Abstract
Objective:
This review describes historical development of selective estrogen receptor modulators (SERMs) and their combination with estrogens, termed a tissue selective estrogen complex (TSEC), and considers the potential for future TSEC development.
Methods:
This narrative review is based on literature identified on PubMed and the TSEC research and development experience of the authors.
Results:
SERMs have estrogenic and antiestrogenic effects in various tissues; however, no single agent has achieved an optimal balance of agonist and antagonist effects for the treatment of menopausal symptoms. Clinically, a number of SERMs protect against osteoporosis and breast cancer but can exacerbate vasomotor symptoms. Estrogens alleviate menopausal hot flushes and genitourinary symptoms as well as reduce bone loss, but the addition of a progestogen to menopausal hormone therapy to protect against endometrial cancer increases vaginal bleeding risk, breast tenderness, and potentially breast cancer. The search for an effective menopausal therapy with better tolerability led to the investigation of TSECs. Clinical development of a TSEC consisting of conjugated estrogens/bazedoxifene increased understanding of the importance of a careful consideration of the combination's components and their respective doses to balance safety and efficacy. Bazedoxifene is an estrogen receptor agonist in bone but an antagonist/degrader in the endometrium, which has contributed to its success as a TSEC component. Other oral TSEC combinations studied thus far have not demonstrated similar endometrial safety.
Conclusions:
Choice of SERM, selection of doses, and clinical trial data evaluating safety and efficacy are key to ensuring safety and adequate therapeutic effect of TSECs for addressing menopausal symptoms.
Keywords: Conjugated estrogens/bazedoxifene, Postmenopause, Selective estrogen receptor modulators, Tissue selective estrogen complex
Over the past 3 decades, preclinical and clinical investigations have expanded our understanding of the physiologic profile and molecular mechanisms of selective estrogen receptor modulators (SERMs), both alone and in combination with estrogens. The combination of estrogens and a SERM is sometimes referred to as a tissue selective estrogen complex (TSEC), although this term is not formally recognized by health authorities. The following review provides a historical perspective on the rationale for evaluating the combination of estrogens and SERMs and an overview of lessons learned during the development of the one approved TSEC combination. In addition, it provides a detailed review of the ongoing and emerging understanding of the importance of the selection and dosing of the individual components that make up a TSEC and the unique effects that may result from different TSEC combinations.
METHODS
This narrative review includes preclinical and clinical studies identified via a search of PubMed through July 2017 using search terms related to the classes of antiestrogens/SERMs, TSECs, and estrogens in menopause and postmenopause. The search also included individual terms for tamoxifen, clomiphene, raloxifene, toremifene, ospemifene, bazedoxifene, ormeloxifene, lasofoxifene, fulvestrant, and tibolone alone and combined with either conjugated estrogens or estradiol. Reviews of the historical development of SERMs were also considered, as were current professional guidelines for management of postmenopausal symptoms. Additional relevant papers were identified by the authors based on their expertise in this area.
SERMs: HISTORICAL PERSPECTIVE AND EVOLVING UNDERSTANDING OF THEIR VARIED EFFECTS
Early “nonsteroidal antiestrogens” (eg, MER25, clomiphene, tamoxifen) were originally investigated as contraceptives; however, it was revealed that they induced, rather than inhibited, ovulation in humans, despite the opposite effects in rats.1-3 Clomiphene was then developed as a treatment for infertility, and tamoxifen, which has also been used in Japan for ovulation induction,4was later “reinvented” as an anticancer agent for breast cancer.2
In the early 1970s, response to tamoxifen by 10 (22%) of 46 postmenopausal women with extensive breast cancer was thought to support its classification as an antiestrogen.5 Preclinical data demonstrated that tumor response to tamoxifen was dependent on estrogen receptor (ER) binding, further supporting its mechanism as estrogen antagonism in the breast and leading to screening breast tumors for the presence of ERs to predict responsiveness to tamoxifen.6 Tamoxifen eventually went on to become a major treatment for ER-positive breast cancer, used as both adjuvant therapy and treatment for metastatic cancer; it has also been used for prevention in high-risk women.2 As adjuvant therapy in women with early-stage, ER-positive breast cancer, tamoxifen was estimated to result in a statistically significant 50% reduction in recurrence and 28% reduction in mortality over 5 years.7
Over time, evidence began to accumulate from preclinical8 and clinical studies9-12 that despite being an estrogen antagonist in the breast, tamoxifen had beneficial agonistic effects on bone. For example, tamoxifen—alone or in combination with estradiol-3-benzoate—significantly reduced bone loss after ovariectomy in rats compared with vehicle-treated animals, based on the ash weight of incinerated femurs after 4 months of treatment.8 Clinical effects on bone were also observed. In a 2-year, randomized, double-blind, placebo-controlled trial of adjuvant tamoxifen in 140 postmenopausal women with a history of axillary node-negative breast cancer, the tamoxifen group had a 0.61% annual increase in lumbar spine bone mineral density (BMD), whereas the placebo group had a 1.0% decrease each year (P < 0.001).9 Another randomized, placebo-controlled study conducted in healthy women who were, on average, about 10 to 12 years postmenopause reported that after 2 years, lumbar spine BMD was 2.9% higher than tamoxifen versus placebo (P < 0.001).10
Unfortunately, bone was not the only tissue in which tamoxifen exhibited agonistic activity—it also stimulated the endometrium. Although tamoxifen had countered estrogen-stimulated increases in uterine wet weight in female rats,8 it stimulated growth of human endometrial tumors implanted into athymic mice.13 In fact, when human breast cancer tumors and endometrial cancer tumors were implanted into the same animal, tamoxifen reduced the former while stimulating growth of the latter, providing evidence that tamoxifen's effects were tissue-specific and not merely species-specific.13 Subsequent clinical trials of tamoxifen reported an increased risk of endometrial cancer.11,12 For example, the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial conducted in women at risk for breast cancer found that although tamoxifen indeed reduced the risk of invasive breast cancer (risk ratio [RR] 0.57; 95% CI, 0.46-0.70) and osteoporotic fracture (RR 0.68; 95% CI, 0.51-0.92), it also was associated with a more than threefold increase in risk of endometrial cancer (RR 3.28, 95% CI, 1.87-6.03), and a more than fivefold increase among those 50 years or older (RR 5.33, 95% CI, 2.47-13.17).12 Thus, recognition grew that tamoxifen was not a pure “antiestrogen,” and that effects were tissue-specific.
Raloxifene was similarly found to have ER antagonist effects in the breast and agonist effects on bone. The Multiple Outcomes of Raloxifene Evaluation (MORE) study in 7,705 postmenopausal women with osteoporosis reported that raloxifene at doses of 60 and 120 mg/d increased lumbar spine and femoral neck BMD by about 2% to 3% and reduced vertebral fracture risk (60 mg: RR 0.7, 95% CI, 0.5-0.8; 120 mg: RR 0.5, 95% CI, 0.4-0.7).14 Women randomized to raloxifene in the MORE trial had a lower risk of invasive breast cancer (RR 0.24, 95% CI, 0.13-0.44, P < 0.001 vs placebo),15 and in contrast with the tamoxifen studies, the risk of endometrial cancer did not appear to be increased.14
To clarify that drugs like raloxifene and tamoxifen had estrogen antagonist activity in some tissues and estrogen agonist activity in others, the term SERMs was introduced and became widely accepted.2,3 Today, SERMs are also known as estrogen agonists/antagonists. The knowledge gleaned from early studies of these agents has since facilitated the development of additional SERMs specifically designed to improve upon their profile of activities.16,17 Available SERMs today each has their own profile of tissue-dependent effects, which typically includes estrogen antagonist activity in breast and estrogen agonist activity in bone, but varying effects on the endometrium (Table 1).1,2,18-29
TABLE 1.
ER agonist and antagonist effects of SERMS on endometrium, breast, and bone, and effects on vasomotor symptoms
| SERM | Endometrium | Breast | Bone | VMS |
| Clomiphene18-20,125 | Antagonist | Antagonist | Agonist | Increase |
| Tamoxifen1,7,11,93 | Agonist | Antagonist | Agonist | Increase |
| Raloxifene1,23,50,90 | Neutral | Antagonist | Agonist | Increase |
| Bazedoxifene1,2,23,24,52,82,95,97,114 | Antagonist | Antagonist | Agonist | Increase |
| Ospemifene2,22 | Mixed agonist/antagonista | Antagonist | Agonist | Increase |
| Toremifene1,21,126 | Neutral | Antagonist | Agonist | Increase |
| Lasofoxifene1,2,23,51,94,107 | Mixed agonist/antagonist | Antagonist | Agonist | Increase |
| Ormeloxifene25-29 | Antagonist or weak agonist | Antagonist | Agonist | Not reported |
ER, estrogen receptor; SERM, selective estrogen receptor modulator; VMS, vasomotor symptoms.
aOspemifene is an ER agonist in the endometrium at high doses and an antagonist at lower doses.
Although they are not typically classified as SERMS, two additional compounds—fulvestrant and tibolone—have mechanisms involving selective effects on the ER. Fulvestrant has been found to be a pure estrogen receptor antagonist that downregulates the estrogen receptor, rather than merely blocking it.30-32 Because of this mechanism, it is sometimes referred to as a selective estrogen receptor degrader (SERD). Tibolone, occasionally referred to as a selective tissue estrogenic activity regulator (STEAR),33 is structurally different from SERMs, but has tissue-selective effects mediated through interactions with estrogen, progesterone, and androgen receptors, as well as regulation of enzymes involved in estrogen metabolism.34 Unlike SERMs, tibolone is effective in treating vasomotor symptoms (VMS).35 It has estrogen agonist effects on bone that are greater than those of raloxifene in postmenopausal women.36,37 Inconsistent results have been reported regarding tibolone's effects on the endometrium and breast of postmenopausal women,36,38-41 based on a limited number of clinical trials available, which were of low to very low quality according to a recent Cochrane review.38
TSEC DEVELOPMENT: RATIONALE AND EVOLVING UNDERSTANDING
In the mid-1980s to late 1990s, it was proposed that SERMs might be used in postmenopausal women to prevent breast cancer, treat osteoporosis, and slow development of atherosclerosis.42 At that time, “estrogen replacement therapy” was thought to provide many of these health benefits, including prevention of osteoporosis, probable reduction in cardiovascular disease risk as well as risk of colorectal cancer and Alzheimer's disease, along with amelioration of hot flushes and vaginal atrophy43,44; however, concerns about breast cancer raised by observational studies were inhibiting use of and adherence to estrogen-based hormone therapy (HT) among postmenopausal women.16,44 It was also during that time that the need to use a progestogen, in women with a uterus, to counter the increase in risk of endometrial hyperplasia and cancer induced by estrogens was confirmed.45,46 However, progestogen-containing HT was associated with vaginal bleeding and breast tenderness, which many women found unacceptable.44,46,47 It was hoped that SERMs might provide an alternative to HT with a better breast safety and overall tolerability profile.16
Thus began the work to identify a SERM for use in postmenopausal women that would have ER agonist effects on bone, lipids, the central nervous system, and the vagina, and ER antagonism (or at least a neutral effect) in the breast and endometrium.17,48 Although raloxifene eventually obtained FDA approval in the United States for prevention of both breast cancer and osteoporosis,49 no SERM to date has achieved a profile conducive to managing menopausal symptoms, particularly because they tend to exacerbate, rather than alleviate, VMS.1,50-53 The question then arose, in the late 1990s, as to whether a combination of SERMs and estrogens might produce estrogen agonist and antagonist effects distinct from that of either component alone and come closer to achieving that goal.54
There was already some precedent for exploring this approach, although it was not widely noted. In the early 1980s, a research group from Finland had published three studies of clomiphene 50 mg/d for 10 days after every 7-week cycle of CE 1.25 mg/d with the hope that clomiphene could be used in lieu of progestins for protection of the endometrium, with lower rates of bleeding and breast tenderness, in postmenopausal women desiring estrogen therapy.55-57 This cyclic combination afforded relief of menopausal symptoms (albeit to a modestly attenuated extent compared with CE alone), reduced ER receptor expression in the endometrium, increased the proportion of women with histologic atrophy of the endometrium, and was associated with low rates of uterine bleeding (lower than cyclical CE/megestrol acetate).55-57 However, there was evidence of endometrial proliferation or hyperplasia in some women treated with this regimen,55,57 whereas no endometrial proliferation or hyperplasia was seen in a comparison group of women treated with CE/megestrol acetate in one trial.57
An estrogen plus SERM “proof of concept” preclinical study evaluated a combination of conjugated estrogens and bazedoxifene (CE/BZA).54 BZA was selected as the SERM component because prior preclinical investigations showed it had favorable effects on bone and lipids with little to no stimulation of the endometrium or breast.17,58 CE was selected because, at that time, it was one of the most widely studied and utilized estrogen therapies for menopausal symptoms. Other preclinical investigations had suggested that at bone-protective doses, BZA might not antagonize the beneficial effects of estrogens on vasomotor instability.17,58 A key finding from the preclinical “proof of concept” investigation in ovariectomized rats was that BZA could completely counter CE's stimulatory effects on the endometrium (based on uterine wet weight) when adequately dosed without attenuating CE's beneficial effects on vasomotor instability, lipids, or bone,54 which supported clinical evaluation of this combination. The term TSEC was introduced in recognition of the fact that the combination of estrogens and a SERM resulted in a blended profile of tissue-selective activity that was different from that of either component alone and from that of traditional estrogen/progestin therapy, therefore warranting a specific classification.
Early clinical explorations of the first TSEC
A phase 2, randomized, double-blind, placebo- and active-controlled, dose-finding study subsequently provided the first indication of CE/BZA's efficacy and safety in postmenopausal women, and demonstrated that careful dose selection would be necessary to achieve the optimal balance of therapeutic effect and endometrial safety.59 This study compared CE 0.3 or 0.625 mg/d combined with BZA 5, 10, or 20 mg/d, with CE 0.3 or 0.625 mg alone, BZA 5 mg alone, CE 0.625 mg/medroxyprogesterone acetate 2.5 mg (CE/MPA), and placebo in 412 healthy postmenopausal women with an average of 4 hot flushes per day. CE alone, especially the higher 0.625 mg dose, increased endometrial thickness evaluated by transvaginal ultrasound (the primary outcome), but this increase was countered in a dose-dependent fashion by BZA. Neither CE 0.3 mg/BZA 20 mg nor CE 0.625 mg/BZA 20 mg significantly increased endometrial thickness relative to placebo, whereas the lower BZA doses provided inadequate endometrial protection, especially when combined with the higher CE 0.625-mg dose. All CE/BZA regimens that used CE 0.625 mg reduced hot flush frequency and severity compared with placebo, whereas combinations that used the lower CE dose (0.3 mg/d) were ineffective when combined with BZA doses of 10 or 20 mg/d. Thus, of the doses studied, CE 0.625 mg/BZA 20 mg provided the best balance of therapeutic efficacy and endometrial protection. At these doses, CE's beneficial effects on vaginal maturation index and bone turnover markers were largely preserved. In addition, CE 0.625 mg/BZA 20 mg was associated with rates of vaginal bleeding/spotting and breast tenderness that were not significantly different from placebo, and lower than with the active comparator CE/MPA.
The phase 2 study showed that, in combination with a BZA dose thought to be adequate to provide endometrial protection, a CE dose of 0.625 mg would retain efficacy against VMS, whereas a CE dose of 0.3 mg would not. To determine if a CE dose somewhere in between might prove to be the lowest effective dose, CE doses of both 0.45 and 0.625 mg were advanced to phase 3 clinical development, along with BZA doses of 10, 20, and 40 mg to better clarify which combination had the best safety and efficacy profile. The first phase 3 study, the Selective estrogens, Menopause, And Response to Therapy (SMART-1) trial, confirmed that BZA 20 mg was the lowest dose that provided endometrial protection (based on incidence of hyperplasia detected by endometrial biopsy at month 12) when added to either CE 0.45 mg or 0.625 mg, and that the higher 40 mg dose of BZA somewhat reduced therapeutic efficacy relative to combinations using lower BZA doses.60,61 CE 0.45 mg/BZA 20 mg and CE 0.625 mg/BZA 20 mg doses were selected for the remaining four phase 3 SMART trials, as they seemed to provide the best balance of endometrial safety while preserving therapeutic effect.60,61
Collectively, the five phase 3 SMART trials showed that CE 0.45 mg/BZA 20 mg and CE 0.625 mg/BZA 20 mg protected the endometrium, such that hyperplasia rates were <1%, comparable to placebo,62 and fulfilled both FDA and European Medicines Agency criteria for endometrial safety.63,64 At those doses, CE's beneficial effects on hot flushes, BMD, and some measures of vaginal atrophy were preserved.61,65-71
ARE OTHER TSEC COMBINATIONS VIABLE?
Clinical trial data: results of studies with other TSECs
The successful development of CE/BZA raises the question as to whether other combinations of a single estrogen or group of estrogens with a SERM might also be viable menopausal therapies. Concurrent with the period of CE/BZA's clinical development, TSEC combinations using raloxifene as the SERM were also investigated clinically. Although there were preliminary indications of efficacy in some of the studies using oral estrogen/SERM combinations, these TSECs generally provided inadequate endometrial protection. Inconsistent efficacy and safety results have been reported thus far with TSEC combinations that have used nonoral estrogens. Additional details from these investigations are summarized below.
TSECs using oral estrogens
In a clinical trial of raloxifene 60 mg/d combined with oral 17β-estradiol 1 mg/d (n = 61) compared with raloxifene alone (n = 62) in postmenopausal women with prior HT use, the TSEC combination significantly (P < 0.001) reduced the frequency of VMS compared with baseline and with raloxifene alone.72 There was no placebo group, and it was known that raloxifene alone may exacerbate VMS50; VMS caused a higher rate of discontinuation in the raloxifene-alone group than in the TSEC group in this study. However, after 1 year of treatment, mean endometrial thickness in the TSEC group was significantly higher than baseline (+0.74 ± 0.28; P < 0.01), whereas no increase was seen with raloxifene alone.72 Two women in the raloxifene/17β-estradiol group were found to have endometrial hyperplasia.72
Similarly, a study of postmenopausal women randomized to raloxifene 60 mg/d alone (n = 7) or in combination with conjugated esterified estrogens 0.312 mg/d (n = 7) found that the latter group had a significant (P < 0.001) reduction in hot flushes compared with baseline (albeit not compared with raloxifene alone), but also exhibited a significant (P < 0.02) increase in endometrial thickness compared with baseline.73
TSECs using nonoral estrogens
A pilot study randomly assigned 60 healthy, nonhysterectomized postmenopausal women with prior HT use to raloxifene 60 mg every other day with either the same dose of transdermal estradiol or a placebo patch for 8 weeks as one part of a larger strategy for transitioning women from estrogen therapy to raloxifene monotherapy.74 During the comparison phase of the study, participant satisfaction increased among those assigned to the TSEC and decreased with raloxifene alone; in addition, the TSEC showed less worsening on the Menopause-Specific Quality of Life Questionnaire vasomotor function domain compared with raloxifene alone. However, the TSEC group showed a mean 0.8 mm increase in endometrial thickness, whereas raloxifene alone showed a 0.9 mm decrease (P = 0.021). Hot flushes were the most frequently reported adverse event, occurring in 37% of the TSEC group and 50% of the raloxifene group (P = 0.297).
In another randomized, placebo-controlled study, 52 postmenopausal women with moderate-to-severe hot flushes were treated with raloxifene 60 mg/d alone or with percutaneous 17β-estradiol 0.5 mg gel, or placebo tablet.75 After 3 months, the TSEC combination was superior to raloxifene alone but not to placebo with regard to improvement in Kupperman index of climacteric symptoms and reduction in hot flush severity. However, in contrast to the study with transdermal estradiol, neither treatment significantly affected endometrial thickness or endometrial proliferation assessed via endometrial biopsy or hysteroscopy.
A 6-month randomized, double-blind, study of raloxifene or placebo in combination with open-label 17β-estradiol vaginal ring releasing 7.5 μg/24 h in 91 postmenopausal women with vaginal atrophy found that the addition of raloxifene did not interfere with the beneficial effects of the vaginal ring, but the study results pointed to no significant efficacy advantage from the addition of raloxifene.76 The TSEC combination was not significantly different from the ring alone in improving signs and symptoms of vaginal atrophy or hot flushes. Changes in endometrial thickness were not significantly different between groups,76 as would be expected because low-dose vaginal estrogens are considered appropriate for administration without the addition of progestogen for endometrial protection.77
Next steps for TSEC combinations: considerations for future development
As previous research has shown, SERMs are not interchangeable and estrogens are not identical, so each specific combination of agents would need to be fully evaluated. Just as each SERM has a unique profile in terms of its ER agonist/antagonist activity, so does each TSEC combination. As demonstrated in the clinical trials, careful evaluation and selection of the doses of the two components of the only approved TSEC (CE/BZA) was necessary to find the doses that balance endometrial protection with therapeutic effect, and to date, no other TSEC combination has been shown to be adequate in clinical trials. Because other TSECs may be developed in the future, lessons learned from the nonclinical and clinical experience to date should help to guide future TSEC development efforts and are outlined below.
Evidence that each TSEC combination has a unique profile
Investigations into the molecular mechanisms of estrogens, SERMs, and eventually TSECs predated and then continued concurrent with clinical development of the first approved TSEC. Each estrogen-ER and SERM-ER complex results in a unique conformation78-81 that affects recruitment of different coactivators (that facilitate ER agonist activity) and corepressors (that facilitate ER antagonist activity).78,80,82 Cofactor recruitment varies in different target tissues based on the concentration of coactivators and corepressors present in those tissues, allowing SERMs to be an antagonist in some tissues and an agonist in other tissues.83 When combined with CE, SERMs may inhibit CE's cofactor recruitment. BZA has been found to be less inhibitive of CE's cofactor recruitment than raloxifene or lasofoxifene,78 suggesting that it may better preserve some of CE's beneficial effects. Inhibition of CE's cofactor recruitment correlates with BZA dose,82 again pointing to the importance of maintaining the relative doses of the estrogen (s) and SERM when balancing efficacy and safety.
As a result of the differences in conformations and recruited cofactors, estrogens and SERMs also differ with regard to gene activation. Furthermore, the gene expression profile of each TSEC is unique and not simply the addition of the individual SERM and estrogen gene expression profiles (Fig. 1).78 For example, when CE and BZA are given together, a distinct heterodimeric ERα conformation results,84 which causes recruitment of corepressors that inhibit ERα activity in uterine and breast tissue82 and recruits coactivators that result in cooperative, maximal activation of gene expression in other tissues.84
FIG. 1.
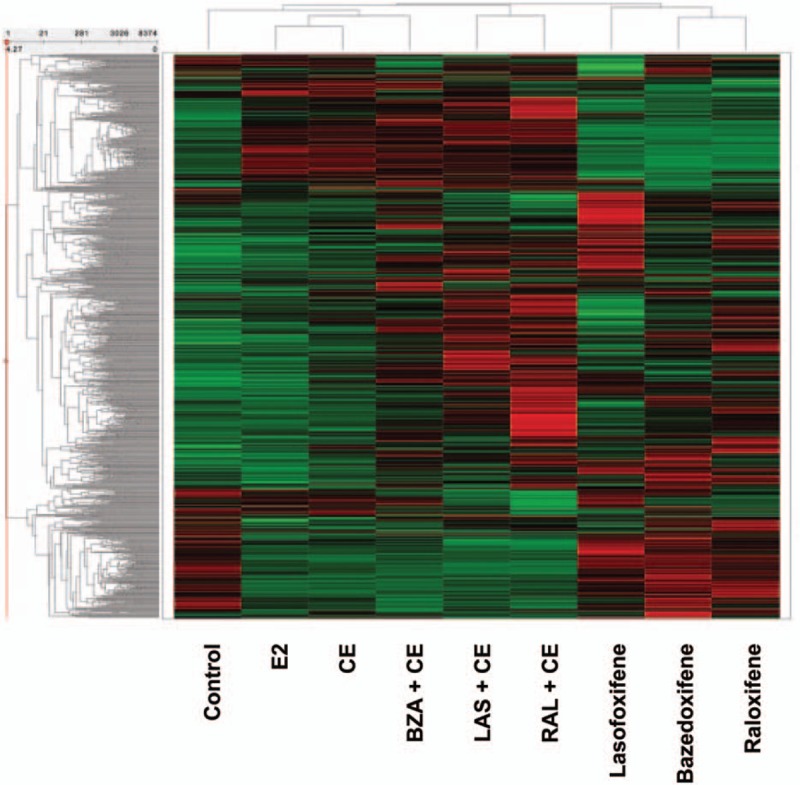
Differential global gene expression profiles of estrogens, SERMs, and their combinations.78 Each row of the heat map represents a specific gene, and each column provides the results for the different treatment sample. Color and intensity indicate the level of gene expression: red represents a higher gene expression level, and green represents a lower expression level. The tree-like dendrograms use hierarchical clustering to show similarities among different genes (left vertical axis) and treatments (top horizontal axis). The calculation settings were done using total number of selected records (8,374, all genes that were significantly regulated by at least one treatment) that used z-score normalized mean of signal intensities for each treatment condition. As shown here, each estrogen, SERM, and TSEC combination has a unique gene expression profile; of the tested TSEC combinations, CE/BZA resulted in a gene expression profile most similar to that of CE or E2. BZA, bazedoxifene; CE, conjugated estrogens; E2, 17β-estradiol; LAS, lasofoxifene; RAL, raloxifene. Reprinted from Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S. Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol 2009;23:74-85, by permission of Oxford University Press.
Cooperative gene expression has also been observed with other TSEC combinations, and is not limited to CE/BZA.84 A combination of 17β-estradiol and BZA induced gene expression at a level similar to that of CE/BZA (Fig. 2A), and combinations of CE with raloxifene, 4-hydroxytamoxifen, and fulvestrant also exhibited cooperative gene expression, though the magnitude of effect, at least with the latter two SERMs, was not as great as that seen with CE/BZA (Fig. 2B).84
FIG. 2.
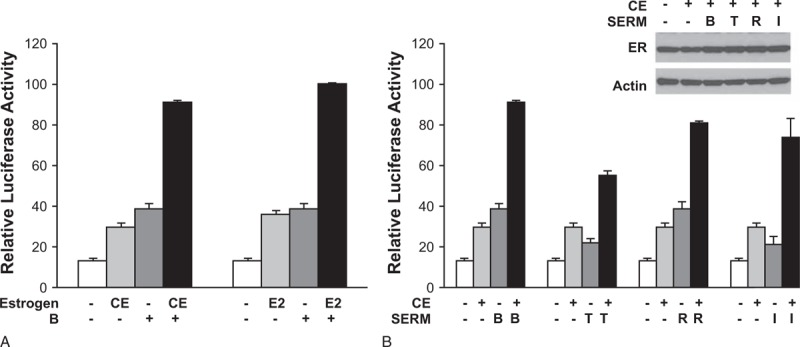
Different estrogens and SERMs synergistically activate chimeric reporter genes.84 Luciferase reporter assays allow measurement of gene expression at a transcriptional level. Such assays make use of the fact that luciferases are a bioluminescent enzyme. The regulatory region of the gene of interest (in this case, variants of the ERα gene) are cloned upstream of the luciferase reporter gene (Luc) and the resulting DNA vector is introduced into cells. A luminometer is then used to measure light emission from any expressed proteins. Luciferase activity correlates with the activity of the gene of interest. Here, specifically, HeLa cells were cotransfected with 1 μg of GERE-Luc reporter gene and 5 ng each of the expression vectors for ERα-G521R and ERα(GSCKV). One day after transfection, cells were treated for 24 hours with different variations of estrogens and SERMs to evaluate gene expression with each agent separately and cooperatively under combination conditions. Panel A depicts the ability of an ERα agonist (either 100 nM CE or 100 nM E2) to stimulate GERE-Luc receptor activity, alone or in combination with 10 nM BZA. Panel B depicts receptor activity after treatment with 100 nM CE, alone or combined with 10 nM of four different SERMs. Data are presented as relative luciferase activity compared with 100 nM E2 + 10 nM BZA-treated samples. Values represent the average ± SEM of three independent experiments. The insert in (B) is a Western blot showing ER expression levels after 24-hour treatment with the indicated ligands. B and BAZ, bazedoxifene; CE, conjugated estrogens; E2, 17β-estradiol; ER, estrogen receptor; I, ICI 182,780 [fulvestrant]; R, raloxifene; SEM, standard error of the mean; SERMs, selective estrogen receptor modulators; T, 4-hydroxytamoxifen. Reprinted from Liu S, Han SJ, CL Smith CL. Cooperative activation of gene expression by agonists and antagonists mediated by estrogen receptor heteroligand dimer complexes. Mol Pharmacol 2013;83(5):1066-1077.
Although the gene expression results with 17β-estradiol and BZA (Fig. 2A) are compelling, it should be noted that components of CE recruit different cofactors than 17β-estradiol alone (Fig. 3).78 The clinical implications of those differences are, however, not completely understood. It may be possible to create a TSEC combination that uses 17β-estradiol rather than CE in combination with a SERM; however, results cannot be assumed to be comparable to those with CE/BZA without clinical testing. Due to the unique profile of each TSEC, each individual TSEC combination needs to be evaluated separately in clinical studies to identify appropriate doses and assess safety and efficacy.
FIG. 3.
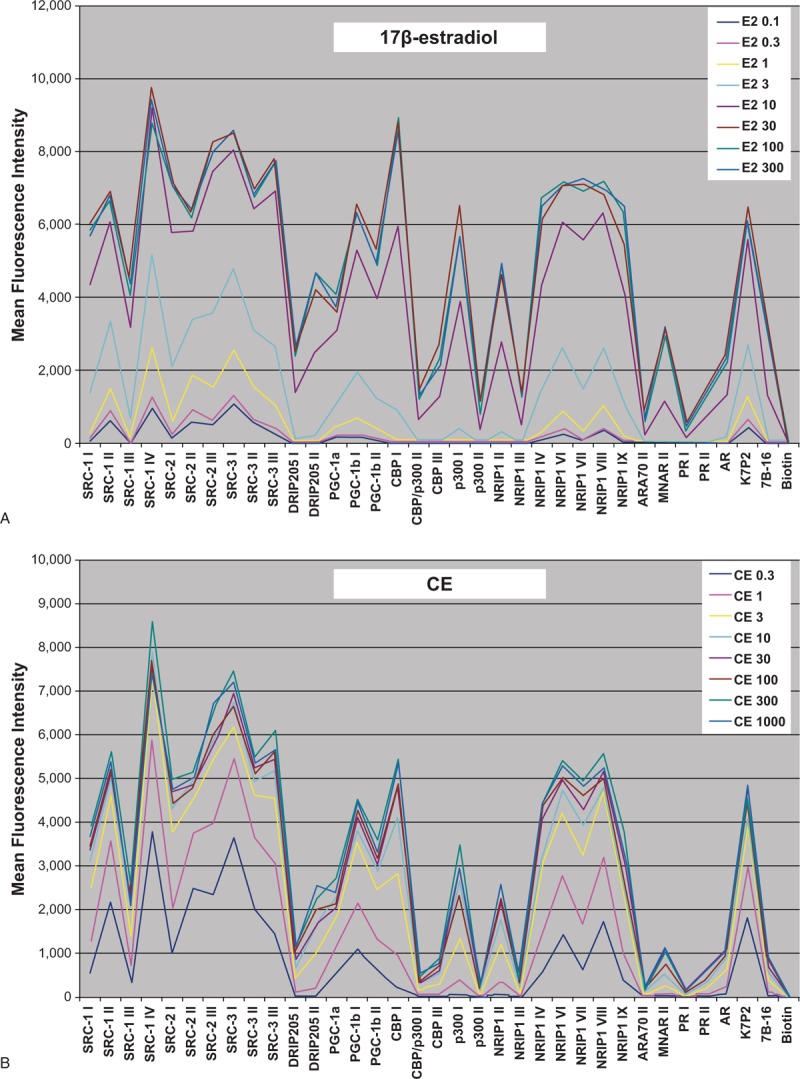
Differential cofactor recruitment for (A) 17β-estradiol and (B) CE.78 In conjunction with ligand binding, the interaction of ERα with coactivators and corepressors modulates gene transcription and influences whether a given ligand (eg, an estrogen or SERM) has agonist or antagonist activity in a particular tissue. Each unique receptor conformation resulting from ligand binding may recruit different coactivators or corepressors. This figure shows a comparison of the binding profiles of ERα-LBD to various different cofactor peptides in the presence of increasing concentrations of 17β-estradiol and CE mix. The multiplex ERα-cofactor peptide recruitment assay was performed in a dose-response mode using 300 nm to 0.1 nm 17β-estradiol (A) or 1000 nm to 0.3 nm of the CE mix (B). The mean fluorescence intensity for each dose (indicative of the magnitude of interaction between the peptide cofactor and ERα) was plotted for 35 of the peptides tested. Note that the cofactor recruitment profile for 17β-estradiol (A) differs from that of CE (B). CE, conjugated estrogens; ER, estrogen receptor; LBD, ligand-binding domain. Reprinted from Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S. Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol 2009;23:74-85, by permission of Oxford University Press.
Endometrial safety is essential to any TSEC combination
As the main function of the SERM component of a TSEC for menopausal symptoms is to provide endometrial protection, this is a critical feature when selecting potential SERM compounds for investigation in clinical trials. Tamoxifen stimulates the endometrium, increasing the risk of hyperplasia and endometrial cancer, whereas raloxifene, lasofoxifene, and ospemifene are more neutral.11,22,50,51,85-94 BZA is an ER antagonist in the endometrium; it not only competitively binds ERs, but also leads to ER degradation in the endometrium.82,95 As part of a TSEC, BZA counters stimulatory effects of CE in the endometrium. Supporting the results of the original proof of concept study described earlier,54 a number of subsequent investigations have similarly reported that CE alone stimulates endometrial proliferation and increases uterine weight in ovariectomized rodents and nonhuman primates, whereas these effects are blocked when BZA is added to CE.82,95-97 One of these studies also looked at TSEC combinations of CE with raloxifene, lasofoxifene, or BZA and found that of the three SERMs, lasofoxifene had the least CE antagonist effects, whereas the difference between BZA and raloxifene was not statistically different based on uterine wet weight (Fig. 4).97
FIG. 4.
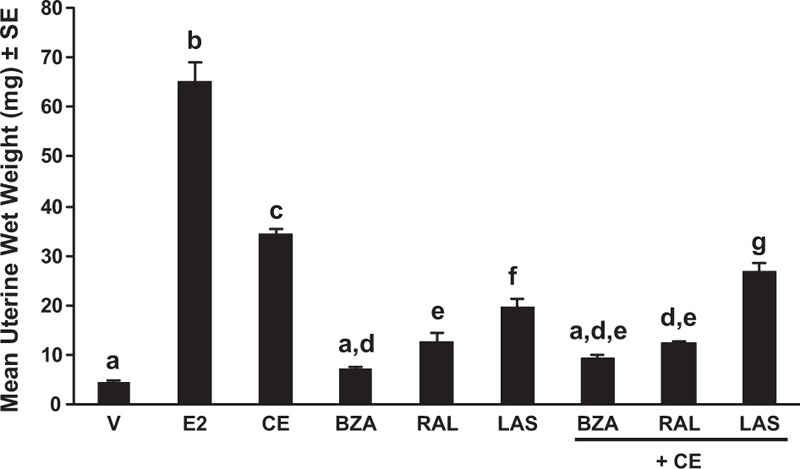
Uterine wet weight after treatment with estrogens, SERMs, and TSECs in ovariectomized C57BL/6 female mice.97 Uterine wet weight after treatment with bazedoxifene (2 mg/kg), raloxifene (10 mg/kg), and lasofoxifene (2 mg/kg) alone and in combination with CE (3 mg/kg) for 14 d (n = 7-10/group) in ovariectomized 4-week old, sexually immature mice. Groups labeled with the same letter are statistically similar (P > 0.05). BZA, bazedoxifene; CE, conjugated estrogens; E2, 17β-estradiol; LAS, lasofoxifene; RAL, raloxifene; SE, standard error; V, vehicle. Reprinted from Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 2009;150:1897-1903, by permission of Oxford University Press.
Recently, investigations into SERM and TSEC effects on endometrial cell gene expression have further elucidated reasons that individual SERMs and TSEC combinations differ in their effects on the endometrium. BZA alone or in combination with 17β-estradiol significantly increased expression of EMX2, which has been found to inhibit proliferation of endometrial cells, and BZA significantly blocked 17β-estradiol-induced increases in expression of HOXA10 messenger RNA, which mediates endometrial cell proliferation and differentiation.98 In contrast, raloxifene/17β-estradiol did not increase EMX2 expression, nor did raloxifene inhibit 17β-estradiol-induced increases in HOXA10 expression.98 BZA alone has been found to decrease expression of leukemia inhibitory factor (LIF), another marker of estrogen's proliferative effects on endometrial cells, whereas other SERMs (raloxifene, tamoxifen, and lasofoxifene) had no significant effect on LIF.98 BZA, lasofoxifene, and raloxifene (but not tamoxifen) were all able to block 17β-estradiol-induced LIF expression.98
Although expression of FGF18 was increased in endometrial adenocarcinoma cells, BZA inhibited FGF18 expression by 13% to 20% in endometrial stromal cells (P < 0.05).99 As fulvestrant was not found to inhibit expression of FGF18, this effect with BZA is likely separate from its ability to degrade the ER, and serves as a possible second mechanism by which it protects the endometrium.99 BZA also suppressed CE-induced increases in FGF18 expression in mice, confirming the in vitro findings in vivo.99
One other SERM that may be an ER antagonist in the endometrium is ormeloxifene, which has been used in India for contraception in premenopausal women and management of dysfunctional uterine bleeding.100,101 Ormeloxifene has been found to inhibit ER binding to coactivator SRC-1 as well as DNA elements, thereby potently antagonizing estrogen-induced gene expression in the endometrium.27,28 When given in combination with 17β-estradiol, ormeloxifene increased interactions of ERα with corepressors RIP140 and NCoR, and like BZA, decreased ERα expression.28 Ormeloxifene modestly increases uterine wet weight in ovariectomized rats, suggesting mild agonist activity, but when given in combination with 17β-estradiol or ethynylestradiol the increase is significantly less than with either estrogen alone, albeit still greater than in control animals.26-29 Ormeloxifene has not been evaluated clinically as part of a TSEC.
Given the myriad of individual effects exhibited by SERMs alone and in combination with estrogen (s), data from well-designed, long-term clinical studies are essential to confirm the endometrial safety profile predicted from preclinical data. Clinical data support the endometrial safety of BZA alone and in combination with CE. As noted earlier, the SMART trials confirmed that CE 0.45 mg/BZA 20 mg and CE 0.625 mg/BZA 20 mg are associated with endometrial hyperplasia rates comparable to placebo.62 Additional long-term safety data on BZA alone in postmenopausal women with osteoporosis showed no increase in risk of endometrial thickness or hyperplasia, and a significantly lower incidence of endometrial cancer compared with placebo at 7 years of follow-up (0.1% vs 0.4%; P = 0.020).102 As discussed earlier, oral TSEC combinations using raloxifene have thus far not demonstrated adequate endometrial safety.72,73
Effects on the breast are another important consideration
Current SERMs are all ER antagonists in the breast (Table 1). Tamoxifen and raloxifene reduce the risk of breast cancer in postmenopausal women.11,15,87,90,103-106 A trial of lasofoxifene in postmenopausal women with osteoporosis also found a reduced risk of invasive breast cancer compared with placebo.107 Tamoxifen and fulvestrant are established breast cancer treatments.7,108-110
Preclinical evidence suggests that BZA antagonizes the effects of estrogens in the breast.24 In several investigations, BZA countered proliferation of MCF-7 breast cancer cells induced by either 17β-estradiol58,111,112 or CE.112,113 BZA was better able to antagonize the effects of CE on these cells than either raloxifene or lasofoxifene.113 Furthermore, BZA inhibited CE-stimulated breast cell proliferation (measured by Ki67 staining revealed by immunocytochemistry or BrdU incorporation measured by ELISA) to lower than that observed in vehicle-treated control glands.112,114 In a study of nonhuman primates, BZA countered CE-induced increases in epithelial density and the proliferation marker Ki67, and also prevented CE-induced lobular enlargement on histopathological analysis of the breast.115
Gene expression analysis showed that BZA selectively antagonized many genes in the breast that are stimulated by CE or 17β-estradiol.112,113 The antagonized CE-regulated genes only partially overlapped with those antagonized by other SERMs (raloxifene and lasofoxifene).113 In a separate investigation, CE/BZA was at least as efficacious in inhibiting the proliferation of MCF-7 breast cancer cells as raloxifene or lasofoxifene in combination with CE, despite the fact that raloxifene and lasofoxifene antagonized expression of more CE-regulated genes.78
In preclinical studies of mammary gland whole mount morphology, CE and 17β-estradiol showed stimulatory effects in breast tissue (increased ductal branch points, increased ductal length and invasion into the fat pad, and/or increased terminal end bud formation), but these effects were blocked when these estrogens were combined with BZA.23,97,114 In one such study looking at the effects of multiple SERMs, BZA and raloxifene, but not lasofoxifene, reversed 17β-estradiol-induced terminal end bud formation.23
As in the endometrium, BZA degrades ERα in breast cells.32,82,111,115 It does so to a greater extent than raloxifene, tamoxifen, or lasofoxifene, and similar to that of the SERD fulvestrant.32,111 In one of these investigations, BZA inhibited the growth of tamoxifen-sensitive and tamoxifen-resistant breast cancer xenografts, and this effect appeared to be independent of its ability to degrade ERα.32
SERM and TSEC effects on bone
With the exception of fulvestrant, all SERMS are ER agonists in bone and protect against postmenopausal bone loss.9,51,52,116,117 BZA and raloxifene are used for prevention of bone loss in postmenopausal women. An in vitro study found that BZA protects osteoblasts from apoptosis induced by homocysteine, the levels of which rise during and after menopause.118 In a 3-year trial of postmenopausal women with osteoporosis, BZA 20 mg reduced the risk of new vertebral fractures by 42% (HR 0.58, 95% CI, 0.38-0.89), as did raloxifene 60 mg (HR 0.58, 95% CI, 0.38-0.89).117 Neither SERM significantly affected the overall incidence of nonvertebral fractures.117
A recent preclinical investigation offers a possible explanation for why BZA had greater benefits with regard to vertebral versus nonvertebral fracture reduction: in ovariectomized mice, BZA, raloxifene, and lasofoxifene all increased trabecular bone mass in the axial skeleton; however, only raloxifene and lasofoxifene increased the thickness and biomechanical strength of cortical bone (although raloxifene also increased its porosity).119 All of these effects were mediated via ERα activation function-1.
Recent preclinical investigations have helped elucidate the mechanisms by which BZA and CE/BZA help protect against bone loss. In one such investigation, both BZA and CE/BZA produced a small increase in ERα in bone tissue, in contrast to BZA's degradation of ERα in the uterus and breast.82 In another, CE/BZA preserved BMD, bone microstructure, and bone quality over 12 months in ovariectomized rats.120 Clinically, it remains unclear whether there is a slight attenuation of benefit when CE and BZA are combined compared with CE alone, but the combination nonetheless slows the bone loss associated with menopause.59,69
SERM and TSEC effects on vaginal tissue
As previously reviewed by Pinkerton and Stanczyck,121 ospemifene is the only currently available SERM that exhibits beneficial ER-agonist effects on vaginal tissue when administered alone. Although most other SERMs have neutral or inconsistent effects on vaginal tissue, tamoxifen may have adverse gynecologic effects including dyspareunia and vaginal dryness, as well as increased risk of uterine cancer.121
In phase 3 trials, ospemifene 60 mg given orally significantly improved vaginal maturation index and pH, and reduced dyspareunia compared with placebo in postmenopausal women with vulvovaginal atrophy.53,122,123 A study comparing vaginal histologic findings in postmenopausal users and nonusers of ospemifene found that ospemifene improved vaginal maturation, increased proliferation index, and increased ERα expression in the vaginal mucosa.124
The approved TSEC combination CE 0.45 mg/BZA 20 mg resulted in significant improvements in vaginal maturation index and vaginal dryness, and increased vaginal lubrication, and improved sexual function compared with placebo in postmenopausal women with vulvovaginal atrophy.66,67
CONCLUSIONS
Early investigations of SERMs led to the critical discovery that these agents were not pure antiestrogens, as initially thought. Rather, they were ER antagonists in some tissues (eg, breast) and ER agonists in other tissues (eg, bone). Subsequent investigations of SERMs have led to the understanding that each has a unique tissue-specific profile of activity, resulting from differences in their SERM-ER conformations, cofactor recruitment, and gene expression. None of the currently available SERMs have, however, been successful as treatments for VMS because they tend to exacerbate them.
This led to consideration of whether combining SERMs with estrogens would result in a blended profile of tissue-specific activity unique from either estrogens or SERMs alone. It was found that estrogen/SERM combinations, termed TSECs, also each exhibit a unique profile with regard to tissue-specific activity and gene expression. Thus, neither the estrogen nor the SERM components are interchangeable, and each pairing requires extensive clinical testing to confirm that it is effective and that undesired effects (eg, endometrial hyperplasia) are not manifested.
Dose-finding studies of the first such approved TSEC, CE/BZA, revealed that it is necessary to carefully select the dose of each component to create the best balance of endometrial protection and therapeutic effects on VMS and bone. This ultimately led to approval of CE 0.45 mg/BZA 20 mg. Combinations using higher or lower doses would impact the endometrial safety and efficacy profile, and it cannot be assumed that other doses or other estrogen/SERM combinations will provide the same results observed with the approved dose of CE/BZA.
Because the SERM component's primary function is to counter the effects of the estrogen component on the endometrium to protect against endometrial hyperplasia and cancer, success of any TSEC combination depends on the SERM acting as an ER antagonist in the endometrium. The lack of a strong ER antagonistic effect on the endometrium has limited the potential of SERMS other than BZA as TSEC components, and studies of oral 17β-estradiol/raloxifene and conjugated esterified estrogens/raloxifene have not demonstrated endometrial safety.
It is possible and perhaps likely that new SERMs and other TSECs will eventually be developed. Based on the lessons learned from the collective experience to date, it is essential that clinical trials be conducted with new TSECs to determine their optimal dose ratio and establish adequate efficacy and safety profiles, especially endometrial safety, before they are used in a clinical setting.
Acknowledgments
Medical writing support was provided by Lauren Cerruto and Diane M. Sloan, PharmD, of Peloton Advantage and was funded by Pfizer.
Footnotes
Financial disclosure/conflict of interest: JHP has received consultant fees from Wyeth/Pfizer, Shionogi Inc., and TherapeuticsMD and has stock options in TherapeuticsMD. MB and DM are employees and shareholders of Pfizer.
REFERENCES
- 1.Pickar JH, MacNeil T, Ohleth K. SERMs: progress and future perspectives. Maturitas 2010; 67:129–138. [DOI] [PubMed] [Google Scholar]
- 2.Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 2013; 8:135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitaker MD. Selective estrogen receptor modulators: from bench to bedside and back. Endocr Pract 2001; 7:113–119. [DOI] [PubMed] [Google Scholar]
- 4.Tsuiki A, Uehara S, Kyono K, et al. Induction of ovulation with an estrogen antagonist, tamoxifen. Tohoku J Exp Med 1984; 144:21–31. [DOI] [PubMed] [Google Scholar]
- 5.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer 1971; 25:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan VC, Jaspan T. Tamoxifen as an anti-tumour agent: oestrogen binding as a predictive test for tumour response. J Endocrinol 1976; 68:453–460. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998; 351:1451–1467. [PubMed] [Google Scholar]
- 8.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat 1987; 10:31–35. [DOI] [PubMed] [Google Scholar]
- 9.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 1992; 326:852–856. [DOI] [PubMed] [Google Scholar]
- 10.Grey AB, Stapleton JP, Evans MC, Tatnell MA, Ames RW, Reid IR. The effect of the antiestrogen tamoxifen on bone mineral density in normal late postmenopausal women. Am J Med 1995; 99:636–641. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005; 97:1652–1662. [DOI] [PubMed] [Google Scholar]
- 13.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res 1988; 48:812–815. [PubMed] [Google Scholar]
- 14.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell DP. The molecular pharmacology of SERMs. Trends Endocrinol Metab 1999; 10:301–311. [DOI] [PubMed] [Google Scholar]
- 17.Komm BS, Lyttle CR. Developing a SERM: stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann N Y Acad Sci 2001; 949:317–326. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez MA, Magee DE, Bryant HU, Turner RT. Clomiphene prevents cancellous bone loss from tibia of ovariectomized rats. Endocrinology 1997; 138:1794–1800. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K, Kato H. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril 1997; 67:256–260. [DOI] [PubMed] [Google Scholar]
- 20.Hecker E, Vegh I, Levy CM, et al. Clinical trial of clomiphene in advanced breast cancer. Eur J Cancer 1974; 10:747–749. [DOI] [PubMed] [Google Scholar]
- 21.Harvey HA, Kimura M, Hajba A. Toremifene: an evaluation of its safety profile. Breast 2006; 15:142–157. [DOI] [PubMed] [Google Scholar]
- 22.Simon JA, Lin VH, Radovich C, Bachmann GA. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause 2013; 20:418–427. [DOI] [PubMed] [Google Scholar]
- 23.Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol 2008; 287:40–46. [DOI] [PubMed] [Google Scholar]
- 24.Santen RJ, Song Y, Wang JP, Yue W. Preclinical breast effects of a tissue selective estrogen complex (TSEC) including conjugated estrogen with bazedoxifene. J Steroid Biochem Mol Biol 2017; 170:61–64. [DOI] [PubMed] [Google Scholar]
- 25.Gara RK, Sundram V, Chauhan SC, Jaggi M. Anti-cancer potential of a novel SERM ormeloxifene. Curr Med Chem 2013; 20:4177–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arshad M, Sengupta S, Sharma S, Ghosh R, Sawlani V, Singh MM. In vitro anti-resorptive activity and prevention of ovariectomy-induced osteoporosis in female Sprague-Dawley rats by ormeloxifene, a selective estrogen receptor modulator. J Steroid Biochem Mol Biol 2004; 91:67–78. [DOI] [PubMed] [Google Scholar]
- 27.Awasthi S, Daverey A, Dwivedi A. Modulation of AP-1 mediated estrogenic response by ormeloxifene in rat uterus. J Steroid Biochem Mol Biol 2007; 104:208–214. [DOI] [PubMed] [Google Scholar]
- 28.Daverey A, Saxena R, Tewari S, Goel SK, Dwivedi A. Expression of estrogen receptor co-regulators SRC-1, RIP140 and NCoR and their interaction with estrogen receptor in rat uterus, under the influence of ormeloxifene. J Steroid Biochem Mol Biol 2009; 116:93–101. [DOI] [PubMed] [Google Scholar]
- 29.Narayana Murthy PS, Sengupta S, Sharma S, Singh MM. Effect of ormeloxifene on ovariectomy-induced bone resorption, osteoclast differentiation and apoptosis and TGF beta-3 expression. J Steroid Biochem Mol Biol 2006; 100:117–128. [DOI] [PubMed] [Google Scholar]
- 30.Carlson RW. The history and mechanism of action of fulvestrant. Clin Breast Cancer 2005; 6 Suppl 1:S5–S8. [DOI] [PubMed] [Google Scholar]
- 31.Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci 1993; 106 (Pt 4):1377–1388. [DOI] [PubMed] [Google Scholar]
- 32.Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res 2013; 19:2420–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubista E, Planellas Gomez JV, Dowsett M, et al. Effect of tibolone on breast cancer cell proliferation in postmenopausal ER+ patients: results from STEM trial. Clin Cancer Res 2007; 13:4185–4190. [DOI] [PubMed] [Google Scholar]
- 34.Kloosterboer HJ. Tissue-selectivity: the mechanism of action of tibolone. Maturitas 2004; 48 Suppl 1:S30–S40. [DOI] [PubMed] [Google Scholar]
- 35.Landgren MB, Bennink HJ, Helmond FA, Engelen S. Dose-response analysis of effects of tibolone on climacteric symptoms. BJOG 2002; 109:1109–1114. [DOI] [PubMed] [Google Scholar]
- 36.Cummings SR, Ettinger B, Delmas PD, et al. The effects of tibolone in older postmenopausal women. N Engl J Med 2008; 359:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delmas PD, Davis SR, Hensen J, Adami S, van Os S, Nijland EA. Effects of tibolone and raloxifene on bone mineral density in osteopenic postmenopausal women. Osteoporos Int 2008; 19:1153–1160. [DOI] [PubMed] [Google Scholar]
- 38.Formoso G, Perrone E, Maltoni S, et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev 2016; 10:Cd008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archer DF, Hendrix S, Gallagher JC, et al. Endometrial effects of tibolone. J Clin Endocrinol Metab 2007; 92:911–918. [DOI] [PubMed] [Google Scholar]
- 40.Bruce D, Robinson J, Rymer J. Long-term effects of tibolone on the endometrium as assessed by bleeding episodes, transvaginal scan and endometrial biopsy. Climacteric 2004; 7:261–266. [DOI] [PubMed] [Google Scholar]
- 41.Ettinger B, Kenemans P, Johnson SR, et al. Endometrial effects of tibolone in elderly, osteoporotic women. Obstet Gynecol 2008; 112:653–659. [DOI] [PubMed] [Google Scholar]
- 42.Jordan VC. The science of selective estrogen receptor modulators: concept to clinical practice. Clin Cancer Res 2006; 12:5010–5013. [DOI] [PubMed] [Google Scholar]
- 43.Burkman RT, Collins JA, Greene RA. Current perspectives on benefits and risks of hormone replacement therapy. Am J Obstet Gynecol 2001; 185:S13–S23. [DOI] [PubMed] [Google Scholar]
- 44.Sarrel PM. Improving adherence to hormone replacement therapy with effective patient-physician communication. Am J Obstet Gynecol 1999; 180:S337–S340. [DOI] [PubMed] [Google Scholar]
- 45.Woodruff JD, Pickar JH. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. The Menopause Study Group. Am J Obstet Gynecol 1994; 170:1213–1223. [DOI] [PubMed] [Google Scholar]
- 46.Pickar JH. The endometrium—from estrogens alone to TSECs. Climacteric 2009; 12:463–477. [DOI] [PubMed] [Google Scholar]
- 47.The North American Menopause Society. Achieving long-term continuance of menopausal ERT/HRT: consensus opinion of The North American Menopause Society. Menopause 1998; 5:69–76. [PubMed] [Google Scholar]
- 48.Taylor HS. Designing the ideal selective estrogen receptor modulator—an achievable goal? Menopause 2009; 16:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evista [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016. [Google Scholar]
- 50.Martino S, Disch D, Dowsett SA, Keech CA, Mershon JL. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452. [DOI] [PubMed] [Google Scholar]
- 51.McClung MR, Siris E, Cummings S, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause 2006; 13:377–386. [DOI] [PubMed] [Google Scholar]
- 52.Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 2008; 23:525–535. [DOI] [PubMed] [Google Scholar]
- 53.Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486. [DOI] [PubMed] [Google Scholar]
- 54.Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology 2008; 149:6084–6091. [DOI] [PubMed] [Google Scholar]
- 55.Kauppila A, Janne O, Kivinen S, et al. Postmenopausal hormone replacement therapy with estrogen periodically supplemented with antiestrogen. Am J Obstet Gynecol 1981; 140:787–792. [DOI] [PubMed] [Google Scholar]
- 56.Kokko E, Janne O, Kauppila A, Vihko R. Cyclic clomiphene citrate treatment lowers cytosol estrogen and progestin receptor concentrations in the endometrium of postmenopausal women on estrogen replacement therapy. J Clin Endocrinol Metab 1981; 52:345–349. [DOI] [PubMed] [Google Scholar]
- 57.Kauppila A, Kivinen S, Leinonen P, Tuimala R, Vihko R, Ylostalo P. Comparison of megestrol acetate and clomiphene citrate as supplemental medication in postmenopausal oestrogen replacement therapy. Arch Gynecol 1983; 234:49–58. [DOI] [PubMed] [Google Scholar]
- 58.Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology 2005; 146:3999–4008. [DOI] [PubMed] [Google Scholar]
- 59.Pickar JH, Lavenberg J, Pan K, Komm BS. Initial investigation into the optimal dose ratio of conjugated estrogens and bazedoxifene: a double-blind, randomized, placebo-controlled phase 2 dose-finding study. Menopause 2018; 25:273–285. [DOI] [PubMed] [Google Scholar]
- 60.Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024. [DOI] [PubMed] [Google Scholar]
- 61.Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92:1025–1038. [DOI] [PubMed] [Google Scholar]
- 62.Mirkin S, Pinkerton JV, Kagan R, et al. Gynecologic safety of conjugated estrogens plus bazedoxifene: pooled analysis of five phase 3 trials. J Womens Health (Larchmt) 2016; 25:431–442. [DOI] [PubMed] [Google Scholar]
- 63.Guidance for Industry. Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms—recommendaitons for clinical evaluation. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071643.pdf Accessed November 17, 2017. [Google Scholar]
- 64.Guideline on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003348.pdf Accessed November 17, 2017. [Google Scholar]
- 65.Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124. [DOI] [PubMed] [Google Scholar]
- 66.Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17:281–289. [DOI] [PubMed] [Google Scholar]
- 67.Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140. [DOI] [PubMed] [Google Scholar]
- 68.Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric 2013; 16:338–346. [DOI] [PubMed] [Google Scholar]
- 69.Pinkerton JV, Harvey JA, Lindsay R, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab 2014; 99:E189–E198. [DOI] [PubMed] [Google Scholar]
- 70.Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052. [DOI] [PubMed] [Google Scholar]
- 71.Gallagher JC, Palacios S, Ryan KA, et al. Effect of conjugated estrogens/bazedoxifene on postmenopausal bone loss: pooled analysis of two randomized trials. Menopause 2016; 23:1083–1091. [DOI] [PubMed] [Google Scholar]
- 72.Stovall DW, Utian WH, Gass ML, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause 2007; 14:510–517. [DOI] [PubMed] [Google Scholar]
- 73.Carranza-Lira S, Gooch AL, Saldivar N, Osterwalder MS. Climacteric symptom control after the addition of low-dose esterified conjugated estrogens to raloxifene standard doses. Int J Fertil Womens Med 2007; 52:93–96. [PubMed] [Google Scholar]
- 74.Davis SR, O’Neill SM, Eden J, et al. Transition from estrogen therapy to raloxifene in postmenopausal women: effects on treatment satisfaction and the endometrium—a pilot study. Menopause 2004; 11:167–175. [DOI] [PubMed] [Google Scholar]
- 75.Valiati B, Capp E, Edelweiss MI, de Freitas FM, Wender MC. Effect of raloxifene and low-dose percutaneous 17beta-estradiol on menopause symptoms and endometrium—a randomized controlled trial. Maturitas 2009; 62:81–84. [DOI] [PubMed] [Google Scholar]
- 76.Pinkerton JV, Shifren JL, La VJ, Rosen A, Roesinger M, Siddhanti S. Influence of raloxifene on the efficacy of an estradiol-releasing ring for treating vaginal atrophy in postmenopausal women. Menopause 2003; 10:45–52. [DOI] [PubMed] [Google Scholar]
- 77.The North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017; 24:728–753. [DOI] [PubMed] [Google Scholar]
- 78.Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S. Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol 2009; 23:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paige LA, Christensen DJ, Gron H, et al. Estrogen receptor (ER) modulators each induce distinct conformational changes in ER alpha and ER beta. Proc Natl Acad Sci USA 1999; 96:3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med 2003; 348:618–629. [DOI] [PubMed] [Google Scholar]
- 81.McDonnell DP, Clemm DL, Hermann T, Goldman ME, Pike JW. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol Endocrinol 1995; 9:659–669. [DOI] [PubMed] [Google Scholar]
- 82.Han SJ, Begum K, Foulds CE, et al. The dual ERalpha inhibitory effects of the tissue-selective estrogen complex for endometrial and breast safety. Mol Pharmacol 2016; 89:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science 2002; 295:2465–2468. [DOI] [PubMed] [Google Scholar]
- 84.Liu S, Han SJ, Smith CL. Cooperative activation of gene expression by agonists and antagonists mediated by estrogen receptor heteroligand dimer complexes. Mol Pharmacol 2013; 83:1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Jamal JH, Dubin NH. The effect of raloxifene on the uterine weight response in immature mice exposed to 17beta-estradiol, 1,1,1-trichloro-2, 2-bis(p-chlorophenyl)ethane, and methoxychlor. Am J Obstet Gynecol 2000; 182:1099–1102. [DOI] [PubMed] [Google Scholar]
- 86.Sikoski P, Register TC, Lees CJ, et al. Effects of two novel selective estrogen receptor modulators, raloxifene, tamoxifen, and ethinyl estradiol on the uterus, vagina and breast in ovariectomized cynomolgus monkeys (Macaca fascicularis). Am J Obstet Gynecol 2007; 196:75–77. [DOI] [PubMed] [Google Scholar]
- 87.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 2002; 360:817–824. [DOI] [PubMed] [Google Scholar]
- 88.Holinka CF, Bressler RS, Zehr DR, Gurpide E. Comparison of effects of estertrol and tamoxifen with those of estriol and estradiol on the immature rat uterus. Biol Reprod 1980; 22:913–926. [DOI] [PubMed] [Google Scholar]
- 89.Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet 1994; 343:1318–1321. [DOI] [PubMed] [Google Scholar]
- 90.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137. [DOI] [PubMed] [Google Scholar]
- 91.Bese T, Kosebay D, Demirkiran F, Arvas M, Bese N, Mandel N. Ultrasonographic appearance of endometrium in postmenopausal breast cancer patients receiving tamoxifen. Eur J Obstet Gynecol Reprod Biol 1996; 67:157–162. [DOI] [PubMed] [Google Scholar]
- 92.Dijkhuizen FP, Brolmann HA, Oddens BJ, Roumen RM, Coebergh JW, Heintz AP. Transvaginal ultrasonography and endometrial changes in postmenopausal breast cancer patients receiving tamoxifen. Maturitas 1996; 25:45–50. [DOI] [PubMed] [Google Scholar]
- 93.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 1994; 86:527–537. [DOI] [PubMed] [Google Scholar]
- 94.Goldstein SR, Neven P, Cummings S, et al. Postmenopausal Evaluation and Risk Reduction With Lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause 2011; 18:17–22. [DOI] [PubMed] [Google Scholar]
- 95.Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model. Menopause 2013; 20:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliva P, Roncoroni C, Radaelli E, et al. Global profiling of TSEC proliferative potential by the use of a reporter mouse for proliferation. Reprod Sci 2013; 20:119–128. [DOI] [PubMed] [Google Scholar]
- 97.Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 2009; 150:1897–1903. [DOI] [PubMed] [Google Scholar]
- 98.Kulak J, Jr, Ferriani RA, Komm BS, Taylor HS. Tissue selective estrogen complexes (TSECs) differentially modulate markers of proliferation and differentiation in endometrial cells. Reprod Sci 2013; 20:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flannery CA, Fleming AG, Choe GH, et al. Endometrial cancer-associated FGF18 expression is reduced by bazedoxifene in human endometrial stromal cells in vitro and in murine endometrium. Endocrinology 2016; 157:3699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ormeloxifene Information from DrugsUpdate. Available at: http://www.drugsupdate.com/generic/view/367/Ormeloxifene Accessed November 13, 2017. [Google Scholar]
- 101.Reference Manual for Oral Contraceptive Pills; Family Planning Division; Ministry of Health and Family Welfare; Government of India. Nirman Bhawan, New Delhi, India: Ministry of Health and Family Welfare; Government of India; 2016. Available at: http://upnrhm.gov.in/site-files/family_planning/4.Manuals_of_FP-2015-16/Oral_Pills_Manual.pdf. [Google Scholar]
- 102.Palacios S, de Villiers TJ, Nardone FC, et al. Assessment of the safety of long-term bazedoxifene treatment on the reproductive tract in postmenopausal women with osteoporosis: results of a 7-year, randomized, placebo-controlled, phase 3 study. Maturitas 2013; 76:81–87. [DOI] [PubMed] [Google Scholar]
- 103.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat 2001; 65:125–134. [DOI] [PubMed] [Google Scholar]
- 104.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761. [DOI] [PubMed] [Google Scholar]
- 105.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741. [DOI] [PubMed] [Google Scholar]
- 106.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila) 2010; 3:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.LaCroix AZ, Powles T, Osborne CK, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst 2010; 102:1706–1715. [DOI] [PubMed] [Google Scholar]
- 108.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 2010; 28:4594–4600. [DOI] [PubMed] [Google Scholar]
- 109.Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2014; 106:djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 2004; 22:1605–1613. [DOI] [PubMed] [Google Scholar]
- 111.Lewis-Wambi JS, Kim H, Curpan R, Grigg R, Sarker MA, Jordan VC. The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor alpha and cyclin D1. Mol Pharmacol 2011; 80:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song Y, Santen RJ, Wang JP, Yue W. Inhibitory effects of a bazedoxifene/conjugated equine estrogen combination on human breast cancer cells in vitro. Endocrinology 2013; 154:656–665. [DOI] [PubMed] [Google Scholar]
- 113.Chang KC, Wang Y, Bodine PV, Nagpal S, Komm BS. Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol 2010; 118:117–124. [DOI] [PubMed] [Google Scholar]
- 114.Song Y, Santen RJ, Wang JP, Yue W. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology 2012; 153:5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ethun KF, Wood CE, Register TC, Cline JM, Appt SE, Clarkson TB. Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys. Menopause 2012; 19:1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology 2000; 141:809–820. [DOI] [PubMed] [Google Scholar]
- 117.Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008; 23:1923–1934. [DOI] [PubMed] [Google Scholar]
- 118.Kanazawa I, Tomita T, Miyazaki S, Ozawa E, Yamamoto LA, Sugimoto T. Bazedoxifene Ameliorates Homocysteine-Induced Apoptosis and Accumulation of Advanced Glycation End Products by Reducing Oxidative Stress in MC3T3-E1 Cells. Calcif Tissue Int 2017; 100:286–297. [DOI] [PubMed] [Google Scholar]
- 119.Borjesson AE, Farman HH, Moverare-Skrtic S, et al. SERMs have substance-specific effects on bone, and these effects are mediated via ERalphaAF-1 in female mice. Am J Physiol Endocrinol Metab 2016; 310:E912–E918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Komm BS, Vlasseros F, Samadfam R, Chouinard L, Smith SY. Skeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized rats. Bone 2011; 49:376–386. [DOI] [PubMed] [Google Scholar]
- 121.Pinkerton JV, Stanczyk FZ. Clinical effects of selective estrogen receptor modulators on vulvar and vaginal atrophy. Menopause 2014; 21:309–319. [DOI] [PubMed] [Google Scholar]
- 122.Portman DJ, Bachmann GA, Simon JA. Ospemifene. a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630. [DOI] [PubMed] [Google Scholar]
- 123.Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene. a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas 2014; 78:91–98. [DOI] [PubMed] [Google Scholar]
- 124.Alvisi S, Baldassarre M, Martelli V, Gava G, Seracchioli R, Meriggiola MC. Effects of ospemifene on vaginal epithelium of post-menopausal women. Gynecol Endocrinol 2017; 33:946–950. [DOI] [PubMed] [Google Scholar]
- 125.Derman SG, Adashi EY. Adverse effects of fertility drugs. Drug Saf 1994; 11:408–421. [DOI] [PubMed] [Google Scholar]
- 126.Kohler PC, Hamm JT, Wiebe VJ, DeGregorio MW, Shemano I, Tormey DC. Phase I study of the tolerance and pharmacokinetics of toremifene in patients with cancer. Breast Cancer Res Treat 1990; 16Suppl:S19–S26. [DOI] [PubMed] [Google Scholar]


