Abstract
Type 2 diabetes mellitus (T2DM) is characterized by the inability of the insulin-producing β-cells to overcome insulin resistance. We previously identified an imprinted region on chromosome 14, the DLK1-MEG3 locus, as being downregulated in islets from humans with T2DM. In this study, using targeted epigenetic modifiers, we prove that increased methylation at the promoter of Meg3 in mouse βTC6 β-cells results in decreased transcription of the maternal transcripts associated with this locus. As a result, the sensitivity of β-cells to cytokine-mediated oxidative stress was increased. Additionally, we demonstrate that an evolutionarily conserved intronic region at the MEG3 locus can function as an enhancer in βTC6 β-cells. Using circular chromosome conformation capture followed by high-throughput sequencing, we demonstrate that the promoter of MEG3 physically interacts with this novel enhancer and other putative regulatory elements in this imprinted region in human islets. Remarkably, this enhancer is bound in an allele-specific manner by the transcription factors FOXA2, PDX1, and NKX2.2. Overall, these data suggest that the intronic MEG3 enhancer plays an important role in the regulation of allele-specific expression at the imprinted DLK1-MEG3 locus in human β-cells, which in turn impacts the sensitivity of β-cells to cytokine-mediated oxidative stress.
Introduction
Diabetes refers to a group of metabolic diseases characterized by an insufficient insulin response to high blood glucose levels. Pancreatic β-cells are critical regulators of glucose homeostasis as they produce, store, and secrete insulin to regulate glucose uptake by peripheral tissues. Their autoimmune destruction or functional decline can lead to type 1 and type 2 diabetes mellitus (T2DM), respectively. Thus, understanding the molecular mechanisms underlying β-cell physiology is fundamental to improving current diabetes treatment strategies.
We previously demonstrated that the imprinted DLK1-MEG3 locus is misregulated in islets from donors with T2DM (1). This locus consists of the paternally active DLK1, RTL1, and DIO3 genes, as well as maternally expressed long noncoding RNAs MEG3, RTL1as, and MEG8, a large microRNA (miRNA) cluster, and several small nucleolar RNAs (2,3). The genes in this locus are active in human β- but not α-cells at very high levels (1,4) and repressed in islets from patients with T2DM (1). This decreased expression correlates with hypermethylation at the MEG3 promoter. Consistent with these human studies, Meg3 expression is decreased in mouse models of type 1 diabetes mellitus and T2DM (5).
Little is known about the mechanism by which imprinting at the DLK1-MEG3 locus is regulated, particularly in human islets and β-cells. Monoallelic expression at this locus is established and maintained through specific methylation patterns at two differentially methylated regions (DMRs), the intergenic IG-DMR and MEG3-DMR, overlapping the promoter of the maternal transcript (6,7). These DMRs are paternally methylated. Although MEG3 promoter hypermethylation and a concomitant decrease in expression have been reported in several human diseases (8–14), a causal relationship between these observations has not been established. In this study, we demonstrate that hypermethylation of this DMR using targeted DNA methylation in mouse βTC6 β-cells causes decreased transcription of Meg3 and that this repressed expression exacerbates β-cell death, consistent with our observation in islets from human donors with T2DM (1). Furthermore, we identified a putative enhancer within an intron of the human MEG3 gene that is bound by transcription factors that are critical for islet function. We demonstrate that this sequence indeed functions as an active enhancer and physically interacts with the MEG3 promoter >16 kb upstream. Intriguingly, this enhancer is bound by islet transcription factors in an allele-specific manner in human islets. Overall, our results suggest an important regulatory function for this newly characterized MEG3 enhancer and provide insights into the mechanism of imprinting at the DLK1-MEG3 locus in β-cells.
Research Design and Methods
Human Islets
Human islets and relevant donor information including age, sex, diabetes status, and BMI were obtained from the Islet Cell Resource Center of the University of Pennsylvania, the National Institute of Diabetes and Digestive and Kidney Diseases–supported Integrated Islet Distribution Program (https://iidp.coh.org), and the National Disease Research Interchange. The donor’s diabetes status was defined by the patient’s medical record and, when available, the hemoglobin A1c (Supplementary Table 1).
Transcription Activator-Like Effector Experiments
Transcription activator-like effectors (TALEs) targeting the mouse Meg3-DMR (mm10 chr12:109,540,635-109,540,653) were designed using an online resource and are as described previously (1). A total of 12 × 106 βTC6 cells in a 10-cm dish were transfected with 12 μg of either TALE wild-type (WT) or mutant plasmids using FuGENE HD Transfection Reagent (Promega). Cells were FACS sorted for GFP+ cells after 72 h on a Diva 206 (University of Pennsylvania Flow Cytometry and Cell Sorting Facility). After sorting, the cells were pooled for total RNA and genomic DNA extraction using an AllPrep DNA/RNA mini kit (Qiagen).
DNA Methylation Analysis
Genomic DNA was isolated using an AllPrep DNA/RNA kit (Qiagen). A total of 325 ng of extracted DNA or unsonicated chromatin input was bisulfite treated with the EpiTect Bisulfite kit (Qiagen) and eluted in 20 μL of Buffer EB. PCR and sequencing primers were designed using PyroMark assay design software version 2.0 (Qiagen) (sequences listed in Supplementary Table 2) to cover CpGs throughout the Meg3-Dlk1 locus. Bisulfite-converted DNA was amplified by PCR using the PyroMark PCR kit (Qiagen) at 95°C for 15 min followed by 45 cycles at 95°C for 15 s, 57°C for 30 s, and 72°C for 15 s. Biotinylated PCR products were immobilized onto streptavidin-coated sepharose beads (GE Healthcare), and DNA strands were separated using PyroMark denaturation solution (Qiagen), washed, and then neutralized using a vacuum preparation station (Qiagen PyroMark Q96 workstation). After annealing the sequencing primer to the immobilized strand, pyrosequencing was performed on the PyroMark Q96 MD (Qiagen) using the PyroMark Gold Q96 CDT kit (Qiagen) according to the manufacturer’s instructions. Data were analyzed using the Pyro Q-CpG software program (Qiagen).
Gene Expression by Quantitative RT-PCR
Primers used to quantify gene expression of Meg3 and Dlk1 in TALE experiments are listed in Supplementary Table 3.
Cell Death Assays
βTC6 cells were seeded in six-well plates at a density of 1 × 106 cells/well. The next day, cells were transfected with 1 mg plasmid and 4 mL Lipofectamine 2000 per well. After 72 h, cells were treated with 20 ng/mL mouse tumor necrosis factor-α, 5 ng/mL mouse interleukin-1b, and 10 ng/mL mouse interferon-γ. Forty-eight hours later, cells were treated with CellROX Deep Red (Thermo Fisher Scientific) according to the manufacturer’s instructions and analyzed for GFP and CellROX fluorescence using an LSRII (BD Biosciences).
Dual Luciferase Reporter Assay: Enhancer Activity
The human MEG3-DMR (hg19, chr14:100,824,307-100,826,452) and MEG3 enhancer (chr14:101,308,419-101,309,405) were subcloned into pGL3-Basic or pGL4.23[luc2/minP] luciferase reporter (Promega). A total of 50,000 βTC6 cells was seeded per well of a 24-well plate and transfected with 500 ng of plasmid DNA and 10 ng of pRL-SV40 (Promega). Cells were harvested 24 h posttransfection and processed for luciferase readout. Experiments were performed in triplicates with four technical replicates per experiment.
Allele-Specific Chromatin Immunoprecipitation PCR
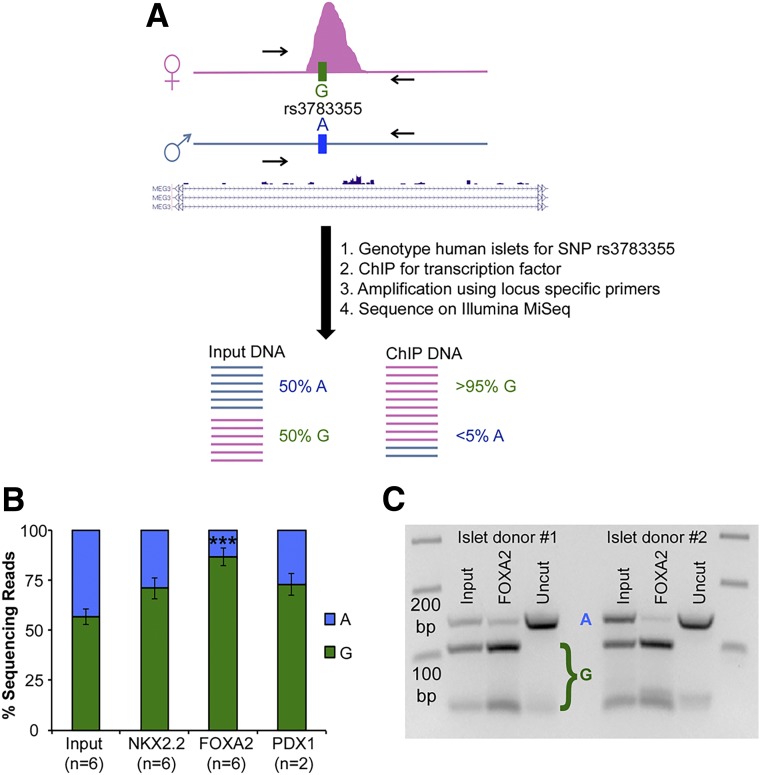
Allele-specific chromatin immunoprecipitation (ChIP) was performed according to the schema in Fig. 4A. A total of 30 ng of genomic DNA from islet donors was used to identify donors heterozygous for the single nucleotide polymorphism (SNP) rs3783355. SNP genotyping was performed using TaqMan SNP Genotyping Assay (C_1259770_10, catalog number 4351379; Thermo Fisher Scientific) and TaqMan Genotyping Master Mix (catalog number 4371353; Thermo Fisher Scientific) on a Stratagene Mx3000P thermocycler. Chromatin was extracted from islets from donors without diabetes as previously described (2). The ChIP antibodies and conditions used for this experiment were described by Charlier et al. (3). The primers used for this experiment are listed in Supplementary Table 4. Following PCR of the input and ChIP DNA, libraries were prepared using the NuGEN Mondrian SP+ system and sequenced on an Illumina MiSeq to obtain ∼200,000 reads per library (sequence read count ranged from 24,568 to 916,096 reads). For the qualitative assay, 300 ng of PCR-amplified input or FOXA2 ChIP DNA was digested using the restriction enzyme BanII. Uncut PCR product (150 bp) and BanII-digested PCR products (113-bp plus 37-bp fragments) were run on a 3% gel to visualize differences.
Figure 4.
Allele-specific transcription factor occupancy at the MEG3 enhancer. A: Schematic representation of the allele-specific ChIP experimental design. Transcription factor ChIP is performed on islets from donors heterozygous for the SNP rs3783355. Following amplification using primers surrounding the SNP, the PCR products are sequenced to quantitatively determine the relative representation of the two alleles. B: Relative amplification (percent sequencing reads) of the rs3783355 alleles as determined by high-throughput sequencing of input, NKX2.2, FOXA2, and PDX1 ChIP DNA from islet donors heterozygous for rs3783355 (G/A). C: The minor allele of rs3783355 alters the recognition sequence of a restriction enzyme, BanII. Following ChIP-PCR to determine FOXA2 occupancy at the MEG3 enhancer, the PCR products were digested with BanII to qualitatively assess the allelic representation of rs3783355. A representative gel of BanII-digested input and FOXA2 ChIP-PCR products from islets from two donors heterozygous for rs3783355 is shown. Data are represented as mean ± SEM. P values calculated using Student t test. ***P < 0.001.
Circular Chromosome Conformation Capture Sequencing
Circular chromosome conformation capture sequencing (4C-Seq) was performed on ∼10,000 islet equivalents of human islets using the enzymes DpnII (first enzyme) and NlaIII (second enzyme) according to Dorrell et al. (4). The libraries were prepared using the BisPCR2 protocol (1). Libraries were sequenced on an Illumina HiSeq on Rapid Run Mode to obtain 100-bp sequences (single-end reads). Five pooled libraries were sequenced per lane, with 20% PhiX supplemented to increase read diversity. Primers used to determine DpnII digestion efficiency and for library preparation are listed in Supplementary Table 5. 4C-Seq reads were analyzed as previously described (15). More than 14 million reads per viewpoint were sorted and aligned to the human genome (hg19). Reads located in fragments flanked by two restriction sites of the same enzyme or in fragments <40 bp were filtered out. Mapped reads were then converted to reads per first enzyme fragment and smoothened using a moving average of 30 fragments per window.
In order to identify significant 4C-Seq interactions, we proceed as previously described (15). Briefly, an average background level was estimated by shuffling the fragments 1,000 times in a 1-Mb window centered on the viewpoint and smoothened as described above. This randomized profile was then used to calculate the P value for each potential target in the observed 4C-Seq distribution by means of Poisson probability function. We considered significant interactions for each 4C-Seq experiment, regions with a Poisson probability <1 × 10−10. In order to take into account biological variability of the primary tissues, only significant interactions that were consistent in at least two of the three replicates were retained.
Results
Promoter Methylation Causes Decreased Expression of MEG3
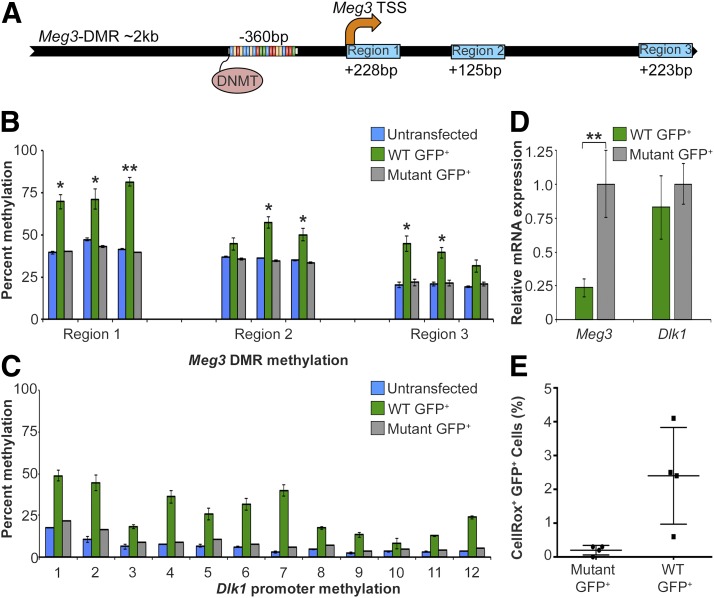
The MEG3 promoter is hypermethylated in pancreatic islets from donors with T2DM compared with donors without diabetes, correlating with a decrease in expression of MEG3 and its associated miRNAs, which are all produced from a single primary transcript originating from a single promoter (1). To test whether increased methylation at this region directly causes a decrease in expression, we used TALEs to target DNA methyltransferases (DNMTs) to the mouse Meg3-DMR sequence 360 bp upstream of the Meg3 transcription start site (TSS) (Fig. 1A and Supplementary Fig. 1A). These TALE-DNMTs are efficient mediators of targeted DNA methylation (16). As a negative control, we introduced an inactivating point mutation into the catalytic domain of DNMT (16,17). We transfected these constructs into βTC6 mouse insulinoma cells and FACS-sorted cells that had been successfully transfected based on their GFP expression. TALE-DNMT–expressing βTC6 cells exhibited an increase in methylation of 20%, along with a 75% decrease in Meg3 transcript levels, demonstrating directly that the activity of the Meg3 locus is controlled by DNA methylation in β-cells. Cells expressing the catalytically inactive version of DNMT showed no changes in DNA methylation or Meg3 RNA levels, verifying that the observed changes were specific to methyltransferase activity and not an artifact of transfection and cell sorting (Fig. 1B and D). To confirm that both transfected cell populations had received comparable amounts of TALE vector, we performed quantitative PCR with primers specific to the TALE construct backbone (Supplementary Fig. 1B).
Figure 1.
Targeted methylation of the Meg3-DMR results in decreased expression and increased β-cell death. βTC6 mouse insulinoma cells were transfected with TALE molecules specific to the mouse Meg3-DMR fused to either a WT or mutant DNMT catalytic domain. Transfected cells were sorted by GFP expression. A: Schema of mouse Meg3-DMR spanning ∼2 kb, depicting TALE-DNMT (mm10 chr12:109,540,635-109,540,653) binding positions relative to the Meg3 TSS. Regions assayed for methylation levels by pyrosequencing are depicted by blue boxes. Percent methylation levels determined by pyrosequencing of CpGs in the Meg3-DMR (mm10 chr12:109,540,014-109,542,041) in untransfected βTC6 (n = 3), WT TALE-DNMT (n = 3), or mutant TALE-DNMT–transfected (n = 2) cells (B) and Dlk1 promoter region (mm10 chr12:109452986-109453847) in untransfected βTC6 cells (n = 2), WT TALE-DNMT GFP+ (n = 3), and mutant TALE-DNMT GFP+ cells (n = 1) (C). *P < 0.05; **P < 0.01. D: Relative expression of Meg3 and Dlk1 measured by quantitative RT-PCR between WT (n = 8) and mutant (n = 7) TALE-DNMT–transfected βTC6 cells, sorted by GFP levels. Data are represented as mean ± SEM. **P < 0.01. E: Percent of WT or mutant TALE-DNMT GFP+ βTC6 cells labeled by CellROX fluorescence, a marker of oxidative stress, following cytokine treatment for 48 h (n = 4). Data are represented as mean ± SEM and P < 0.02.
TALE-DNMT constructs can have proximal off-target effects (16). To characterize possible nonspecific targets of TALE binding and methyltransferase activity, we profiled DNA methylation at CpG islands near the Meg3-DMR. The IG-DMR of the Dlk1-Meg3 locus is located 16 kb upstream of the Meg3 TSS and also paternally methylated in both human and mouse β-cells (1). However, we found this region to be fully methylated and thus uninformative in βTC6 cells (Supplementary Fig. 1C). Next, we tested the methylation levels of the Dlk1 promoter, located ∼87 kb upstream of the Meg3 TSS. This region demonstrated a 20% increase in methylation in cells transfected with WT TALE-DNMT compared with cells transfected with the mutant construct, but no change in expression, suggesting possible chromatin looping of the Dlk1 promoter to the Meg3 promoter DMR (Fig. 1C and E). We observed no difference in methylation levels at two CpG islands close to the Dio3 gene ∼650 kb and 737 kb downstream of the Meg3 TSS and found no change in DNA methylation status at this locus (Supplementary Fig. 2).
Several targets of the miRNAs encoded by the Meg3 locus, such as islet amyloid polypeptide p53-induced nuclear protein 1 (TP53INP1), function in controlling the sensitivity of β-cells to metabolic stress and proapoptotic stimuli (1). Therefore, we hypothesized that the decreased expression of the miRNAs encoded by the Meg3 locus would lead to increased susceptibility of β-cells to proapoptotic stimuli. To test this, we measured oxidative stress, as a marker upstream of cell death, in response to inflammatory cytokine treatment in TALE-DNMT–transfected β-cells (18). We found a significant increase in oxidative stress in TALE-DNMT–expressing cells relative to the control group (Fig. 1E), indicating that decreasing expression of the Meg3 locus and its associated miRNAs does indeed increase susceptibility to cytokines. This finding is consistent with the dramatic downregulation of this cluster of miRNAs in islets from individuals with T2DM (1), which correlates with the increased sensitivity of T2DM β-cells to metabolic and apoptotic stress. In summary, using targeted epigenetic modifiers, we have shown that hypermethylation of the Meg3 promoter causes decreased production of the RNA transcripts produced by this locus and a subsequent increase in cytokine-mediated β-cell death.
Characterization of a Novel Enhancer in MEG3
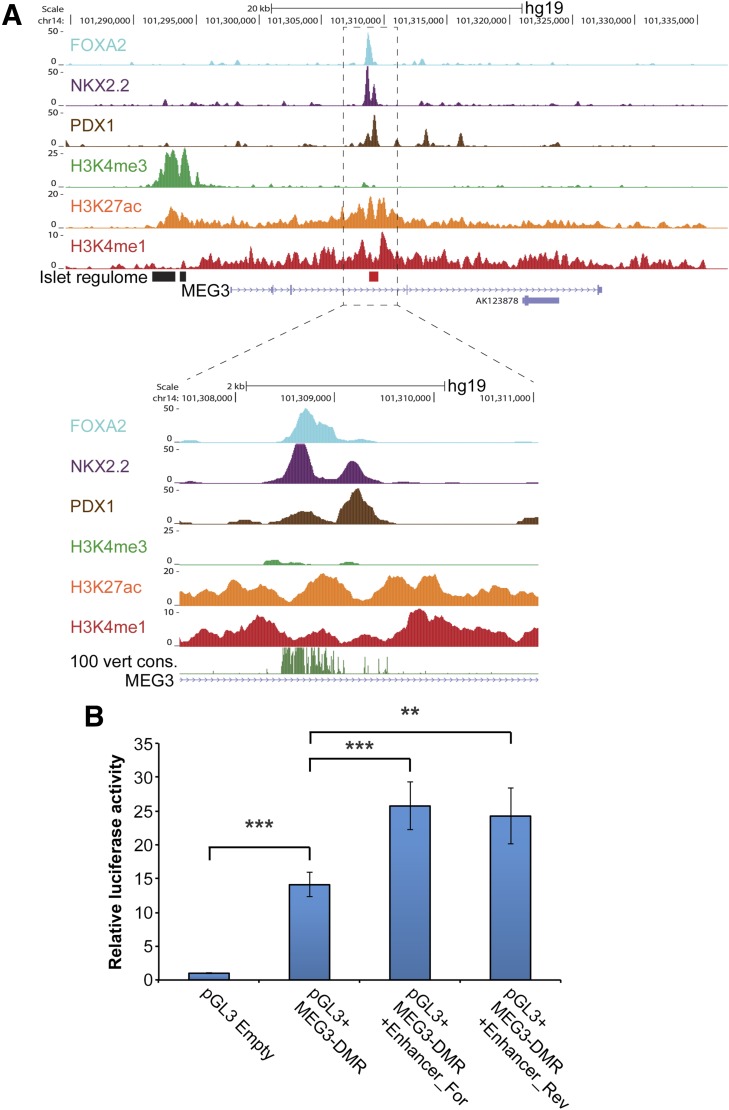
From a previous study that profiled regulatory elements based on a comparative analysis of expression data, transcription factor binding data, and chromatin marks from human islets and FACS-sorted β-cells (15), we identified a putative enhancer within an intron of the MEG3 gene. This region, located ∼16 kb downstream of the MEG3 TSS, is bound by transcription factors critical to islet development and function and marked by histone modifications that correlate with active enhancer activity (19) (Fig. 2A). Similar H3K27ac enrichment at this region has been observed in a human lung fibroblast cell line, but not in other human cell lines (20) (Supplementary Fig. 3A). We hypothesized that this putative intronic MEG3 enhancer may be a critical regulator of monoallelic expression at the DLK1-MEG3 locus.
Figure 2.
Characteristics of a novel intronic enhancer human islet. A: The chromatin landscape for a putative enhancer in an intron of MEG3 is shown with human islet ChIP sequencing tracks for histone modification marks associated with enhancers (H3K4me1 and H3K27ac) and active promoters (H3K4me3). Occupancy of islet transcription factors PDX1, NKX2.2, and FOXA2 at the putative enhancer is also shown. Data retrieved from Pasquali et al. (15). B: Activity of the MEG3 enhancer was validated by luciferase reporter assays. pGL3 vectors with the MEG3-DMR and the enhancer sequence in either its native (For) or reverse (Rev) orientation were transfected into βTC6 cells. Data are represented as mean ± SEM. n = 3. P values calculated by Student t test. **P < 0.1 × 10−3; ***P < 0.1 × 10−5.
First, we sought to validate the activity of the putative enhancer using luciferase reporter assays. The MEG3-DMR promoter sequence itself increased luciferase activity by 14-fold, validating its strong activity in β-cells (1) (Fig. 2B). Addition of the intronic enhancer, in either orientation, further doubled luciferase activity in mouse βTC6 β-cells (Fig. 2B). We additionally validated that the MEG3 enhancer increased luciferase activity of an unrelated promoter in β-cells by fivefold (Supplementary Fig. 3B). Thus, this intronic region of the MEG3 gene can function as an enhancer in β-cells.
Long-range Interactions of the MEG3 Promoter and Enhancer
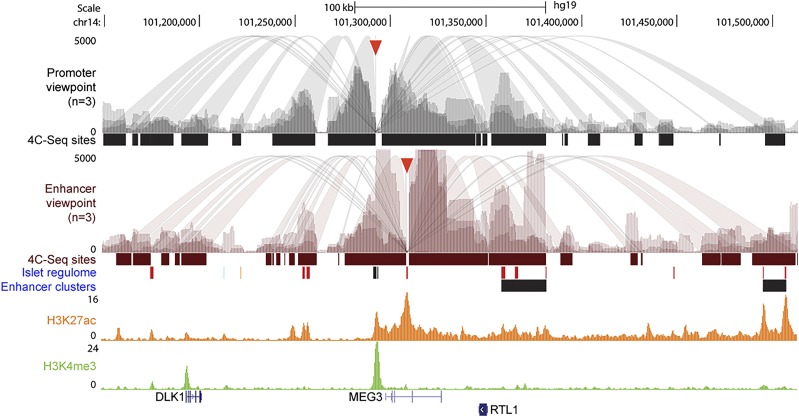
We hypothesized that the MEG3 enhancer may physically interact with the promoter to facilitate transcription of the maternal noncoding RNA transcript. To determine long-range interactions between the MEG3 promoter and the newly characterized enhancer, we undertook 4C-Seq (21). This technique provides an unbiased sampling of all interacting partners of a selected region of interest (the viewpoint). We performed 4C-Seq using either the MEG3 promoter or enhancer as viewpoints separately, using human islets from three independent donors. This reciprocal approach allowed us to test the hypothesis that the MEG3 promoter and enhancer interact with each other while also identifying potential other chromatin interactions of these regulatory elements. To date, no information regarding the chromatin confirmation at the DLK1-MEG3 locus has been reported in any tissue or species.
Consistent with our hypothesis, we found that the MEG3 promoter displayed frequent interactions with the enhancer, and vice versa, in three biological replicates (Fig. 3). Strikingly, the promoter and enhancer shared many of their long-range interactions, including with the paternally expressed DLK1 gene. Additionally, these regulatory elements made contacts with other putative enhancers within the imprinted domain, including an enhancer that lies intergenic to DLK1 and MEG3, as well as an enhancer cluster downstream of MEG3 that overlaps with the small nucleolar RNA transcript, MEG8. However, some interactions were unique to each viewpoint. One such example is the interaction between the MEG3 promoter and an enhancer cluster upstream of DLK1. Thus, using 4C-Seq, we have mapped the long-range interactions of the MEG3 promoter and enhancer in human islets and established that the intronic enhancer in the MEG3 gene 16 kb downstream of the TSS loops back to the MEG3 promoter. These findings also provide a plausible explanation for the increased DNA methylation we saw at the Dlk1 promoter when we performed targeted methylation of the Meg3 promoter DMR (Fig. 1), as the two regions are in frequent physical contact in β-cells.
Figure 3.
The MEG3 promoter and enhancer interact with other putative enhancers within the DLK1-MEG3–imprinted region. Genome browser image of the selective interactions of the MEG3 promoter and enhancer within 350 kb of the MEG3 promoter. 4C-Seq was performed using the MEG3 promoter and enhancer, respectively, as viewpoints (indicated with red triangles) using three human islet donors (represented by different shaded tracks) per viewpoint. Bars under each track represent the significant interaction sites (P < 1 × 10−8). The MEG3 promoter and enhancer make frequent contact with putative enhancers within the imprinted locus. ChIP sequencing for the histone modification marks and the putative enhancer identification was performed by Pasquali et al. (15).
Allele-Specific Transcription Factor Binding at the MEG3 Enhancer
In mouse midgestation embryos, the open chromatin landscape of the Meg3 promoter is restricted to the active maternal allele (22,23). Similarly, in mouse embryonic fibroblasts, binding of the insulator protein CTCF to the Meg3-DMR is restricted to the maternal chromosome (24). To test whether the islet transcription factors that bind the MEG3 enhancer are similarly restricted to a single allele, we performed allele-specific ChIP-PCR for FOXA2, NKX2.2, and PDX1. In order to differentiate the two alleles, we screened donor islets for heterozygosity of rs3783355, a common SNP that lies within the enhancer region (Fig. 4). Importantly, this SNP does not overlap with the consensus binding motifs for any of the three transcription factors being tested. Using islets from donors heterozygous for rs3783355, we performed ChIP-PCR and then high-throughput sequencing to determine the relative abundance of the two alleles. We anticipated that the input material would have an equal representation of both alleles, whereas allele-specific transcription factor binding would result in a preferential amplification of a single allele (schema outlined in Fig. 4A). As predicted, we observed a roughly equal number of reads for each allele in the input material for all samples, whereas the transcription factor binding was skewed toward one allele, with FOXA2 demonstrating the strongest allelic bias (Fig. 4B).
Additionally, for an alternate, qualitative readout of allele-specific transcription factor binding, we took advantage of the fact that rs3783355 lies in the recognition site for the restriction enzyme BanII. As expected, the input samples had both the uncut and digested fragments, corresponding to the A and G alleles, respectively. Conversely, the FOXA2 transcription factor ChIP samples primarily contained the digested (G allele) fragments with minimal uncut (A allele) fragments (Fig. 4C), confirming allele-specific occupancy by FOXA2.
These two methods independently verify that the islet-active transcription factors FOXA2, NKX2.2, and PDX1 that bind to the MEG3 enhancer do so in a monoallelic fashion. However, as we cannot obtain genetic information regarding the parents of the organ donors who provided islets for our study, we cannot determine which allele is inherited maternally or paternally. Nevertheless, our results suggest that the enhancer is regulated in an allele-specific manner in human islets, with the most plausible explanation that it is bound by the transcription factors on the active, maternally inherited allele.
Discussion
We have previously reported hypermethylation of the MEG3 promoter and a concomitant decrease in expression of the maternal RNAs including a cluster of 54 miRNAs in islets from donors with T2DM (1), but had not established a causal link between these observations. To circumvent any confounding nonspecific effects of the use of global DNA demethylating agents like 5-aza-2-deoxycytidine, we used TALE molecules fused to a DNMT to specifically direct methylation to the Meg3-DMR in mouse β-cells. Using this targeted approach, we demonstrate that increased methylation of the Meg3-DMR in β-cells does in fact cause decreased Meg3 expression. This approach also enabled us to expand upon our previous observation that targets of the miRNAs in this locus are related to β-cell death and apoptosis, providing a functional validation of our previous results.
We also observe an increase in methylation at the promoter of the paternally expressed Dlk1. This change in Dlk1 methylation levels is likely the result of the three-dimensional chromatin architecture of this region, as we also demonstrate that the DLK1 and MEG3 promoters physically interact in human islets using our chromatin conformation capture analysis. The fact that the MEG3 regulatory elements make physical contact with DLK1 suggests that these interactions occur in trans between the two parental chromosomes or, alternatively, that the cis interaction is constitutive on both alleles, but gene activation is determined by other trans-acting determinants, such as transcription factor binding. Allele-specific occupancy of transcription factors and histone marks has been demonstrated at cis-regulatory elements of several imprinted regions, including the Meg3-DMR (22,23,25). Our data suggest that the novel MEG3 enhancer is also bound by islet transcription factors in an allele-specific manner in human islets and thereby contributes to monoallelic gene expression.
Our characterization of this novel enhancer is particularly noteworthy in light of the observation that a SNP located ∼2.5 kb upstream of this MEG3 enhancer in the human locus is correlated with risk for type 1 diabetes mellitus. The SNP identified as the risk variant lies within the same intron of MEG3 as the enhancer characterized in our study (26) and is part of the same linkage disequilibrium block. It remains to be determined whether this SNP and other variants in linkage disequilibrium with it confer risk for type 1 diabetes mellitus by altering regulation of the genes in the imprinted locus by affecting the enhancer region characterized in this study. This is indeed supported by evidence that in general, disease variants identified by genome-wide association studies are frequently found in cis-regulatory elements (27,28).
It is likely that other unidentified regulators contribute to the control of imprinting at this locus. Recent studies have suggested that similar to other nuclear long noncoding RNAs, MEG3 directly interacts with the polycomb repressive complex (PRC2) in embryonic stem cells to guide the repressive histone modification mark H3K27me3 to its target sites (29,30). A careful characterization of MEG3-PRC2 complex targets in adult pancreatic islets will provide better insights into the role of this silencing complex in mediating allelic expression at this locus. Additionally, although islet transcription factors bound by both the promoter and the enhancer, such as NKX2.2, are likely mediators of the looping interaction between these regions, it is possible that architectural elements unexplored in this current study are also involved in stabilizing this interaction.
Overall, our results demonstrate that targeted methylation of the MEG3-DMR is sufficient to repress the locus and increase β-cell susceptibility to cytokines and provide evidence for the role of a novel enhancer in the regulation of imprinting at the DLK1-MEG3 locus in human cells. These results extend our understanding of allelic expression of genes in this important locus and thus its misregulation in T2DM and other diseases.
Supplementary Material
Article Information
Acknowledgments. The authors thank the University of Pennsylvania Diabetes Research Center for the use of the Functional Genomics Core.
Funding. This work was supported by the National Institutes of Health (R01-DK-088383 and UC4-DK-104119). The University of Pennsylvania Diabetes Research Center Functional Genomics Core was supported by the National Institutes of Health (P30-DK-19525).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. V.K. wrote the manuscript and researched data. M.L.G., M.R.-R., K.O., Y.J.W., J.Z., and L.P. researched data. K.H.K. contributed to discussion and reviewed and edited the manuscript. K.H.K. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0682/-/DC1.
References
- 1.Kameswaran V, Bramswig NC, McKenna LB, et al. . Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab 2014;19:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet 2008;24:306–316 [DOI] [PubMed] [Google Scholar]
- 3.Charlier C, Segers K, Wagenaar D, et al. . Human-ovine comparative sequencing of a 250-kb imprinted domain encompassing the callipyge (clpg) locus and identification of six imprinted transcripts: DLK1, DAT, GTL2, PEG11, antiPEG11, and MEG8. Genome Res 2001;11:850–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorrell C, Schug J, Lin CF, et al. . Transcriptomes of the major human pancreatic cell types. Diabetologia 2011;54:2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You L, Wang N, Yin D, et al. . Downregulation of long noncoding RNA Meg3 affects insulin synthesis and secretion in mouse pancreatic beta cells. J Cell Physiol 2016;231:852–862 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt JV, Matteson PG, Jones BK, Guan XJ, Tilghman SM. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev 2000;14:1997–2002 [PMC free article] [PubMed] [Google Scholar]
- 7.Takada S, Paulsen M, Tevendale M, et al. . Epigenetic analysis of the Dlk1-Gtl2 imprinted domain on mouse chromosome 12: implications for imprinting control from comparison with Igf2-H19. Hum Mol Genet 2002;11:77–86 [DOI] [PubMed] [Google Scholar]
- 8.Benetatos L, Dasoula A, Hatzimichael E, Georgiou I, Syrrou M, Bourantas KL. Promoter hypermethylation of the MEG3 (DLK1/MEG3) imprinted gene in multiple myeloma. Clin Lymphoma Myeloma 2008;8:171–175 [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Dahle D, Zhou Y, Zhang X, Klibanski A. Hypermethylation of the promoter region is associated with the loss of MEG3 gene expression in human pituitary tumors. J Clin Endocrinol Metab 2005;90:2179–2186 [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Dong Z, Liu S, et al. . Promoter hypermethylation-mediated downregulation of miR-770 and its host gene MEG3, a long non-coding RNA, in the development of gastric cardia adenocarcinoma. Mol Carcinog 2017;56:1924–1934 [DOI] [PubMed] [Google Scholar]
- 11.Sun M, Xia R, Jin F, et al. . Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol 2014;35:1065–1073 [DOI] [PubMed] [Google Scholar]
- 12.Peng W, Si S, Zhang Q, et al. . Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res 2015;34:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol 2015;36:4851–4859 [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther 2016;17:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, et al. . Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet 2014;46:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein DL, Le Lay JE, Ruano EG, Kaestner KH. TALE-mediated epigenetic suppression of CDKN2A increases replication in human fibroblasts. J Clin Invest 2015;125:1998–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JY, Pu MT, Hirasawa R, et al. . Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol Cell Biol 2007;27:8748–8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A 2002;99:12363–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creyghton MP, Cheng AW, Welstead GG, et al. . Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 2010;107:21931–21936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature 2012;489:57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z, Tavoosidana G, Sjölinder M, et al. . Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet 2006;38:1341–1347 [DOI] [PubMed] [Google Scholar]
- 22.Carr MS, Yevtodiyenko A, Schmidt CL, Schmidt JV. Allele-specific histone modifications regulate expression of the Dlk1-Gtl2 imprinted domain. Genomics 2007;89:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurray EN, Schmidt JV. Identification of imprinting regulators at the Meg3 differentially methylated region. Genomics 2012;100:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S, Ferguson-Smith AC, Schultz RM, Bartolomei MS. Nonallelic transcriptional roles of CTCF and cohesins at imprinted loci. Mol Cell Biol 2011;31:3094–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verona RI, Thorvaldsen JL, Reese KJ, Bartolomei MS. The transcriptional status but not the imprinting control region determines allele-specific histone modifications at the imprinted H19 locus. Mol Cell Biol 2008;28:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace C, Smyth DJ, Maisuria-Armer M, Walker NM, Todd JA, Clayton DG. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet 2010;42:68–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakabe NJ, Savic D, Nobrega MA. Transcriptional enhancers in development and disease. Genome Biol 2012;13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature 2009;461:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Ohsumi TK, Kung JT, et al. . Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell 2010;40:939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko S, Bonasio R, Saldaña-Meyer R, et al. . Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell 2014;53:290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.