Abstract
Membrane-type matrix metalloproteinase 1 (MT1-MMP) plays a critical role in extracellular matrix remodeling under both physiological and pathological conditions. However, the mechanisms controlling its activity on the cell surface remain poorly understood. In this study, we demonstrate that MT1-MMP is regulated by endocytosis. First, we determined that Con A induces proMMP-2 activation in HT1080 cells by shifting endogenous MT1-MMP from intracellular compartments to cell surface. This phenotype was mimicked by the cytoplasmic truncation mutant MT1ΔC with more robust pro-MMP-2 activation and cell surface expression than wild-type MT1-MMP in transfected cells. MT1ΔC was subsequently shown to be resistant to Con A treatment whereas MT1-MMP remains competent, suggesting that Con A regulates MT1-MMP activity through cytoplasmic domain-dependent trafficking. Indeed, MT1-MMP was colocalized with clathrin on the plasma membrane and with endosomal antigen 1 in endosomes. Internalization experiments revealed that MT1-MMP is internalized rapidly in clathrin-coated vesicles whereas MT1ΔC remains on cell surface. Coexpression of a dominant negative mutant of dynamin, K44A, resulted in elevation of MT1-MMP activity by interfering with the endocytic process. Thus, MT1-MMP is regulated by dynamin-dependent endocytosis in clathrin-coated pits through its cytoplasmic domain.
Keywords: clathrin‖gelatinase A‖MMP‖MT1-MMP
Matrix metalloproteinases (MMPs), a family of zinc-dependent proteolytic enzymes capable of degrading components of the extracellular matrix (ECM), are regulated by diverse mechanisms at the levels of transcription, translation, posttranslational modification, zymogen activation, and inhibition by endogenous inhibitors to achieve precise remodeling of the ECM during normal development (1–3). Dysregulated MMP activity has been demonstrated in degenerative diseases such as cancer, arthritis, and cardiovascular diseases (4–8). Comprised of 25 members, MMPs are classified as either soluble or integral membrane proteinase depending the absence or presence of type I or II transmembrane domains or glycosylphosphatidylinositol anchor (3, 9, 10). Although a minority, the membrane-type MMPs (MT-MMPs) have gained considerable attention partly because of the abnormality associated with MT1-MMP knockout mice that sharply contrasts with the minimal phenotype exhibited by mice generated similarly for secretory MMPs (11–13). Anchored on the plasma membrane, the MT-MMPs are mobilized by cells to specialized membrane domains where ECM components are proteolyzed efficiently (14, 15). Recent works (11, 12, 16–19) have concluded that MT1-MMP is more destructive than not only those secretory MMPs, but also its own soluble form, suggesting that the MT-MMPs may be regulated differently at the cell surface. In this study, we hypothesize that membrane trafficking regulates MT1-MMP activity on cell surface. We demonstrate that MT1-MMP is internalized in clathrin-coated vesicles to early endosomes through a dynamin-dependent process and that the blockade of internalization enhances MT1-MMP activity on the cell surface.
Materials and Methods
Cell Culture.
HT1080, Madin–Darby canine kidney (MDCK), COS, Chinese hamster ovary, SKBr3, SKOV3, and HEK293 cells have been described (20) or were gifts from Ping-Yi Law (University of Minnesota) or Mike Xu (Fox Chase Cancer Center, Philadelphia) and maintained as described (20). Cell culture media and supplements were purchased from Life Technologies (Rockville, MD).
Immunological Reagents.
Rabbit anti-MT1-MMP antibody (Ab3) has been described (15). Proteinase inhibitors, Con A, and secondary Ab conjugates were from Sigma. Anti-dynamin, clathrin, and early endosome antigen-1 (EEA1) Abs were purchased from Transduction Laboratories (Lexington, KY). BB-94 was a gift from British Biotechnology (Oxford, U.K.).
Expression Constructs and Transfection.
Wild-type (wt) and C-terminally truncated MT1-MMP constructs have been detailed (21). Expression constructs for wt rat dynamin and its K44A mutant were generous gifts from the Lefkowitz laboratory (Duke University, Durham, NC) (22). The DNA constructs were transfected into various cells by Lipofectamine (Life Technologies). Stable clones were selected in the presence of G418. Screening for stable clones were carried out by Western analysis of the cell lysates or zymographic analysis of pro-MMP-2 activation as described below.
Western Blotting, Immunoprecipitation, and Gelatin Zymography.
These procedures have been described (20, 21). In brief, serum-free media supplemented with purified pro-MMP-2 or 5% FBS was added to cells. After the indicated time period, conditioned media were collected and cleared of cell debris by centrifugation and analyzed by SDS/PAGE impregnated with gelatin (1 mg/ml) as described (20, 21). For immunoprecipitation and Western blot, cells were lysed in RIPA buffer (250 μl/50 mM Tris, pH 7.5/150 mM NaCl/0.25% sodium deoxycholate/0.1% Nonidet P-40/10 μM leupeptin/0.1 μM 5-p-amidinophenylmethanesulfonyl fluoride/1 μM aprotinin). The lysates were centrifuged (14,000 × g, 20 min). The resulting supernatants were immunoprecipated and blotted as described (18). Cell surface biotinylation and detections were carried out as suggested by the manufacturer (Pierce) (23).
Immunostaining and Confocal Microscopy.
For internalization experiments, cells seeded on coverslips in 6-well plates were washed three times with PBS and shifted to 4°C. Anti-MT1-MMP Ab was added to the cells at 0.2 μg/ml for 2 h. Ab was subsequently removed and cells were washed before being shifted to 37°C for the indicated time. Cells were then fixed and stained with FITC-conjugated secondary Ab. For colocalization experiments, cells were labeled with either anti-clathrin, dynamin, or EEA1 Abs as the first Ab followed by rhodamine- or FITC- conjugated secondary Abs (Jackson ImmunoResearch). Confocal microscopy was carried out in the Biomedical Image Processing laboratories at the University of Minnesota as described (10).
Results
Con A Stimulates Pro-MMP-2 Activation by Increasing Cell Surface Expression of MT1-MMP.
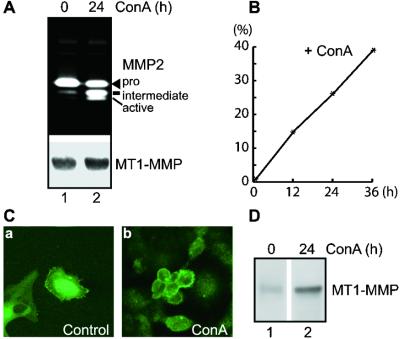
HT1080 cells derived from human fibrosarcoma secreted mostly the pro form of MMP-2 when cultured under serum-free conditions, but converted a substantial portion of pro-MMP-2 into active forms when treated with Con A (Fig. 1 A Upper and B) (24). To understand the mechanism of ConA-mediated pro-MMP-2 activation, we characterized the primary activator of pro-MMP-2 in HT-1080 cells, namely, MT1-MMP (25). The expression level of MT1-MMP protein did not change significantly with Con A treatment, nor did the pattern of MT1-MMP activation (Fig. 1A Lower) as reported (26). We next considered the possibility that Con A may alter the cellular localization of MT1-MMP. Immunofluorescent confocal microscopy revealed that MT1-MMP protein in unstimulated HT1080 cells is localized inside cells (Fig. 1Ca), and thus is unable to access and activate pro-MMP-2 on the cell surface (Fig. 1A Upper; ref. 27). On the other hand, ConA treatment dramatically shifted MT1-MMP from the intracellular compartment to the cell surface where pro-MMP-2 is activated (Fig. 1C, b vs. a), arguing that Con A mediates cellular activation of pro-MMP-2 by mobilizing MT1-MMP from intracellular compartment to cell surface. To confirm the shift in the localization of MT1-MMP, we analyzed cell surface MT1-MMP from HT1080 cells with or without Con A treatment by biotinylation coupled with immunoprecipitation and blotting with alkaline phosphatase-conjugated streptavidin. As shown in Fig. 1D, HT1080 cells treated with Con A have more MT1-MMP protein on the cell surface than the unstimulated cells (Fig. 1D, lane 2 vs. 1), arguing that trafficking, rather than expression, of MT1-MMP may play a predominant role in regulating its activity at the cell surface.
Figure 1.
Con A stimulates pro-MMP-2 activation by increasing cell surface presentation of MT1-MMP. (A) Stimulation of pro-MMP-2 activation by Con A in HT1080 cells. Confluent HT1080 cells were either treated in serum-free DMEM alone (lane 1) or with Con A (50 μg/ml, lane 2) for 24 h. Supernatants were analyzed by gelatin zymography (Upper), and cell lysates were analyzed by Western blotting using anti-MT1-MMP Ab (Ab3) as described (Lower) (15, 21). (B) Time course of Con A-stimulated pro-MMP-2 activation in HT1080 cells. HT1080 cells were stimulated with Con A (50 μg/ml) for 0, 12, 24, and 36 h and analyzed by zymography as described in A. Percentages of pro-MMP-2 activation was calculated as active + intermediate/pro + intermediate + active species as determined by densitometry (18) and plotted against incubation time (x axis). (C) Shift of MT1-MMP from intracellular compartment to cell surface by Con A treatment. HT1080 cells grown on coverslips in 6-well plates were either left untreated (a) or treated with Con A (50 μg/ml, b) for 24 h. Cells were then permabilized, stained with anti-MT1-MMP Ab, and scanned by confocal microscopy as described in Materials and Methods. Note that HT1080 cells are all positive for MT1-MMP staining but with varying degree of intensity as shown in a; and they responded to Con A at varying degree as well as shown in b. (D) Accumulation of MT1-MMP in Con A-treated HT1080 determined by cell surface biotinylation. HT1080 cells cultured in the absence (lane 1) or presence (lane 2) of Con A (50 μg/ml) were biotinylated, lysed, and immunoprecipitated with anti-MT1-MMP Ab (Ab3), fractionated on SDS/PAGE, transferred to poly(vinylidene difluoride) membrane, and blotted with alkaline phosphatase-conjugated streptavidin (22). Note the enhancement of MT1-MMP in Con A-treated cells (lane 2 vs. 1).
Deletion of MT1-MMP Cytoplasmic Domain Enhances Its Cell Surface Localization with Increased Activation of Pro-MMP-2.
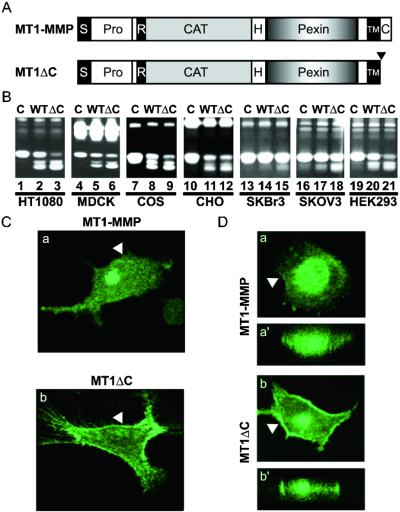
We rationalized that the cytoplasmic domain might bridge the ectoenzyme to the cellular machinery and, thus, might regulate MT1-MMP trafficking. Therefore, we analyzed the truncation mutant MT1ΔC as depicted in Fig. 2A. As shown in Fig. 2B, MT1ΔC expressed more robust activity in processing pro-MMP-2 than the wt construct when transfected into HT1080, MDCK, COS, Chinese hamster ovary, and HEK293 cells (lane 3 vs. 2, 6 vs. 5, 9 vs. 8, 12 vs. 11, and 21 vs. 20), but to a lesser degree in SKBr3 and SKOV3 cells (lane 15 vs. 14 and 18 vs. 17). Reminiscent of Con A treatments shown in Fig. 1A, these results raise the possibility that removal of the cytoplasmic domain promotes cell surface accumulation of MT1-MMP. Indeed, MT1ΔC exhibited more surface expression than its wt counterpart as documented by confocal microscopy (Fig. 2 C and D). Mimicking the endogenous MT1-MMP in Con A-treated HT1080 cells (Fig. 1Cb), the transfected MT1ΔC is primarily localized in the plasma membrane (Fig. 2Cb, arrowhead), in contrast to the perinuclear localization of the wt MT1-MMP (Fig. 2Ca). Similarly, in MDCK cells, a significant portion of MT1ΔC is expressed on the cell surface whereas the wt molecules are mostly intracellular (Fig. 2D b vs. a for z-sections, (arrowhead) and b′ vs. a′ for vertical scans of the same cells). These data suggest that the cytoplasmic domain is required for the observed intracellular localization of MT1-MMP.
Figure 2.
Regulation of MT1-MMP activity by its cytosolic domain. (A) Schematic illustrations of MT1-MMP and MT1ΔC. Full-length MT1-MMP is depicted with signal peptide (s), prodomain (pro), furin cleavage site (R), catalytic domain (cat), hinge region (H), hemopexin-like domain (Pexin), transmembrane domain (TM), and cytoplasmic domain (C) as described (21). MT1ΔC contains a truncation of the 20-residue cytoplasmic domain downstream of the transmembrane domain as indicated by the downward arrowhead (21). (B) Cytoplasmic domain negatively regulates MT1-MMP activity. HT1080 (lanes 1–3), MDCK (lanes 4–6), COS (lanes 7–9), Chinese hamster ovary (CHO) (lanes 10–12), SKBr3 (lanes 13–15), SKOV3 (lanes 16–18), and HEK293 (lanes 19–21) cells were transfected with control vector (C, lanes 1, 4, 7, 10, 13, 16, and 19), MT1-MMP (WT, lanes 2, 5, 8, 11, 14, 17, and 20) or MT1ΔC (ΔC, lanes 3, 6, 9, 12, 15, 18, and 21). Cellular activation of pro-MMP-2 was analyzed by zymography as described in Fig. 1. Note that varying degree of activation for pro-MMP-2 between the cell lines were observed, but the ΔC mutant consistently exhibited higher activity than the wt molecule. (C and D) Cellular localizations of transfected MT1-MMP and MT1ΔC in HT1080 (C) and MDCK (D) cells. Serial Z-sections for MT1-MMP (Ca) and MT1ΔC (Cb) were acquired by confocal microscopy and one from each scan was presented to show differences in distribution between the wt and cytosolic domain truncation mutant. (D) Vertical scans were performed on the corresponding cells and shown in a′ and b′. The arrowheads mark the plasma membrane. Note the general lack of intracellular signals for MT1ΔC vs. the prominent intracellular signal for the wt molecule.
The Cytoplasmic Domain of MT1-MMP Is Required for Con A-Stimulated Enhancement of Pro-MMP-2 Activation.
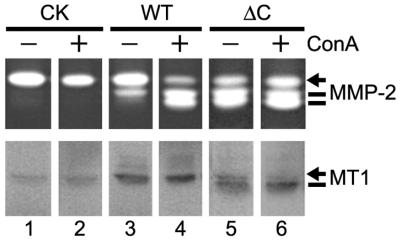
The apparent phenotypic similarity between endogenous MT1-MMP in Con A-treated cells and exogenous MT1ΔC in transfected cells (Figs. 1 and 2) suggests that Con A may act through the cytoplasmic domain of MT1-MMP. To test this idea, we incubated MDCK cells stably transfected by control vector, wt MT1-MMP and MT1ΔC with Con A. As shown in Fig. 3, Con A stimulated the activation of pro-MMP-2 in MT1-MMP transfected cells without significantly affecting its expression (lane 4 vs. 3, Upper and Lower). MDCK cells transfected with control vector failed to activate pro-MMP-2 with or without Con A treatment, indicating that the Con A effect is MT1-MMP dependent (Fig. 3, lane 2 vs. 1). On the other hand, MDCK cells transfected with MT1ΔC exhibited robust pro-MMP-2 activation but failed to respond to Con A treatment for further enhancement (Fig. 3, lane 6 vs. 5). In fact, Con A failed to enhance MT1ΔC activity in a time-course study, even at early time points when pro-MMP-2 activation was <50% (data not shown). These data suggest that the cytoplasmic domain is required for Con A-stimulated enhancement of MT1-MMP activity.
Figure 3.
Cytoplasmic domain is required for Con A-mediated stimulation of pro-MMP-2 activation. MDCK cells were transfected with control vector (lanes 1 and 2), MT1-MMP (lanes 3 and 4), and MT1-MMPΔC (lanes 5 and 6) for 4 h before being washed three times with PBS and replenished with fresh culture media. Fresh media containing 5% FBS was added to the cells (24 h later) either alone (lanes 1, 3, and 5) or with Con A (50 μg/ml). The conditioned media were harvested and analyzed by zymography (12 h later) (5 μl/lane Upper). The cells were lysed and analyzed by Western blotting using anti-MT1-MMP Ab (Lower). Note that MT1-MMPΔC is smaller in size because of the truncation of the cytoplasmic domain. The weak signals from control-transfected cells may be endogenous MT1-MMP (21).
Colocalization Between MT1-MMP and Markers for Endocytosis.
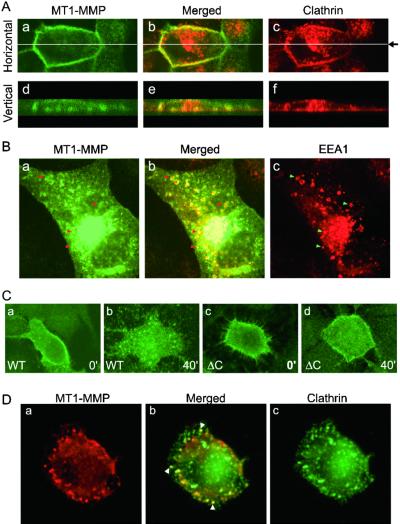
The apparent accumulation of MT1-MMP on the cell surface because of either Con A treatment or cytoplasmic domain truncation suggests that MT1-MMP may be regulated through vesicular trafficking as a membrane protein. One major mechanism of regulation for cell membrane protein is endocytosis through clathrin-coated pits (28). In fact, it has been reported that Con A blocks clathrin-mediated endocytosis (29). To investigate this possibility, we attempted to colocalize MT1-MMP with clathrin on the plasma membrane. To avoid the overwhelming signal from intracellular MT1-MMP, we labeled MT1-MMP transfected cells with anti-MT1-MMP Ab at 4°C, before fixation, permeabilization and staining with Ab against the heavy chain of clathrin. Despite heterogeneity associated with the expression of transfected MT1-MMP and endogenous clathrin, we observed general colocalization between MT1-MMP and clathrin on plasma membrane. As shown in Fig. 4A, MT1-MMP can be observed clearly on the cell surface (a). In the same cell, clathrin can also be detected on the plasma membrane and in intracellular compartments (Fig. 4Ac). Merging the images for both MT1-MMP and clathrin demonstrates that MT1-MMP and clathrin colocalizes on the plasma membrane (Fig. 4Ab). Fig. 4A d–f are vertical scans, which further depict the colocalization of clathrin and MT1-MMP on the plasma membrane.
Figure 4.
Colocalization of MT1-MMP, clathrin, and EEA1 and cytoplasmic domain-dependent internalization. (A) MT1-MMP and clathrin colocalization. MDCK cells transfected with MT1-MMP (1 μg) were incubated with rabbit anti-MT1-MMP Ab at 4°C, followed by fixation and staining with mouse anti-clathrin Ab as described in Materials and Methods). MT1-MMP (green, a and d) and clathrin (red, c and f) were detected by confocal microscopy with horizontal (Upper, a-c) and vertical (Lower, d-f) scans. Two pictures were then merged and presented as b and e. Note that the expression of clathrin and MT1-MMP are heterogeneous in nature and a representative field is presented to depict clathrin inside the plasma membrane and MT1-MMP on the other side of the plasma membrane (b and e). (B) MT1-MMP in endosomes. Cells transfected as in A were fixed, permabilized, and stained with anti-MT1-MMP Ab (see Aa) or anti-EEA1 Ab (c). The same secondary Abs were used for the detections as in A. Note that the signal for intracellular MT1-MMP overwhelms its presence on plasma membrane (a). The typical ring-type endosomes are clearly visible as orange color and selectively marked by arrows (a–c). (C) Differential internalization for MT1-MMP and MT1-MMPΔC. HT1080 cells were transfected with MT1-MMP (a and b) or MT1-MMPΔC (c and d). These cells were labeled with anti-MT1-MMP Ab at 4°C for 2 h, 24 h later. The Ab was removed and cells were washed three times with cold PBS before being shifted to 37°C for commencement of the internalization program. Cells were fixed at 0 (a and c) or 40 min (b and d) and stained with FITC-conjugated goat anti-rabbit secondary Ab. Note that the wt MT1-MMP internalized into endosome-like structures in 40 min (b), whereas MT1-MMPΔC failed to be internalized appreciably (d). (D) Clathrin colocalizes with internalized MT1-MMP. HT1080 cells transfected with MT1-MMP were labeled with anti-MT1-MMP Ab and allowed to internalize for 20 min as described in C. Cells were then fixed, permeabilized, and stained with anti-clathrin Ab, followed by secondary Abs conjugated with FITC or rhodamine. MT1-MMP (red, a) and clathrin (green, c) were detected by confocal microscopy. The merged panel (b) depicts colocalization between clathrin and MT1-MMP (arrowheads).
Colocalization between MT1-MMP and clathrin raises the possibility that MT1-MMP be internalized in clathrin-coated pits to maintain a relatively low level on cell surface as shown above (Fig. 1Ca; Fig. 2 C and D). To test this possibility, we attempted to localize MT1-MMP in early endosomes by staining cells with MT1-MMP and EEA1, a marker for early endosomes (30), under permeabilized conditions. As expected, the EEA1 Ab labeled classic endosomal structures within the cells, but not the cell surfaces (Fig. 4Bc). Anti-MT1-MMP Ab, on the other hand, stained mostly intracellular structures (arrows), with only modest signals on cell surface (Fig. 4Ba). When merged, it is apparent that most of the EEA1-positive endosomes are also positive for MT1-MMP, supporting the notion that MT1-MMP may be internalized into endosomes through clathrin-coated vesicles.
wt MT1-MMP, Not Its Cytoplasmic Domain Truncated Form, Is Internalized.
The apparent colocalization between MT1-MMP and clathrin and EEA1 suggests that MT1-MMP may be internalized by endocytosis. In preliminary studies, we characterized the internalization process by first labeling cells with anti-MT1-MMP Ab at 4°C and then shifting the cells to 37°C to commence internalization for 0, 10, 30, 60, 80, and 120 min. At the indicated time points, cells were fixed, permeabilized, and stained with FITC-conjugated goat anti-rabbit secondary Ab. Most of the cell surface labeling signals disappeared in ≈60 min with corresponding appearance of intracellular signals (data not shown, also see Fig. 4). This seemingly rapid rate of internalization would potentially explain the poor accumulation of wt MT1-MMP on cell surface (Figs. 1C and 2 C and D). To compare the rate of internalization for wt and cytoplasmic domain truncated MT1-MMPs, we performed cell surface labeling and internalization experiments as described above. As shown in Fig. 4C, wt MT1-MMP was internalized into endosomal-like structures (b vs. a) in 40 min, whereas MT1ΔC remained mostly on the plasma membrane (d vs. c). To confirm that MT1-MMP is internalized in clathrin-coated pits, we double-stained cells in the internalization experiments with MT1-MMP and clathrin. As shown in Fig. 4D, most of MT1-MMP internalized within 20 min remained in vesicles coated with clathrin (arrows in b), arguing that MT1-MMP is internalized in clathrin-coated pits. At 40 min, however, most of the internalized MT1-MMP had been routed to vesicles negative for clathrin (data not shown). Together, these data argue strongly that MT1-MMP is internalized in clathrin-coated vesicles and its cytoplasmic domain down-regulates its activity on cell surface through endocytosis.
Regulation of MT1-MMP by Dynamin.
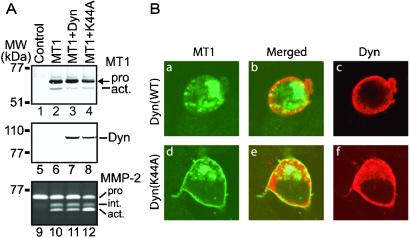
For protein internalized through clathrin-coated vesicles, the scission of newly formed vesicles from the plasma membrane requires dynamin, a large GTPase implicated in clathrin-mediated endocytosis (22, 28, 31). To test whether dynamin plays any role in MT1-MMP internalization, we cotransfected the wt dynamin and the K44A dominant-negative mutant with MT1-MMP into various cell lines. Shown in Fig. 5A are representative data obtained from transfected MDCK cells. Western blot analysis using anti-dynamin or MT1-MMP Abs revealed equivalent levels of expression from the MT1-MMP or dynamin plasmids, respectively, among the transiently transfected cells, indicating similar efficiencies of transfection (Fig. 5A, lanes 2–4 and 7 and 8). The K44A dominant negative mutant of dynamin, not its wt, enhanced MT1–MMP-dependent activation of pro-MMP-2 (Fig. 5A, lane 12 vs. 10 and 11). Furthermore, the K44A mutant did not alter the expression or processing of MT1-MMP significantly (Fig. 5, lane 4 vs. 3), although the cells transfected with dynamin or K44A appeared to have less active MT1-MMP (Fig. 5A, lane 2 vs. 3 and 4). We tested the effects of dynamin K44A on MT1-MMP in other cells such as HEK293, Neuro2A, COS, and HT1080, similar enhancements were observed (data not shown). Mechanistically, we demonstrated that K44A, but not wt dynamin, enhanced the expression of wt MT1-MMP on plasma membrane as shown in Fig. 5B (d vs. a), by interfering with dynamin-mediated endocytosis of MT1-MMP. Taken together, we concluded that MT1-MMP is negatively regulated by its cytoplasmic domain through dynamin-dependent endocytosis in clathrin-coated pits.
Figure 5.
Dynamin K44A stimulates MT1-MMP-dependent pro-MMP-2 activation by blocking endocytosis. (A) K44A increases MT1-MMP activity in MDCK cells. MDCK cells were transfected with control vector alone (lanes 1, 5, and 9) or MT1-MMP expression vector (lanes 2–4, 6–8, and 10–12) plus dynamin (lanes 3, 7, and 11), dynamin K44A (lanes 4, 8, and 12). Cells were washed 24 h later with PBS and replenished with fresh serum-free media supplemented with recombinant MMP-2. After an additional 24-h incubation, conditioned media were harvested and analyzed by zymography (lanes 9–12), and cells were lysed and analyzed for MT1-MMP (lanes 1–4) or dynamin (lanes 5–8). Note that K44A significantly enhances pro-MMP-2 activation without enhancing the amount of MT1-MMP expression or processing (lane 12 vs. 10; 4 vs. 2). (B) Blockade of MT1-MMP endocytosis by dynamin K44A. MT1-MMP was cotransfected with dynamin (a-c) or K44A (d-f) into N2A cells. Cells were fixed and stained with anti-MT1-MMP and anti-dynamin Abs as described in Fig. 4 legend. MT1-MMP (a and d) and dynamin (c and f) were obtained by confocal microscopy. The merged pictures (b and e) depict any colocalization. Note that dynamin K44A, not its wt, increases MT1-MMP expression on plasma membrane (d vs. a).
Discussion
As the first MMP identified with a transmembrane domain, MT1-MMP provides a unique paradigm for MMP-dependent ECM proteolysis. It confers invasive and migratory phenotypes to cells of endothelial, epithelial, and fibroblastic origins (7, 14–17, 32–35). Furthermore, it has been reported that its cytoplasmic and transmembrane domains target MT1-MMP to specialized cellular structures of invading or migrating cells (14, 15), highlighting the importance of domains unique to the membrane-type MMPs. In this study, we present evidence that the cytoplasmic domain of MT1-MMP regulates its expression on the cell surface through dynamin-dependent endocytosis in clathrin-coated pits. This endocytic pathway could provide a versatile mechanism for cells to regulate MT1-MMP activity under both physiological and pathological conditions.
Cytoplasmic Domains of MT-MMPs.
There are six MT-MMPs reported so far that can be classified into two subgroups depending on their anchoring mechanisms (36). The first subgroup includes MT1-, MT2-, MT3-, and MT5-MMPs with a single path transmembrane domain in a type I topology (Nout/Cin) (7, 20, 37, 38). The second subgroup has two members, MT4 and MT6-MMPs, anchored via a glycosylphosphatidylinositol anchor (18, 39, 40). Although the GPI-anchored MMPs contain no cytoplasmic domains, the type I MT-MMPs all contain a 20-residue cytoplasmic tail. Interestingly, the cytoplasmic domain of MT1-MMP appears to be divergent at the amino acid level from those of MT2, MT3, and MT5-MMPs, raising the possibility that MT1-MMP may be regulated differently (9). It would be of interest to see whether endocytosis also regulates the activity of the other type I MT-MMPs, as we demonstrated here for MT1-MMP. Compared to other mechanisms of regulation, endocytosis offers fine-tuning of MT-MMP activity on the cell surface, perhaps a common theme for other membrane-associated proteolysis. Indeed, the tumor necrosis factor α (TNF-α)-converting enzyme has been shown to be down-regulated by endocytosis (41). However, it is not clear whether the cytoplasmic domain of the TNF-α-converting enzyme is involved in the internalization process as we observed for MT1-MMP. Future work to compare and contrast the internalization processes for these two distinct families of membrane-anchored metalloproteianses should shed light on the regulation of cell surface proteolysis.
Functional Consequence of MT1-MMP Internalization.
We have shown here that internalization provides a mechanism to down-regulate MT1-MMP activity. Blockade of internalization by three different means, i.e., Con A treatment (Fig. 1), cytoplasmic domain deletion (Fig. 2), and cotransfection with dominant negative mutant of dynamin K44A (29) (Fig. 5), all enhanced the cell surface presentation of MT1-MMP accompanied by elevated pro-MMP-2 activation, the classic function for MT1-MMP (7, 25). The fate of the internalized MT1-MMP remains unresolved. Recent reports (15, 19, 23, 26, 42) suggest that MT1-MMP may be processed into a 45-kDa form that is devoid of its catalytic domain and unable to activate pro-MMP-2. It would be interesting to determine whether the internalized MT1-MMP is processed into the 45-kDa form or simply routed to the lysosome for destruction as reported for internalized TIMP-2 (42). Either process would lead to a down-regulation of MT1-MMP activity and thus offer a potential explanation for our results in this article. Taken together, we suggest that the default pathway for MT1-MMP trafficked to cell surface is to be internalized to keep its activity intracellularly (Fig. 1). Therefore, it is possible that a transient increase in MT1-MMP activity could be achieved by temporary inhibition of MT1-MMP endocytosis when cells are programmed, stimulated, or activated to remodel their extracellular matrix (11, 12, 17). On the other hand, excessive MT1-MMP activity may be disposed rapidly by enhancing its internalization through the same cytoplasmic domain—e.g., when active remodeling events involving MT1-MMP are terminated. In essence, an “on/off” or “up/down” switch might have been built into the cytoplasmic domain to regulate MT1-MMP activity, in addition to its purported function for targeting to the invasive front (14, 15). Thus, the cytoplasmic domain regulates MT1-MMP mediated proteolysis by controlling not only its localization, but also its steady-state concentration at the cell surface.
Acknowledgments
We thank Drs. Lefkowitz and Ahn at Duke University for providing the dynamin constructs and Drs. Birkadal-Hanson and Strongin for discussions. This work was supported by National Institutes of Health Grant CA76308 and American Heart Association Grant-In-Aid 9750197N (to D.P.) and the Academy of Finland (J.K-O.).
Abbreviations
- MT-MMP
membrane-type matrix metalloproteinase
- MMP
matrix metalloproteinase
- ECM
extracellular matrix
- EEA1
early endosomal antigen 1
- wt
wild type
- MDCK
Madin–Darby canine kidney
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Werb Z. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 2.Massova I, Kotra L P, Fridman R, Mobashery S. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- 3.Nagase H, Woessner J F., Jr J Biol Chem. 1999;274:21491–21994. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 4.Celentano D C, Frishman W H. J Clin Pharmacol. 1997;37:991–1000. doi: 10.1002/j.1552-4604.1997.tb04278.x. [DOI] [PubMed] [Google Scholar]
- 5.Basset P, Bellocq J P, Wolf C, Stoll I, Hutin P, Limacher J M, Podhajcer O L, Chenard M P, Rio M C, Chambon P. Nature (London) 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- 6.Buttner F H, Chubinskaya S, Margerie D, Huch K, Flechtenmacher J, Cole A A, Kuettner K E, Bartnik E. Arthritis Rheum. 1997;40:704–709. doi: 10.1002/art.1780400415. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. Nature (London) 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 8.Stamenkovic I. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 9.Pei D. Cell Res. 1999;9:291–303. doi: 10.1038/sj.cr.7290028. [DOI] [PubMed] [Google Scholar]
- 10.Pei D, Kang T, Qi H. J Biol Chem. 2000;275:33988–33997. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Apte S S, Soininen R, Cao R, Baaklini G Y, Rauser R W, Wang J, Cao Y, Tryggvason K. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. . (First Published March 28, 2000; 10.1073/pnas.060037197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov S A, Mankani M, Robey P G, Poole A R, Pidoux I, et al. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro S D. Matrix Biol. 1997;15:527–533. doi: 10.1016/s0945-053x(97)90027-5. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara H, Howard L, Thompson E W, Sato H, Seiki M, Yeh Y, Chen W T. Proc Natl Acad Sci USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehti K, Valtanen H, Wickstrom S, Lohi J, Keski-Oja J. J Biol Chem. 2000;275:15006–15013. doi: 10.1074/jbc.M910220199. [DOI] [PubMed] [Google Scholar]
- 16.Hotary K, Allen E, Punturieri A, Yana I, Weiss S J. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraoka N, Allen E, Apel I J, Gyetko M R, Weiss S J. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 18.Kang T, Yi J, Yang W, Wang X, Jiang A, Pei D. FASEB J. 2000;14:2559–2568. doi: 10.1096/fj.00-0269com. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Barrantes S, Toth M, Bernardo M M, Yurkova M, Gervasi D C, Raz Y, Sang Q A, Fridman R. J Biol Chem. 2000;275:12080–12089. doi: 10.1074/jbc.275.16.12080. [DOI] [PubMed] [Google Scholar]
- 20.Pei D. J Biol Chem. 1999;274:8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- 21.Pei D, Weiss S J. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- 22.Ahn S, Maudsley S, Luttrell L M, Lefkowitz R J, Daaka Y. J Biol Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 23.Lehti K, Lohi J, Valtanen H, Keski-Oja J. Biochem J. 1998;334:345–353. doi: 10.1042/bj3340345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overall C M, Sodek J. J Biol Chem. 1990;265:21141–21151. [PubMed] [Google Scholar]
- 25.Strongin A Y, Collier I, Bannikov G, Marmer B L, Grant G A, Goldberg G I. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 26.Lohi J, Lehti K, Westermarck J, Kahari V M, Keski-Oja J. Eur J Biochem. 1996;239:239–247. doi: 10.1111/j.1432-1033.1996.0239u.x. [DOI] [PubMed] [Google Scholar]
- 27.Azzam H S, Thompson E W. Cancer Res. 1992;52:4540–4544. [PubMed] [Google Scholar]
- 28.Pearse B M, Robinson M S. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 29.Luttrell L M, Daaka Y, Della Rocca G J, Lefkowitz R J. J Biol Chem. 1997;272:31648–31656. doi: 10.1074/jbc.272.50.31648. [DOI] [PubMed] [Google Scholar]
- 30.Patki V, Virbasius J, Lane W S, Toh B H, Shpetner H S, Corvera S. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks B, Stowell M H, Vallis Y, Mills I G, Gibson A, Hopkins C R, McMahon H T. Nature (London) 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 32.Deryugina E I, Bourdon M A, Jungwirth K, Smith J W, Strongin A Y. Int J Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 33.Ellerbroek S M, Fishman D A, Kearns A S, Bafetti L M, Stack M S. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- 34.Haas T L, Davis S J, Madri J A. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- 35.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang T, Yi J, Guo A, Wang X, Overall C, Jiang W, Elde R, Borregaard N, Pei D. J Biol Chem. 2001;276:21960–21968. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- 37.Takino T, Sato H, Shinagawa A, Seiki M. J Biol Chem. 1995;270:23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]
- 38.Will H, Atkinson S J, Butler G S, Smith B, Murphy G. J Biol Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 39.Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. J Biol Chem. 1999;274:34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- 40.Kojima S, Itoh Y, Matsumoto S, Masuho Y, Seiki M. FEBS Lett. 2000;480:142–146. doi: 10.1016/s0014-5793(00)01919-0. [DOI] [PubMed] [Google Scholar]
- 41.Doedens J R, Black R A. J Biol Chem. 2000;275:14598–14607. doi: 10.1074/jbc.275.19.14598. [DOI] [PubMed] [Google Scholar]
- 42.Maquoi E, Frankenne F, Baramova E, Munaut C, Sounni N E, Remacle A, Noel A, Murphy G, Foidart J M. J Biol Chem. 2000;275:11368–11378. doi: 10.1074/jbc.275.15.11368. [DOI] [PubMed] [Google Scholar]