ABSTRACT
Plants rely on lateral roots to explore their soil environment and to maximize their uptake of essential minerals and water. Here we present evidence that the receptor kinases XIP1/CEPR1 and CEPR2 regulate both the initiation of lateral root primordia and emergence of lateral roots locally in the root, while also controlling lateral root extension in response to shoot-derived sucrose in Arabidopsis plants. In addition, mutation of both of these receptors prevents seedlings from responding to sucrose in the media, resulting in longer lateral roots. These results, combined with previous data, establish XIP1/CEPR1 and CEPR2-dependent roles in short- and long-distance pathways regulating different stages of lateral root growth.
KEYWORDS: receptor like kinase, lateral root development, Arabidopsis, sucrose
Introduction
Plants use their root system for access to minerals and water, and for tethering into the soil. In Arabidopsis thaliana, the root system is composed of a central primary root, which is initiated during embryonic development, and lateral roots (LRs), which form post-embryonically and whose number and growth rate are greatly influenced by environmental cues.1,2 For a plant to access available nutrients and water in the soil environment, it first must sense their presence, decide if it has the resources to invest into expanding its root system, and then respond accordingly. Furthermore, the ability of a plant to optimize available nutrients depends on the branching pattern of its roots,3,4 and, as the majority of the root system in eudicots is composed of LRs,4 understanding the mechanisms behind the environmental control of initiation and extension of LRs is paramount.
Many of the factors important in triggering LR initiation, emergence and extension have been elucidated, and have been reviewed in depth elsewhere.5,6 Briefly, auxin accumulation in the protoxylem of the basal meristem of the root primes small groups of cells7,8. These cells are triggered to re-enter mitosis to produce lateral root primordia,9 which can mature to LRs after emergence from the primary root. Conditions that alter the frequency and extent to which LRs initiate and emerge include nutrient availability10 and gravitropic signals,11 among others.6 Lateral root extension, on the other hand, has been less thoroughly studied, and factors that control the degree of lateral root outgrowth are less well known. The molecular pathways behind these pre-programmed behaviors rely on a complex interplay of numerous short- and long-range signaling molecules in both the root and shoot.6
Sucrose, the major mobile form of carbon in plants, is made in photosynthesizing tissues and is transported via the phloem to sink tissues where it is used for growth or storage.12,13 There is evidence that sucrose may also serve as a distinct signal separate from its role as a source of energy.13-15 Sucrose signaling has been associated with regulation of starch synthesis, photosynthesis, root growth, and nitrogen metabolism among other processes.13,16-18 The precise cellular mechanisms through which sucrose produces these effects have not been discerned, as separating the increase in available energy that sucrose supplementation provides from its signaling component has been difficult. It has been noted that in most cases, the addition of sucrose to media increases growth; however, plants are able to hold variables such as lateral root density and lateral root primordium position constant across many genotypes and conditions, indicating a master control mechanism that balances available resources to appropriate growth.19
Recently, a model has been proposed to coordinate root-derived environmental signals and shoot-mediated control of growth.20 Briefly, upon sensing low nitrogen availability within one part of the root system, C-TERMINALLY ENCODED PEPTIDEs (CEPs) are produced and transported to the shoot, where they are perceived by the receptor-like kinases XIP1/CEPR1 and CEPR2, which leads to the up-regulation of nitrogen transporters in the portion of the root system exposed to ample nitrogen.20 CEPs have also been associated with control of lateral root number,21 nodulation,22 and total root size.23 Here we extend the current model20 by using grafting experiments to show that the density of LR primordia and the emergence of LRs is controlled locally in the root by these two receptors. Furthermore, based on the hypersensitivity phenotype of the double mutants to sucrose, we propose that available photoassimilates influence lateral root extension. We conclude that both short-range root-specific and long-range signals are perceived by XIP1/CEPR1 and CEPR2 to regulate growth of LRs.
Results
We characterized the root phenotypes of XIP1/CEPR1 and CEPR2 single and double mutants. We expected the LR to primary root (PR) length ratio to be higher in the double mutants than in controls (Figure S2 in20), however, when the seedlings were grown on ½ MS media without sucrose, we observed that xip1/cepr1:cepr2 double mutants have lower LR/PR length ratios (Figure 1A). We typically do not use sucrose in plate assays as it is not found in normal soils, and because sucrose artificially increases plant growth. Based on our results, we hypothesized that the double mutant might be sensitive to sucrose, and when we supplemented the media with 1% sucrose, the LR/PR length ratio in the double mutant was significantly above the wild-type ratio (Figure 1A). When the sucrose sensitivity of the various genotypes was determined, the double mutant produced a ten-fold and twenty-fold increase in average LR length and LR to PR length, respectively, whereas the other genotypes have a two-fold increase (Figure 1B). This sensitivity could be due to: (i) a shorter primary root length with supplemental sucrose; (ii) the production of fewer LRs when grown without sucrose; (iii) a large increase of LR length in response to sucrose in the media; (iv) the production of many short roots in response to sucrose; or (v) a combination of these four scenarios. We found that the primary root length increased in all genotypes, but all genotypes were equally sensitive to sucrose in the media (Figure 1B and Figure S1A). The density of emerged LRs, as well as the density of LRP, was significantly increased in the double mutant plants when grown on media with and without supplemental sucrose (Figure 2A and B). While sucrose does produce a larger percentage of emerged LRs (from ~ 20%, of total to about 30%, Figure S1B), this trend was present in all genotypes. Furthermore, this increase is not due to an increase in any one stage of LRP, but an increase of LR initiation events, LRP progression, and emergence (Figure S1B). The reason for the sensitivity to sucrose is apparent when the LR length to PR length ratio, or the average LR lengths are examined: the LR length in double mutant plants is shorter than that of controls when there is no supplemented sucrose in the media, but is above wild-type levels with 1% sucrose in the media (Figure 2C and 2D). Consequently, while there is an increase in lateral root density in the double mutant, it occurs under both conditions, and the addition of sucrose increases the amount of LR extension, producing a sucrose hypersensitive phenotype.
Figure 1.
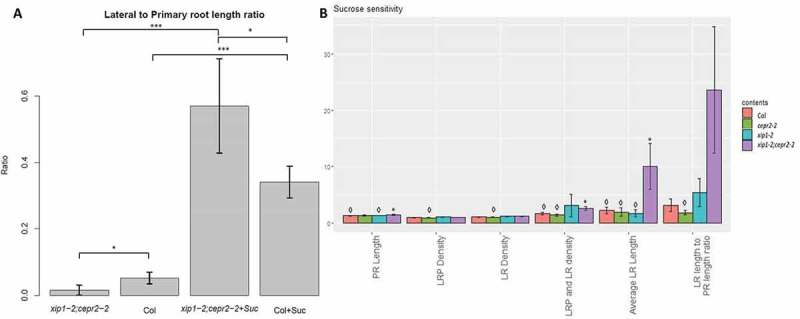
(A) Double mutant plants have smaller ratios of lateral to primary root length when grown without sucrose, but have larger ratios when grown in the presence of sucrose. (Pairwise Wilcox Test with Bonferroni correction, * – p < 0.05; ** – p < 0.01; *** – p < 0.005, error bars represent standard error, n = 25 for mutants, n = 120 for Col). (B) xip1-2;cepr2-2 seedlings show sensitivity to sucrose with respect to LR extension. The ratio of values between seedlings grown on 0.5X MS with 1% sucrose versus those grown on 0.5X MS media only. (N = 4, 3, 2, 4 experiments respectively for each genotype, with each experiment representing 5–25 plants; student T-test, * – p < 0.05 compared to Col, ◊ – p < 0.05 compared to xip1-2;cepr2-2).
Figure 2.
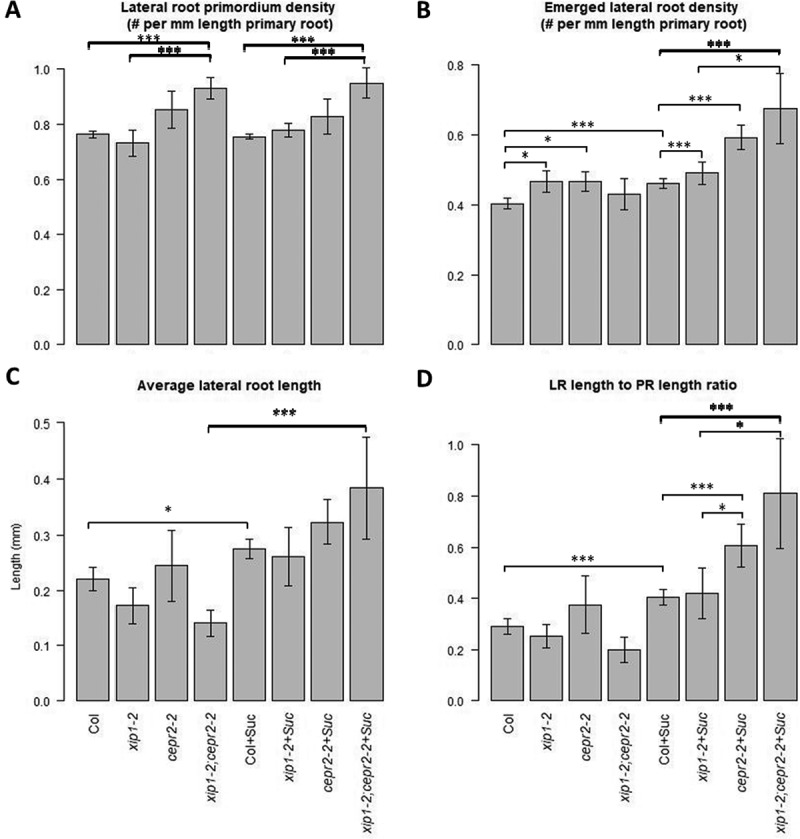
(A) The density of lateral root primordia was increased in double mutant lines grown with and without sucrose. (B) Emerged lateral root density was higher in the single mutants when plants were grown without sucrose. When sucrose was present in the media, all genotypes have significantly higher densities. (C) When plants are grown without added sucrose, average lateral root length is decreased in xip1-2/cepr1-1 and double mutant plants but not significantly. Adding sucrose to the media results in longer average LRs, with the double mutant having longer, but not significantly longer, LRs compared to Col. (D) The ratio between lateral root length and primary root length is lower in the double mutant grown without sucrose, but not significantly so. The double mutant and cepr2-2 single mutants produce roots with a significantly higher LR length to PR length ratios (Square root transformed values followed by Kruskal-Wallis Rank Sum Test followed by pairwise comparisons using Wilcoxon rank sum test and Benjamini & Hochberg correction; * – p < 0.05; ** – P < 0.01; *** – P < 0.005, error bars represent standard error; n ≥ 15).
To determine if this sensitivity was specific to sucrose, or if other saccharides have the same effect, we tested the effects of glucose, fructose, a combination of these two, and the non-metabolizable saccharide mannitol as an osmotic control (Figure S2). All genotypes produced shorter primary roots with long lateral roots compared to the no-saccharide and metabolizable saccharide conditions (Figure S2). Concurrently, the plants produced longer lateral roots in all genotypes when grown on mannitol when compared to no additive, however the saccharides produced much longer LR roots in the double mutants (Figure S2). We observed that the double mutant is sensitive to all four saccharide treatments, indicating that the double mutant cannot properly assess the amount of energy available to it in the form of saccharides (Figure S3).
Since XIP1/CEPR1 and CEPR2 are proposed to function in long-distance signaling from roots to shoots (20), we tested if the LR phenotypes were dependent on signals from the shoot or were root-autonomous. We performed grafting experiments between control and double mutant plants, and found that plants with xip1-2/cepr1-1;cepr2-2 or xip1-2/cepr1-1;cepr2-3 rootstock have an increased density of LRP and emerged LRs (Figure 3). This result indicates that XIP1/CEPR1 and CEPR2-modulated LR initiation and emergence is controlled by the root genotype, therefore the two receptors function in short-range control of both LR initiation and emergence.
Figure 3.
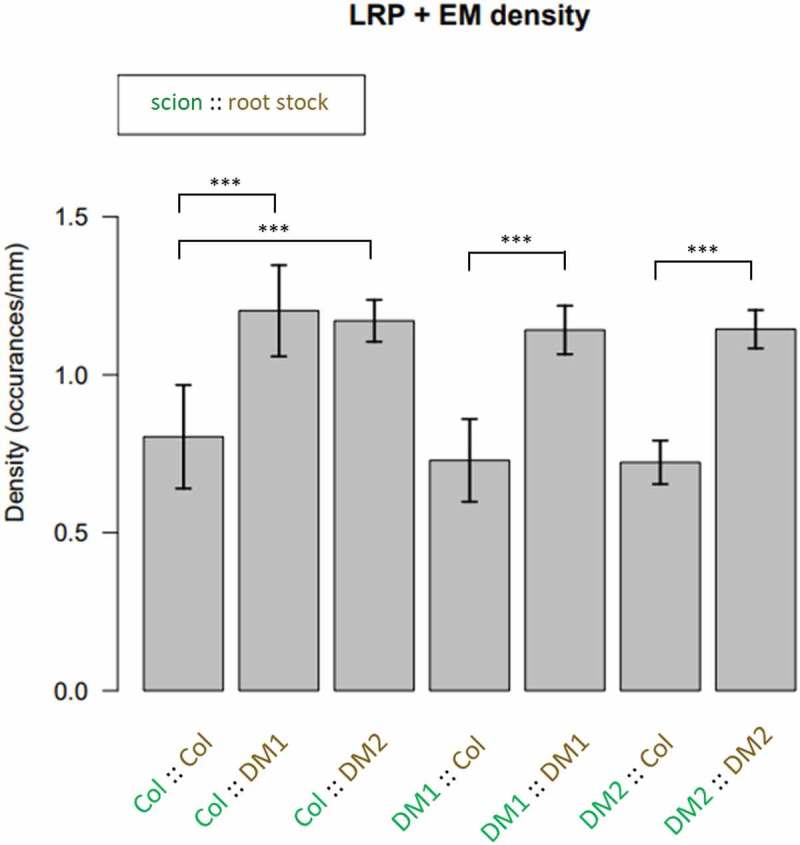
Lateral root primordia and emerged root density were significantly increased in the rootstock from the double mutants (DM1 → xip1-2:cepr2-3 and DM2 → xip1-2:cepr2-2) independently from which shootstock they were connected to. (ANOVA, followed by pairwise T test with Bonferroni correction; * – p < 0.05; ** – P < 0.01; *** – P < 0.005, error bars represent standard error, n = 9–22).
Discussion
To maximize the overall ability of the plant to grow and reproduce as efficiently as possible, its root system has to integrate a plethora of environmental cues, as well as reference its internal energy stores, before proceeding to grow. Root systems have a complex response to availability of nitrogen in their immediate environment. For example, plants suppress LRs in regions with very low nitrogen concentrations, and increase LR initiation in areas with more accessible nitrogen.24 Previously, XIP1/CEPR1 and CEPR2 were identified as the primary receptors for several CEPs, a class of short peptide hormones.20 These researchers proposed a model where, upon sensing a lack of nitrogen in one portion of the root system, CEPs are produced, and travel long-distance through the xylem into the shoot, where they are perceived by the LRR RLKs CEPR1 and CEPR2, activating a shoot-to-root signaling pathway that drives the upregulation of nitrogen transporters in portions of the root system where nitrogen is available.20 Since XIP1/CEPR2 are expressed in the phloem pole pericycle cells,21,25 while lateral root primordia initiate at the xylem pole pericycle (see Figure 4, arrows), a short-range signal spanning one or more cells would be required to functionally link these expression domains.
Figure 4.
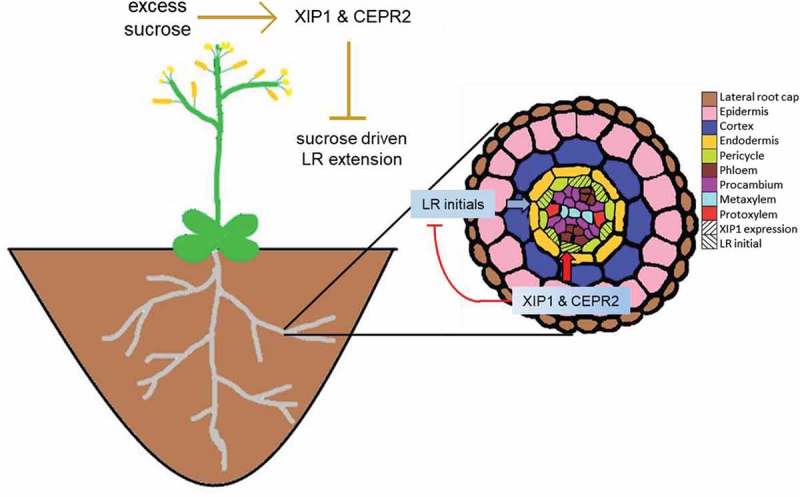
Model for XIP1/CEPR1 and CEPR2 action. In the shoot, XIP1/CEPR1 and CEPR2 act to control the extension of lateral roots in response to increased sucrose. In the root, the two receptors serve to inhibit lateral root initiation.
Our model is consistent with results that demonstrate CEP5 plays a specific role in the initiation of LRs: CEP5 is expressed in the phloem pole-associated pericycle, and is present at the base of growing LRP, and when the peptide is overexpressed or supplemented to the media, the LR density is decreased.21 In addition, mutants in XIP1/CEPR1 appear to decrease the LR density.21 We note that their growth conditions differ from ours, and when we also supplement our media with 0.1% myo-inositol, we do observe that xip1-1/cepr1-2 produces a lower density of LRs compared to controls (Figure S4).
We propose that XIP1/CEPR1 and CEPR2 function to suppress LR initiation and emergence locally in the root (Figure 4). Our model of local suppression fits well with previous observations20 that there was inhibition of nitrogen acquisition mechanisms in regions of the root exposed to low nitrogen conditions. In addition, our data suggests the presence of a long distance signal that is controlled by these two LRK-RLKs that connects to energy availability, in the form of saccharides, to growth, as is evident in the sucrose sensitivity of lateral root length. The phenotypes of double mutants of XIP1/CEPR1 and CEPR2 show that these receptors may function to control LR extension dependent on sucrose availability, thereby keeping the plant from overinvesting into longer LRs. Previous analysis demonstrated that excess sucrose in the media is perceived in the shoot, which could drive growth of both LRs and PRs.26 Adding our data to this finding suggests XIP1/CEPR1 and CEPR2 may also be involved in the control of LR outgrowth in the shoot in response to the carbon status of the plant. The shoot is the source of photosynthetically derived carbon, and given evidence that nitrogen acquisition is already controlled by these two receptors from the shoot,20 the aerial tissues should be the location at which carbon status could be assessed.
Several questions are raised by our results. First, does the induction of LRP and LR emergence indicate an increased rate of initial priming of founder cells, or is it simply due to increased activation of those cells in the differentiation zone of the root? Auxin plays a key role in this pathway,7 and as many genes that affect auxin transport and synthesis have been defined, identifying connections between these receptors and auxin synthesis or transport will be important. Secondly, how do these two receptors influence a plant’s developmental program in response to excess energy in the form of sucrose? These two mechanisms could be linked as there is evidence that sucrose in the media increases auxin levels.27 The location of this control is also in question, as shoot derived sucrose drives lateral root extension by action in the shoot,26 and given that XIP1/CEPR1 and CEPR2 drive nitrogen responses from the shoot20 they may have a similar role in responses to sucrose.26
Our results highlight the importance of the interaction between root-controlled lateral root initiation and emergence, and the regulation of sugar production in shoots, and their root and shoot control through XIP1/CEPR1 and CEPR2.
Material and methods
Plants and growth conditions
The xip1-1/cepr1-2, xip1-2/cepr1-1 mutants were published previously,20,25 and the CEPR2 T-DNA insertion mutants were obtained from TAIR ABRC (cepr2-2 -SALK_081193; cepr2-3 – SALK_014533, genotyping primers are in TableS1). While the xip1-2 allele is in the Nossen ecotype, we have been introgressing the double mutants into Columbia, the parent ecotype of the SALK lines, and therefore used Columbia as a control in our experiments. We note that for all measurements, Nossen was indistinguishable from Columbia (Figure S5).
Plants were grown on plates with agar media containing ½ MS media (per liter 2.15g MS salts, Gibco Laboratories- 11117–074), 0.5 g MES (Sigma- M2933), pH 5.8, and 3.75g agar (Sigma- A1296), which was supplemented with 1% sucrose (Mallinckrodt- 8360) fructose (Fisher- L96–212), D-glucose (Spectrum- D1017) or myoinositol (Sigma- I3011). Seeds were surface sterilized using 0.1% Triton (Aldrich- 23,472–9) in 70% ethanol, rinsed three times with 95% ethanol, then plated, three genotypes per plate, 15 plants per plate, and stratified for 2 days at 4°C, then moved to a growth room at 22°C, with a 16h/8h day/night light cycle.
Analysis of cepr2 alleles
Seedlings of Col, cepr2-2 and cepr2-3 were grown on plates, and total RNA was extracted using Trizol (Fisher-15596018). cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (ThermoFisher- K1621), using oligoT primers (included in the kit). Amplification PCR and sequencing was performed using CEPR2_E1_F and pROK2_salk for cepr2-2, and CEPR2_SEQ_F5 and pROK2_salk for cepr2-3 (Table S1). Based on our RT-PCR and sequencing results, we believe the cepr2-2 and cepr2-3 alleles are nulls. The cepr2-2 allele produces mRNAs that include the T-DNA upstream of the original translation initiation site, which results in several new upstream ORFs. The mRNA produced from the cepr2-3 allele encodes a frameshift and subsequent early stop codon around the T-DNA insertion site, and therefore would encode a truncated protein. Since cepr2-3 is a null, and cepr2-2 acts the same as cepr2-3 in our assays, we conclude cepr2-2 is also a null.
Grafting
Five days after moving plants from stratification, grafting was performed based on a modified protocol28. Briefly, a horizontal cut was made in the seedling hypocotyl using a razor blade, then the corresponding shoot/root stocks were joined. Two to four days later, joined plants were examined and any adventitious roots that developed were trimmed. To make sure that the graft had resulted in a solid joining of shoot to root, only plants that could be wholly lifted by the shoot, determined when microscope slides were made, were analyzed. This experiment was performed twice with between 5 and 15 plants for every graft genotype.
Lateral root primordia staging
Roots were scored for LRP and emerged lateral roots at 16 days after stratification. Plates were scanned, and primary and lateral root lengths were measured using ImageJ.29 Seedlings were fixed and cleared as described,9 and root staging was scored.9
Statistical analysis
Statistical analyses were performed in R.30 ANOVA was the preferred method for significance calling, however when the assumption of data normality and homogeneity of variance were not met, as determined by Shapiro-Wilk Normality Test and Levene’s test for homogeneity of variance across groups, the Kruskal-Wallis Rank Sum Test followed by Wilcoxon Rank Sum tests were performed as described in the figure legends. Each experiment was repeated two or more times.
Funding Statement
This research was supported by NSF IOS 1257316 (awarded to FET), and ID was funded by NSF IGERT DGE-0114420.
Acknowledgments
Thanks to the members of the Tax lab for comments on the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the author.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Bellini C, Pacurar DI, Perrone I.. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014;65 639–666. doi: 10.1146/annurev-arplant-050213-035645. [DOI] [PubMed] [Google Scholar]
- 2.Du Y, Scheres B.. Lateral root formation and the multiple roles of auxin. J Exp Bot. 2017; 69(2):155–167. [DOI] [PubMed] [Google Scholar]
- 3.López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003. June 1;6(3):280–287. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson JA, Rasmussen A, Traini R, Voß U, Sturrock C, Mooney SJ, Wells DM, Bennett MJ. Branching out in roots: uncovering form, function, and regulation. Plant Physiol. 2014;166(2):538–550. doi: 10.1104/pp.114.245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor-Teeples M, Lanctot A, Nemhauser JL. As above, so below: auxin’s role in lateral organ development. Dev Biol. 2016. November 1;419(1):156–164. doi: 10.1016/j.ydbio.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung J, McCouch S. Getting to the roots of it: genetic and hormonal control of root architecture. Front Plant Sci. 2013. June 18;4(186). (in English). doi: 10.3389/fpls.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ten Tusscher KH, Laskowski M. Periodic lateral root priming, what makes it tick. Plant Cell. 2017;29(3):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xuan W, Audenaert D, Parizot B, Möller BK, Njo MF, De Rybel B, De Rop G, Van Isterdael G, Mähönen AP, Vanneste S, et al. Root cap-derived auxin pre-patterns the longitudinal axis of the Arabidopsis root. Curr Biol. 2015. May 18;25(10):1381–1388. doi: 10.1016/j.cub.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124(1):33–44. [DOI] [PubMed] [Google Scholar]
- 10.Walch-Liu PIA, Ivanov II, Filleur S, Gan Y, Remans T, Forde BG. Nitrogen regulation of root branching. Ann Bot. 2006;97(5):875–881. doi: 10.1093/aob/mcj601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian H, De Smet I, Ding Z. Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci. 2014. July 1;19(7):426–431. doi: 10.1016/j.tplants.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Truernit E. Plant physiology: the importance of sucrose transporters. Curr Biol. 2001. March 6;11(5):R169–R171. [DOI] [PubMed] [Google Scholar]
- 13.Wind J, Smeekens S, Hanson J. Sucrose: metabolite and signaling molecule. Phytochemistry. 2010. October 1;71(14–15):1610–1614. doi: 10.1016/j.phytochem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Lastdrager J, Hanson J, Smeekens S. Sugar signals and the control of plant growth and development. J Exp Bot. 2014. March;65(3):799–807. (in eng). doi: 10.1093/jxb/ert474. [DOI] [PubMed] [Google Scholar]
- 15.Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14 Suppl(suppl 1):SS185–S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horacio P, Martinez-Noel G. Sucrose signaling in plants: a world yet to be explored. Plant Signal Behav. 2013. March;8(3):e23316 (in eng). doi: 10.4161/psb.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulpice R, Nikoloski Z, Tschoep H, Antonio C, Kleessen S, Larhlimi A, Selbig J, Ishihara H, Gibon Y, Fernie AR, et al Impact of the carbon and nitrogen supply on relationships and connectivity between metabolism and biomass in a broad panel of Arabidopsis accessions. Plant Physiol. 2013;162(1):347–363. doi: 10.1104/pp.112.210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kircher S, Schopfer P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci. 2012;109(28):11217–11221. doi: 10.1073/pnas.1203746109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mudgil Y, Karve A, Teixeira PJPL, Jiang K, Tunc-Ozdemir M, Jones AM. Photosynthate regulation of the root system architecture mediated by the heterotrimeric G protein complex in Arabidopsis. Front Plant Sci. 2016;7. doi: 10.3389/fpls.2016.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science. 2014;346(6207):343–346. doi: 10.1126/science.1257800. [DOI] [PubMed] [Google Scholar]
- 21.Roberts I, Smith S, Stes E, De Rybel B, Staes A, Van De Cotte B, Njo MF, Dedeyne L, Demol H, Lavenus J, et al CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. J Exp Bot. 2016;67(16):4889–4899. doi: 10.1093/jxb/erw231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J Exp Bot. 2013;64(17):5395–5409. doi: 10.1093/jxb/ert369. [DOI] [PubMed] [Google Scholar]
- 23.Delay C, Imin N, Djordjevic MA. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. J Exp Bot. 2013;64(17):5383–5394. doi: 10.1093/jxb/ert332. [DOI] [PubMed] [Google Scholar]
- 24.Vidal EA, Gutiérrez RA. A systems view of nitrogen nutrient and metabolite responses in Arabidopsis. Curr Opin Plant Biol. 2008;11(5):521–529. doi: 10.1016/j.pbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Bryan AC, Obaidi A, Wierzba M, Tax FE. XYLEM INTERMIXED WITH PHLOEM1, a leucine-rich repeat receptor-like kinase required for stem growth and vascular development in Arabidopsis thaliana. Planta. 2012. January;235(1):111–122. (in eng). doi: 10.1007/s00425-011-1489-6. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor DR, Deak KI, Ingram PA, Malamy JE. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell. 2008;20(10):2643–2660. doi: 10.1105/tpc.107.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilley JLS, Gee CW, Sairanen I, Ljung K, Nemhauser JL. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012. October;160(4):2261–2270. doi: 10.1104/pp.112.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnbull CGN, Booker JP, Leyser H. Micrografting techniques for testing long‐distance signalling in Arabidopsis. Plant J. 2002;32(2):255–262. [DOI] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012. July;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


