Supplemental Digital Content is available in the text
Keywords: 17β-Estradiol, Blood viscosity, FSH, Leucocyte count, Menopausal status, Neutrophil-to-lymphocyte ratio
Abstract
Objective:
The multidisciplinary Estrogenic Regulation of Muscle Apoptosis (ERMA) study was designed to reveal how hormonal differences over the menopausal stages affect the physiological and psychological functioning of middle-aged women. This paper describes the protocol and nonrespondent analysis of ERMA and novel findings on menopausal differences in blood count variables and their association with female sex hormones.
Methods:
Women aged 47 to 55 years were assigned to pre, early peri, late peri, and postmenopausal groups based on follicle-stimulating hormone (FSH) and bleeding diary. Multivariate linear regression models were constructed to estimate the association of 17β-estradiol (E2) and FSH with the blood count variables.
Results:
In all, 3,064 women returned the prequestionnaire (ERMA phase one), 1,393 donated blood samples and were assigned to the relevant menopausal group (phase two), and 914 completed phase three, which included physiological and psychological measurements. Nonrespondents were more likely than respondents to be obese, whereas the menopausal groups showed no mean differences in body mass index. Blood count variables, while being within clinical reference values, showed significant differences between groups. E2 and FSH were associated with the white blood cell (WBC) count and neutrophil-to-lymphocyte ratio.
Conclusions:
The ERMA study was successful in recruiting and characterizing the menopausal status of a cohort sample of middle-aged women. The significant group differences found in the blood count variables and their associations with E2 and FSH verifies menopause-associated changes in WBC composition potentially being an early sign of low-grade inflammation that may develop later in life.
The difference in the hormonal aging process in men and women may contribute to the sexual dimorphism observed for multiple traits. Compared with men, women's middle age is characterized by changes in the hormonal milieu that take place over a rather short period, namely during the transition from reproductive age to postmenopause, during which periodical estrogen production by the ovaries ceases and is replaced by a very low constant level of circulating 17β-estradiol (E2) and an elevated level of follicle-stimulating hormone (FSH). Consequently, postmenopausal women develop estrogen deficiency and become more prone to impairment in physiological1,2 and cognitive3 functions than premenopausal women. In addition, views on women's psychological functioning in middle age are conflicting: it is seen either as a time of crisis or as a time of high-level psychological functioning,4,5 thus warranting further studies.
Positive associations with estrogen have been reported for functions of the nervous,6 immune,7,8 cardiovascular,9 and musculoskeletal10,11 systems. For example, menopausal age has been found to hallmark the faster decrements observed in age-related female skeletal muscle function compared with the situation in same-age men.12 Further support for the positive role of estrogen in muscle health, for example, muscle mass, strength, power and twitch torque, and decreased fat infiltration, has been found in several studies investigating postmenopausal estrogen-based hormone therapy (EHT) users in comparison with nonusers,13-19 and in the rare longitudinal studies investigating menopause transition.2,20
Changes in female hormone homeostasis are initiated years before the final menstrual period, with high interindividual variation in the length and duration of the menopause transition, making menopause extremely difficult to predict. Thus, consensus is lacking on the causality of the effects of natural E2 withdrawal caused by depletion of ovarian function on muscular decrements and other health concerns. However, studies of postmenopausal women with or without hormone therapy (HT) and of rodent models with ovariectomy (OVX) have shown that E2 participates in the regulation of different molecular signaling pathways that influence muscle composition and performance characteristics.13,21-23 In addition to the effects of E2 on muscular signaling pathways, a positive association between white blood cell (WBC) count and sarcopenia was recently observed in postmenopausal women.24 Furthermore, the age-dependent sexual dimorphism and menopausal age-associated change in WBC count and composition was proposed to underlie the sex difference in inflammation, immunity, and related diseases.25 These results warrant further studies to ascertain whether E2 or other hormones associated with menopause are involved instead of aging alone.
The aim of this paper is to describe the multidisciplinary Estrogenic Regulation of Muscle Apoptosis (ERMA) study and its implementation, present the results of the nonrespondent analysis, describe the menopausal characteristics and anthropometrics of the collected age-cohort, and report on menopausal differences in blood count variables and the associations between blood count variables and systemic hormones in a population sample of 47 to 55-year-old women. The primary aim of the ERMA study is to investigate the molecular mechanisms affected by the menopausal withdrawal of E2, especially in skeletal muscle, by means of repeated muscle biopsy sampling of women in a core-ERMA group. To obtain access to such a biologically valuable longitudinal sample, which will go through natural menopause during the follow-up period and which lacks potential confounders that could mask the determination of menopause or affect its timing or course, a large age cohort of 47 to 55-year-old women (base-ERMA group) has been recruited. The purpose of the base-ERMA group is to satisfy the multidisciplinary aims of the study in order to find out how specific health-related functional abilities (body and muscle composition; physical performance; physical activity; cognitive capacity; and psychological functioning), are associated with the different menopausal stages in mid-life women and how these abilities change during the transition from the peri to postmenopausal state. The ERMA participants have been carefully characterized to represent different menopausal stages (pre, early and late peri, and postmenopausal), of which the women in the early and late perimenopausal stages are followed up over the menopause transition until they become postmenopausal. When the ongoing follow-up period is complete, the core-ERMA group will be used to investigate skeletal muscle apoptosis as one of the potential molecular level mechanisms that has been suggested to affect the menopause-related decline in physical performance. Meanwhile, this paper describes in detail the study design and implementation, and reports on the nonrespondent analysis and the menopausal differences in blood count measures and their associations with systemic E2 and FSH in middle-aged women in the base-ERMA population.
METHODS
Study design
The ERMA study is a population-based cohort study with both cross-sectional and longitudinal study designs. Our ultimate aim is to investigate the molecular and other mechanisms affected by the menopausal withdrawal of E2. To obtain access to such a valuable sample, which will go through natural menopause during the follow-up period, a large age cohort needed to be recruited. The ERMA cohort comprises women aged 47 to 55 years, living in the city of Jyväskylä and neighboring municipalities, in Finland. According to the Finnish Health 2000 population survey (https://www.thl.fi/en/web/thl-biobank/for-researchers/sample-collections/health-2000-and-2011-surveys, National Public Health Institute, Finland), the target age group was expected to fall into the four menopausal stages—premenopause, early perimenopause, late perimenopause, and postmenopause—in approximately equal proportions (28%, 22%, 26%, and 24%, respectively). The ERMA cross-sectional study includes all four menopausal groups, and the ERMA longitudinal follow-up study design includes the two perimenopausal groups to be followed up until the final menstrual period. The sample and contact information were both drawn from the Population Information System administered by the Population Register Center (http://vrk.fi/en). To avoid self-exclusion and to obtain as representative population sample as possible for phase one of the study, the invitation letter did not contain information on the exclusion criteria. Instead, a stepwise exclusion procedure (Table 1) was applied to serve the multidisciplinary aims of the study. For instance, to achieve the biological research aims, the exclusion of more participants from the physical performance tests (for safety reasons) than from the questionnaire-based measurements and basic laboratory tests produced a healthier core group with fewer potential confounding factors. As potential confounders, which may affect systemic E2 levels or psychological or physical function, or modify systemic inflammation stage, all potentially affecting the molecular mechanisms of interest, we considered estrogen-containing medications, bilateral oophorectomy, pregnancy, lactation, polycystic ovary syndrome, severe obesity, and musculoskeletal disorders. This scheme accompanied with hormone measurements and menstrual bleeding diaries enabled us to provide reliable categorization into the four menopausal groups. Based on information obtained through prequestionnaires (See PDF, Supplemental Digital Data 1, which provides English translation of the prequestionnaire mailed together with the research information letter to the study participants), participants having potential confounders that might preclude determination of menopausal status or that might affect skeletal muscle function were excluded. At this first exclusion step, as a potential confounder, we considered conditions or medications affecting ovarian function, obesity, and chronic diseases, or medications affecting muscle function. These confounders were inspected based on the responses participants provided in the prequestionnaire form and were rechecked after invitation to the blood draw. The second exclusion step occurred at phase two, in which participants having conditions or using medications potentially affecting daily mental or physical function, or systemic hormone or inflammatory status were excluded (Table 1). Further, some measures, such as muscle biopsies, were also limited to the core-ERMA group to limit the potential physical discomfort to as small a sample size as possible.
TABLE 1.
Stepwise exclusion procedure and exclusion criteria
| Phase of exclusion | Reasons for exclusion |
| Phase one: After receiving prequestionnaire and informed consent based on prequestionnaire data | Conditions or use of medications affecting ovarian function, obesity and chronic disease, or medication affecting muscle function including: |
| Use of estrogen-containing contraceptives or other medication during the past 3 months | |
| Bilateral oophorectomy | |
| Current pregnancy or lactating | |
| Polycystic ovary syndrome or other condition affecting ovarian function | |
| Body mass index (BMI) >35 kg/m2 (based on self-reported height and weight) | |
| Any musculoskeletal disorders seriously affecting everyday physical activity | |
| Phase two: During/after first laboratory visit based on health screening form | Conditions or use of medications affecting daily mental or physical function or systemic hormone or inflammatory status including: |
| Type I diabetes or type II diabetes with insulin medication | |
| Crohn's disease or colitis ulcerosa | |
| Asthma or other condition with continuous cortisone medication | |
| Serious mental illness | |
| Rheumatic disease with continuous anti-inflammatory or cytotoxic medication or other musculoskeletal disorders seriously affecting everyday physical activity | |
| Being on continuous prescribed anti-inflammatory medication | |
| Cancer diagnosed less than 5 years ago or under current cytotoxic treatment or estrogen suppressor medication | |
| Phase three: During/after second laboratory visit | Meeting any of the phase one or two exclusion criteria between the first and second laboratory visit. In addition, safe participation for each participant was considered at each physiological measurement |
Participants were first approached by a postal inquiry with an invitation letter, a research information letter (see PDF, Supplemental Digital Data 2, which provides English translation of the research information letter mailed to the study participants), a general consent form for participation in the ERMA study and for the use of prequestionnaire survey data for research purposes, and a prequestionnaire (see PDF, Supplemental Digital Data 1) to evaluate the person's willingness and potential eligibility for the study. All women were asked to return the prequestionnaire even if they did not consent to participate in other phases of the ERMA study. The invitation package was mailed a second time to the participants if the response was not received within 6 weeks of the initial postal enquiry. If no response was received after second postal enquiry, a participant was considered as nonrespondent.
Eligible participants who passed phase one were invited to the phase two laboratory visit during which they gave their informed consent to participate in phases two and three of the ERMA study, filled in the health screen questionnaire (see PDF, Supplemental Digital Data 3, which provides English translation of the health screen), and gave fasting blood samples. The health screen questionnaire was designed according to American College of Sports Medicine guidelines26 with some modifications to enable estimation of safe participation for each of the physical performance measurements to be conducted at the phase three laboratory visit. Before the phase two laboratory visit, participants also kept a menstrual diary for at least 12 weeks and were advised to keep the diary for the duration of the ERMA study.
The phase three laboratory visit included an examination and interview by a nurse and/or study physician and a set of laboratory measurements to assess body composition, physical performance, and psychological and cognitive abilities. Participants who had reported arrhythmia or some other unclear medical condition at the phase two laboratory visit underwent a medical examination (including resting electrocardiogram [ECG] and blood count) by a physician to ascertain their safe participation in physical performance measurements. Participants also brought the full baseline questionnaire (see PDF, Supplemental Digital Data 4, which provides English translation of the full baseline questionnaire), which they had filled in at home. The research assistant went through the questionnaire, and participants were given the opportunity to supplement their answers if needed. After the phase three laboratory visit, participants’ physical activity was objectively assessed by 7-day use of an accelerometer and accompanying physical activity diary.
The early and late perimenopausal women in the natural menopausal state (ie, no progestogen-releasing hormonal contraceptive coil or other progestogen-containing contraception or other conditions affecting ovarian function or menstrual bleeding pattern) formed the core-ERMA group, who were asked to participate in additional laboratory visits for assessment of muscle composition by computed tomography scanning and for muscle biopsy sampling.
All participants assigned to the early and late perimenopausal groups were invited to participate in the ongoing follow-up arm of the ERMA study (phase four). They are to be followed until they reach the postmenopausal stage. Thereafter, all the baseline measurements will be repeated. During the follow-up, the women keep a menstrual diary and visit our laboratory at 3 to 6-month intervals until they become postmenopausal. During these visits, questionnaires are filled in, fasting blood samples are taken, body composition is measured, and the objective physical activity assessment is repeated.
Ethics approval and consent to participate
The Ethics Committee of the Central Finland Health Care District approved the ERMA study in 2014 (K-S shp Dnro U/2014). The study participants gave two separate signed consents. Those who responded to the original request to participate in the study signed a consent to allow the use for research purposes and in publications of their health-related information collected by the prequestionnaire (phase one). An additional informed consent, explaining the possible risks and personal benefits associated with the laboratory examinations and giving permission for the use of the data for research purposes and in publications was signed by the participants at the laboratory before any sampling or measurements were conducted (phase two). Sixty percent of the participants also signed the biobank consent allowing their blood samples to be stored in the Central Finland Biobank and used for biobank studies as described in the Finnish Biobank Law (Law 688/2012). Altogether, the study protocol followed good clinical and scientific practice and the declaration of Helsinki.
Biosampling
Fasting blood samples were taken from the antecubital vein in a supine position from all the ERMA participants between 7:00 and 10:00 am during the phase two laboratory visit. Fasting blood samples are also being taken from the participants in the follow-up study arm during each subsequent follow-up visit and during end measurements. Blood count was performed from the whole blood sample immediately after sampling (Sysmex KX-21N, Sysmex Corp., Japan). An additional whole blood sample was taken and stored for DNA and RNA isolation. The samples of participants with a regular or predictable menstrual cycle were collected during the first 5 days of the menstrual cycle, as these contain the lowest E2 concentrations. For serum separation, whole blood was left to clot for 30 minutes at room temperature and centrifuged at 2,200x g before aliquoting and storing the sera at −80°C.
Muscle biopsies were obtained under local anaesthesia from m. vastus lateralis. Visible connective tissue, adipose tissue, or blood was removed, after which two parts of the biopsy, to be used for the biochemical and molecular biological analyses, were snap frozen in liquid nitrogen. A third part of the biopsy sample, to be used in the microscopical analyses, was embedded in Tissue Tek compound on the cork and quickly frozen in isopentane cooled in liquid nitrogen (−160°C). All samples are stored at −80°C pending analysis. Participants at increased risk for hemorrhage were excluded from the muscle biopsy sampling.
Definition of menopausal stage and group assignments
Assignment to the different menopausal groups was determined following the slightly modified Stages of Reproductive Aging Workshop +10 guidelines,27 which take systemic hormone status and self-reported menstrual cycle into account. Menstrual cycle was assessed based on the menstrual diary for 6 to 12 months. FSH and E2 levels were immunoassayed using IMMULITE 2000 XPi (Siemens Healthcare Diagnostics, UK). The analytical sensitivity limit provided by the manufacture is 0.1 IU/L for FSH and 0.055 nmol/L for E2. Intra-assay precision values for the lower end of the detection range are 6.8 ± 0.2 IU/L (mean ± SD) with 2.9% coefficient of variation (CV) for FSH and 0.33 ± 0.03 nmol/L with 9.9% CV for E2. Corresponding precision values for higher end of the detection range are 103 ± 3.2 IU/L, CV 3.1% for FSH and 6.6 ± 0.32 nmol/L, CV 4.9% for E2. Due to high pulsatile variability of the E2 level, only the FSH level was used together with self-reported menstrual cycle for defining menopausal stage. For participants whose menstrual bleeding pattern was not natural, that is, they had undergone hysterectomy or were using progesterone-containing contraceptives, the group assignment was solely based on the systemic FSH level, but with a more stringent cut-off value than used for participants with natural menstrual bleeding (Table 2).
TABLE 2.
Assignment to menopausal groups
| Group | If menstrual cycle information was available (core-ERMA) | If menstrual cycle information was not available (hysterectomised women and progestogen-based contraception users) |
| Premenopausal | FSH < 9.5 IU/L | FSH < 15 IU/L |
| or | ||
| FSH < 17 IU/L and regular menstrual cycle | ||
| Early perimenopausal | FSH 17-25 IU/L | FSH 15-25 IU/L |
| or | ||
| FSH > 9.5 IU/L and irregular menstrual cycle | ||
| Late perimenopausal | FSH 25-30 IU/L | FSH 25-39 IU/L |
| or | ||
| FSH > 30 IU/L and occasional menstrual bleeding during past 3 months | ||
| Postmenopausal | FSH > 30 IU/L and no menstrual bleeding during past 6 months | FSH > 39 IU/L |
| or | ||
| FSH > 39 IU/L and no menstrual bleeding during past 3 months | ||
| or | ||
| very high FSH (>130 IU/L) even if occasional bleeding still occurs |
ERMA, Estrogenic Regulation of Muscle Apoptosis; FSH, follicle-stimulating hormone.
Questionnaire surveys
Three separate questionnaire surveys were conducted. The prequestionnaire in phase one assessed medical and gynecological issues, use of hormonal contraception, pelvic floor dysfunctions, and self-reported weight and height (see PDF, Supplemental Digital Data 1, which provides English translation). The health screen questionnaire in phase two assessed health and safety for participation in the physiological performance measurements (see PDF, Supplemental Digital Data 3, which provides English translation). The full baseline questionnaire in phase three assessed, for example, sociodemographic variables, diseases and medication, psychological functioning, gynecological status, and reproductive health and history, menopausal symptoms, life habits (sleeping, smoking, alcohol consumption, and eating habits), current physical activity, life-long physical activity history, and physical activity over the past 12 months (see PDF, Supplemental Digital Data 4, which provides English translation). This survey is repeated at each follow-up laboratory visit.
Psychological and cognitive functioning
Psychological and cognitive functioning was measured during the phase three laboratory visit by the following questionnaires and tests. “Personality traits” were assessed using an abbreviated version of the Eysenck Personality Inventory (19 items, nine for extraversion and ten for neuroticism).28,29 “Mental well-being” was measured using several questionnaires: depression was assessed using the Centre for Epidemiological Studies Depression Scale (CES-D, with 20 items, four subscales),30 life satisfaction using the Satisfaction with Life Scale (with five items),31 and positive and negative affectivity using the Internationally Reliable Short form of the Positive and Negative Affect Schedule (PANAS; with 10 adjectives: five for positive affect and five for negative affect).32 “Mid-life crisis” was assessed with questions about the occurrence of a mid-life crisis (no; yes, somewhat; yes, clearly, what kind of?) and the occurrence of a crisis at an earlier stage in life (no; yes, when?).33 “Self-perceived aging” was assessed as subjective age (in years, followed by the open-ended question “why?”) and as desired age (in years, followed by the open-ended question “why?”).34 We also used the Attitudes towards Own Aging subscale (with five items) from the Philadelphia Geriatric Center Morale Scale35,36 (see also33). “Executive function” was assessed by the Trail Making Tests B and B-A.37 “Verbal fluency” was measured by the animal naming test,38 “working memory” by the digit span39 and word list tests,40 and “visual memory” by family pictures.40
Physical activity
Physical activity was measured subjectively and objectively. Subjective measures included assessment of “current physical activity” using the 7-point scale ranging from household chores to competitive sports,16,41 and with questions assessing leisure-time and work-associated physical activity pattern.42,43 “Physical activity over the past 12 months” was assessed with the modified Kuopio Ischemic Heart Disease Risk Factor Study Questionnaire.44,45 “Life-long physical activity history” was assessed by asking about regular participation in independent leisure-time physical activities, organized competitive sports, or other supervised physical activities at different stages of life.46
“Objective physical activity” was measured with GT3X+ and wGT3X+ ActiGraph accelerometers (Pensacola, FL). Participants were instructed to wear the monitors on their right hip for 7 consecutive days during their waking hours except while bathing or doing other water-based activities. They were also provided with a diary and instructed to record their wake-up time, working hours, and periods when the monitor was removed for longer than 30 minutes. Raw acceleration data were collected at 60 Hz, filtered and converted into 60-second epoch counts. A customized Excel-based program was used for further data analysis. Daily number of steps and mean time spent at different physical activity intensities were calculated for each participant. Tri-axial vector magnitude cut-points of 450 cpm, two 690 cpm, and six 166 cpm were used to separate different levels of intensity of physical activity, for example, sedentary, light, moderate, and vigorous activity.47,48
Health assessments
Participants’ overall health was first evaluated based on questionnaire surveys and assessed in more detail during a medical examination performed by a nurse practitioner, or, for unclear medical conditions, by a physician, at the beginning of the phase three laboratory visit. The presence of chronic conditions and use of prescribed medication were confirmed according to a prestructured questionnaire and current prescriptions. Self-rated health was assessed as subjective current health (five scales from very good to very poor), subjective health over the past year (five scales from improved considerably to become much worse), and as subjective health compared to the health of peers (5 scales from considerably better to considerably worse or do not know).49
Anthropometrics and body composition
Anthropometrics and body composition were measured between 7:00 and 10:00 am after overnight fasting. Body weight was measured with a beam scale and height by a stadiometer with the participant wearing only undergarments. Body mass index (BMI) was calculated as weight (kg) per height squared (m2). Waist circumference was measured midway between the superior iliac spine and the lower rib margin, and hip circumference at the level of the greater trochanters. Total body composition was assessed by both dual x-ray absorptiometry (DXA, LUNAR; GE Healthcare, Chicago, IL),50 and with a multifrequency bioelectrical impedance analyzer (InBody 720; Biospace, Seoul, Korea). Bone traits including bone mineral content and areal bone mineral density of the whole body and femoral neck were measured using DXA. In addition, in the core-ERMA group, muscle cross-sectional area and muscle composition were measured by quantitative computed tomography from mid-thigh and lower leg.16
Physical performance measurements
Hand grip force was measured on the dominant side by fixing the arm to the armrest of the chair with the elbow flexed at 90°.16 Participants were instructed to squeeze the handle as forcefully as possible. The contraction was maintained for 2 to 3 seconds, and the peak value taken for analysis. Maximal isometric knee extension force was measured in a sitting position from the side of the dominant hand with a custom-made dynamometer chair (Good Strength; Metitur Oy, Palokka, Finland) at a knee angle of 60° from full extension.16 Participants were encouraged to extend the knee to produce maximal force. Maximal knee extension torque (Nm) was calculated: force × (chair lever arm × cos30°).51 Lower body muscle power, that is, the ability of the neuromuscular system to produce the greatest possible force as fast as possible, was assessed as the height that the participant was able to elevate her body's center of gravity during a vertical jump (vertical jumping height) on a contact mat. Flight time (t) was measured, and vertical jumping height (cm) was calculated: (g × t2) ÷ 8 × 100.18,52 In the muscle strength and power tests, three to five maximal efforts were performed, and the effort with the highest value was taken as the result.
Maximal walking speed was assessed over 10 m in a laboratory corridor. Five meters were allowed for acceleration, and the time was measured with photocells. The faster of two trials was taken as the result.16 Dual task cost during walking was assessed with a 10-m maximal walking speed test and a concurrent word-naming cognitive task. The 6-minute walking test was used to assess submaximal exercise tolerance and aerobic capacity. The test was performed on a 20-m indoor track, and participants were instructed to complete as many laps as possible within 6 minutes.53 The distance walked, heart rate, and perceived exersion (Borg scale54) were measured.
In addition, maximal voluntary isometric plantarflexion strength (MVC), Achilles tendon and gastrocnemius muscle architecture, and cortical silent period were measured in the core-ERMA group only. MVC was measured using a custom-made dynamometer (University of Jyväskylä). Plantarflexor muscle strength was assessed both using voluntary effort and by using supramaximal (150% of maximum M-wave) intensity electrical stimulation15 which yields twitch characteristics, postactivation potentiation, and voluntary activation level (calculated as VA[%] = 100 × (1 − Tinterpolated/Tcontrol).55 Achilles tendon thickness and length, and medial gastrocnemius muscle fascicle length and pennation angles were assessed using ultrasonography (Aloka α10, Aloka Japan). The cortical silent period, reflecting spinal and motor cortex inhibitory mechanisms, was measured using transcranial magnetic stimulation (TMS).56 While the participant was sustaining constant dorsiflexion torque (at 20%, 40%, and 60% of maximal dorsiflexion torque with rest periods in between) in a seated position, a series of TMS pulses at 6-second intervals was elicited to the representation area of the tibialis anterior (TA) muscle on the primary motor cortex. At each torque level, the duration of the silent period after the motor-evoked potential is analyzed from the TA electromyographic signal.57
Statistics
Data are presented as mean ± SD for continuous variables and percentage for categories. Data analyses were carried out using chi-square test or Student's t test when comparing the two groups (nonrespondent analysis) and using one-way analysis of variance (ANOVA, P for trend shown in tables) with post hoc Bonferroni test (significant P values are shown in the text) when investigating differences between the menopausal groups. Kruskal-Wallis test was used to investigate differences in E2 and FSH between the menopausal groups, because due to the nature of the menopause, the distribution of these hormones is skewed over the menopausal groups. To estimate the association of E2 and FSH with the blood count variables, multivariate linear regression models were constructed. In particular, separate models were constructed to test if one or both of the explanatory variables, E2 and FSH, associate with criterion variable hemoglobin, red blood cell (RBC) count, white blood cell (WBC) count, or neutrophil-to-lymphocyte ratio (NLR). To provide estimation of the effect size both η2 (for group mean comparisons) and R2 values (for multivariate linear regression models) were calculated. Data analysis was carried out using IBM SPSS Statistics software version 24 (Chicago, IL), and the level of significance was set at P < 0.05.
RESULTS
Study flow and enrollment of the study participants
Figure 1 shows the flow chart of the study. The collection of cross-sectional ERMA data has proceeded in three phases followed by a phase four follow-up study for the perimenopausal women.
FIG. 1.
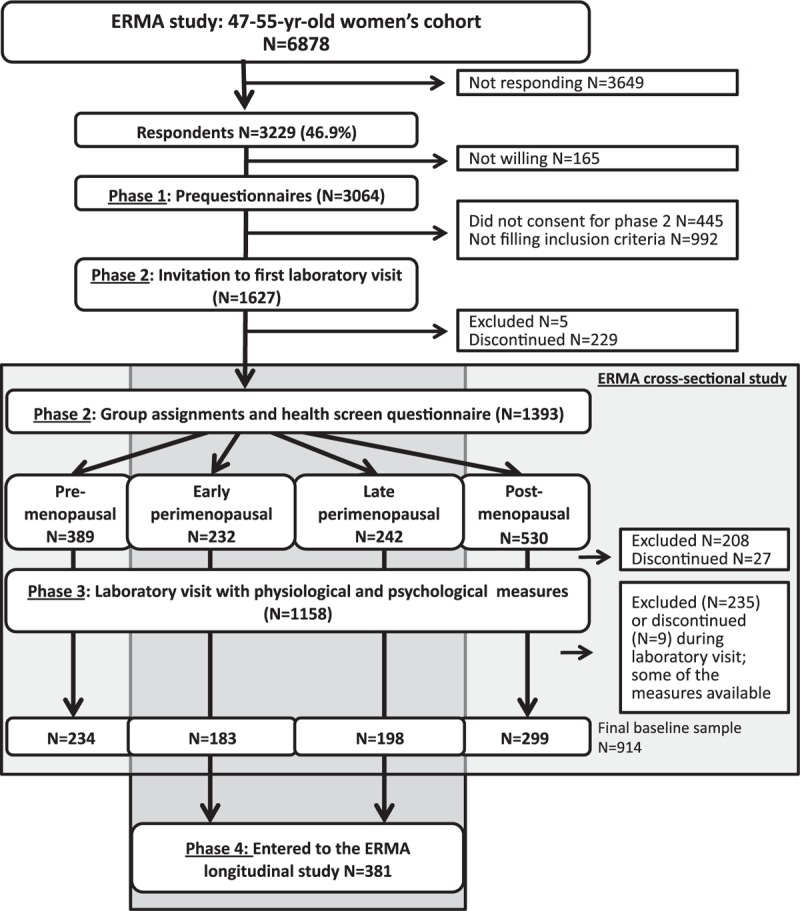
Flow chart of the recruitment process.
Of the women in the total age cohort, 82% (n = 6,878) were sent a postal invitation to participate in the ERMA study. Of this number, 3,649 did not respond (response rate 47%) and 165 were unwilling to participate. A further 1,437, who had delivered the phase one prequestionnaire data, were excluded (unwilling n = 445, and not eligible n = 992) from other phases. In phase two, a further 469 participants were excluded (not eligible n = 213, and discontinued n = 256). The total number of participants in the phase two laboratory visit, which included blood sampling and the health screen questionnaire, was 1,393, representing 43.1% of the responded, and 85.6% of the 1,672 women who were invited to participate at phase two. Of these, 389 were premenopausal, 232 early postmenopausal, 242 late perimenopausal, and 530 postmenopausal. However, of these 1,393 participants who had been assigned to the menopausal groups, 208 were later excluded based on issues reported in the health screen questionnaire and a further 27 discontinued participation before the phase three physiological and psychological measurements. In connection with the phase three physiological measurements, an additional 235 participants were excluded and nine discontinued participation. After all exclusions and dropouts, 234 premenopausal, 183 early perimenopausal, 198 late perimenopausal, and 299 postmenopausal women remained in the cross-sectional study, of whom the perimenopausal women (n = 381) entered the phase four longitudinal follow-up study.
In all, 1,440 women were excluded from all or some parts of the ERMA study (number does not include participants who did not consent or discontinued). The reasons for exclusion are summarized in Table 3. Of the participants, 90 were excluded due to more than one reason. To indicate the prevalence of each reason, each individual reason is counted separately; thus, the total number of the reasons given for exclusion in Table 3 is higher than the number of excluded participants. During the data collection, it became evident that recruiting the pre and postmenopausal groups was a faster process than filling the perimenopausal groups. Therefore, after each group had over 200 participants, we started to exclude women who had reported regular menstruation (premenopausal) or being 12 months or more from their last menstruation (postmenopausal) in the prequestionnaire collected at phase one (n = 568, 19% of those who provided consent for prequestionnaire and were willing to participate in other phases of the study). Nevertheless, we ended up with more women eligible for the pre or postmenopausal groups than either of the perimenopausal groups.
TABLE 3.
Summary of the reasons for exclusion.
| Exclusion criteria | Number of excluded participants | Prevalence of the criteria |
| Pre or postmenopausal group full | 568 | 18.54% |
| Use of hormone therapy | 440 | 14.36% |
| Use of E2-containing contraceptives | 64 | 2.09% |
| Bilateral oophorectomy | 53 | 1.73% |
| Polycystic ovarian syndrome | 20 | 0.65% |
| BMI > 35 | 131 | 4.28% |
| Having diseases or medications seriously affecting daily mental or physical function or influencing inflammatory status | ||
| Total number | 251 | 8.19% |
| Rates for specific reasons: | ||
| Crohn's disease | 7 | 0.23% |
| MS disease | 7 | 0.23% |
| Cancer | 18 | 0.59% |
| Musculoskeletal disease | 69 | 2.25% |
| Mental disease | 13 | 0.42% |
| Diabetes | 10 | 0.33% |
| Cardiovascular issues | 23 | 0.75% |
| Other disease | 18 | 0.59% |
| Continuous cortisone or inflammatory drug treatment | 93 | 3.04% |
In phase one, 1,437 women were excluded based on the prequestionnaire (47% of providers). The prevalence of each reason for exclusion is calculated for the 3,064 women who provided a consent in phase one.
MS, multiple sclerosis.
The second most common reason for exclusion was the use of EHT, which was being used by 14% of the 3,064 women providing prequestionnaire data. The third most common reason (8%) was having diseases or medications seriously affecting daily mental or physical function or influencing inflammatory status. Four percent were excluded owing to BMI >35. The prevalence of other reasons for exclusion was as follows: use of E2-containing contraceptives 2%, bilateral oophorectomy 1.7%, and polycystic ovarian syndrome 0.7%.
Nonrespondent analysis
The nonrespondent analysis concerned those who gave consent in phase one to the use of their prequestionnaire data. Of these, 992 were excluded, 445 did not consent to participate in phase two of the study, 229 discontinued before phase two, and 1,393 were included in phase two. The nonrespondent group was thus formed from the participants who did not consent or discontinued before phase two of the study (n = 674). The responders comprise the participants who were included in phase two (n = 1,393). However, to avoid potential bias due to under-reporting of obesity, which may influence gynecological status, participants who did not report their height and weight, thereby preventing the calculation of BMI in the prequestionnaire, were excluded from the analysis. In all, 655 of those who declined to participate after phase one and 1,386 of those who gave blood samples for group assignment were included in the nonrespondent analyses (n = 2,041; Table 4).
TABLE 4.
Nonrespondent analyses
| Nonrespondents (n = 655) | Respondents (n = 1,386) | Pa | |
| Age, y | 51.2 | 51.1 | 0.804 |
| Height, cm | 164.8 | 165.5 | 0.015 |
| Weight, kg | 71.6 | 69.5 | 0.001 |
| BMI, kg/m2 | <0.001 | ||
| Underweight (<18.5) | 0.5% | 0.2% | |
| Normal weight (18.5-24.99) | 41.8% | 45.1% | |
| Overweight (25.0-29.45) | 35.1% | 40.0% | |
| Obese (>29.5) | 22.6% | 14.6% | |
| Gynecological status | |||
| Hysterectomyb | 12.1% | 9.5% | 0.076 |
| Uni or bilateral oophorectomyb | 6.2% | 4.1% | 0.045 |
| Regular menstrual cycleb | 19.5% | 16.5% | 0.099 |
| Irregular menstrual cycleb | 35.7% | 38.9% | 0.192 |
| No hormonal contraceptionb | 68.0% | 60.0% | <0.001 |
| Progestogenic contraceptionb | 28.4% | 38.8% | <0.001 |
BMI, body mass index.
aData analyses were carried out using Student's t test for continuous variables and chi-square test for categorical variables.
bMissing data: hysterectomy (n = 3), oophorectomy (n = 7), regular menstrual cycle (n = 87), irregular menstrual cycle (n = 205), no hormonal contraception (n = 8), progestogenic contraception (n = 8).
Nonrespondents were more likely to be obese, were more often oophorectomized, and were less likely to use hormonal contraception than the women participating in the ERMA study. There were no differences in the reported regularity of the menstrual cycle or in the prevalence of hysterectomy between respondents and nonrespondents.
Anthropometrics and systemic hormone levels of the study participants
Age, self-reported height and weight, calculated BMI, and the measured systemic E2 and FSH levels are presented in Table 5. As expected, the premenopausal women were on average the youngest, with increasing age across the peri and postmenopausal groups. The age range in all groups was wide (47 to 54 years in premenopausal, and 47 to 55 years in all other groups). Of the 47 to 49-year-old participants, 45.9% were premenopausal, 20.2% were early perimenopausal, 11.8% were late perimenopausal, and 22.1% were postmenopausal. While, of the 50 to 52-year-old participants, 25.6% were premenopausal, 18.3% were early perimenopausal, 21.3% were late perimenopausal, and 34.8% were postmenopausal. And finally, of the 53 to 55-year-old participants, 7.7% were premenopausal, 9.2% were early perimenopausal, 18.3% were late perimenopausal, and 64.8% were postmenopausal. No differences were observed between the groups in height, weight, or BMI. The differences between groups in systemic E2 and FSH were as expected.
TABLE 5.
Age, anthropometrics and systemic hormone status of the study participants (base-ERMA group)
| Premenopausal (n = 389) | Early perimenopausal (n = 232) | Late perimenopausal (n = 242) | Postmenopausal (n = 530) | P for trend | |
| Age, ya | 49.7 ± 1.7 | 50.3 ± 1.8 | 51.1 ± 1.9 | 51.7 ± 2.1 | <0.001 |
| Height, cmb | 165.6 ± 5.4 | 165.3 ± 5.7 | 165.4 ± 5.8 | 165.5 ± 5.7 | 0.893 |
| Weight, kgb | 69.9 ± 10.6 | 69.5 ± 10.9 | 70.4 ± 10.8 | 68.8 ± 11.0 | 0.233 |
| BMI, kg/m2b | 25.5 ± 3.5 | 25.4 ± 3.7 | 25.7 ± 3.7 | 25.1 ± 3.7 | 0.140 |
| Normal weight | 43.2% | 46.1% | 40.7% | 48.7% | |
| Overweight | 42.9% | 38.8% | 43.2% | 37.1% | |
| Obese | 14.0% | 15.1% | 16.2% | 14.3% | |
| E2, nmol/L | 0.6 ± 0.6 | 0.4 ± 0.3 | 0.3 ± 0.2 | 0.2 ± 0.1 | <0.001 |
| FSH, IU/La | 7.6 ± 3.5 | 16.9 ± 4.7 | 43.8 ± 19.7 | 82.6 ± 30.5 | <0.001 |
Values are mean ± standard deviations expect the frequencies of the weight categories within each menopausal group that are presented as as percentage and highlighted with italics.
BMI = body mass index, E2 = 17β-estradiol, ERMA, Estrogenic Regulation of Muscle Apoptosis; FSH = follicle-stimulating hormone.
aKruskal-Wallis test was used.
bDue to uncompleted prequestionnaires, missing values were found for height (post, n = 1), weight (pre, n = 2; late peri, n = 1; post, n = 4), and BMI (pre, n = 2; late peri, n = 1; post, n = 4).
The blood count of the study participants
The results of the basic blood count are presented in Table 6. Significant group differences were found for hemoglobin, hematocrit, erythrocytes (RBCs), thrombocytes (platelets), total leucocytes (WBCs), lymphocytes (small WBCs), neutrophils (large WBCs), and for the NLR. The Bonferroni post hoc test to localize group-wise differences revealed differences in hemoglobin and hematocrit between the postmenopausal and all other menopausal groups (P < 0.05 for all comparisons). The number of RBCs differed only between the early perimenopausal and postmenopausal groups (P = 0.037), and number of platelets only between the premenopausal and postmenopausal groups (P = 0.033). The total WBC count differed between the premenopausal and early peri, late peri, and postmenopausal groups (P = 0.001, P = 0.015, and P < 0.001, respectively). In addition, the late perimenopausal group differed from the postmenopausal group (P = 0.018). For small WBCs, the significant group difference was observed between the pre and postmenopausal groups (P = 0.001), and for large WBCs the premenopausal group differed from late peri and postmenopausal groups (P < 0.001 for both comparisons). In addition, the early and late perimenopausal groups differed from the postmenopausal group (P < 0.001 in both comparisons), but not from each other. The postmenopausal group differed in NLR from all other groups (P ≤ 0.001 for all comparisons) and the late perimenopausal group differed from the premenopausal women (P = 0.001).
TABLE 6.
Basic blood count in the different menopausal groups
| Premenopausal (n = 388) | Early perimenopausal (n = 232) | Late perimenopausal (n = 242) | Postmenopausal (n = 527) | P for trend | Effect size, η2 | |
| Hemoglobin, g/L | 133.54 ± 10.03 | 133.34 ± 9.49 | 133.21 ± 9.34 | 135.33 ± 8.29 | 0.002 | 0.011 |
| Hematocrit | 39.75 ± 2.59 | 39.57 ± 2.57 | 39.78 ± 2.61 | 40.26 ± 2.35 | 0.001 | 0.012 |
| RBC (×1012/L) | 4.49 ± 0.33 | 4.47 ± 0.33 | 4.51 ± 0.33 | 4.54 ± 0.31 | 0.020 | 0.007 |
| MCV | 88.69 ± 4.11 | 88.70 ± 4.1 | 88.48 ± 4.1 | 88.73 ± 3.48 | 0.867 | 0.001 |
| MCH | 29.86 ± 1.83 | 29.95 ± 1.76 | 29.68 ± 1.71 | 29.92 ± 1.42 | 0.240 | 0.003 |
| Platelets (×109/L) | 263.70 ± 59.33 | 261.66 ± 59.32 | 262.88 ± 51.93 | 253.27 ± 53.71 | 0.020 | 0.007 |
| WBC (×109/L) | 5.81 ± 1.47 | 5.36 ± 1.29 | 5.47 ± 1.49 | 5.15 ± 1.29 | <0.001 | 0.036 |
| Small WBC (%) | 33.67 ± 19.85 | 35.62 ± 27.70 | 37.39 ± 26.76 | 39.95 ± 23.43 | 0.001 | 0.012 |
| Medium WBC (%) | 10.34 ± 2.0 | 10.53 ± 3.47 | 10.40 ± 3.72 | 10.37 ± 3.95 | 0.925 | <0.001 |
| Large WBC (%) | 57.15 ± 8.01 | 56.15 ± 7.91 | 54.28 ± 8.81 | 51.57 ± 9.21 | <0.001 | 0.071 |
| NLR | 1.91 ± 0.76 | 1.81 ± 0.65 | 1.69 ± 0.76 | 1.48 ± 0.71 | <0.001 | 0.058 |
Values are mean ± standard deviation.
MCH, mean corpuscular hemoglobin; platelets, B-thrombocyte count; MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; RBC, red blood cell, B-erythrocytes; WBC, B-leucocyte count; small WBC, L-lymphocyte count; medium-sized WBCs, L-monocyte count; large WBC, L-neutrophil count.
Association of 17β-estradiol and follicle-stimulating hormone with blood count variables
The association of E2 and FSH with hemoglobin, RBC, WBC, and NLR was investigated using multinomial linear regression (Table 7). No associations were found for E2 or FSH with hemoglobin and RBC count. Both E2 and FSH were associated with the WBC count and NLR, explaining 5.6% and 7.7% of the variation, respectively (P < 0.001 for both models).
TABLE 7.
Linear regression models for hemoglobin, red blood cell (RBC) count, white blood cell (WBC) count, and neutrophil-to-lymphocyte ratio (NLR)
| B | SE | Standardized beta | P | |
| Hemoglobin: model P = 0.070; R2 = 0.004 | ||||
| E2, nmol/L | −0.533 | 0.658 | −0.024 | 0.417 |
| FSH, IU/L | 0.012 | 0.007 | 0.048 | 0.100 |
| RBC count: model P = 0.024; R2 = 0.005 | ||||
| E2, nmol/L | −0.031 | 0.023 | −0.040 | 0.177 |
| FSH, IU/L | 0.000 | 0.000 | 0.048 | 0.102 |
| WBC count: model P = 0.001; R2 = 0.056 | ||||
| E2, nmol/L | 0.472 | 0.097 | 0.139 | <0.001 |
| FSH, IU/L | −0.005 | 0.001 | −0.145 | <0.001 |
| NLR: model P < 0.001; R2 = 0.077 | ||||
| E2, nmol/L | 0.234 | 0.051 | 0.129 | <0.001 |
| FSH, IU/L | −0.004 | 0.001 | −0.199 | <0.001 |
E2, 17β-estradiol; FSH, follicle-stimulating hormone.
DISCUSSION
This paper describes the protocol, implementation, and nonrespondent analysis of the ERMA study, and also some basic group characteristics and novel findings using the data collection of the base-ERMA group. The cross-sectional data collection was completed in November, 2016, and follow-up study continues.
The ERMA study was successfully launched by inviting 6,878 Finnish women aged 47 to 55 years to participate either in the postal prequestionnaire (phase one) alone or also in the subsequent phase two and three data collections. The perimenopausal women were also invited to participate in the longitudinal study. Of the whole age cohort, 82% were approached by postal invitation and 47% responded. It is quite common that only a small portion of the invited group responds to the postal invitations. For instance, in another Finnish study with similar age group and study theme, only 25% responded to the first postal invitation, and after two reminders the response rate was 52%.58 Therefore, our response rate (47%, accrued after one reminder) can be considered as expected and was quite near to our a priori estimation. Slightly more pre and postmenopausal woman than perimenopausal women were interested in participating in all phases of the study. This is perhaps due to reluctance to engage in the longitudinal study, which was offered only to the perimenopausal women. However, we cannot exclude the possibility that perimenopausal women may experience more menopausal symptoms than the other groups and thus be less willing to participate. Our pre-expectation for the distribution of women across the menopausal groups, based on the Finnish Health 2000 population survey (https://www.thl.fi/en/web/thl-biobank/for-researchers/sample-collections/health-2000-and-2011-surveys, National Public Health Institute, Finland), was 28% premenopausal, 22% early perimenopausal, 26% late perimenopausal, and 24% postmenopausal. We were able to assign 1,393 women to the corresponding menopausal groups: 28% premenopausal, 17% early postmenopausal, 17% late perimenopausal, and 38% postmenopausal. Thus, our sample contains a somewhat larger proportion of postmenopausal women than expected. The difference, however, had leveled off in the final sample (n = 914), which finished all three phases of the ERMA study, including the physiological and psychological measurements in phase three, and comprised 26% premenopausal, 20% early perimenopausal, 22% late perimenopausal, and 33% postmenopausal women.
The use of stepwise inclusion criteria (phases one to three shown in Fig. 1) enabled us to obtain subpopulations of a size suited to the multidisciplinary demands of the study. Only 14% of responders were excluded due to current use of EHT and only 2% due to use of E2-containing contraceptives. In 2003, the proportion of HT users in Finland was 21% among over 45-year-old women (consensus statement on menopausal hormonal treatment, the Finnish Medical Society Duodecim and the Academy of Finland, https://www.duodecim.fi/). Further population-wide studies are needed to verify whether the use of HT has declined during the past 10 to 13 years, or whether HT users were simply less interested in participating in ERMA. Other common exclusion criteria were diseases or medication that could affect psychological or physical function or inflammatory status (8%), thereby potentially confounding our aim of investigating the role of menopause in predisposing to decrements in function or increment in women with aging-associated low-grade inflammation.
It is also important to note that severely obese women (self-reported BMI >35) were excluded in phase one. The prevalence of severe obesity in our study population was 4.3%, which can be considered low compared with the findings in large world-wide population studies.59 It is highly possible that severely or morbidly obese persons are unwilling to participate in studies which they consider potentially too demanding on themselves. While the invitation to participate in the study did not include any explicit exclusion criteria, we did ask respondents to report their height and weight; this may have been understood as an indication that severe overweight would restrict participation. It may also be that severely obese women are uncomfortable about sharing their anthropometric measures and thus did not want to return the prequestionnaire. Another indication of a higher self-exclusion rate among obese than nonobese women emerged from the nonrespondent analysis. The nonrespondent group, that is, those who consented only for phase one or discontinued after phase one, contained a higher portion of obese (BMI > 30) women than the respondent group (22.6% vs 14.6%, respectively). Recently, a European-wide study found 17.1% of the 40 to 59-year-old population to be obese, whereas the prevalence of obesity among adults in Finland was 18.7%.59 Thus, given that the most severely obese (BMI > 35) were already excluded from our study, the obesity prevalence in the ERMA population can be considered representative of the Finnish general population. In addition to obesity, the nonrespondent analyses showed that the women who did not participate in phase two or three of ERMA had more often been oophorectomized and were thus less likely to use hormonal contraception. We do not consider these differences to prevent generalization of the results to the healthy middle-aged female population in Finland, despite the under-representation of severely obese women in the study.
The women assigned into the four menopausal groups were on average similar in their weight, height, and BMI calculated from self-reported measures. Although the age range of the ERMA participants is rather narrow, there was a clear increment in mean age through pre to postmenopausal group. However, all ages were represented within each menopausal group, emphasizing a great interindividual variation in the timing of menopause. On the basis of the E2 and the FSH serum concentrations, our group assignment was successful, even though a menstrual diary was not available for all the women. For instance, although women who have had a hysterectomy do not bleed, they nevertheless continue to have cycling hormones, and women using progestogen-based contraception may have almost no bleeding at all. The highest values for E2 and lowest for FSH were observed, as expected, in the premenopausal group. The E2 group means decrease and the FSH group means increase from the pre to postmenopausal group, which follows the known trend towards postmenopause for FSH secretion from the pituitary gland to intensify with the cessation of ovarian function.60
Basic blood counts were taken from all participants during the phase two laboratory visit. We observed slightly higher hemoglobin, hematocrit, and RBC count among the postmenopausal women compared with the other menopausal groups, which could be a reflection of the cessation of menstruation. Normal menstrual periods involve blood loss of up to 80 mL, but only abnormal uterine bleeding, has been shown to cause anemia in nonmenopausal women.61 The mean values observed in our study did not indicate the presence of anemia in any of the menopausal groups, and therefore the clinical significance of the observed group differences remains unclear. Blood rheological characteristics are, however, dependent on hemoglobin, hematocrit, and RBC count and size, and have been associated with aging and increased disease risk.62 Higher whole blood viscosity, of which an increment in hematocrit is one of the primary surrogate measurements, has been independently linked to many cardiovascular risk factors.63 Therefore, the observed increase in hematocrit values associated with postmenopausal status may be interpreted as an early indicator of increased health risks, warranting further studies on the links between the menopause transition and change in female sex steroids as a cause of change in blood rheological characteristics and thus as a mediator of these increased health risks.
We also observed a lower total WBC count (leucocytes) in postmenopausal women compared with the other menopausal groups. Of the WBCs, the proportion of large WBCs (neutrophils) was lower and the proportion of small WBCs (lymphocytes) higher in the postmenopausal than other menopausal groups. In addition, the NLR diminished from the pre to peri to postmenonopausal groups. Therefore, the broad picture drawn from our findings is that a trend favoring small over larger WBCs is associated with menopause. The total WBC and subtype cell counts were within the clinical reference values in each menopausal group. Consequently, the presence of acute infections or inflammation is implausible. This, in turn, means that it is unlikely that the observed group differences in WBCs are biased by acute or chronic diseases causing elevated inflammation. Lymphocytes have great capacity to secrete proinflammatory cytokines, thereby playing a pivotal role in the development of inflammaging.64 Thus, it is reasonable to hypothesize that pre to postmenopausal drift favoring lymphocytes over neutrophil leucocytes reflects aging or menopause-associated low-grade inflammation, or is possibly an early sign of the future inflammaging.
An earlier study has shown clear sexual dimorphism between females and males in WBC composition,25 a finding that potentially explains sex differences in the incidence of chronic diseases.8 The NLR, in particular, showed higher values in women than in men before age 50, but flipped-over after age 50, being thereafter lower in women than men. The authors proposed that change in menopausal E2 levels would underlie the NLR flip-over, but were not able to measure E2 in their study. We used multiple linear regression to ascertain the association of E2 levels with NLR, and found that, keeping FSH constant, a 1-unit increment in E2 would lead to 0.26-unit increment in NLR. Therefore, the menopausal drop in E2 seems to be directly associated with lower NLR. At first sight, our result would seem to conflict with the hypothesis that loss of E2 predisposes to diseases. Indeed, an elevated NLR has been identified as a disease activity marker for several diseases.65,66 Furthermore, NLR positively correlates with aging in a healthy population.67 It was recently proposed that normal NLR values lie between 0.78 and 3.53 in a general population in good health.68 If so, then the group means of the NLRs observed in the ERMA study, which varied from 1.9 in the premenopausal to 1.5 in the postmenopausal women, were well within normal values. The fact that our study population is healthy and relatively young may potentially explain why we do not see an aging or disease-associated increment in NLR. Furthermore, a very similar drop in NLR between ages 46 to 55 was observed by Chen et al25 in a cross-sectional study comparing age groups formed from 18 to 93-year-old women. NLR rose again in those aged 60+, potentially indicating the association between high NLR and disease risk to occur later in life. The clinical explanation for the drop in NLR around the age of menopause remains unknown.
CONCLUSIONS
The ERMA study has successfully gathered a representative cohort sample of 47 to 55-year-old women and determined their menopausal status as pre, early peri, late peri, or postmenopausal. The ERMA study has recently ended the recruitment stage, and is currently following up the perimenopausal women and analyzing the cross-sectional data. Therefore, more findings will be reported in the future. In this paper, we report our finding of significant differences in the blood count variables between the menopausal groups and provide evidence that E2 and FSH are associated with the WBC count and NLR. These findings are the first steps towards understanding how menopause-associated hormonal differences are associated with the age-dependent sexual dimorphism observed for many diseases.
Supplementary Material
Acknowledgments
We are grateful to all the ERMA participants who contributed their time, information, and biological samples. In addition, we thank the staff members at the Faculty of Sport and Health Sciences and at the LIKES Research Centre for Physical Activity and Health who helped with this study.
Footnotes
Funding/support: This research is supported by the Academy of Finland (VK: Grant nro 275323, EKL: nro 309504), PANINI (SS: Horizon 2020—the Framework Programme for Research and Innovation, Marie Sklodowska-Curie Actions, ITN, ref 15-0667), and the Juho Vainio Foundation (EKL).
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1.da Camara SM, Zunzunegui MV, Pirkle C, Moreira MA, Maciel AC. Menopausal status and physical performance in middle aged women: a cross-sectional community-based study in Northeast Brazil. PLoS One 2015; 10:e0119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurina LM, Gulati M, Everson-Rose SA, et al. The effect of menopause on grip and pinch strength: results from the Chicago, Illinois, site of the Study of Women's Health Across the Nation. Am J Epidemiol 2004; 160:484–491. [DOI] [PubMed] [Google Scholar]
- 3.Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. Evidence for cognitive aging in midlife women: Study of Women's Health Across the Nation. PLoS One 2017; 12:e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helson R, Soto CJ. Up and down in middle age: monotonic and nonmonotonic changes in roles, status, and personality. J Pers Soc Psychol 2005; 89:194–204. [DOI] [PubMed] [Google Scholar]
- 5.Lachman ME, Teshale S, Agrigoroaei S. Midlife as a pivotal period in the life course: balancing growth and decline at the crossroads of youth and old age. Int J Behav Dev 2015; 39:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999; 20:279–307. [DOI] [PubMed] [Google Scholar]
- 7.Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: changes in the immune system: a review. Maturitas 2010; 67:316–320. [DOI] [PubMed] [Google Scholar]
- 8.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol 2015; 294:102–110. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999; 340:1801–1811. [DOI] [PubMed] [Google Scholar]
- 10.Sipilä S, Narici M, Kjaer M, et al. Sex hormones and skeletal muscle weakness. Biogerontology 2013; 14:231–245. [DOI] [PubMed] [Google Scholar]
- 11.Sipilä S, Finni T, Kovanen V. Estrogen influences on neuromuscular function in postmenopausal women. Calcif Tissue Int 2015; 96:222–233. [DOI] [PubMed] [Google Scholar]
- 12.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993; 84:95–98. [DOI] [PubMed] [Google Scholar]
- 13.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci 2009; 64:1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pöllänen E, Sipilä S, Alén M, et al. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre- and postmenopausal women. Aging Cell 2011; 10:650–660. [DOI] [PubMed] [Google Scholar]
- 15.Finni T, Noorkoiv M, Pöllänen E, et al. Muscle function in monozygotic female twin pairs discordant for hormone replacement therapy. Muscle Nerve 2011; 44:769–775. [DOI] [PubMed] [Google Scholar]
- 16.Ronkainen PH, Kovanen V, Alen M, et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol 2009; 107:25–33. [DOI] [PubMed] [Google Scholar]
- 17.Taaffe DR, Sipilä S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging 2005; 25:297–304. [DOI] [PubMed] [Google Scholar]
- 18.Sipilä S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001; 101:147–157. [PubMed] [Google Scholar]
- 19.Qaisar R, Renaud G, Hedstrom Y, et al. Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J Physiol 2013; 591:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Khoudary SR, McClure CK, VoPham T, et al. Longitudinal assessment of the menopausal transition, endogenous sex hormones, and perception of physical functioning: the Study of Women's Health Across the Nation. J Gerontol A Biol Sci Med Sci 2014; 69:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronkainen PH, Pöllänen E, Alen M, et al. Global gene expression profiles in skeletal muscle of monozygotic female twins discordant for hormone replacement therapy. Aging Cell 2010; 9:1098–1110. [DOI] [PubMed] [Google Scholar]
- 22.Pöllänen E, Ronkainen PH, Suominen H, et al. Muscular transcriptome in postmenopausal women with or without hormone replacement. Rejuvenation Res 2007; 10:485–500. [DOI] [PubMed] [Google Scholar]
- 23.Pöllänen E, Fey V, Törmäkangas T, et al. Power training and postmenopausal hormone therapy affect transcriptional control of specific co-regulated gene clusters in skeletal muscle. Age (Dordr) 2010; 32:347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung T, Shim J, Lee Y. Association between leukocyte count and sarcopenia in postmenopausal women: The Korean National Health and Nutrition Examination Survey. Maturitas 2016; 84:89–93. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Zhang Y, Zhao G, et al. Difference in leukocyte composition between women before and after menopausal age, and distinct sexual dimorphism. PLoS One 2016; 11:e0162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007; 39:1423–1434. [DOI] [PubMed] [Google Scholar]
- 27.Harlow SD, Gass M, Hall JE, et al. STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012; 97:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Floderus B. Psycho-social factors in relation to coronary heart disease and associated risk factors. Stockholm; 1974. [Google Scholar]
- 29.Viken RJ, Rose RJ, Kaprio J, Koskenvuo M. A developmental genetic analysis of adult personality: extraversion and neuroticism from 18 to 59 years of age. J Pers Soc Psychol 1994; 66:722–730. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D Scale. Appl Psychol Measurement 1977; 1:385–401. [Google Scholar]
- 31.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life scale. J Pers Assess 1985; 49:71–75. [DOI] [PubMed] [Google Scholar]
- 32.Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS). J Cross-Cultural Psychol 2007; 38:227–242. [Google Scholar]
- 33.Pulkkinen L. KK. Keski-ikä elämänvaiheena [Middle age as a stage of life]: Jyväskylä: Jyväaskylän yliopisto; 2010. [Google Scholar]
- 34.Uotinen V, Rantanen T, Suutama T, Ruoppila I. Change in subjective age among older people over an eight-year follow-up: ‘getting older and feeling younger?’. Exp Aging Res 2006; 32:381–393. [DOI] [PubMed] [Google Scholar]
- 35.Levy BR, Slade MD, Kunkel SR, Kasl SV. Longevity increased by positive self-perceptions of aging. J Pers Soc Psychol 2002; 83:261–270. [DOI] [PubMed] [Google Scholar]
- 36.Liang J, Bollen KA. The structure of the Philadelphia Geriatric Center Morale scale: a reinterpretation. J Gerontol 1983; 38:181–189. [DOI] [PubMed] [Google Scholar]
- 37.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Motor Skills 1958; 8:271–276. [Google Scholar]
- 38.Johnson-Selfridge MT, Zalewski C, Aboudarham JF. The relationship between ethnicity and word fluency. Arch Clin Neuropsychol 1998; 13:319–325. [PubMed] [Google Scholar]
- 39.Richardson JTE. Measures of short-term memory: a historical review. Cortex 2007; 43:635–650. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Memory Scale: Third Edition Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 41.Hirvensalo M, Lampinen P, Rantanen T. Physical exercise in old age: an eight-year follow-up study on involvement, motives, and obstacles among persons age 65-84. 1998;6:157-168. [Google Scholar]
- 42.Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes (Lond) 2008; 32:353–361. [DOI] [PubMed] [Google Scholar]
- 43.Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA 1998; 279:440–444. [DOI] [PubMed] [Google Scholar]
- 44.Lakka TA, Salonen JT. The Physical Activity Questionnaires of the Kuopio Ischemic Heart Disease Study (KIHD). Med Sci Sports Exerc 1997; 29 Suppl:46–58. [Google Scholar]
- 45.Rottensteiner M, Leskinen T, Niskanen E, et al. Physical activity, fitness, glucose homeostasis, and brain morphology in twins. Med Sci Sports Exerc 2015; 47:509–518. [DOI] [PubMed] [Google Scholar]
- 46.Hirvensalo M, Lintunen T, Rantanen T. The continuity of physical activity: a retrospective and prospective study among older people. Scand J Med Sci Sports 2000; 10:37–41. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 2011; 14:411–416. [DOI] [PubMed] [Google Scholar]
- 48.Laakkonen EK, Kulmala J, Aukee P, et al. Female reproductive factors are associated with objectively measured physical activity in middle-aged women. PLoS One 2017; 12:e0172054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leinonen R, Heikkinen E, Jylha M. Self-rated health and self-assessed change in health in elderly men and women: a five-year longitudinal study. Soc Sci Med 1998; 46:591–597. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Ploeg GE, Withers RT, Laforgia J. Percent body fat via DEXA: comparison with a four-compartment model. J Appl Physiol 2003; 94:499–506. [DOI] [PubMed] [Google Scholar]
- 51.Sipilä S, Multanen J, Kallinen M, Era P, Suominen H. Effects of strength and endurance training on isometric muscle strength and walking speed in elderly women. Acta Physiol Scand 1996; 156:457–464. [DOI] [PubMed] [Google Scholar]
- 52.Bosco C, Luhtanen P, Komi PV. A simple method for measurement of mechanical power in jumping. Eur J Appl Physiol Occup Physiol 1983; 50:273–282. [DOI] [PubMed] [Google Scholar]
- 53.Anonymous ATS Statement. Am J Respir Crit Care Med 2002; 166:111–117. [DOI] [PubMed] [Google Scholar]
- 54.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381. [PubMed] [Google Scholar]
- 55.Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 1995; 18:593–600. [DOI] [PubMed] [Google Scholar]
- 56.Epstein CM. How far can TMS coils be optimized? A partial resolution. Brain Stimul 2013; 6:15. [DOI] [PubMed] [Google Scholar]
- 57.Saisanen L, Julkunen P, Niskanen E, et al. Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J Clin Neurophysiol 2008; 25:367–372. [DOI] [PubMed] [Google Scholar]
- 58.Mansikkamäki K, Raitanen J, Malila N, et al. Physical activity and menopause-related quality of life: a population-based cross-sectional study. Maturitas 2015; 80:69–74. [DOI] [PubMed] [Google Scholar]
- 59.Marques A, Peralta M, Naia A, Loureiro N, de Matos MG. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur J Public Health 2018; 28:295–300. [DOI] [PubMed] [Google Scholar]
- 60.MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin Endocrinol (Oxf) 1992; 36:339–345. [DOI] [PubMed] [Google Scholar]
- 61.Marret H, Fauconnier A, Chabbert-Buffet N, et al. Clinical practice guidelines on menorrhagia: management of abnormal uterine bleeding before menopause. Eur J Obstet Gynecol Reprod Biol 2010; 152:133–137. [DOI] [PubMed] [Google Scholar]
- 62.Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. J Geriatr Cardiol 2013; 10:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeong S, Cho Y, Duey M, Rosenson R. Cardiovascular risks of anemia correction with erythrocyte stimulating agents: should blood viscosity be monitored for risk assessment? Cardiovasc Drugs Ther 2010; 24:151–160. [DOI] [PubMed] [Google Scholar]
- 64.Xia S, Zhang X, Zheng S, et al. An update on inflammaging: mechanisms, prevention, and treatment. J Immunol Res 2016; 2016:8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bisgaard AK, Pihl-Jensen G, Frederiksen JL. The neutrophil-to-lymphocyte ratio as disease actvity marker in multiple sclerosis and optic neuritis. Mult Scler Relat Disord 2017; 18:213–217. [DOI] [PubMed] [Google Scholar]
- 66.Suh B, Shin DW, Kwon H, et al. Elevated neutrophil to lymphocyte ratio and ischemic stroke risk in generally healthy adults. PLoS One 2017; 12:e0183706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Chen Q, Luo X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal 2015; 29:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forget P, Khalifa C, Defour J, Latinne D, Van Pel M, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes 2017; 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


