 5 in the Supplement).
Figure 1. Proportion of Individuals With Acute Kidney Injury (AKI) by Acetaminophen Exposure and Dose.
A, The proportion (percentage) of individuals with early postoperative AKI is shown for those administered no acetaminophen or at least 1 dose of acetaminophen in the first 48 hours after surgery. B, The proportion (percentage) of individuals with early postoperative AKI is shown for those with no acetaminophen administered in the first 48 hours after surgery and for the lowest (<40 mg/kg), middle (40-80 mg/kg), and highest (>80 mg/kg) cumulative doses of acetaminophen in the first 48 hours after surgery. P values are from the Pearson χ2 test across all groups.
aP < .001 across all 4 groups.
bP = .42 across all 4 groups.
Table 2. Risk Factors for Acute Kidney Injury (AKI) in the Primary and Validation Cohortsa.
| Variable | Primary Cohort (n = 666) | Validation Cohort (n = 333) | ||||
|---|---|---|---|---|---|---|
| No AKI (n = 325) | AKI (n = 341) | Unadjusted P Value | No AKI (n = 171) | AKI (n = 162) | Unadjusted P Value | |
| Acetaminophen dose, median (IQR), mg/kg | 78 (43-104) | 47 (16-88) | <.001 | 70 (45-94) | 60 (40-87) | .03 |
| Age, median (IQR), mo | 15.2 (4.5-70.9) | 5.5 (3.3-16.6) | <.001 | 39.8 (5.3-203.9) | 6.7 (2.9-46.9) | <.001 |
| Female sex, No. (%) | 171 (52.6) | 157 (46.0) | .09 | 95 (55.6) | 77 (47.5) | .14 |
| Weight, median (IQR), kg | 9.2 (5.6-20.2) | 6.1 (4.7-9.3) | <.001 | 13.7 (5.3-62.0) | 6.5 (4.4-16.9) | <.001 |
| Serum creatinine, median (IQR), mg/dL | ||||||
| Preoperative baseline | 0.43 (0.36-0.52) | 0.26 (0.21-0.34) | <.001 | 0.40 (0.30-0.80) | 0.30 (0.20-0.50) | <.001 |
| Postoperative peak | 0.52 (0.44-0.62) | 0.54 (0.45-0.71) | .005 | 0.50 (0.40-0.80) | 0.60 (0.50-1.00) | .004 |
| CPB time, median (IQR), minb | 80 (51-111) | 99 (68-132) | <.001 | 85 (32-123) | 110 (41-170) | .01 |
| RDW, median (IQR), %c | 13.2 (12.7-14.4) | 14.0 (13.2-15.3) | <.001 | 13.9 (13.2-15.3) | 15.4 (14.2-16.8) | <.001 |
| Postoperative hypotension, No. (%) | 238 (73.2) | 280 (82.1) | .006 | 74 (43.3) | 85 (52.5) | .09 |
| High-risk nephrotoxins, No. (%)d | ||||||
| 0 | 158 (48.6) | 192 (56.3) | .02 | 52 (30.4) | 72 (44.4) | .002 |
| 1 | 148 (45.5) | 117 (34.3) | 77 (45.0) | 73 (45.1) | ||
| 2 | 18 (5.5) | 29 (8.5) | 35 (20.5) | 12 (7.4) | ||
| ≥3 | 1 (0.3) | 3 (0.9) | 7 (4.1) | 5 (3.1) | ||
| Moderate-risk nephrotoxins, No. (%) | ||||||
| 0 | 17 (5.2) | 13 (3.8) | .20 | 6 (3.5) | 2 (1.2) | .36 |
| 1 | 151 (46.5) | 147 (43.1) | 21 (12.3) | 14 (8.6) | ||
| 2 | 128 (39.4) | 134 (39.3) | 54 (31.6) | 54 (33.3) | ||
| ≥3 | 29 (8.9) | 47 (13.8) | 90 (52.6) | 92 (56.8) | ||
| RACHS score, No./total No. (%)e | ||||||
| 1 | 93/320 (29.1) | 50/316 (15.8) | <.001 | 57/143 (39.9) | 31/130 (23.8) | .02 |
| 2 | 123/320 (38.4) | 133/316 (42.1) | 52/143 (36.4) | 60/130 (46.2) | ||
| ≥3 | 104/320 (32.5) | 133/316 (42.1) | 34/143 (23.8) | 39/130 (30.0) | ||
Abbreviations: CPB, cardiopulmonary bypass; IQR, interquartile range; RACHS, Risk Adjustment for Congenital Heart Surgery; RDW, red blood cell distribution width.
SI conversion factor: To convert creatinine level to micromoles per liter, multiply by 88.4.
P values are from the univariate Wilcoxon rank sum test for continuous variables and from the univariate Pearson χ2 test for categorical variables.
Time available for 665 in the primary cohort and all 333 in the validation cohort.
Value available for 613 in the primary cohort and 329 in the validation cohort.
See the Population and Electronic Health Record Data Extraction subsection of the Methods for examples of nephrotoxins.
Score available for 636 in the primary cohort and 273 in the validation cohort.
Figure 2. Multivariable Analyses of Acute Kidney Injury (AKI) Among the Primary and Validation Cohorts.
Shown are the odds ratios (ORs) and 95% CIs for each of the clinical variables in the logistic regression analysis for association with acute kidney injury in the primary cohort (A) and validation cohort (B). Odds ratios are for the following: weight-adjusted acetaminophen dose (per each additional 10 mg/kg), age (75th vs 25th percentile for each cohort), cardiopulmonary bypass (CPB) time (per additional 50 minutes), red blood cells distribution width (RDW) (per additional 1%), postoperative hypotension (present vs absent), high-risk nephrotoxins (≥1 vs none), moderate-risk nephrotoxins (1, 2, and ≥3 vs none [see the Population and Electronic Health Record Data Extraction subsection of the Methods for examples of nephrotoxins]), and Risk Adjustment for Congenital Heart Surgery (RACHS) score (2 and ≥3 vs 1). Point estimates and 95% CIs are shown to the right of each plot.
Figure 3. Adjusted Odds Ratios for Acute Kidney Injury (AKI) by Acetaminophen Dose.
Odds of AKI in the primary cohort (A) and validation cohort (B) as a function of weight-adjusted acetaminophen dose are shown, adjusting for age, cardiopulmonary bypass time, red blood cell distribution width, postoperative hypotension, nephrotoxin exposure, and Risk Adjustment for Congenital Heart Surgery score, which were set to their reference or median values. Shaded areas indicate 95% CIs.
For the validation cohort, 18 (5.4%) had no acetaminophen exposure, and there was no significant difference in AKI incidence between those with vs without acetaminophen exposure (Figure 1A). Comparing those with and without AKI, the median early postoperative acetaminophen dose was again higher among those without AKI than among those with AKI (Table 2). Younger age, longer CPB time, higher RDW, fewer high-risk nephrotoxins, and higher RACHS score were also associated with postoperative AKI. When stratified by AKI stage, those with no AKI again had the highest median weight-adjusted acetaminophen dose (70 [IQR, 45-94] mg/kg). Those with stage 1 AKI had the lowest median dose (50 [IQR, 26-76] mg/kg), and acetaminophen doses were similar in those with stage 2 and stage 3 AKI as in those with no AKI (67 [IQR, 43-88] and 66 [IQR, 45-87] mg/kg, respectively; P = .02). Those with no acetaminophen exposure again had the highest rates of AKI, with decreasing incidence in the lowest, middle, and highest acetaminophen dose groups, without statistical significance (Figure 1B). In multivariable analysis adjusting for age, CPB time, RDW, postoperative hypotension, nephrotoxin exposure, and RACHS score, postoperative acetaminophen had a protective association against AKI, with an adjusted OR of 0.91 (95% CI, 0.84-0.99) per each additional 10 mg/kg (Figure 2). The association between continuous acetaminophen dose and adjusted odds for AKI in the validation cohort is shown in Figure 3. When those with no acetaminophen exposure were excluded, acetaminophen exposure remained protective (OR, 0.90 [95% CI, 0.82-0.99] per each additional 10 mg/kg; P = .03).
Pooled Subgroup Analysis
The pooled subgroup analysis included a total of 310 individuals with a RACHS score of 3 or higher from both clinical sites (237 from the primary cohort and 73 from the validation cohort). After adjusting for clinical site and the covariates included in the adjusted analyses of the full cohorts, acetaminophen dose (in milligrams per kilogram) in this subgroup again showed a protective association against AKI (OR, 0.86 [95% CI, 0.81-0.93] per each additional 10 mg/kg; eFigure in the Supplement).
Discussion
We present the finding of a protective association of acetaminophen against AKI in children who undergo cardiac surgery. The weight-adjusted total dose of acetaminophen given in the first 48 hours after surgery was inversely correlated with the incidence of AKI in the primary cohort using the dichotomous indicator of acetaminophen exposure and acetaminophen dose as a continuous variable and after adjusting for potential confounders, including age, CPB time, RDW, postoperative hypotension, nephrotoxin exposure, and RACHS score. Given this novel finding, we sought to confirm these observations in an independent data set, in which after adjusting for confounding variables that were not balanced across acetaminophen exposure groups the protective association of acetaminophen was again observed. Subgroup analyses of only those patients who received acetaminophen and those with a RACHS score of 3 or higher, representing more clinically homogeneous patients, yielded consistent results.
The hypothesis that acetaminophen may be protective against AKI in the setting of cardiac surgery stemmed from several observations. In a case-control study30 of adults undergoing cardiac surgery, the 10 individuals with postoperative AKI had higher intraoperative peak free hemoglobin concentrations than risk-matched controls (289.0 vs 104.4 mg/dL, P = .01 [to convert to grams per liter, multiply by 0.01]). This association was replicated in a cohort study29 of 60 children undergoing surgery requiring CPB, where those with AKI had a greater increase in plasma hemoglobin concentration (53.9 vs 38.3 mg/dL, P = .03 [to convert to grams per liter, multiply by 0.01]), and an increase in plasma hemoglobin was an independent risk factor for AKI (OR, 1.02 [95% CI, 1.00-1.03]) per each additional 1 mg/dL). The mechanism whereby free hemoglobin leads to nephrotoxicity is postulated to be through lipid peroxidation, catalyzed by oxidized cell-free hemoglobin, a process that can be inhibited by acetaminophen.32,40
Cell-free hemoglobin was also associated with death in a retrospective observational study31 of critically ill adults with sepsis, and acetaminophen exposure had a protective association with in-hospital mortality. In the subset of patients with no detectable plasma hemoglobin, there was no protective association of acetaminophen. This potential protective association of acetaminophen was evaluated prospectively in the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis (ACROSS) trial.33 That single-center, randomized, placebo-controlled trial of acetaminophen (1 g enterally every 6 hours for 3 days) found no difference in the primary end point, F2-isoprostane levels at day 3 (a measure of oxidative injury), or in hospital mortality, but it demonstrated that acetaminophen treatment affected secondary outcomes. Specifically, acetaminophen lowered F2-isoprostane levels at day 2 (24 vs 36 pg/mL, P = .047) and serum creatinine levels at day 3 (1.0 vs 1.3 mg/dL, P = .039). In another randomized trial41 that included 700 adult ICU patients with fever, there was no difference in the primary outcome (ICU-free days) and no difference in the peak creatinine level in the first week after randomization. This negative result may indicate that acetaminophen has protective associations only in specific patient populations.
There have been 2 pilot studies of the effect of acetaminophen on lipid peroxidation in patients undergoing CPB. In a prospective double-blind clinical trial,42 before initiation of CPB, 60 adult patients were treated with 4 doses of intravenous acetaminophen over 24 hours (1 g for those >50 kg and 15 mg/kg for those <50 kg) or placebo. The primary outcome was oxidative stress, measured by plasma and urine F2-isoprostane and isofuran values. The acetaminophen treatment group had smaller increases in plasma isofuran concentrations compared with the placebo group, although there were no significant differences between groups in plasma F2-isoprostane levels, urinary markers of lipid peroxidation, or AKI. The second pilot study43 randomized 30 children to be treated with 4 doses of intravenous acetaminophen (15 mg/kg for age ≥2 years and 12.5 mg/kg for age <2 years) or placebo, beginning before the start of CPB. As in the adult trial, acetaminophen attenuated increases in plasma isofuran levels, but there were no differences between the intervention and control groups in F2-isoprostane levels, AKI prevalence, AKI severity, or the AKI biomarker neutrophil gelatinase–associated lipocalin (NGAL). There were several limitations to these pilot studies, including limited power because of the small number of participants and administration of 1 or more doses of acetaminophen before and during CPB, when circulating concentration of the drug may not have reached therapeutic levels because of hemodilution. These studies were not definitive in determining the potential role of acetaminophen to protect against AKI in patients with elevated cell-free hemoglobin.
Our results indicate a modest influence of acetaminophen on AKI. Specifically, we saw an approximately 10% reduction in AKI risk for each additional 10 mg/kg administered in the first 48 hours after surgery (one typical dose of acetaminophen). In the subgroup analysis of those with a RACHS score of 3 or higher, we observed a 14% reduction per 10 mg/kg of additional acetaminophen. The ideal dose for renal protection, while avoiding dose-related hepatotoxicity or other adverse events, has not been established. There may be several sources of interindividual variability contributing to differences in the exposure and response to acetaminophen in both adults and children.44,45 Further delineation of individualized therapeutic concentrations through a precision medicine approach may be required to maximize benefit and minimize risk of acetaminophen use.
Limitations
Our study has several limitations. This was a retrospective observational study. Therefore, causality between acetaminophen exposure and reduced incidence of AKI cannot be established. The apparent protective association of acetaminophen may be owing to residual confounding. Patients with acetaminophen exposure had fewer risk factors for AKI. Furthermore, these patients may have the most frequent serum creatinine testing, increasing AKI detection. We attempted to control for this potential confounding through multivariable analysis, adjusting for factors like age, CPB time, and RACHS score. Despite inclusion of these variables, acetaminophen remained protective. Our analyses of the sensitivity of the data to unmeasured confounding indicate that our data are robust. Those with AKI had fewer exposures to highly nephrotoxic drugs, which is an observation we have seen in multiple cohorts3,37 and may be owing to avoidance of these drugs among high-risk patients. Another limitation of our study stems from the definition of AKI. We used the consensus KDIGO definition based on serum creatinine values but did not define AKI based on urine output (which may be inaccurately represented in these retrospective data sets) or biomarkers, such as NGAL or cystatin C.46 Our initial finding from one tertiary referral center was validated in independent data from a second tertiary referral center. However, these results may not be generalizable to institutions with a different patient mix, operative procedures, and postoperative care.
Conclusions
In 2 independent cohorts of children undergoing cardiac surgery, we observed a dose-dependent inverse association between postoperative receipt of acetaminophen and development of AKI. Taken together with other retrospective and prospective studies showing protective associations of acetaminophen against AKI in several conditions associated with release of cell-free hemoglobin, these findings provide a compelling rationale to justify prospective evaluation of acetaminophen for prevention of AKI in children undergoing CPB.
eTable 1. Nephrotoxic Medications
eTable 2. Primary Diagnoses of the Primary Cohort
eTable 3. Primary Diagnoses of the Validation Cohort
eTable 4. Clinical Characteristics of Acetaminophen Exposed vs Unexposed in the Primary and Validation Cohorts
eTable 5. Bias-Corrected Adjusted Odds Ratios From Sensitivity Analysis of Unmeasured Confounding Effects
eAppendix. Supplemental Sensitivity Analysis of Unmeasured Confounding Effects on the Association of Acetaminophen Exposure and Acute Kidney Injury Risk
eFigure. Subgroup Analysis of RACHS Score ≥3
References
- 1.Sutherland SM, Ji J, Sheikhi FH, et al. . AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8(10):1661-1669. doi: 10.2215/CJN.00270113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028-1035. doi: 10.1038/sj.ki.5002231 [DOI] [PubMed] [Google Scholar]
- 3.McGregor TL, Jones DP, Wang L, et al. . Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis. 2016;67(3):384-390. doi: 10.1053/j.ajkd.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey D, Phan V, Litalien C, et al. . Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8(1):29-35. doi: 10.1097/01.pcc.0000256612.40265.67 [DOI] [PubMed] [Google Scholar]
- 5.Moghal NE, Brocklebank JT, Meadow SR. A review of acute renal failure in children: incidence, etiology and outcome. Clin Nephrol. 1998;49(2):91-95. [PubMed] [Google Scholar]
- 6.Selewski DT, Cornell TT, Heung M, et al. . Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med. 2014;40(10):1481-1488. doi: 10.1007/s00134-014-3391-8 [DOI] [PubMed] [Google Scholar]
- 7.Acute Kidney Injury Work Group Kidney Disease: Improving Global Outcomes (KDIGO): KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138. doi: 10.1038/kisup.2012.6 [DOI] [Google Scholar]
- 8.Alkandari O, Eddington KA, Hyder A, et al. . Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. doi: 10.1186/cc10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soler YA, Nieves-Plaza M, Prieto M, García-De Jesús R, Suárez-Rivera M. Pediatric Risk, Injury, Failure, Loss, End-Stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: a prospective study. Pediatr Crit Care Med. 2013;14(4):e189-e195. doi: 10.1097/PCC.0b013e3182745675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccinni P, Cruz DN, Gramaticopolo S, et al. ; NEFROINT Investigators . Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol. 2011;77(11):1072-1083. [PubMed] [Google Scholar]
- 11.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933-939. doi: 10.1097/CCM.0b013e3181cd12e1 [DOI] [PubMed] [Google Scholar]
- 12.Hsu CN, Chen HL, Tain YL. Epidemiology and outcomes of community-acquired and hospital-acquired acute kidney injury in children and adolescents [published online November 20, 2017]. Pediatr Res. doi: 10.1038/pr.2017.262 [DOI] [PubMed] [Google Scholar]
- 13.Watkins SC, Williamson K, Davidson M, Donahue BS. Long-term mortality associated with acute kidney injury in children following congenital cardiac surgery. Paediatr Anaesth. 2014;24(9):919-926. doi: 10.1111/pan.12419 [DOI] [PubMed] [Google Scholar]
- 14.Soni M, Piggott KD, DeCampli W, et al. . Are we overdiagnosing acute kidney injury in pediatric patients following cardiac surgery? World J Pediatr Congenit Heart Surg. 2015;6(4):496-501. doi: 10.1177/2150135115593129 [DOI] [PubMed] [Google Scholar]
- 15.Miklaszewska M, Korohoda P, Sobczak A, et al. . Acute kidney injury in a single pediatric intensive care unit in Poland: a retrospective study. Kidney Blood Press Res. 2014;39(1):28-39. doi: 10.1159/000355774 [DOI] [PubMed] [Google Scholar]
- 16.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92(3):751-756. doi: 10.1016/j.kint.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 17.Li S, Krawczeski CD, Zappitelli M, et al. ; TRIBE-AKI Consortium . Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493-1499. doi: 10.1097/CCM.0b013e31821201d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcaraz AJ, Gil-Ruiz MA, Castillo A, et al. . Postoperative neutrophil gelatinase–associated lipocalin predicts acute kidney injury after pediatric cardiac surgery. Pediatr Crit Care Med. 2014;15(2):121-130. doi: 10.1097/PCC.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 19.Hazle MA, Gajarski RJ, Aiyagari R, et al. . Urinary biomarkers and renal near-infrared spectroscopy predict intensive care unit outcomes after cardiac surgery in infants younger than 6 months of age. J Thorac Cardiovasc Surg. 2013;146(4):861-867.e1. doi: 10.1016/j.jtcvs.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lex DJ, Tóth R, Cserép Z, et al. . A comparison of the systems for the identification of postoperative acute kidney injury in pediatric cardiac patients. Ann Thorac Surg. 2014;97(1):202-210. doi: 10.1016/j.athoracsur.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 21.Meersch M, Schmidt C, Van Aken H, et al. . Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One. 2014;9(10):e110865. doi: 10.1371/journal.pone.0110865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil-Ruiz Gil-Esparza MA, Alcaraz Romero AJ, Romero Otero A, et al. . Prognostic relevance of early AKI according to pRIFLE criteria in children undergoing cardiac surgery. Pediatr Nephrol. 2014;29(7):1265-1272. doi: 10.1007/s00467-014-2757-z [DOI] [PubMed] [Google Scholar]
- 23.Ruf B, Bonelli V, Balling G, et al. . Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case-control study. Crit Care. 2015;19:27. doi: 10.1186/s13054-015-0760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto K, Toda Y, Iwasaki T, et al. . Urinary albumin levels predict development of acute kidney injury after pediatric cardiac surgery: a prospective observational study. J Cardiothorac Vasc Anesth. 2016;30(1):64-68. doi: 10.1053/j.jvca.2015.05.194 [DOI] [PubMed] [Google Scholar]
- 25.Park SK, Hur M, Kim E, et al. . Risk factors for acute kidney injury after congenital cardiac surgery in infants and children: a retrospective observational study. PLoS One. 2016;11(11):e0166328. doi: 10.1371/journal.pone.0166328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano D, Ito A, Yamada A, et al. . Independent risk factors and 2-year outcomes of acute kidney injury after surgery for congenital heart disease. Am J Nephrol. 2017;46(3):204-209. doi: 10.1159/000480358 [DOI] [PubMed] [Google Scholar]
- 27.Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass–associated acute kidney injury. J Am Coll Cardiol. 2010;55(19):2024-2033. doi: 10.1016/j.jacc.2009.12.046 [DOI] [PubMed] [Google Scholar]
- 28.Vercaemst L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: a review in search of a treatment algorithm. J Extra Corpor Technol. 2008;40(4):257-267. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim-Campbell N, Gretchen C, Callaway C, et al. . Cell-free plasma hemoglobin and male gender are risk factors for acute kidney injury in low risk children undergoing cardiopulmonary bypass. Crit Care Med. 2017;45(11):e1123-e1130. doi: 10.1097/CCM.0000000000002703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billings FT IV, Ball SK, Roberts LJ II, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50(11):1480-1487. doi: 10.1016/j.freeradbiomed.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janz DR, Bastarache JA, Peterson JF, et al. . Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41(3):784-790. doi: 10.1097/CCM.0b013e3182741a54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutaud O, Moore KP, Reeder BJ, et al. . Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A. 2010;107(6):2699-2704. doi: 10.1073/pnas.0910174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janz DR, Bastarache JA, Rice TW, et al. ; Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis Study Group . Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis trial. Crit Care Med. 2015;43(3):534-541. doi: 10.1097/CCM.0000000000000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Heart Association Pediatric Advanced Life Support (PALS) Provider Manual. Dallas, TX: American Heart Association; 2017. [Google Scholar]
- 35.James MT, Hobson CE, Darmon M, et al. ; Acute Dialysis Quality Initiative (ADQI) Consensus Group . Applications for detection of acute kidney injury using electronic medical records and clinical information systems: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Health Dis. 2016;3:9. doi: 10.1186/s40697-016-0100-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SW, Yu MY, Lee H, et al. . Risk factors for acute kidney injury and in-hospital mortality in patients receiving extracorporeal membrane oxygenation. PLoS One. 2015;10(10):e0140674. doi: 10.1371/journal.pone.0140674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, McGregor TL, Jones DP, et al. . Electronic health record–based predictive models for acute kidney injury screening in pediatric inpatients. Pediatr Res. 2017;82(3):465-473. doi: 10.1038/pr.2017.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110-118. doi: 10.1067/mtc.2002.119064 [DOI] [PubMed] [Google Scholar]
- 39.Rubin DB. Multiple imputations in sample surveys: a phenomenological Bayesian approach to nonresponse (with discussion). https://ww2.amstat.org/sections/srms/Proceedings/papers/1978_004.pdf. Published 2017. Accessed August 4, 2017.
- 40.Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387(2):273-280. doi: 10.1006/abbi.2000.2232 [DOI] [PubMed] [Google Scholar]
- 41.Young P, Saxena M, Bellomo R, et al. ; HEAT Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med. 2015;373(23):2215-2224. doi: 10.1056/NEJMoa1508375 [DOI] [PubMed] [Google Scholar]
- 42.Billings FT IV, Petracek MR, Roberts LJ II, Pretorius M. Perioperative intravenous acetaminophen attenuates lipid peroxidation in adults undergoing cardiopulmonary bypass: a randomized clinical trial. PLoS One. 2015;10(2):e0117625. doi: 10.1371/journal.pone.0117625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson SA, Zaccagni H, Bichell DP, et al. . Acetaminophen attenuates lipid peroxidation in children undergoing cardiopulmonary bypass. Pediatr Crit Care Med. 2014;15(6):503-510. doi: 10.1097/PCC.0000000000000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Court MH, Zhu Z, Masse G, et al. . Race, gender, and genetic polymorphism contribute to variability in acetaminophen pharmacokinetics, metabolism, and protein-adduct concentrations in healthy African-American and European-American volunteers. J Pharmacol Exp Ther. 2017;362(3):431-440. doi: 10.1124/jpet.117.242107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allegaert K, Vanhaesebrouck S, Verbesselt R, van den Anker JN. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31(4):411-415. doi: 10.1097/FTD.0b013e3181a8cc0a [DOI] [PubMed] [Google Scholar]
- 46.Toda Y, Sugimoto K. AKI after pediatric cardiac surgery for congenital heart diseases: recent developments in diagnostic criteria and early diagnosis by biomarkers. J Intensive Care. 2017;5:49. doi: 10.1186/s40560-017-0242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Nephrotoxic Medications
eTable 2. Primary Diagnoses of the Primary Cohort
eTable 3. Primary Diagnoses of the Validation Cohort
eTable 4. Clinical Characteristics of Acetaminophen Exposed vs Unexposed in the Primary and Validation Cohorts
eTable 5. Bias-Corrected Adjusted Odds Ratios From Sensitivity Analysis of Unmeasured Confounding Effects
eAppendix. Supplemental Sensitivity Analysis of Unmeasured Confounding Effects on the Association of Acetaminophen Exposure and Acute Kidney Injury Risk
eFigure. Subgroup Analysis of RACHS Score ≥3