Abstract
Triclocarban (TCC) is an antimicrobial agent routinely detected in surface waters which has been hypothesized to interact with the vertebrate endocrine system. The present study examined the effects of TCC alone and in combination with the model endocrine disruptor 17β-trenbolone (TRB) on fish reproductive function. Adult Pimephales promelas were continuously exposed to either 1 or 5 μg TCC/L, 0.5 μg TRB/L or a mixture (MIX) of 5 μg TCC and 0.5 μg TRB/L for 22 d and a variety of reproductive and endocrine-related endpoints were examined. Cumulative fecundity was significantly reduced in fathead minnows exposed to TRB, MIX or 5 μg TCC/L. Exposure to 1 μg TCC/L had no effect on reproduction. In general both TRB and MIX treatments caused similar physiological effects, evoking significant reductions in female plasma vitellogenin, estradiol, and testosterone, and significant increases in male plasma estradiol. Based on analysis of the ovarian transcriptome, there were potential pathway impacts that were common to both TRB and TCC-containing treatment groups. However, in most cases, those pathways were more plausibly linked to differences in reproductive status than to androgen-specific functions. Overall, TCC was reproductively toxic to fish at concentrations at or near those that have been measured in surface water. There was little evidence that TCC elicits reproductive toxicity through a specific mode of endocrine or reproductive action, nor that it could augment the androgenic effects of TRB. Nonetheless, the relatively small margin of safety between some measured environmental concentrations and effect concentrations suggest concern is warranted.
Keywords: Adverse outcome pathway, contaminants of emerging concern, reproductive toxicity, toxicity mechanisms, endocrine disrupting compounds
INTRODUCTION
Triclocarban (TCC) is a chlorinated biphenyl ether that has been used for more than 50 years as an antimicrobial agent in consumer products such as soaps, deodorants, skin creams and toothpaste [1, 2]. Almost 500,000 kg of TCC are used on an annual basis in the USA [3–5]. Found in a variety of matrices, including wastewater treatment plant (WWTP) effluents and biosolids, surface waters and sediments, it is one of the most frequently detected personal care products in the environment, [1–3, 6]. Environmental concentrations of TCC can be substantial; for example, TCC has been reported at a concentration as high as 6.75 μg/L in surface water [3]. Mean/median surface water concentrations typically fall in the 0.1–0.2 μg/L range [3]. In addition, bioaccumulation of TCC from WWTP discharges and/ or sediments has been reported in algae and benthic macroinvertebrates [7, 8], and a log bioconcentration factor of about 2.8 has been determined for TCC in fish [9].
Despite its large production volume, broad distribution in aquatic environments, and at least some propensity to bioaccumulate, comparatively little is known concerning the potential toxicity of TCC to aquatic animals [1, 2]. In short-term toxicity tests, invertebrates seem to be more sensitive to TCC than fish with a 48-h EC50 value of 3.1 μg/L for Ceriodaphnia dubia, compared to 96-h LC50 values around 100 μg/L for rainbow trout (Oncorhynchus mykiss) and bluegill (Lepomus macrochirus) (http://www.aciscience.org/docs/Triclocarban_HPV_Robust_Study_Summaries.pdf). In chronic tests with invertebrates, lowest-observable effect concentrations of 4.7 and 0.13 μg TCC/L were reported for Daphnia magna and mysid shrimp reproduction, respectively (http://www.aciscience.org/docs/Triclocarban_HPV_Robust_Study_Summaries.pdf). Exposure to concentrations up to 1.6 μg TCC/L were reported to reduce aggression in sexually mature male fathead minnows, but had no significant effects on secondary sex characteristics, histopathology, or circulating vitellogenin concentrations in adults, nor growth or escape performance in larval fathead minnows [10].
A number of studies have suggested that TCC has the potential to cause toxicity through interactions with vertebrate endocrine systems [11]. Specifically, it has been postulated that TCC may exhibit a new mode of endocrine disruption, augmentation of endogenous hormonal activities [12, 13]. This stemmed from TCCs apparent ability to enhance hormone-dependent induction of estrogen receptor (ER) and androgen receptor (AR)-dependent gene expression in vitro [12–15]. In vivo studies in rats have supported the in vitro evidence that TCC augments, but does not directly elicit, androgen action [13, 16]. Similarly, in a previous study with fathead minnow, TCC (10 μg/L) enhanced 17β trenbolone (TRB)-induced formation of nuptial tubercles (a male secondary sex characteristic) in females exposed to mixtures of the two chemicals for 21 d [17]. Augmentation of estrogenic action has also been reported in vivo. Specifically, TCC enhanced overexpression of aromatase B (cyp19a1b) in zebrafish (Danio rerio) embryos exposed to exogenous estrogen [18]. Thus, there appears to be a growing body of evidence that TCC may augment the activity of androgens and/or estrogens.
To date, however, the mechanism(s) by which this apparent augmentation is achieved is unclear. Triclocarban does not appear to compete for binding to the AR, at least at concentrations <200 μM, nor does it directly activate cAMP/PKA-mediated signaling [13]. At least one study has indicated that TCC is capable of activating ER alpha (ERα) and down-stream transcription products in vitro and in vivo [19]. However, two other studies reported no evidence of direct activation of ERα [12, 20]. Data on whether TCC in combination with testosterone increases immunoreactive AR abundance compared to testosterone alone were equivocal [13]. High throughput bioassay screening identified TCC as a potent inhibitor of recombinant soluble epoxide hydrolase in a cell free system [20, 21]. However, TCC’s major metabolites do not show similar activity and no alterations in plasma biomarkers of soluble epoxide hydrolase inhibition were detected in rats exposed topically to TCC [21]. Furthermore, there is no clear connection between soluble epoxide hydrolase inhibition and augmentation of androgenic or estrogenic activity. It has been suggested that perhaps TCC sensitizes proteins that act as co-regulators of AR or ER activity [12]; however, that is largely speculative at the present time. Given the established occurrence of TCC in aquatic environments and its potential to affect endocrine function, further characterization of the chemical’s effects in aquatic vertebrates could help address current uncertainties concerning TCC’s possible risks and aid the development of novel adverse outcome pathways that may be relevant for other contaminants.
In an effort to fill some of the current knowledge gaps relative to TCC, we conducted a 22-d reproduction study with the fathead minnow (Pimephales promelas). In contrast with a previous study reported by Ankley et al. [17], the present experiment evaluated a broader range of endpoints and employed a paired-spawning rather than group-spawning design [22]. The study included evaluation of a number of apical endpoints directly relevant to assessing risk (egg production, fertility), as well as several pathway-specific responses reflective of endocrine function (e.g., gonad histology, plasma concentrations of vitellogenin and sex steroids, targeted gene expression). These endpoints were complemented with an unsupervised microarray analysis of the effects of TCC on the ovarian transcriptome. The data were evaluated to answer several key questions: first, whether exposure to TCC could be linked with an ecologically-relevant adverse outcome, i.e., impaired reproduction; second, whether the present study provided additional support for augmentation of androgen action as an endocrine-disrupting mode of action for TCC; and third, whether there were any novel and/or plausible linkages between changes in the ovarian transcriptome and the apical responses observed in females that could support adverse outcome pathway development and/or further hypothesis-driven testing.
METHODS
Chemical and test organisms
Solvent-free, saturated aqueous solutions of TCC (Sigma-Aldrich; 99% purity) and TRB (Sigma-Aldrich; >95% purity) were prepared in UV-treated, filtered, Lake Superior water through continuous stirring in a 20 L glass carboy. Reproductively mature (5–6 month old) fish used for the research were obtained from an on-site culture unit at the US EPA Mid-Continent Ecology Division (Duluth, MN, USA) and displayed prominent secondary sex characteristics (i.e., tubercles and dorsal fat pads on males and ovipositors on females). All laboratory procedures involving fathead minnows were reviewed and approved by the Animal Care and Use Committee in accordance with Animal Welfare Act and interagency Research Animal Committee guidelines.
Experimental design
Fathead minnows were exposed for 22 d to target test concentrations of 0 (Lake Superior Water control), TCC (1 and 5 μg/L), TRB (0.5 μg/L), or a mixture (MIX) of the two chemicals (5 μg TCC and 0.5 μg TRB/L). Test concentrations were selected based on a previous study [17] in which exposure to 10 μg TCC/L caused significant mortality, particularly in males, while exposure to 5 μg/L did not (Supplementary Figure S.0; [17]). Concentrations of TRB were chosen to match concentrations that had been used in a series of TRB mixture experiments [17].
Fathead minnows were randomly paired (one male, one female) and placed in tanks at a density of two pairs per tank with six replicate tanks per treatment (n=12 males, 12 females per treatment). Pairs were separated by a water permeable mesh divider and each pair had its own breeding substrate. The fish were held in the test system, receiving UV-treated, filtered Lake Superior (control) water only, for a 21 d acclimation period during which the fecundity and fertility of each pair were assessed daily. After 21 d of acclimation, exposures were initiated with pairs that had spawned successfully during acclimation by pumping diluted test chemicals to 20 L tanks containing 10 L of water, at a flow rate of approximately 45 ml/minute, to achieve the appropriate test concentrations. The animals were held at 25±0.5 °C under a 16:8 L:D photoperiod and were fed frozen adult brine shrimp (Artemia) to satiation twice per day during both acclimation and exposure.
Over the course of the experiment, the total number of eggs spawned and the number of fertile eggs (determined via inspection under a microscope at the time of egg counting) produced by each pair were recorded daily. Water concentrations of TCC and TRB were determined in stock solutions and all exposure tanks every two to three days. General water quality characteristics (mean ± SD) measured regularly over the course of the exposure were: temperature, 24.8 ± 0.7 °C; pH, 7.87 ± 0.04; dissolved oxygen, 6.8 ± 0.35 mg/L with no significant differences between treatment groups.
At the conclusion of the experiment, the fish were anaesthetized with buffered tricaine methane sulfonate (sodium bicarbonate, 200 mg/L; MS-222 100 mg/L). Immediately after anesthetization, blood was collected from the caudal vasculature using heparinized microhematocrit tubes. Plasma was separated by centrifugation and stored at −80 °C until extracted and analyzed. Body mass and secondary sex characteristics (i.e., nuptial tubercles) were evaluated, and liver, gonads, brains, and pituitary were removed. Nuptial tubercles were enumerated and scored based on their relative size [23, 24]. Gonads were weighed and divided into several pieces. A subsample (≈5–10 mg) was used for an ex vivo steroid production assay. The remaining gonad mass was divided into approximately equal thirds (females) or halves (males) with one portion preserved in Davidson’s fixative for later histological examination, one portion preserved in RNAlater® (Sigma-Aldrich), and one portion (ovaries only) snap-frozen in liquid nitrogen. Brains and pituitaries, collected separately using fine forceps, were each transferred to pre-weighed tubes containing RNAlater®. Samples in RNAlater were stored at −20 °C until total RNA was extracted (gonads only in the present study). Snap-frozen samples were stored at −80 °C. All dissection tools were washed with RNAseZap (Ambion) between samples to prevent cross-contamination or sample degradation by RNAses. Following collection of plasma and tissues, the remainder of each fish was wrapped in solvent-rinsed and dried aluminum foil and stored frozen at −20°C until extracted and analyzed for tissue concentrations of TCC.
Analytical methods
Water concentrations of TCC were determined using a modification of the high-pressure liquid chromatography (HPLC) method described by Ying et al. [25] with diode array detection (265 nm). Water samples (100 μl) were directly injected onto a Zorbax SB C18 column (2.1 × 75 mm; Agilent, Santa Clara, CA, USA) and eluted isocratically with 75% acetonitrile/25% water at a flow-rate of 0.2 mL/min and column temperature of 21 °C. An external standard method of quantification with a six point curve was used. Lake Superior water (method) blanks, spiked water samples, and duplicates from one or more exposure tanks were analyzed with each set of samples. The detection limit for TCC in water was 0.9 μg/L and there was 86±15% agreement among duplicate analyses (mean ± SD; n=48)
Concentrations of TRB were determined using direct injection HPLC with fluorescence detection. Water samples (100–500 μl) were injected onto a Synergi-Hydro RP-C18 column (3 × 75 mm; Phenomenex, Torrance, CA, USA) and eluted isocratically with 70% methanol/water at a flow rate of 0.25 mL/min at 30 °C. The TRB was detected using excitation and emission wavelengths of 364 and 460 nm, respectively, and concentrations determined with an external standard method of quantification. The detection limit for the analysis was typically 50 ng/L. As for TCC, Lake Superior water (method) blanks, spiked–water samples, and duplicates from one or more of the exposure tanks were analyzed with each set of samples. Average (±SD) agreement among duplicate determinations was 100 ± 4.8% (n=15 duplicate analyses).
Bioconcentration factor estimation
Carcasses (sans blood, gonad, liver, pituitary, and brain) from n=5 males and n=5 females per treatment were randomly selected for determination of tissue concentrations of TCC and bioconcentration factor (BCF) estimation. Each fish was thawed, cut into two or three pieces and then placed in 50 ml round bottom glass centrifuge tube. Samples were homogenized in 14 ml acetonitrile using an Ultra-Turrax T27 tissue homogenizer (Janke and Kunkel, Germany). Homogenates were centrifuged at 3010xg for 20 min at −10°C. Supernatants were transferred to 40 ml glass centrifuge tubes, evaporated to 10 ml under a gentle stream of nitrogen and then frozen for at least 1 h. Extracts were thawed, quantitatively transferred to new tubes and evaporated to 5 ml. They were then diluted 1:1 with HPLC grade water before being injected into the HPLC (50 μl) and analyzed as described above for water samples. Average (SD) spike recoveries (n=7) for tissue spiked with TCC and carried through the extraction and analysis procedure was 97.3% (15.8%). Detection limits were 333 ng/g for females and 75 ng/g for males due to variations in overall body size and composition. Bioconcentration factors were estimated by dividing the measured tissue concentration by the corresponding tank-specific measured water concentration.
Steroid and vitellogenin measurements
Ex vivo steroid production assays were conducted using methods adapted from McMaster et al. [26], as described previously by Martinovic et al. [27]. Briefly, subsamples of gonad tissue from each fish were transferred to wells of a 48-well plate (Falcon 35–3078, Beckton Dickinson, Franklin Lakes, NJ, USA) containing 500 ml of Medium 199 (M2520; Sigma) supplemented with 0.1 mM isobutylmethylxanthine (IBMX; Sigma I7018) and 1 mg 25-hydroxycholesterol (Sigma)/ml on ice. Samples were incubated at 25 °C overnight (16.5 hr), after which media from each well was transferred to a microcentrifuge tube and stored at −20 °C until steroid hormones were extracted and analyzed. The mass of each gonad sample was weighed after collection of the media. Average (± SD) sample masses were 9.3 ± 3.5 mg for ovaries and 5.1 ± 2.5 mg for testes. Wells containing supplemented medium but no tissue were incubated, sampled, and analyzed along with experimental samples to serve as assay blanks. Steroids were extracted from medium samples (ex vivo) or plasma samples by liquid-liquid extraction with diethyl ether and quantified by radioimmunoassay [23, 24]. With the exception of cases where plasma volumes were limiting, both plasma E2 and T concentrations were quantified. Plasma concentrations of the estrogen-inducible egg yolk precursor protein, vitellogenin (Vtg), were quantified using an enzyme-linked immunosorbent assay with a polyclonal antibody to fathead minnow Vtg and purified fathead minnow Vtg as a standard [24, 28].
Histology
Gonads preserved for histological analyses were embedded, stained, sectioned and analyzed as described previously [29]. Briefly, gonads were embedded in paraffin, sectioned longitudinally at 4 to 5 μm in a step-wise fashion, and stained with hematoxylin and eosin. A total of three sections were assessed from each gonad sample, beginning at the midline and then at 50-μm intervals following the first section. Pathology findings for the specimens were assigned a severity score (1 – 4, minimal to severe) based on a qualitative analyses of the amount of affected gonad tissue compared to the controls. Due to cost considerations, only ovaries from the TCC5 and control treatments (n=12 per group) were evaluated histologically.
RNA extraction and gene expression measurements
Total RNA was extracted from ovary and testis tissue using RNeasy kits (Qiagen, Valencia, CA). RNA quantity was determined spectrophotometrically (Nanodrop ND-1000, Nanodrop Technologies, Wilmington, DE) and quality was evaluated based on 260/280 ratios (all samples in acceptable range between 1.8 and 2.2). Total RNA samples were diluted to 10 ng/μl and stored at −80 °C until analyzed.
Relative transcript abundance of several genes involved in endocrine function was determined in gonadal tissue from males and females, with each sex analyzed independently on separate plates. These included mRNAs coding for enzymes involved in steroid synthesis: cholesterol side-chain cleavage (cyp11a), 17-alpha-hydroxylase/17, 20 lyase (cyp17), aromatase (cyp19a1a), 3β-hydroxysteroid dehydrogenase (3βhsd), 11β-hydroxysteroid dehydrogenase (11βhsd), and 17β hydroxysteroid dehydrogenase (17βhsd). Five additional transcripts measured included steroidogenic acute regulatory protein (star), Vtg receptor (vtgr), follicle-stimulating hormone receptor (fshr), luteinizing hormone receptor (lhr), and androgen receptor (ar). Quantitative real-time polymerase chain reaction (QPCR) assays were conducted with a one-step procedure using Taqman RNA-to-CT 1-Step Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s recommended protocol. Briefly, template RNA (2.0 μl at 10 ng/μl) was combined with 300 nM forward and reverse primers (Supplementary Table S.1), 150 nM probe, and 2x Master Mix in a 12-μl reaction. Samples were amplified over 40 cycles (melt 94°C for 20 s, anneal and extend 58°C for 60 s) using a 7500 Real Time PCR System (Applied Biosystems), and the relative transcript abundance was estimated as described by Villeneuve et al. [30] based on a standard curve generated by analyzing multiple dilutions of a gene-specific RNA standard, without correction for amplification efficiency. Dilution of the amplicon used as a standard was optimized to yield a standard curve with threshold cycle numbers ranging from approximately 15 to 35. Average amplification efficiencies for RNA standards were all greater than 0.90, with the exception of 11βhsd (0.88 ± 0.07) and star (0.87 ± 0.06). Sample sizes for the QPCR analyses were n=12 per treatment, with the exception of the TCC5 treatment for females, which was n=10.
Microarray analysis
Ovary RNA samples from 24 fish (n=5 for all treatments except TRB alone where n=4) were selected for microarray analysis. Each sample was hybridized to a custom fathead minnow 15,000 oligonucleotide microarray (GEO Platform Accession GPL10259) purchased from Agilent Technologies (Palo Alto, CA). Hybridization was conducted according to the manufacturer’s guidelines for One-Color Microarray-Based Gene Expression Analysis (version 5.7) using 1 μg of total RNA. Complementary DNA synthesis, cRNA labeling, amplification, and hybridization were performed following the manufacturer’s kits and protocols (Quick Amp Labeling kit; One-Color Microarray-Based Gene Expression Analysis version 5.7; Agilent). Scanning was conducted at 5 μm resolution using an Axon GenePix 4000B Microarray Scanner (Molecular Devices Inc., Concord, Ontario, Canada). Data were extracted from microarray images using Agilent Feature Extraction Software. Text versions of the raw and normalized data have been deposited to the Gene Expression Omnibus (GEO: http://www.ncbi.nlm.nih.gov/geo/; Accession GSE64291). The microarrays uniformly exceeded the quality control metric “gNegCtrlAveNetSignal (metric values exceeded the upper limit of 40), suggesting that higher than normal background may have limited sensitivity to detect low abundance transcripts. Otherwise, QC metrics were within the recommended ranges. To further evaluate the quality of the microarray data, relative abundance of seven transcripts evaluated by QPCR (those for which unique microarray probes could be identified) were compared with microarray results for those same genes (supplementary Figure S.1). To control for differences resulting from analysis of different sample sizes and/or statistical subpopulations, the QPCR:microarray comparison was limited to results for the same (n=4 or 5) individual fish per treatment evaluated in the microarray analysis. Consequently, plots shown in supplementary Figure S.1 do not necessarily match the treatment-dependent pattern and statistical results shown for QPCR.
Data analyses
Targeted biological data were tested for normality and homogeneity of variance using a Kolmogorov-Smirnov test and Levene’s test. When data were normally distributed and variance was homogenous, one-way analysis of variance (ANOVA) was used to test for differences across treatments and a post-hoc Duncan’s test was applied to determine which groups differed (p ≤ 0.05). When data did not conform to parametric assumptions, they were either transformed (e.g., log 10) and analyzed via one-way ANOVA when transformed data conformed with parametric assumptions, or analyzed using a non-parametric Kruskall–Wallis test (p ≤ 0.05) followed by a multiple comparisons of mean ranks for all groups (Statistica 12, Dell, Tulsa, OK, USA). Factorial ANOVA was initially employed to test for tank effects, but no significant tank effects were detected, therefore individual fish were considered the unit of replication for the purposes of one-way ANOVA or the Kruskall-Wallace test.
Microarray data were normalized using quantile normalization implemented in GeneSpring GX (Agilent Technologies, Santa Clara, CA, USA). While there are many options for microarray data normalization, quantile normalization is widely used and recognized as one of the most effective and simple general approaches for data normalization [31]. Determination of differential expression between each chemical treatment and the control group was evaluated using two approaches. First, significance analysis of microarray (SAM), implemented using MultiExperiment Viewer (MeV) v. 4.7.1 [32] was employed for higher stringency determination of the differential expression of individual transcripts. For each chemical treatment (TCC1, TCC5, TRB, and MIX), a two class, unpaired test using all unique permutations (252 for TCC1, TCC5, and MIX treatments, 126 for TRB) was used to compare treated versus control. Delta was set to 0.5 and the median number of estimated false significant genes was calculated for each treatment. In addition to the more stringent SAM analysis, pair-wise t-tests employing a critical p-value set to 0.05, assuming unequal group variances (Welch approximation), with no multiple test correction was applied to identify a greater number of potential differentially expressed genes. After applying the t-test-based statistical criteria, an additional 1.5x fold-change criteria was applied such that only microarray features that were up- or down-regulated by a factor of at least 1.5-fold were considered. The more liberal criteria (t-test p<0.05, fold change >1.5) was applied for functional analysis based on the assumption that false positives would be randomly distributed among functional annotation groups. Finally, the group of genes identified as differentially expressed across the five treatment groups using one-way analysis of variance (ANOVA; p<0.05, no multiple testing correction; no fold-change cut off) were used for principal components analysis (PCA). For PCA, expression data were median-centered (per gene) and then PCA was implemented using PC-ORD version 5.31 (MjM Software, Gleneden Beach, Oregon, USA). A total of 759 differentially expressed genes and n=24 samples (n=5 for all treatments except TRB, n=4) were used in the PCA. Statistical significance of the separation of treatment groups along PC1 and PC2 were evaluated by conducting one-way ANOVA on the PC scores, followed by a post-hoc Duncan’s multiple range-test (p<0.05). Statistical over- or under-representation of functional pathway annotations including KEGG (KEGG [Kyoto Encylcopedia of Genes and Genomes; http://www.genome.jp/kegg/]) and REACTOME (www.reactome.org) within the various low stringency gene lists (i.e., those based on pair-wise t-tests; p<0.05, fold change > 1.5) were evaluated using the Set Analyzer tool in CTDbase (ctdbase.org; [33]). Human homolog gene symbols corresponding to features on the fathead minnow microarray were submitted (see Table S.2) and the “Enriched pathways” analysis was applied using a 41237 gene background (default in ctdbase). Up- and down-regulated features for each treatment were considered independently and pathways were defined as significantly enriched when corrected p-value was < 0.05.
RESULTS
Chemical exposure
Water concentrations of TCC in the test tanks remained stable and close to the target concentrations of 1 and 5 μg/L throughout the course of the 22-d reproduction assay (Table 1). Mean water concentrations in the six replicate tanks at each TCC treatment were similar, with mean coefficients of variation (across the duration of the test) of 8.6 and 4.8%, respectively for the 1 and 5 μg/L treatments. The mean ± SD percent recovery of TCC from spiked water samples was 87 ± 6.2 (n = 6) and the percentage agreement among duplicate water samples collected from exposure tanks was 96 ± 3.4 (n = 18). No TRB was detected in method blanks, control water, or treatment tanks containing only TCC. The bioconcentration factor (BCF) for TCC ranged from about 300 (males from the 1 μg/L treatment) to 3,200 (males from the 5 μg/L treatment), indicating potential for the chemical to bioaccumulate (Table 1).
Table 1.
Summary of water and tissue concentrations following 22 d of continuous exposure to 1 (TCC1) or 5 (TCC5) μg triclocarban/L, 0.5 μg trenbolone/L (TRB), or a mixture (MIX) of 5 μg TCC and 0.5 μg TRB/L.
| Measured Water Concentration | TCC Tissue Concentration (ng/g) 3 | TCC BCF 4 | ||||
|---|---|---|---|---|---|---|
| Nominal Treatment | TCC (μg/L) 1 | TRB (ng/L) 2 | Male | Female | Male | Female |
| Control | ND 5 | ND 6 | ND 6 | ND | NA 7 | NA |
| TCC1 | 1.1 (0.07) | ND | 323 (36) | 496 (104) | 293 | 451 |
| TCC5 | 5.6 (0.20) | ND | 17,973 (7281) | 14,921 (3429) | 3209 | 2664 |
| TRB | ND | 601 (198) | ND | ND | NA | NA |
| MIX | 5.3 (0.29) | 549 (137) | 20,041 (5012) | 18,617 (9973) | 3781 | 3513 |
Mean (SD) for six measurements over the 22-d test.
Mean (SD) for nine measurements over the 22-d test.
Mean (SD) of carcass (sans blood, gonad, and liver); ng/g wet weight; n=5.
Bioconcentration factor
ND; not detectable (0.9 μg/L for water; 75–330 ng/g for tissue).
ND; not detectable (190 ng/L).
ND; not detectable (0.9 μg/L for water; 75–330 ng/g for tissue).
Survival and reproduction
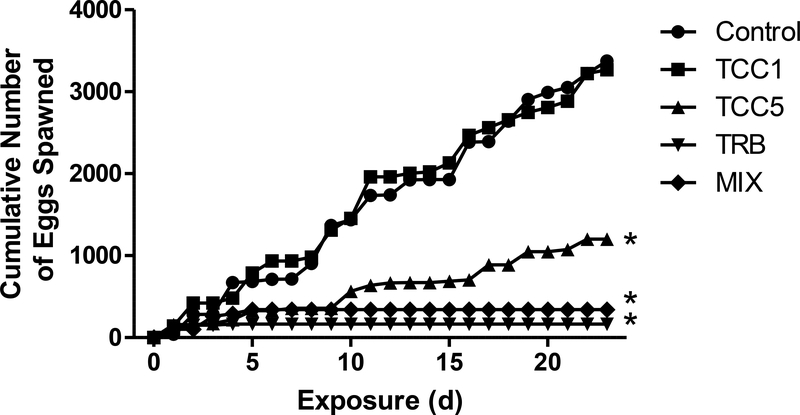
No mortalities or abnormal behaviors were observed. Fecundity of the fish was significantly decreased by exposure to 5 μg TCC/L and in TRB-containing treatments (Figure 1). Fish exposed to 0.5 μg TRB/L alone or in the mixture with 5 μg TCC/L ceased spawning. Fish exposed to 5 μg TCC/L continued to spawn. However, cumulative egg production by fish from the TCC5 treatment was less than one-half that observed in the control fish. No significant difference was found in fecundity between the control fish and those exposed to 1 μg TCC/L. The decrease in total fecundity associated with TCC exposure was related both to significant reduction in the number of spawns (mean ± SE)—3.6 ± 0.5, 4.2 ± 0.6, and 1.8 ± 0.7—in the control, low, and high treatments, respectively, and to the number of eggs per spawn (mean ± SE)—78 ± 13, 74 ± 14, and 36 ± 12—in the control, low and high treatments, respectively (Supplementary Figure S.2). Among females exposed to TRB, only 25% (MIX) or 33% (TRB) of the females spawned, and only one of those fish spawned more than once. Mean eggs per spawn (mean ± SE) were 21 ± 11 in the TRB treatment and 14 ± 9 in the MIX treatment (Supplementary Figure S.2). Fertility (>95%) was not affected by exposure to TCC, TRB, or the combination of the two (data not shown).
Figure 1.
Cumulative number of eggs spawned following 22 d of continuous exposure to 1 (TCC1) or 5 (TCC5) μg triclocarban/L, 0.5 μg 17β-trenbolone/L (TRB), or a mixture (MIX) of 5 μg TCC and 0.5 μg TRB/L. Each line represents the cumulative fecundity of 12 pairs of fish for each treatment. Asterisks indicate a significant difference from the control (p ≤ 0.05).
Morphological endpoints
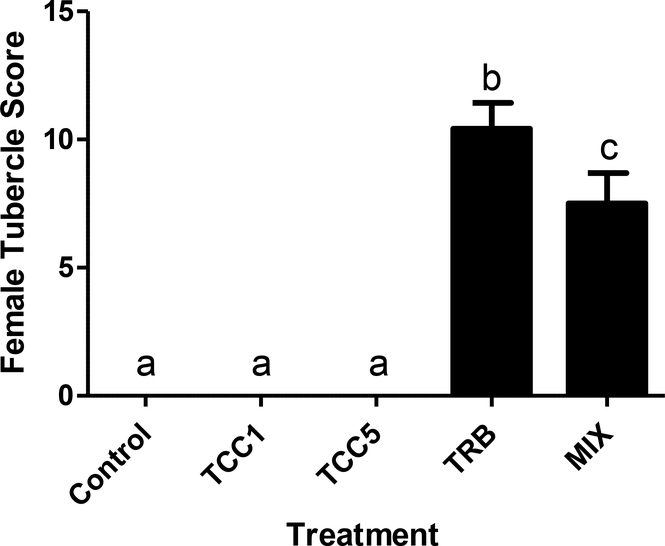
Twenty two days of continuous exposure to TCC (1 or 5 μg/L) had no significant effect on body mass (data not shown) or GSI of either males or females (Supplementary Figure S.3). Mean female body weights were about 20% greater in the TRB-containing treatments than in the other three treatments. However, that was not the case for males, nor was GSI significantly impacted in males or females exposed to TRB alone or in combination with 5 μg TCC/L. The secondary sex characteristics (nuptial tubercle scores) of males were unaffected by either TCC or TRB exposures (Supplementary Figure S.4). As expected, 22 d of exposure to 0.5 μg TRB/L caused phenotypic masculinization of females, as evidenced by a significant increase in tubercle scores (Figure 2). Tubercle formation was less prominent in the females co-exposed to 0.5 μg TRB and 5 μg TCC/L, but was still markedly greater than in both control fish and those exposed to TCC alone (Figure 3). Triclocarban alone did not cause any detectable phenotypic masculinization of female fathead minnows.
Figure 2.
Tubercle score of female fathead minnows following 22 d of continuous exposure to 1 (TCC1) or 5 (TCC5) μg triclocarban/L, 0.5 μg 17β-trenbolone/L (TRB), or a mixture (MIX) of 5 μg TCC and 0.5 μg TRB/L. Bars on graph represent mean (± SE; n=12). Letters indicate statistically significant differences between treatments (p ≤ 0.05).
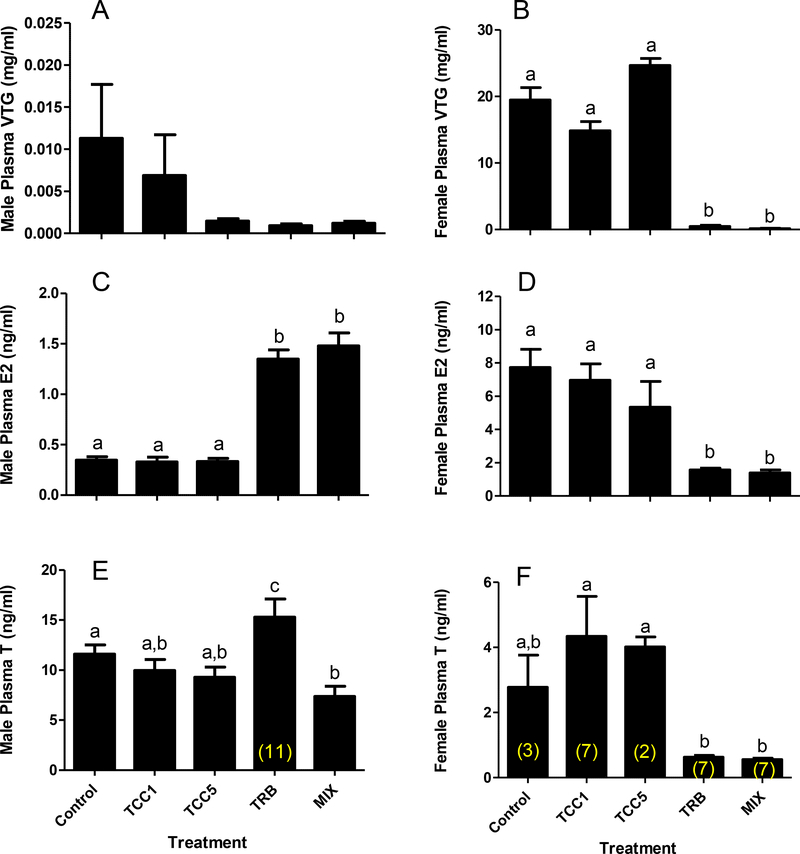
Figure 3.
Fathead minnow (A) male plasma vitellogenin (VTG), (B) female plasma VTG, (C) male plasma 17β-estradiol (E2), (D) female plasma E2, (E) male plasma testosterone (T), (F) female plasma T following 22 d of continuous exposure to 1 (TCC1) or 5 (TCC5) μg triclocarban/L, 0.5 μg 17β-trenbolone/L (TRB), or a mixture (MIX) of 5 μg TCC and 0.5 μg TRB/L. Bars on graph represent mean (± SE; n=12 except where indicated in parentheses on respective columns). Letters indicate statistically significant differences between treatments (p ≤ 0.05).
Steroids and vitellogenin
Plasma Vtg concentrations were low to nondetectable in male fathead minnows, and not affected by TCC or TRB exposure (Figure 3A). Plasma Vtg concentrations in females were not affected by exposure to TCC. However, they were significantly reduced by exposure to TRB alone and in combination with TCC (Figure 3B). In males, exposure to TRB alone resulted in significant increases in plasma E2 and T concentrations (Figure 3C, 3E), but when combined with 5 μg TCC/L, only the impact on E2 was evident. Exposure to TCC alone did not impact circulating concentrations of E2 or T. Both E2 and T concentrations were significantly reduced in females exposed to TRB-containing treatments, but unaffected in females exposed to TCC alone (Figure 3D, 3F). With the exception of ex vivo T production in females (Supplementary Figure S.5), ex vivo steroid production results did not mirror the impacts on circulating steroids. No significant effect on ex vivo E2 production was detected in ovary tissue. Furthermore, testosterone production by testes tissue collected from males exposed to 5 μg TCC/L was significantly greater than that of control males.
Ovarian histology
Histological examination of the ovaries from fish exposed to 5 μg TCC/L revealed an increase in preovulatory atretic follicles compared to the control fish. Preovulatory atretic follicles averaged 9.5% of vitellogenic follicles in the TCC-exposed ovaries compared to 0.4% in the control ovaries (Supplementary Figure S.6). Two other abnormalities of unknown significance observed more frequently in the fish exposed to 5 μg TCC/L were a mottled staining pattern in the vitelline envelope of otherwise normal-appearing oocytes and a “ballooning” of the vitelline envelope (Supplementary Figure S.6).
Targeted gene expression
Overall, 22 d of exposure to TCC, TRB, or a combination of the two had little effect on the relative abundance of the transcripts examined in testes using QPCR (Supplementary Figure S.7). Among the seven targets examined (cyp11a, cyp17, star, 11βhsd, 3βhsd, 17βhsd, and ar) only star showed significant differential expression among treatments, with expression being significantly lower in the MIX treatment group than in fish exposed to TCC5. However, star expression was not significantly different from control for either treatment.
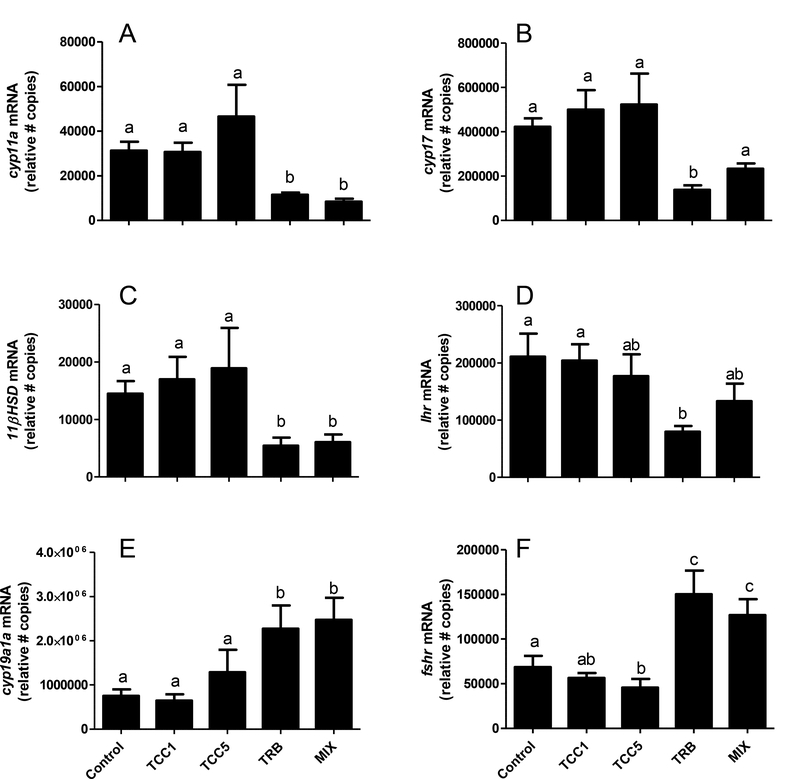
Effects on gene expression were much more prominent in ovary, although the significant impacts were predominantly associated with TRB-containing treatments. Exposure to TRB caused significant reductions in the abundance of transcripts coding for cyp11a, cyp17, 11βhsd, and lhr (Figure 4A-D). A similar trend was typically observed in fish exposed to the mixture of TRB and TCC, but in the case of cyp17 and lhr the more modest reductions caused by the mixture were not statistically significant (Figure 4B,D). Expression of cyp19a1a and fshr was significantly elevated in females exposed to TRB-containing treatments (Figure 4E,F). Expression of fshr was also significantly lower in the ovaries of females exposed to 5 μg TCC/L, although that trend did not hold for cyp19a1a. Relative abundance of vtgr, ar, 3βhsd, and star transcripts was unaffected by TCC, TRB, or MIX (Supplementary Figure S.8).
Figure 4.
Relative transcript abundance of (A) cholesterol side-chain cleavage (cyp11a), (B) 17-alpha-hydroxylase/17, 20 lyase (cyp17), (C) 11β-hydroxysteroid dehydrogenase (11βhsd), (D) luteinizing hormone receptor (lhr), (E) aromatase (cyp19a1a), (F) follicle-stimulating hormone receptor (fshr) in ovarian tissue from adult female fathead minnows following 22 d of continuous exposure to 1 (TCC1) or 5 (TCC5) μg triclocarban/L, 0.5 μg 17β-trenbolone/L (TRB), or a mixture (MIX) of 5 μg TCC and 0.5 μg TRB/L. Bars on graph represent mean (± SE; n=12 except TCC5, n=10). Letters indicate statistically significant differences between treatments (p ≤ 0.05).
Overall impact on ovarian transcriptome
Using the more stringent SAM criteria, differential gene expression relative to controls was only identified for two of the four treatments examined, TRB (Supplementary Table S.2) and TCC5 (Supplementary Table S.3). In the case of TRB exposed females, 99 genes were identified as differentially expressed, 98 of which were down-regulated compared to controls. Exposure to TCC5 had much less impact, with only 7 genes identified as differentially expressed relative to controls using SAM. Notably, no differentially expressed genes were detected using SAM for ovary tissue from fish exposed to the MIX treatment.
None of the differentially expressed genes detected by stringent SAM analysis were immediately remarkable or obvious as potential biomarkers of TRB or TCC exposure. Furthermore, given that effects on the transcriptome were evaluated 22 days after the onset of exposure, it could not be determined whether transcriptome-level responses observed were direct effects of the chemical exposures or secondary effects reflecting biological changes distantly removed from the initial impact of the chemicals. Consequently, no specific efforts to verify the individual gene expression responses by QPCR or other orthogonal methods were made. Nonetheless, to help assess the overall validity of the microarray data, microarray results for seven genes were compared with corresponding QPCR results obtained as part of the targeted analyses conducted for the study (Supplementary Figure S.1). Restricting the analysis to just the subset of samples that were evaluated by microarray, there was fairly strong qualitative agreement between QPCR and microarray results. While median normalized intensities and variability varied somewhat between the two methods, the general trends across treatment were consistent.
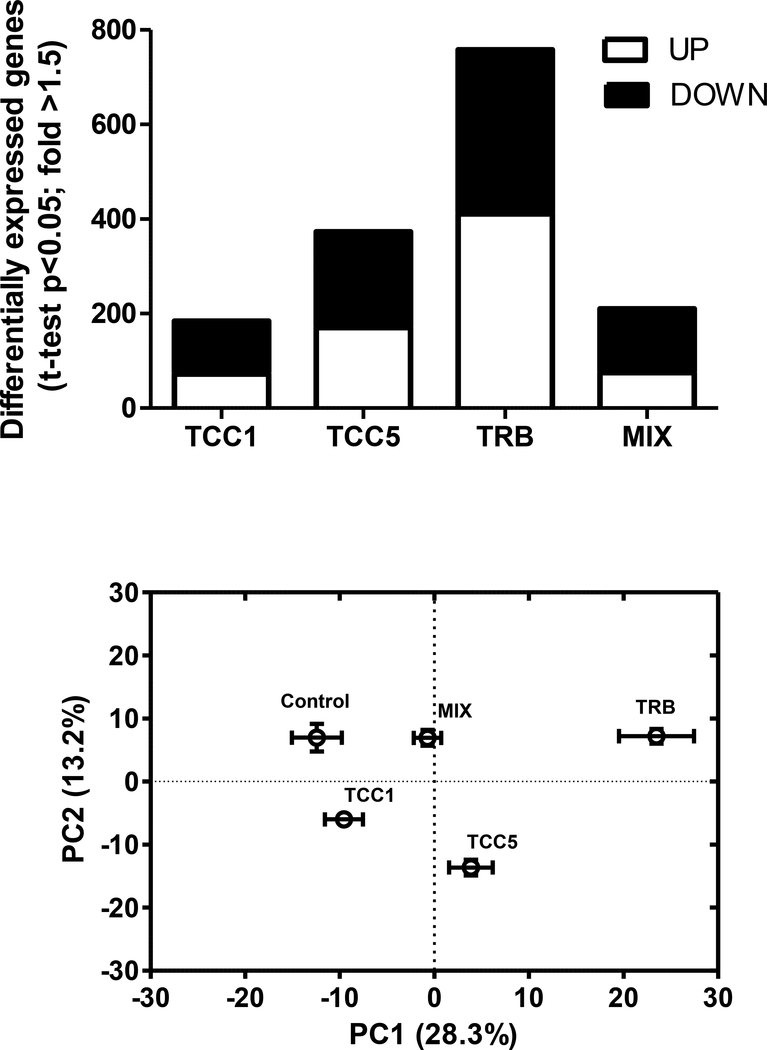
Applying a less stringent statistical criteria, pair-wise t-tests (p<0.05; no multiple-testing correction; fold-change up or down >1.5), provided a general sense of the overall impact of the treatments on the ovarian transcriptome (although this allowed a much greater false discovery rate). Based on pair-wise t-tests (fold >1.5), the number of putative differentially expressed genes increased from 185 to 373 with increasing concentration of TCC (Figure 5A; Supplementary Tables S.4, S.5). The greatest number of putative differentially expressed genes were detected for fish exposed to TRB (Figure 5A; Supplementary Table S.6), while the ovaries of fish exposed to the MIX treatment had fewer putative differentially expressed genes than those exposed to either treatment (TRB or TCC5) alone (Figure 5A; Supplementary Table S.7). Results of a PCA conducted using the set of 759 genes identified as differentially expressed across the five treatments based on one-way ANOVA (p<0.05; no multiple testing correction; Supplementary Table S.8) suggested a similar profile. Treatments were displaced along PC1, relative to control, in the same order as their total number of putative differentially expressed genes; i.e., TCC1 nearest to control, followed by MIX, TCC5, and TRB. Only the TCC1 and TCC5 treatments were significantly displaced from controls along PC2 (Figure 5B), suggesting the genes driving the loadings along that axis may be more characteristic of TCC exposure, and that the TRB-influenced expression profile may have either dominated over or countered the TCC-influenced expression in the MIX treatment.
Figure 5.
(A) Number of putative differentially expressed genes in fathead minnow ovary tissue, relative to controls, following 22 d of continuous exposure to 1 (TCC1) or 5 (TCC5) μg triclocarban/L, 0.5 μg 17β-trenbolone/L (TRB), or a mixture (MIX) of 5 μg triclocarban and 0.5 μg TRB/L. Significance based on pair-wise t-test, p<0.05, with no multiple testing correction. White portion of bar indicates genes whose expression was up-regulated relative to controls. Black portion of the bar indicates genes whose expression was down-regulated relative to controls. (B) Principal component analysis (PCA) scores plots. (A) PCA conducted on the group of 759 genes identified as differentially expressed among all five treatments examined in the study (one-way ANOVA, uncorrected p<0.05; Supplementary Table S.8). Error bars represent SEM for principal components (PCs) 1 and 2, respectively (n=5 for all treatments except TRB, n=4).
Transcriptomics – functional analyses
Pathway enrichment analysis using the CTDbase set analyzer tool (http://ctdbase.org/tools/analyzer.go; [33]) was employed to examine the potential functional significance of the gene expression changes detected by microarray (Supplementary Table S.10). Among the genes up-regulated in the ovaries of fish exposed to TCC, TRB, or MIX, enrichment in specific functional pathways was only detected in the case of the TCC5 and TRB treatments. Exposure to both TRB and TCC5 yielded up-regulation of genes associated with transmembrane transport of small molecules, largely coding for cross-membrane ion transporters and channel proteins. Genes annotated as being associated with disease and DNA repair were also up-regulated in the TCC5 group, while in the TRB group additional pathways associated with up-regulated genes included axon guidance, tight junction, gonadotrophin releasing hormone signaling, and insulin signaling.
In general, despite relatively even proportions of up- versus down-regulated genes in each treatment group, down-regulated genes showed much greater enrichment within annotated functional pathways. Five significantly enriched pathway annotations were associated with genes down-regulated in the ovaries of fish from the TCC1 and MIX treatment groups, while nine and 16 pathway annotations enriched in the down-regulated gene lists were identified for the TCC5 and TRB treatments, respectively (Supplementary Table S.10). Overall, there was relatively little overlap in the enriched, down-regulated pathways among treatments. Beyond the fairly broad term, metabolism, the only pathway annotation term enriched across all treatments (TCC1, TCC5, TRB, and MIX) was complement and coagulation cascades. Down-regulation of genes associated with hemostasis was common to TCC1 and TRB while down-regulation of immune system related genes was common to TCC5 and TRB. Only the TRB group showed significant down-regulation of genes enriched in steroid hormone and endocrine-related calcium signaling, while only genes down-regulated in the MIX treatment were enriched in oocyte meiosis related genes. Likewise, genes associated with extracellular matrix organization were only statistically enriched among those down-regulated in the TCC5 treatment.
DISCUSSION
Triclocarban’s impact on fish reproduction
In ecotoxicology, risks are typically framed at the population level and assessed in terms of potential impacts on survival, development/growth, and reproduction. Previous studies have established TCC to be acutely toxic to fish at concentrations on the order of 100 μg/L (96 h LC50; http://www.aciscience.org/docs/Triclocarban_HPV_Robust_Study_Summaries.pdf). In a previous study by Ankley et al., fathead minnows exposed to 10 μg TCC/L for up to 21 d showed signs of overt toxicity with reduced feeding, reduced mobility, and death observed, particularly among males (See supplementary Figure S.0; [17]). Schultz et al. [10] reported significant reductions in aggression in males exposed to 1.6 μg TCC/L for 21 d. However, the consequences of such behavioral effects are more difficult to translate into potential consequences for a population, and thus rarely used for risk assessment. The present study represents the first to define a LOEC for adverse reproductive effects of TCC in fish. Significant reductions in cumulative fecundity were observed in fathead minnows exposed to 5 μg TCC/L, but not 1 μg TCC/L for 22 d (Figure 2). Collectively, these results indicate that, in chronic exposures, TCC exerts reproductive effects at 5 μg/L, which is just two-fold lower than that at which signs of overt toxicity have been observed [17].
Based on estimated annual loadings and co-occurrence with triclosan, median and mean predicted concentrations in streams nationwide in the US were estimated to be 0.109 and 0.213 μg TCC/L, respectively [3]. Measured concentrations in surface waters throughout Minnesota were reported to range from 0.02 to 0.416 μg TCC/L [34]. Another survey of river water samples across the US reported the 95th percentile of TCC concentrations to be 0.038 μg/L upstream of WWTPs and 0.250 μg/L downstream [5]. Thus, it would appear that the margin of safety relative to adverse reproductive effects in fathead minnows or other fish of similar sensitivity may be as little as a factor of 20 in many aquatic environments throughout the US, particularly those directly impacted by WWTP discharges. Furthermore, ambient concentrations in excess of 5 μg/L, presumably associated with spills or leakage of untreated sewage, have been documented [3]. It is recognized that the dilution water used in the present study (i.e., UV treated, filtered, Lake Superior water) is not representative of all potential waterbodies into which TCC is discharged. Site-specific variations in water quality parameters and environmental conditions may significantly influence the fate, availability, and/or overall toxicity of TCC. Nonetheless, based on these data, it would appear that TCC poses a potential risk to freshwater fish.
Evidence for augmentation of androgen action
The present experiments included co-exposures to TRB as a means to further evaluate TCC’s purported ability to augment effects of exogenous androgens. Based on previous studies [35, 36], and a proposed adverse outcome pathway linking AR agonism to reproductive dysfunction, a number of prototypical responses were expected in females exposed to TRB. These included reductions in circulating testosterone, estradiol, and Vtg, as well as overall reductions in cumulative fecundity (https://aopkb.org/aopwiki/index.php/Aop:23; aop:23). While a causal role in reproductive impairment has not been established, phenotypic masculinization of females is another prototypical response to strong androgens that has been frequently used as an indicator of exposure to AR agonists [17] (https://aopkb.org/aopwiki/index.php/Event:25). Finally, it was previously hypothesized that females exposed to TRB mount a compensatory response aimed at offsetting a perceived overabundance of androgen by reducing androgen synthesis through lower cyp11a expression in theca cells, while maximizing flux of androgenic precursors to estradiol synthesis by increasing cyp19a1a expression in granulosa cells [36]. These anticipated responses to TRB exposure provided a set of targeted endpoints that could be used to probe the question of whether TCC could augment the effects of an AR agonist.
Responses of the females to 22 d of continuous exposure to 0.5 μg TRB/L were consistent with both the adverse outcome pathway and previous investigations. As in previous studies [35], plasma estradiol, testosterone, and Vtg were all reduced in the females exposed to TRB (Figure 3) and cumulative fecundity was reduced (Figure 1). Likewise, significant phenotypic masculinization of females was observed (Figure 2). Even at the molecular level, the targeted gene expression responses were as predicted, with reductions in expression of cyp11a, cyp17, 11βhsd, and lhr which could all be plausibly linked with an effort to reduce androgen production (e.g., T, 11-ketotestosterone), but increases in cyp19a1a and fshr which would both serve to help maximize E2 production in an attempt to maintain vitellogenesis. All these data suggest the fish were responding as expected to an androgenic chemical.
In terms of the impact of TCC co-exposure on the anticipated responses to AR agonism, a significant augmentation effect was not evident. Reproductive output in terms of cumulative eggs spawned, eggs per spawn, and number of spawns per female for fish exposed to TRB alone were not significantly different than for those exposed to the MIX treatment (Figure 1; Supplementary Figure S.2). Reductions in circulating estradiol, testosterone, or Vtg concentrations were not significantly greater in the females exposed to the mixture than in those exposed to TRB alone (Figure 3). Likewise, with the exception of somewhat lesser impacts on cyp17 and lhr expression, targeted gene expression responses in females did not differ markedly between the TRB and MIX treatments (Figure 4). In no case did TCC, by itself, elicit a response that would be viewed as prototypical of an AR agonist.
Similarly, no augmentation of androgenic effects on secondary sex characteristics was evident. Morphologically, both TRB and the mixture of TRB and TCC5 induced significant tubercle formation in females. However, tubercle scores for females exposed to the MIX were significantly less than those for females exposed to TRB only (Figure 2) suggesting, if anything, a modest anti-androgenic effect. However, previous study reported no evidence for an anti-androgenic action of TCC. Specifically, co-exposure to 5 μg TCC/L and TRB for 21 d did not significantly alter (augment or suppress) mean tubercle score in females in comparison to the TRB only group [17]. In previous work, significant enhancement of TRB-induced tubercle formation was only observed when fish were co-exposed to TRB and 10 μg TCC/L [17], a concentration of TCC that caused signs of overt toxicity, particularly in males [17].
As expected, TRB exposure had less impact on males than females. The most notable impacts were increased circulating estradiol and testosterone in the exposed males. Co-exposure with 5 μg TCC/L did not significantly modulate the impact of TRB exposure on circulating estradiol, but testosterone concentrations in the MIX-exposed males were significantly lower than in the males exposed to TRB alone. However, in contrast with the hypothesis that TCC can augment androgenic activity, these data were more suggestive of a potential anti-androgenic action than augmentation. At least one recent study suggests TCC may impair steroid biosynthesis in vitro [37]. However, the concentrations tested in H295R cells were in excess of 150 μg/L [37], so it is unclear whether those effects would be relevant in vivo given that similar concentrations cause overt toxicity in fish. Overall, the targeted endpoints examined at the phenotypic, physiological, and molecular level provided no compelling evidence that TCC could specifically augment or otherwise modulate the effects of TRB as a prototypical androgen receptor agonist.
As an additional line of evidence, we examined the relative similarity of the ovarian transcriptome response to each concentration of TCC alone, TRB alone, or the MIX. Augmentation of androgenic effects of an AR agonist, which had been previously observed in vivo and in vitro [12–17] following co-exposure to TCC, was not evident in the overall transcriptomic response of female fathead minnows that had been continuously exposed to the MIX for 22 d. In terms of total differentially expressed genes, the MIX yielded less than either the TCC5 or TRB treatments alone (Figure 5A). Based on PCA, the MIX did not yield an overall profile of differentially expressed transcripts that was shifted toward greater similarity to that of TRB alone (Figure 5B). At the pathway level, there were no enriched pathways common to the genes up-regulated in the TRB, TCC5, and/or MIX groups. Complement and coagulation cascades and the general term metabolism were common among genes down-regulated in the TRB, TCC5, and MIX treatments, but a plausible linkage to augmentation of androgen action was not evident. Likewise, a link between the down-regulation of genes associated with immune function in both the TRB and TCC5 groups did not suggest a plausible augmentation, nor were any of the down-regulated genes associated with the immune function enrichment common to both groups. Among the low stringency lists of differentially expressed genes identified for each the TCC5, TRB, and MIX treatments, there were 17 genes impacted by all three treatments (Supplementary Table S.9). If further apical evidence for augmentation warranted, these represent potential targets for follow-up mechanistic investigation, but at present nothing more than speculative associations to androgenic effects could be drawn. Finally, there were no cases where the MIX treatment elicited a greater fold-change in expression (up or down) than TCC5 or TRB alone (Supplementary Figure S.9), a pattern that could be interpreted as augmentation.
Given that the greatest concentration tested in the present study, either individually or in combination with TRB, was 5 μg TCC/L, we cannot rule out the possibility that the concentration was inadequate to produce a clear “augmentation” response. However, overall our results do not seem to support the idea that AR augmentation, if indeed it occurs in fish in vivo, has much relevance from a risk assessment perspective. The apparent TCC-mediated augmentation of TRB-induced tubercle formation in females reported by Ankley et al. [17], was only significant at exposure concentrations greater (i.e., 9.8 μg TCC/L) than those shown to cause significant reproductive impairment and equal to those causing symptoms of more systemic toxicity. We note that co-exposure to 5 mg ammonia/L has also been shown to augment TRB-induced tubercle formation in female fish [17]. Furthermore, the reproductive LOEC for fathead minnow, determined in the present study, was similar to that determined in a 21 d reproduction assay with Daphnia magna (4.7 μg TCC/L; http://www.aciscience.org/docs/Triclocarban_HPV_Robust_Study_Summaries.pdf). In light of evidence that a non-endocrine-specific stressor like ammonia can augment TRB-induced tubercle formation and that the potency of TCC to cause reproductive impairment in fish is similar to its potency to elicit reproductive effects in a crustacean lacking a functional AR [38], it would appear likely that the effects of TCC on fathead minnow reproduction were caused by a more generalized toxic effect, rather than a specific AR augmentation mode of action. Therefore, detailed elucidation of TCC’s putative ability to augment AR-dependent activities may have little value for aquatic ecological risk assessment.
Apical effects and alterations to the ovarian transcriptome
Beyond consideration of potential AR augmentation, the transcriptomic results were considered in light of the apical effects of TCC on exposed females. Other than the non-specific “metabolism” term, the only pathway universally enriched in the three TCC-containing treatments was complement and coagulation cascades which refers to closely linked processes of activating innate immune response and factors that promote blood clotting [39]. The specificity of this pathway response to TCC is doubtful given that the same pathway was also down-regulated in the TRB group. It seems more likely that down-regulation of complement and coagulation cascade-related gene expression was associated with reduced ovulation and spawning in the TCC5, TRB, and MIX treatments. Ovulation in mammals is accompanied by an increase in vascular permeability followed by coagulation and clot formation [40–42]. Likewise, in rainbow trout strong up-regulation of genes coding for inflammation, coagulation, vasodilation, and angiogenesis just prior to ovulation has been reported [43]. Thus, down-regulation of coagulation and complement cascades in females that aren’t spawning might be expected. Based on the same reasoning, the reduced ovulation and spawning associated with the TRB and TCC5 treatments could also explain the enriched down-regulation of genes associated with immune system (TCC5) and innate immune system (TRB) as well as hemostasis (TRB). However, the enrichment of down-regulated coagulation and complement cascades and hemostasis-related genes in the TCC1 treatment group, which did not have reduced cumulative fecundity and spawning, raises some question about this interpretation. The possibility that TCC impacts coagulation-related gene expression in ovaries in a manner independent of ovulatory state cannot be ruled out.
In addition to evaluating the transcriptomics in light of reproductive activity, for the TCC5 treatment, results could also be considered in the context of histological changes. Increased numbers of preovulatory atretic follicles are a common histological observation in female fathead minnows whose spawning activity is impaired. In particular, previous fathead minnow reproduction studies with 17β-trenbolone have reported significant increases in their abundance [35]. One notable pathway down-regulated in the ovaries of females exposed to the TCC5 treatment was extracellular matrix organization which included down-regulation of bone morphogenetic protein 4 (BMP4). Treatment of rat ovaries with antibodies to BMP4 has been shown to produce increased cellular apoptosis and disruption of normal ovarian tissue morphology over time [44]. Consequently, down-regulation of BMP4 and related genes involved in extracellular matrix organization could at least plausibly be linked with some of the observed histological changes including increased atresia and “ballooning” of the vitelline envelope. Overall, based on a single set of observations following 22 d of continuous exposure it is difficult to infer whether the observed alterations in the ovarian transcriptome are the consequence of impaired spawning and the associated apical changes in the structure and state of the ovary tissue or whether those altered structures and states are a consequence of transcriptional changes. The occurrence of some similarities in the pathway responses to TCC1 treatment, relative those in the TCC5 and TRB treatments suggest the possibility that ovarian function may be impacted even at concentrations that did not cause overt reductions in fecundity. However, without histological evidence for the TCC1 treatment, this possibility could not be explored more thoroughly in the current study.
Implications for risk assessment
Results of the present study have several implications related to TCC’s potential risk to aquatic organisms. First, the data demonstrate that the margin of safety relative to TCC’s ability to act as a reproductive toxicant in fish is likely <50 in many aquatic environments, particularly downstream of wastewater treatment plant discharges. Environmental concentrations exceeding the reproductive effect concentration determined in the present study have been reported [3]. Second, given that reproductive toxicity was observed at a concentration just two-fold lower than that which caused indications of overt toxicity and just five-fold greater than a reproductive NOEC, it is rather questionable whether TCC represents a specifically-acting reproductive toxicant. Rather, the data suggest that TCC can elicit a more systemic toxicity that impacts reproductive functions via generalized stress to the organism, or at least in addition to effects on the reproductive organs. This was in general agreement with the fact that the present study provided little compelling evidence for any augmentation of the effects of TRB, when fish were exposed to a mixture of TRB and TCC. Overall, at the transcriptomic level, the MIX did not induce a gene expression profile similar to that elicited by either of its constituents, TRB or TCC5. Neither the examination of individual high confidence differentially expressed genes (based on SAM) nor potentially over-represented pathway annotations (based on putative differentially expressed genes identified using less stringent t-tests) provided immediate insight into TCC’s mode of action, although some of the targets and pathways identified could be a starting point for follow-up investigations. Overall, TCC does appear to pose a risk to fish as a surface water contaminant but our data provide no support for its purported action as a new class of endocrine disrupting chemical, at least at concentrations markedly lower than those exerting more generalized toxicity.
Supplementary Material
Acknowledgments:
The authors thank L. Thomas and S. Seidl for additional technical support and J. Haselman for critical feedback on the manuscript. Funding for this work was provided in part by the US EPA Chemical for Sustainability Research Program. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The contents of this manuscript neither constitute, nor necessarily reflect US EPA policy.
REFERENCES
- [1].Chalew TE, Halden RU. 2009. Environmental Exposure of Aquatic and Terrestrial Biota to Triclosan and Triclocarban. J Am Water Works Assoc 45:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brausch JM, Rand GM. 2011. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82:1518–1532. [DOI] [PubMed] [Google Scholar]
- [3].Halden RU, Paull DH. 2005. Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ Sci Techno 39:1420–1426. [DOI] [PubMed] [Google Scholar]
- [4].Perencevich EN, Wong MT, Harris AD. 2001. National and regional assessment of the antibacterial soap market: a step toward determining the impact of prevalent antibacterial soaps. Am J Infect Control 29:281–283. [DOI] [PubMed] [Google Scholar]
- [5].Sapkota A, Heidler J, Halden RU. 2007. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res 103:21–29. [DOI] [PubMed] [Google Scholar]
- [6].Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211. [DOI] [PubMed] [Google Scholar]
- [7].Coogan MA, Edziyie RE, La Point TW, Venables BJ. 2007. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67:1911–1918. [DOI] [PubMed] [Google Scholar]
- [8].Higgins CP, Paesani ZJ, Chalew TE, Halden RU. 2009. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ Toxicol Chem 28:2580–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schebb NH, Flores I, Kurobe T, Franze B, Ranganathan A, Hammock BD, Teh SJ. 2011. Bioconcentration, metabolism and excretion of triclocarban in larval Qurt medaka (Oryzias latipes). Aquat Toxicol 105:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schultz MM, Bartell SE, Schoenfuss HL. 2012. Effects of Triclosan and Triclocarban, Two Ubiquitous Environmental Contaminants, on Anatomy, Physiology, and Behavior of the Fathead Minnow (Pimephales promelas). Arch Environ Contam Toxicol 63:114–124. [DOI] [PubMed] [Google Scholar]
- [11].Witorsch RJ, Thomas JA. 2010. Personal care products and endocrine disruption: A critical review of the literature. Crit. Review Toxicol 40 Suppl 3:1–30. [DOI] [PubMed] [Google Scholar]
- [12].Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DP, Gee SJ, Hammock BD. 2008. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect 116:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. 2008. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology 149:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blake LS, Martinovic D, Gray LE, Jr., Wilson VS, Regal RR, Villeneuve DL, Ankley GT. 2010. Characterization of the androgen-sensitive MDA-kb2 cell line for assessing complex environmental mixtures. Environ Toxicol Chem 29:1367–1376. [DOI] [PubMed] [Google Scholar]
- [15].Christen V, Crettaz P, Oberli-Schrammli A, Fent K. 2010. Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere 81:1245–1252. [DOI] [PubMed] [Google Scholar]
- [16].Duleba AJ, Ahmed MI, Sun M, Gao AC, Villanueva J, Conley AJ, Turgeon JL, Benirschke K, Gee NA, Chen J, Green PG, Lasley BL. 2011. Effects of triclocarban on intact immature male rat: augmentation of androgen action. Reprod Sci 18:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ankley GT, Jensen KM, Kahl MD, Durhan EJ, Makynen EA, Cavallin JE, Martinovic D, Wehmas LC, Mueller ND, Villeneuve DL. 2010. Use of chemical mixtures to differentiate mechanisms of endocrine action in a small fish model. Aquat Toxicol 99:389–396. [DOI] [PubMed] [Google Scholar]
- [18].Chung E, Genco MC, Megrelis L, Ruderman JV. 2011. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. PNAS 108:17732–17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yueh MF, Li T, Evans RM, Hammock B, Tukey RH. 2012. Triclocarban Mediates Induction of Xenobiotic Metabolism through Activation of the Constitutive Androstane Receptor and the Estrogen Receptor Alpha. PLoS One 7:e37705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morisseau C, Merzlikin O, Lin A, He G, Feng W, Padilla I, Denison MS, Pessah IN, Hammock BD. 2009. Toxicology in the fast lane: application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Health Perspect 117:1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. 2011. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol 45:3109–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li Z, Villeneuve DL, Jensen KM, Ankley GT, Watanabe KH. 2011. A computational model for asynchronous oocyte growth dynamics in a batch-spawning fish. Can J Fish Aquat Sci 68:1528–1538. [Google Scholar]
- [23].Jensen K, Korte J, Kahl M, Pasha M, Ankley G. 2001. Aspects of the basic reproductive biology and endocrinology in the fathead minnow (Pimephales promelas). Compar Biochem Phsyiol Part C 128:127–141. [DOI] [PubMed] [Google Scholar]
- [24].USEPA. 2002. A short-term method for assessing the reproductive and developmental toxicity of endocrine-disrupting chemicals using the fathead minnow (Pimephales promelas). Vol EPA-600/R-01/067 United States Environmental Protection Agency, Cincinnati, OH. [Google Scholar]
- [25].Ying GG, Yu XY, Kookana RS. 2007. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ Pollut 150:300–305. [DOI] [PubMed] [Google Scholar]
- [26].McMaster MEMK, Jardine JJ, Robinson RD, Van Der Kraak GJ. 1995. Protocol for measuring in vitro steroid production by fish gonadal tissue. Canadian Technical Report of Fisheries and Aquatic Sciences 1961. 1961:1–78. [Google Scholar]
- [27].Martinovic D, Blake LS, Durhan EJ, Greene KJ, Kahl MD, Jensen KM, Makynen EA, Villeneuve DL, Ankley GT. 2008. Reproductive toxicity of vinclozolin in the fathead minnow: confirming an anti-androgenic mode of action. Environ Toxicol Chem 27:478–488. [DOI] [PubMed] [Google Scholar]
- [28].Korte JJ, Kahl MD, Jensen KM, Mumtaz SP, Parks LG, LeBlanc GA, Ankley GT. 2000. Fathead minnow vitellogenin: complementary DNA sequence and messenger RNA and protein expression after 17B-estradiol treatment. Environ Toxicol Chem 19:972–981. [Google Scholar]
- [29].Leino R, Jensen K, Ankley G. 2005. Gonadal histology and characteristic histopathology associated with endocrine disruption in the adult fathead minnow. Environ Toxicol Pharmacol 19:85–98. [DOI] [PubMed] [Google Scholar]
- [30].Villeneuve DL, Blake LS, Brodin JD, Greene KJ, Knoebl I, Miracle AL, Martinovic D, Ankley GT. 2007. Transcription of key genes regulating gonadal steroidogenesis in control and ketoconazole- or vinclozolin-exposed fathead minnows. Toxicol Sci 98:395–407. [DOI] [PubMed] [Google Scholar]
- [31].Reimers M 2010. Making informed choices about microarray data analysis. PLoS computational biology 6:e1000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378. [DOI] [PubMed] [Google Scholar]
- [33].Davis AP, Gondin CJ, Lennon-Hopkins K, Saraceni-Richards C, Sciaky D, King BL, Wiegers TC, Mattingly CJ. 2014. The comparative toxicogenomics database’s 10 year anniversary: update 2015. Nucleic Acids Res. Piii: gku935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee KE, Langer SK, Barber LB, Writer JH, Ferrey ML, Schoenfuss HL, Furlong ET, Foreman WT, Gray JL, ReVello RC, Martinovic D, Woodruff OP, Keefe SH, Brown GK, Taylor HE, Ferrer I, Thurman EM. 2011. Endocrine active chemicals, pharmaceuticals, and other chemicals of concern in surface waters, waste-water treatment plant effluent, and bed sediment and biological characteristics in selected streams, Minnesota - design, methods, and data 2009. [Google Scholar]
- [35].Ankley G, Jensen K, Makynen E, Kahl M, Korte J, Hornung M, Henry T, Denny J, Leino R, Wilson V, Cardon M, Hartig P, Gray L. 2003. Effects of the androgenic growth promoter 17-b-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem 22:1350–1360. [PubMed] [Google Scholar]
- [36].Ekman DR, Villeneuve DL, Teng Q, Ralston-Hooper KJ, Martinovic-Weigelt D, Kahl MD, Jensen KM, Durhan EJ, Makynen EA, Ankley GT, Collette TW. 2011. Use of gene expression, biochemical and metabolite profiles to enhance exposure and effects assessment of the model androgen 17beta-trenbolone in fish. Environ Toxicol Chem. [DOI] [PubMed] [Google Scholar]
- [37].Tonoli D, Fürstenberger C, Boccard J, Hochstrasser D, Jeanneret F, Odermatt A, Rudaz S. 2015. Steroidomic footprinting based on ultra-high performance liquid chromatography coupled with qualitative and quantitative high-resolution mass spectrometry for the evaluation of endocrine disrupting chemicals in H295R cells. Chem Res Toxicol 38:955–966. [DOI] [PubMed] [Google Scholar]
- [38].Thomson SA, Baldwin WS, Wang YH, Kwon G, Leblanc GA. 2009. Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genomics 10:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Markiewski MM, Nilsson B, Nilsson Ekdahl K, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol 28:184–192. [DOI] [PubMed] [Google Scholar]
- [40].Parr EL. 1974. Histological examination of the rat ovarian follicle wall prior to ovulation. Biol Reprod 11:483–503. [DOI] [PubMed] [Google Scholar]
- [41].Gerdes U, Gåfvels M, Bergh A, Cajander S. 1992. Localized increases in ovarian vascular permeability and leucocyte accumulation after induced ovulation in rabbits. J Reprod Fert 95:539–550. [DOI] [PubMed] [Google Scholar]
- [42].Hosseini G, Liu J, de Agostini AI. 1996. Characterization and hormonal modulation of anticoagulant heparan sulfate proteoglycans synthesized by rat ovarian granulosa cells. J Biol Chem 271:22090–22099. [DOI] [PubMed] [Google Scholar]
- [43].Bobe J, Nguyen T, Fostier A. 2009. Ovarian function of the trout preovulatory ovary: new insights from recent gene expression studies. Compar Biochem Physiol Pt A 153:63–68. [DOI] [PubMed] [Google Scholar]
- [44].Nilsson EE, Skinner MK. 2003. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 69:1265–1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.