ABSTRACT
Most land plants rely on root symbioses to complement or improve their mineral nutrition. Recent researches have put forward that mycorrhizal fungi efficiently absorb and transfer potassium (K+) from the soil to host plant roots, but the molecular mechanisms involved are not completely elucidated yet. We have recently revealed that K+ is likely released from the fungal Hartig net to the plant by TOK channels in the ectomycorrhizal model Hebeloma cylindrosporum – Pinus pinaster. H. cylindrosporum harbours three TOK members. Herein, we report that one of them, HcTOK1, has similar features than the yeast ScTOK1. Moreover, we propose a role for this channel in the transport of K+ from the medium to ectomycorrhizal roots under K+ starvation.
Keywords: ectomycorrhizal symbiosis, ion homeostasis, plant nutrition, potassium transport, subcellular localization, TOK channel
Text
Potassium (K+) is one of the main macronutrients for plants, but its low availability often hampers the absorption by roots.1 Plants can face this constraint by establishing mycorrhizal symbioses, in which the fungus overcomes the lack of K+ with efficient soil exploration and specialized transport systems at both uptake and release sites of symbiotic structures.2–4 In ectomycorrhizal symbiosis, the fungus differentiates three pseudo-tissues called extra-radical hyphae involved in nutrient acquisition, mantle isolating the roots from the soil, and Hartig net where trophic exchanges take place with plant epidermal and cortical cells.5 In the symbiotic model between the ectomycorrhizal fungus Hebeloma cylindrosporum and the host plant Pinus pinaster, the Na+-K+ symporter HcTrk1 acts as an uptake mechanism in extra-radical and mantle hyphae,6,7 whereas secretion of K+ to colonized roots is still barely known. Excitingly, we have recently described three tandem-pore outward-rectifying K+ (TOK) channels of H. cylindrosporum,8 of which at least one, HcTOK2.2, is likely to transfer K+ from Hartig net hyphae to the symbiotic interface. Another channel, named HcTOK1, is phylogenetically close to the only member from Saccharomyces cerevisiae, ScTOK1, and shows similarities in its transport properties.8 In the present study, we describe the sub-cellular localization of HcTOK1 and provide additional information about its possible role in symbiotic transport of K+ within ectomycorrhizas under K+-limiting conditions.
The S. cerevisiae strain ply232 was transformed with the pFL61 plasmid9 containing a HcTOK1-eGFP fusion, which led to a strong fluorescence signal at the plasma membrane (Figure 1). To study the K+ homeostasis of H. cylindrosporum when this outward-rectifying channel was overexpressed, two transgenic lines transformed with HcTOK1-OE constructs already described in Guerrero-Galán et al.8 along with two control lines (wild type h7 and empty pPZP-133 vector CT) were cultured in a K+-rich medium (N6, described in Louche et al., 2010).10 After two weeks, fungal mycelia were transferred to the same medium but without K+ (N6-K, described in Garcia et al., 2014)7 and sampled 0, 12, 24 and 48 hours later. Tissues were dried, weighted and mineralized in 0.1 N HCl for 24 h before measuring the K+ and Na+ contents by atomic absorption spectrophotometry (SpectrAA 220 FS, Varian). The HcTOK1-OE2 line, which showed the strongest overexpression of HcTOK1,8 displayed a higher K+ accumulation than the two control lines (Figure 2), but no change in Na+ contents (data not shown). The same lines were tested in symbiosis with Pinus pinaster, in comparison to non-inoculated plants as an additional control (Figure 3). Seedlings were cultured in vitro for two months before harvest in low K+ medium and analyzed as described in Garcia et al.7 Inoculation increased the K+ nutrition of all the seedlings, compared to non-mycorrhizal controls. Moreover, plants inoculated with the lines overexpressing HcTOK1 accumulated more K+ in shoots but not in roots, indicating that translocation of K+ was increased in these plants (Figure 3). On the contrary, the Na+ contents of seedlings did not change (data not shown). The overexpression of HcTOK1 did not affect the biomass of fungal cultures, nor that of the inoculated seedlings, compared to the corresponding controls (data not shown).
Figure 1.
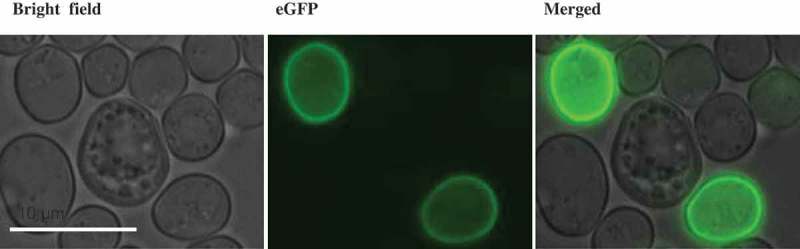
Subcellular localization of HcTOK1 channels in Saccharomyces cerevisiae.
A fluorescence signal, obtained with HcTOK1-cDNA-eGFP fusions expressed in Saccharomyces cerevisiae, is observed at the plasma membrane.
Figure 2.
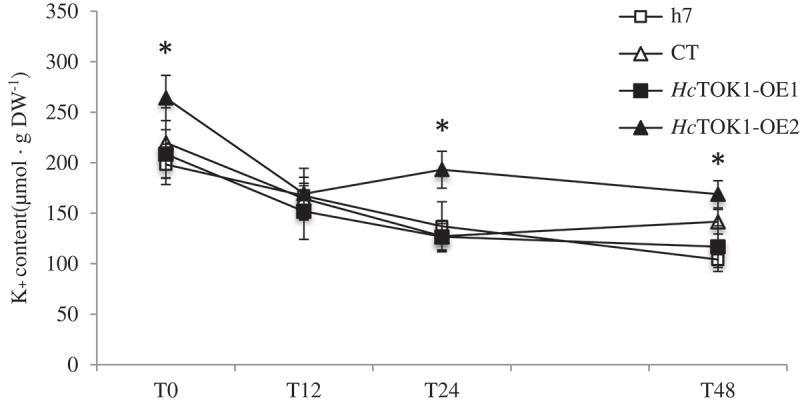
Potassium accumulation in HcTOK1-overexpressing lines of Hebeloma cylindrosporum.
Potassium contents of Hebeloma cylindrosporum h7 and empty pPZP133 (CT) controls and two lines overexpressing HcTOK1 cDNA (HcTOK1-OE1 and -OE2) in K+ starvation kinetics. Mycelia were cultured 2 weeks in complete N6 medium and then transferred to an N6 medium without K+. Samples were harvested at 0, 12, 24 and 48 hours (T0, T12, T24 and T48). Overexpression of HcTOK1 induced an accumulation of K+ in the fungus. *Significant difference compared to controls (Wilcoxon-Mann-Whitney test; p < 0.05). n = 6–9.
Figure 3.
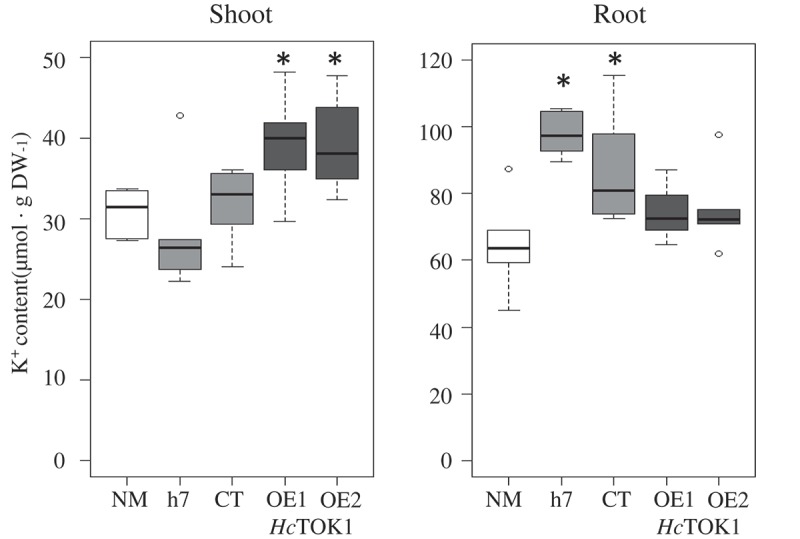
Distribution of potassium in Pinus pinaster shoots and roots colonized with HcTOK1-overexpressing fungal lines.
Distribution of K+ contents in Pinus pinaster seedlings non-inoculated (NM) or inoculated with Hebeloma cylindrosporum, after 2 months of in vitro culture in K+ starvation conditions (0.05 mM). Mycorrhizal plants were inoculated with h7 and CT controls and the two HcTOK1-OE lines. In low K+ conditions, plants inoculated with H. cylindrosporum displayed a greater K+ content, particularly in roots. Overexpression of HcTOK1 channel enhanced the transfer of K+ to the shoot. *Significant difference compared to NM controls (Wilcoxon-Mann-Whitney test; p < 0.05). n = 5–6.
The channel HcTOK1 is unambiguously localized at the plasma membrane, as previously suggested by results obtained by yeast complementation and two-electrode voltage-clamp in Guerrero-Galán et al.8 The same distribution pattern has been found for ScTOK1.11 HcTOK1 is expressed at very low levels in H. cylindrosporum, compared to the other two channels of the family, HcTOK2.1 and HcTOK2.2.8 The two overexpressing lines chosen for this study, HcTOK1-OE1 and -OE2, show different levels of induction (40X and 100X, respectively), and the one with the strongest activity induced an accumulation of K+ in the fungus. Even though this result may seem contradictory with the already described role of HcTOK1 as an outward-rectifying channel,8 it actually fits well in the description of the yeast ScTOK1 channel. Indeed, Maresova et al.11 demonstrated that overexpressing ScTOK1 hyperpolarized the plasma membrane. In this situation, even when the K+ concentration gradient is strong, the activity of the channel would be blocked, as its activation relies on depolarization. Moreover, under hyperpolarizing membrane potentials, HcTOK1, as ScTOK1,12,13 shows a slight inward current, that would be responsible for higher K+ contents in HcTOK1-OE2 mycelium compared to control strains.8 This behavior was conserved along the K+-starvation kinetic assay, in which the overexpression of HcTOK1 did not imply a faster loss of K+. The fact that Na+ contents did not vary in the fungus support the idea that HcTOK1 is selective for K+.
In vitro inoculation of P. pinaster with HcTOK1-OE lines resulted in a higher K+ content in shoots, compared to inoculated and non-inoculated controls. Two hypotheses could explain this result. First, K+ accumulation in the HcTOK1-OE2 line (and presumably, also in HcTOK1-OE1) would imply that plants are directly interacting with a greater “source” of K+ than plants mycorrhized with control lines when cultured under K+ starvation. In this case, the root would act itself as a “sink”, maintaining a stronger medium-fungus-plant flux. Second, HcTOK1 is overexpressed in all fungal hyphae types which could lead to a dual role: K+ accumulates in extra-radical hyphae and its transfer towards plant roots through the Hartig net increases.
In conclusion, we revealed that HcTOK1 is localized at the plasma membrane, shows similar physiological properties than ScTOK1, and is probably a key regulator of K+ homeostasis in the fungus at the cellular level.14 Furthermore, its role as uptake channel, albeit not strong, might be complementary to that of putative high affinity K+ transporters (unpublished results). For now, the study of TOK channels has led to a detailed description of the TOK1 subfamily, well conserved in all fungi. It will be interesting to focus future researches in analyzing members of the TOK2 subfamily, described in our recent publication, which presents new functional properties and could have unexpected roles not only in the physiology of the fungus, but also in ectomycorrhizal symbiosis.8
Acknowledgments
We thank the French Ministry of Higher Education and Research for financing the fellowships of C.G.G., K.G. and G.H. K.G. was also supported by the U.S. Department of Agriculture (USDA NIFA 2017-67014-26530).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
References
- 1.Zörb C, Senbayramb M, Peiter E.. Potassium in agriculture–status and perspectives. J Plant Physiol. 2014;171:656–669. doi: 10.1016/j.jplph.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Garcia K, Zimmermann SD.. The role of mycorrhizal associations in plant potassium nutrition. Front Plant Sci. 2014;5:337. doi: 10.3389/fpls.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia K, Doidy J, Zimmermann SD, Wipf D, Courty PE. Take a trip through the plant and fungal transportome of mycorrhiza. Trends Plant Sci. 2016;21:937–950. doi: 10.1016/j.tplants.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Garcia K, Chasman D, Roy S, Ane J-M. Physiological responses and gene coexpression network of mycorrhizal roots under K+ deprivation. Plant Physiol. 2017;173:1811–1823. doi: 10.1104/pp.16.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SE, Read DJ. Mycorrhizal Symbiosis. 3rd Edn. New York, NY: Academic Press; 2008. ISBN: 9780123705266. [Google Scholar]
- 6.Corratgé C, Zimmermann S, Lambilliotte R, Plassard C, Marmeisse R, Thibaud JB, Lacombe B, Sentenac H. Molecular and functional characterization of a Na+-K+ transporter from the Trk family in the ectomycorrhizal fungus Hebeloma cylindrosporum. J Biol Chem. 2007;282:26 057–26 066. doi: 10.1074/jbc.M611613200. [DOI] [PubMed] [Google Scholar]
- 7.Garcia K, Delteil A, Conéjéro G, Becquer A, Plassard C, Sentenac H, Zimmermann SD. Potassium nutrition of ectomycorrhizal Pinus pinaster: overexpression of the Hebeloma cylindrosporum HcTrk1 transporter affects the translocation of both K+ and phosphorus in the host plant. New Phytol. 2014;201:951–960. doi: 10.1111/nph.12603. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero-Galán C, Delteil A, Garcia K, Houdinet G, Conéjéro G, Gaillard I, Sentenac H, Zimmermann SD. Plant potassium nutrition in ectomycorrhizal symbiosis: properties and roles of the three fungal TOK potassium channels in Hebeloma cylindrosporum. Environ Microbiol. 2018. doi: 10.1111/1462-2920.14122. [DOI] [PubMed] [Google Scholar]
- 9.Minet M, Dufour M-E, Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992;2:417–422. doi: 10.1046/j.1365-313X.1992.t01-38-00999.x. [DOI] [PubMed] [Google Scholar]
- 10.Louche J, Ali MA, Cloutier-Hurteau B, Sauvage FX, Quiquampoix H, Plassard C. Efficiency of acid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol Ecol. 2010;73:323–335. doi: 10.1111/j.1574-6941.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 11.Maresova L, Urbankova E, Gaskova D, Sychrova H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006;6:1039–1046. doi: 10.1111/j.1567-1364.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 12.Fairman C, Zhou XL, Kung C. Potassium uptake through the TOK1 K+ channel in the budding yeast. J Membr Biol. 1999;168:149–157. doi: 10.1007/s002329900505. [DOI] [PubMed] [Google Scholar]
- 13.Bertl A, Ramos J, Ludwig J, Lichtenberg-Fraté H, Reid J, Bihler H, Calero F, Martínez P, Ljungdahl PO. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2 and tok1 null mutations. Mol Microbiol. 2003;47:767–780. doi: 10.1046/j.1365-2958.2003.03335.x. [DOI] [PubMed] [Google Scholar]
- 14.Volkov V. Quantitative description of ion transport via plasma membrane of yeast and small cells. Front Plant Sci. 2015;6:425. doi: 10.3389/fpls.2015.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]


