Abstract
The role of interferons, either pathogenic or protective, during autoimmune diabetes remains controversial. Herein, we examine the progression of diabetes in NOD mice lacking the type I (IFNAR) or type II (IFNGR) interferon receptor and, for the first time, in mice deficient in both receptors (double knockout [DKO]). All mice were bred, maintained, and monitored in a single specific pathogen-free facility with high female and low male diabetes incidence. Our expectation was that removal of interferon signaling would reduce autoimmune destruction. However, examination of diabetes incidence in the IFNAR- and IFNGR-deficient NOD mice showed a reduction in females and an increase in males. In DKO mice, diabetes occurred only in female mice, at decreased incidence and with delayed kinetics. These results show that interferons act as both positive and negative modulators of type 1 diabetes disease risk dependent on sex.
Introduction
The role of interferon (IFN) signaling in the development of autoimmune diabetes (type 1 diabetes [T1D]) has been studied for more than 30 years (1). However, a clear understanding of the role of type I (IFN-I) and II (IFN-II) IFNs in progression or regulation of the disease is elusive. This is due to the number of different systems used to study IFNs in T1D and the variations found among different mouse facilities, in which the incidence of diabetes varies greatly.
A long line of evidence links IFN-I to promoting T1D. IFN-I expression can be detected in the islets of Langerhans during T1D in rodents and humans (2–4). Treatment of NOD mice with blocking monoclonal antibodies against the IFN-I receptor (IFNAR) delays and reduces T1D incidence in female mice (5). T1D is a clinical complication after IFN-I therapy for treatment of viral infections and cancers (6). In contrast to all of this suggestive evidence, mice rendered deficient in IFNAR show reduction of IFN signatures in the islets but develop T1D normally (7).
The role of IFN-γ in T1D progression is perplexing. Genetic ablation of IFN-γ or the IFN-γ receptor (IFNGR) in the NOD mouse leads to phenotypes that range from no effect (Ifngr1−/−), to mild delay (Ifngr2−/−), and to delay and reduction (Ifng−/−) (8–10).
Despite these diverse results among the published studies, two essential findings demonstrate that IFN signaling and/or IFN-inducible genes are required for T1D incidence. First, NOD mice deficient in the interferon regulatory factor-1, an IFN transcriptional regulator that acts through interferon-stimulated response elements, never develop T1D or detectable leukocytic infiltration into the islets (11). Second, NOD mice deficient in STAT-1, an essential transcription factor immediately downstream of both IFNAR and IFNGR, are also protected from T1D (12).
For these reasons, we have generated a new set of IFN-deficient mice on a pure NOD background. These have all been bred and cohoused in the same high diabetes incidence animal facility in which the control of environmental pathogens is strictly enforced. Our hypotheses were that IFN signaling would increase T1D incidence in all NOD mice and that the effect of removing the two receptors would be additive or multiplicative. Therefore, deletion of a single IFN receptor would partially delay disease, but deletion of two IFN receptors would prevent disease. Reported here is the evaluation of diabetes incidence and leukocytic infiltration status of male and female NOD.Ifnar1−/−, NOD.Ifngr1−/−, and NOD.Ifnar1−/−×Ifngr1−/− mice. In contrast to our hypothesis, the effects of single IFN-deficient mice vary depending on the sex of the mice in our colony, whereas double IFN–deficient female mice still developed diabetes albeit at lower incidence and with delayed kinetics.
Research Design and Methods
Mice
NOD mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in our animal facility. NOD.Ifnar1−/− mice were generated by backcrossing 129S2.Ifnar1tm1Agt mice for seven generations until homogenously NOD. NOD.Ifngr1−/− mice were generated by backcrossing B6.Ifngr1f/f mice for seven generations until genetically identical to NOD mice. Mice were then crossed to NOD.FVB-Tg(EIIa-cre)C5379Lmgd/J (The Jackson Laboratory) mice to generate a germline Ifngr1−/− deficiency. NOD.DKO (double knockout) mice were generated by intercrossing NOD.Ifnar1−/− and NOD.Ifngr1−/− mice. All backcrosses were monitored by PCR evaluation of 115 microsatellites at each generation (speed congenics). Mice were considered backcrossed when all tested microsatellites were derived from NOD and not 129 or B6 based on reference mice from The Jackson Laboratory.
Blood glucose was monitored weekly. After two consecutive readings of >250 mg/dL (Chemstrip 2GP; Roche Diagnostics, Indianapolis, IN), mice were considered diabetic. For diabetes transfer experiments, spleens were isolated from NOD or NOD.DKO mice at 8–10 weeks of age. Single-cell suspensions were generated using mechanical disruption. Spleen cells were strained over a 70-µm filter and resuspended in pyrogen-free PBS (Thermo Fisher). Cells (107) were injected into recipient NOD.129S7(B6)-Rag1tm1Mom/J (NOD.Rag1−/−; The Jackson Laboratory) mice intraperitoneally, and mice were monitored for diabetes.
All mice were bred and maintained under specific pathogen-free conditions in our animal facility. All experiments were approved by the Division of Comparative Medicine of Washington University School of Medicine in St. Louis (Association for Assessment and Accreditation of Laboratory Animal Care, accreditation number A3381-01). Incidence and statistical analysis was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA).
Islet Isolation, Flow Cytometry, and Histology
Islets were harvested as described previously (13) and dispersed using Cell Dissociation Solution Non-enzymatic (Sigma-Aldrich, St. Louis, MO) for 5 min at 37°C. Single-cell suspensions were incubated in PBS (pH 7.4) having 1% BSA (Sigma-Aldrich) and 50% 2.4G2 conditioned DMEM at 4°C for 15 min to block Fc receptors. Cells were stained with fluorescently labeled antibodies and analyzed using the FACSCanto II (BD Biosciences, San Jose, CA). The BV510/APC anti-CD45 (30-F11) was purchased from BioLegend (San Diego, CA). The fluorescein isothiocyanate/allophycocyanin anti-CD3e (145-2C11) was purchased from BD Biosciences. Pacific Blue I-Ag7 (AG2.42.7) was purified and conjugated in our laboratory (Pacific Blue Antibody Labeling Kit; Invitrogen). For histology, pancreata were isolated, fixed in 10% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. All flow cytometry data were analyzed using FlowJo software (FlowJo, Ashland, OR).
Results
Loss of IFN Signaling Affects T1D Incidence Differentially Based on Sex
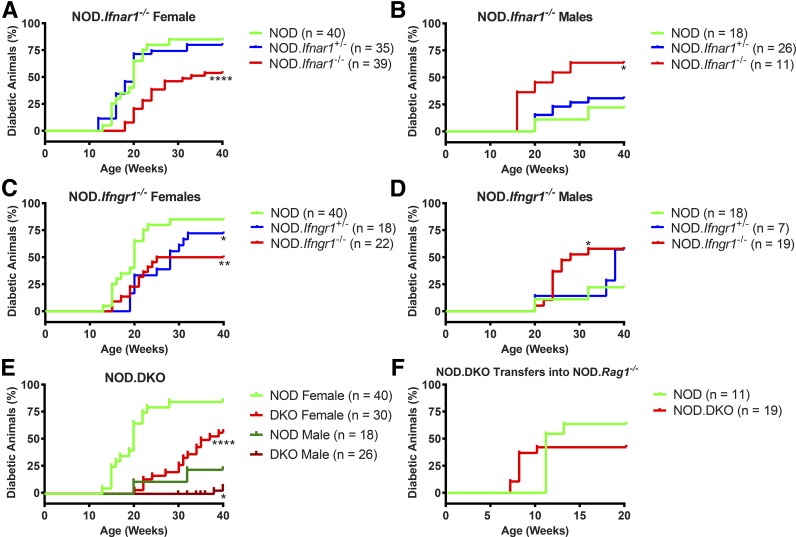
Three different IFN receptor–deficient mice were generated on the NOD background: Ifnar1−/− (IFNAR), Ifngr1−/− (IFNGR), and Ifnar1−/−×Ifngr1−/− (DKO) (Fig. 1). Male and female mice of all three genotypes were observed for the development of hyperglycemia up to 40 weeks of age. Diabetes development in female NOD mice was 85%. It was significantly delayed in NOD.Ifnar1−/− and NOD.Ifngr1−/− female mice, with a final incidence of 54% and 50%, respectively (Fig. 1A and C). The incidence in male NOD mice in our colony was 23% (Fig. 1B), but increased to 63% in NOD.Ifnar1−/− and to 58% in NOD.Ifngr1−/− male mice (Fig. 1B and D).
Figure 1.
Incidence of diabetes in NOD mice is influenced by IFN receptor signaling. A–E: Mice of the indicated genotypes and sex were monitored for hyperglycemia until 40 weeks of age. The mean number (n) of mice examined are indicated on the graphs. For all three IFN-deficient genotypes, two independent groups of mice were monitored at least 6 months apart, and the data of the experiments are pooled. Control NOD mice were monitored throughout the study in two (males) or three (females) different groups, and incidences were pooled for the analysis. We observed no change in incidence in our wild-type NOD colony over the course of the experiments. F: Spleens were isolated from 10- to 12-week-old NOD or NOD.DKO mice, and 107 splenocytes were transferred into NOD.Rag1−/− mice. Mice were monitored for diabetes for the indicated weeks after transfer. Comparison of survival curves was performed using the Mantel-Cox log-rank test. All survival curve comparisons are of the indicated genotype against the control NOD mice. *P = 0.0332, **P = 0.0021; ****P < 0.0001.
Surprisingly, female mice double deficient for IFNAR and IFNGR (NOD.DKO) had a T1D incidence of 50% at 40 weeks, albeit with a much slower progression in time. Male NOD.DKO mice had a T1D incidence of only 6% (2 of 34 mice). Transfer of the spleen cells isolated from NOD or NOD.DKO females into NOD.Rag1−/− mice led to equivalent diabetes incidence.
Analysis of Inflammatory Leukocyte Infiltration Into IFN Receptor–Deficient Mice
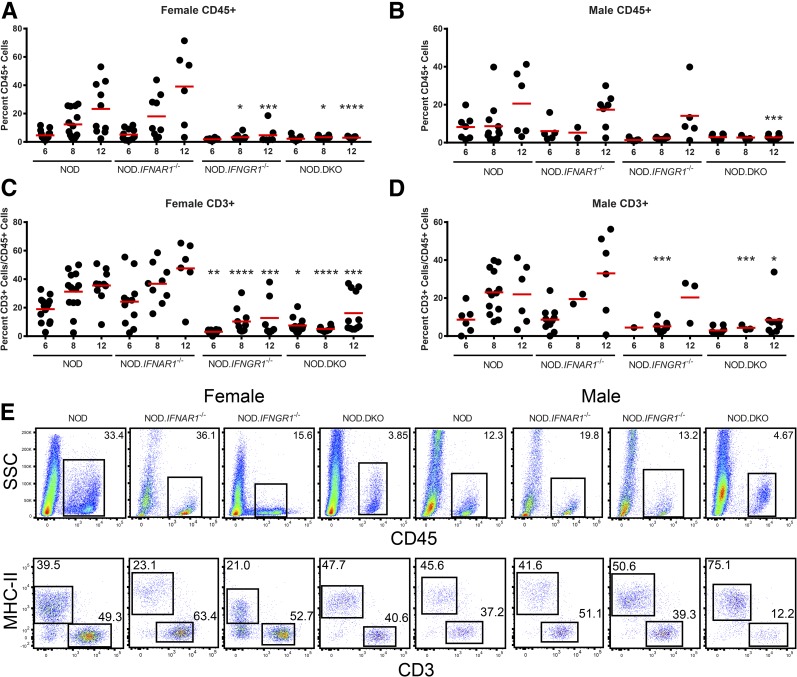
In normal mice, macrophages are the major leukocyte throughout the life of the mouse (13). During NOD diabetes, there is an entry of additional leukocytes into islets that increases in number and complexity over time (14). Figure 2A and B show the progressive entry of CD45+ cells that takes place in the NOD mouse. Leukocyte infiltration in NOD.Ifnar1−/− mice follows a similar pattern as in NOD mice. The female NOD.Ifngr1−/− and NOD.DKO mice had a significant reduction in the percentage of CD45+ cells at 8 and 12 weeks of age (Fig. 2A). In male mice, only 12-week-old NOD.DKO mice differed significantly from their aged-matched NOD controls in the number of CD45+ cells infiltrating into islets (Fig. 2B).
Figure 2.
Flow cytometry analysis of islet-infiltrating leukocytes reveals different patterns of infiltration in IFN receptor–deficient mice. Flow cytometry analysis was performed on isolated islets of Langerhans taken from mice of the indicated genotype, sex, and age in weeks. A and B: The percentage of islet cells that were CD45+ are shown. C and D: The percentage of CD3+ cells in the islets was plotted as a percentage of the CD45+ cells. Scatter plots show the analysis of individual mice and the mean for each group. E: Representative flow cytometry plots are shown for the indicated phenotype and sex at 12 weeks of age. P values were calculated using an ordinary one-way ANOVA, followed by uncorrected Fisher least significant difference comparing all columns. *P = 0.0332, **P = 0.0021, ***P = 0.0002, and ****P < 0.0001. Where indicated, significance is from the marked column to the same age NOD control.
The number of CD3+ calculated as a percentage of the total CD45+ cells did not show a difference between the NOD and NOD.Ifnar1−/− female or male mice (Fig. 2C and D). Both showed a progressive increase in CD3+ cells. There was an increase in the percentage of CD3+ T cells over time in NOD.Ifngr1−/− and NOD.DKO mice (Fig. 2C). In male mice, the CD3+ T cells were reduced in NOD.Ifngr1−/− at 8 weeks of age and in NOD.DKO mice at 8 and 12 weeks of age (Fig. 2D). Representative flow cytometric evaluations performed at 12 weeks of age for both sexes and all strains are shown in Fig. 2E.
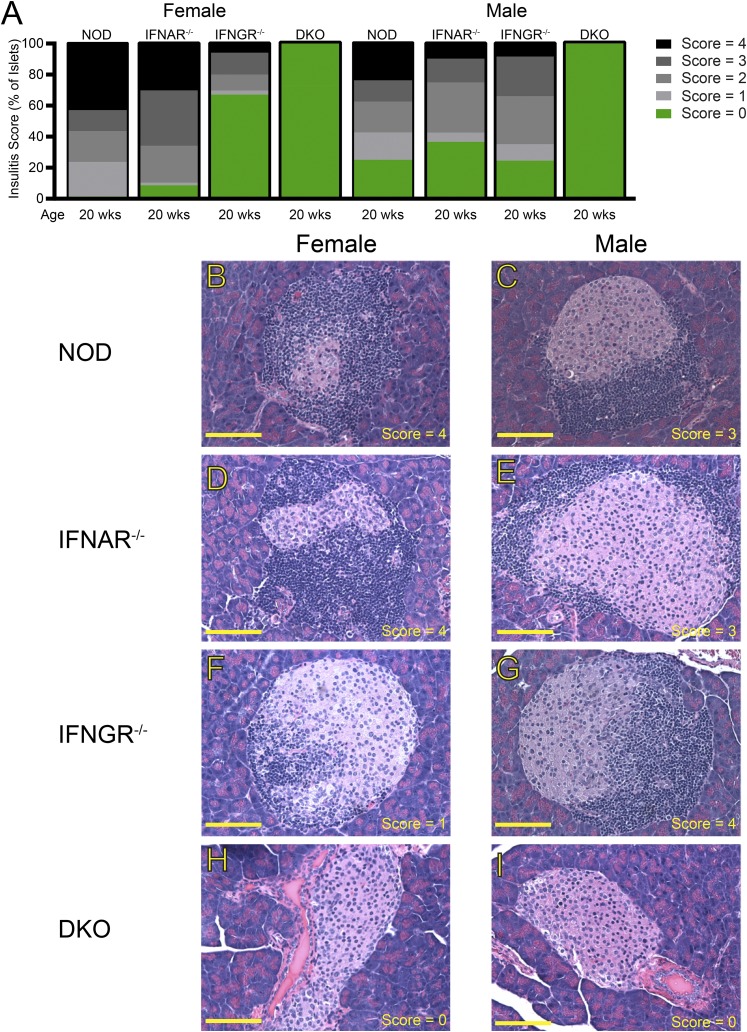
At 20 weeks of age, the islets of NOD mice had changes mostly consistent of insulitic or peri-insulitic lesions (Fig. 3A–C). Most of the islets of female NOD.Ifnar1−/− mice contained large peri-insulitic lesions or a destructive insulitis (Fig. 3A and D). Most of the islets in male NOD.Ifnar1−/− mice also showed large peri-insulitis or destructive insulitis (Fig. 3A and E). Female NOD.Ifngr1−/− were less affected, containing mostly smaller peri-insulitic lesions, and ∼67% of islets were normal (Fig. 3A and F). Male NOD.Ifngr1−/− had more extensive leukocytic lesions, and only ∼24% were clean (Fig. 3A and G). All islets from NOD.DKO mice were normal at 20 weeks of age, consistent with their slow progression (Fig. 3A and I). By 40 weeks of age, the females of all the three strains and the males of the single knockouts had similar pathology. No discernible lesions were detected in more than 169 examined islets in male NOD.DKO mice at 40 weeks.
Figure 3.
Histological analysis reveals islet infiltration status of the IFN receptor–deficient mice. A: Summary of histological analysis of islets of Langerhans from the indicated mice at the indicated ages. Results were compiled from three to eight mice per group. B–I: Representative photomicrographs of hematoxylin and eosin–stained pancreatic sections taken from the indicated mice at the indicated ages. Photomicrographs are representative of 59–169 individual islets per group. Scale bars = 100 µm.
Discussion
In brief, our results suggest that IFNs affect T1D incidence via at least two pathways. First, deficiency of IFN-γ delays the entry of inflammatory leukocytes into the islets of NOD mice. Second, deficiency of IFN-I or -II signaling alters the regulation of diabetes in a sex-dependent manner, leading to a reduction of incidence in females and an increase in males.
The results seen in our NOD.Ifnar1−/− female mice are different from those published by another group (7). Their results showed no difference in diabetes incidence between female NOD and NOD.Ifnar1−/− mice. This occurred despite the reduction in IFN-I signatures in the islets of the NOD.Ifnar1−/− mice. These divergent results could be explained by differences in local environmental conditions or perhaps by genetic drifts in NOD colonies, which are known to occur (15).
Our results with NOD.DKO mice are puzzling compared with those of the NOD.Stat1−/− and NOD.Irf1−/− mice. Neither of these mice developed diabetes or inflammatory infiltrates in their islets (11,12). Our expectation was that the NOD.DKO would phenocopy these mice. Instead, our results suggest that there is another pathway (or pathways) that leads to activation of STAT1 and IRF1 during T1D. Type III IFNs (IFN-λ) can activate STAT1, but thus far they have been described as mucosal surface cytokines that protect the intestines and lung from infection (16). Cytokines of the interleukin (IL)-6 family, such as IL-6 and IL-27, signal through STAT1/STAT3 (17). However, the IL-6–deficient NOD mouse (Stock #029264; The Jackson Laboratory) has a normal incidence of T1D, and there is no published link between IL-27 and normal NOD diabetes.
We draw several conclusions from the histological examination. First, even though the NOD.Ifnar1−/− mice have different disease outcomes than their NOD counterparts (females had a decrease in incidence, whereas males showed an increase) (Fig. 1), the histological lesions between the two are indistinguishable. This suggests that the effect of IFN-I in modulating the incidence of diabetes may not be through the recruitment of inflammatory cells but through some undetermined regulatory activity in the islets. Concerning IFN-γ, the first CD4 T cells that enter islets produce the cytokine, which likely promotes the recruitment and/or accumulation of other lymphocytes. In the female NOD.Ifngr−/− mice, the reduction in numbers of leukocytes and delay in T-cell entry probably accounts for the reduction and delay of T1D incidence. In male mice, despite the delay of leukocyte entry, the incidence of T1D is increased, reinforcing the suggestion of an IFN-γ–mediated negative regulation in male diabetogenesis (18). This result also suggests that the immune activation status of infiltrating leukocytes is more of a determinant of T1D outcome than simply the absolute number of cells that enter islets. Leukocytic infiltration occurs in the female DKO mice that can cause diabetes, but the kinetics are slower than in NOD.Ifngr−/− mice. The absence of both IFN receptors prevents leukocyte accumulation in and around the islets of the male DKO mice.
Sexual dimorphism of NOD diabetes incidence in different animal colonies around the world is well documented (19); however, not all colonies display this sex bias (12,19). In addition, gnotobiotic mice lack sexual dimorphism, and selective bacterial colonization of germ-free mice reduces diabetes incidence selectively in males and not females (18,20). It has been proposed that the protection of NOD males from diabetes by microbiota is partly mediated by induction of a strong IFN signature that negatively regulates diabetogenesis in male but not female mice. Our data agree with this conclusion: in the absence of IFNGR signaling, male mice become more susceptible to T1D. The results obtained from the NOD.Ifnar1−/− suggest that IFN-I may also contribute to protection from diabetes in male NOD mice, an issue that will need to be explored further.
In sum, the data reported here demonstrate that the sex bias observed in NOD mice colonies like ours is normalized by single IFN receptor deficiency. Surprisingly, female NOD mice still developed T1D in the absence of both IFN-I and -II receptors, whereas male mice had almost no incidence. This study, although mostly descriptive, provides a foundation for future work that will be needed to understand the role of these two interferon receptors in enhancing or limiting T1D incidence depending on sex and, possibly, housing environment.
Article Information
Acknowledgments. The authors thank Katherine E. Frederick (Washington University in St. Louis) for maintaining the mouse colony.
Funding. This work was funded by grants provided by the National Institute of Diabetes and Digestive and Kidney Diseases (DK-058177) and National Institute of Allergy and Infectious Diseases (AI-14551). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The laboratory receives general support from the Kilo Diabetes & Vascular Research Foundation (Special Award).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.A.C. and E.R.U. designed and performed experiments, collected and analyzed data, and wrote and edited the manuscript. N.D.B. and K.N. designed and performed experiments and collected and analyzed data. J.A.C. and E.R.U. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Campbell IL, Wong GH, Schrader JW, Harrison LC. Interferon-γ enhances the expression of the major histocompatibility class I antigens on mouse pancreatic beta cells. Diabetes 1985;34:1205–1209 [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Yuang J, Goddard A, et al. . Interferon expression in the pancreases of patients with type I diabetes. Diabetes 1995;44:658–664 [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Hultgren B, Dybdal N, Stewart TA. Islet expression of interferon-alpha precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity 1994;1:469–478 [DOI] [PubMed] [Google Scholar]
- 4.Foulis AK, Farquharson MA, Meager A. Immunoreactive α-interferon in insulin-secreting β cells in type 1 diabetes mellitus. Lancet 1987;2:1423–1427 [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 2008;105:12439–12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, Kawasaki E, Imagawa A, et al.; Research Committee on Type 1 Diabetes of the Japan Diabetes Society . Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care 2011;34:2084–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quah HS, Miranda-Hernandez S, Khoo A, et al. . Deficiency in type I interferon signaling prevents the early interferon-induced gene signature in pancreatic islets but not type 1 diabetes in NOD mice. Diabetes 2014;63:1032–1040 [DOI] [PubMed] [Google Scholar]
- 8.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes 1996;45:812–817 [DOI] [PubMed] [Google Scholar]
- 9.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J Immunol 2000;164:3919–3923 [DOI] [PubMed] [Google Scholar]
- 10.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes 2000;49:2007–2011 [DOI] [PubMed] [Google Scholar]
- 11.Nakazawa T, Satoh J, Takahashi K, et al. . Complete suppression of insulitis and diabetes in NOD mice lacking interferon regulatory factor-1. J Autoimmun 2001;17:119–125 [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Kim HS, Chung KW, et al. . Essential role for signal transducer and activator of transcription-1 in pancreatic beta-cell death and autoimmune type 1 diabetes of nonobese diabetic mice. Diabetes 2007;56:2561–2568 [DOI] [PubMed] [Google Scholar]
- 13.Calderon B, Carrero JA, Ferris ST, et al. . The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 2015;212:1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 2014;41:657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simecek P, Churchill GA, Yang H, et al. . Genetic analysis of substrain divergence in non-obese diabetic (NOD) mice. G3 (Bethesda) 2015;5:771–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wack A, Terczyńska-Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol 2015;16:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirahara K, Onodera A, Villarino AV, et al. . Asymmetric action of STAT transcription factors drives transcriptional outputs and cytokine specificity. Immunity 2015;42:877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurkovetskiy L, Burrows M, Khan AA, et al. . Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunol Today 1993;14:193–196 [DOI] [PubMed] [Google Scholar]
- 20.Wen L, Ley RE, Volchkov PY, et al. . Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008;455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]