Abstract
Background:
Routine atrioventricular optimization (AVO) has not been shown to improve outcomes with cardiac resynchronization therapy (CRT). However, more recently subgroup analyses of multicenter CRT trials have identified electrocardiographic or lead positions associated with benefit from AVO. Therefore, the purpose of this analysis was to evaluate whether interventricular electrical delay modifies the impact of AVO on reverse remodeling with CRT.
Methods:
This substudy of the SMART-AV trial (SMARTDELAY Determined AV Optimization) included 275 subjects who were randomized to either an electrogram-based AVO (SmartDelay) or nominal atrioventricular delay (120 ms). Interventricular delay was defined as the time between the peaks of the right ventricular (RV) and left ventricular (LV) electrograms (RV-LV duration). CRT response was defined prospectively as a >15% reduction in LV end-systolic volume from implant to 6 months.
Results:
The cohort was 68% men, with a mean age of 65±11 years and LV ejection fraction of 28±8%. Longer RV-LV durations were significantly associated with CRT response (P<0.01) for the entire cohort. Moreover, the benefit of AVO increased as RV-LV duration prolonged. At the longest quartile, there was a 4.26× greater odds of a remodeling response compared with nominal atrioventricular delays (P=0.010).
Conclusions:
Baseline interventricular delay predicted CRT response. At long RV-LV durations, AVO can increase the likelihood of reverse remodeling with CRT. AVO and LV lead location optimized to maximize interventricular delay may work synergistically to increase CRT response.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00874445.
Keywords: cardiac resynchronization therapy, heart failure, heart ventricles, treatment outcome
See Editorial by Rickard and Varma
WHAT IS KNOWN?
Routine atrioventricular (AV) optimization for cardiac resynchronization therapy (CRT) is no longer recommended because the results of multicenter randomized trials have been disappointing.
Anatomic position of the left ventricular (LV) lead for CRT has limited utility to predict response. However, pacing at sites of late mechanical or electrical delay are associated with improved outcomes. Both prolonged LV delay (QLV interval, onset of QRS to LV) and interventricular delay (right ventricular [RV]-LV duration) are associated with better CRT response rates.
WHAT THE STUDY ADDS?
AV optimization, using an algorithm that promotes fusions with intrinsic conduction and maximizes acute hemodynamic response (SmartDelay), is associated with improved CRT response compared with nominal AV delay programming when pacing at sites with long RV-LV durations. There is no benefit of (AV) optimization at pacing sites with short RV-LV durations.
The CRT nonresponder rate can be decreased significantly by choosing pacing sites or pacing electrodes of multipolar leads with long RV-LV duration (>70 ms) and using AV optimization.
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with symptomatic heart failure (HF), left ventricular (LV) systolic dysfunction, and QRS prolongation. CRT results in LV reverse remodeling, as well as improvement in clinical outcomes, including reductions in HF hospitalizations and mortality.1–5 Despite the consistently observed benefits of CRT in randomized clinical trials, many patients remain classified as nonresponders.1,6 Multiple factors have been identified impacting the response rate, including patient characteristics (eg, sex and pathogenesis of HF) and electrocardiographic properties, such as QRS duration and morphology. In addition to these nonmodifiable clinical characteristics, procedural factors can also affect response rates. For example, pacing at LV sites with late electrical or mechanical activation has been associated with better outcomes.7–18 In contrast, early enthusiasm has waned for the role of optimizing atrioventricular (AV) timing may play in improving responder rates because of disappointing results from multicenter randomized trials.19–22
Recent studies have indicated that atrioventricular optimization (AVO) may be useful in certain subgroups of patients with CRT. For instance, LV-only pacing with AVO may be beneficial among patients with left bundle branch block (LBBB) and normal AV conduction times.23 LV delay at the LV pacing site also identifies a subgroup that is associated with improved outcomes with AVO during biventricular pacing.24 Prolonged interventricular delay, as measured by the difference in activation time between the right ventricular (RV) and LV leads, is associated with better CRT response.17–19 The present study was designed to assess whether AVO further improves the benefit of pacing at long RV-LV durations.
Methods
The present analysis is a substudy of the SMART-AV trial (SMARTDELAY Determined AV Optimization). SMART-AV was a multicenter randomized trial of AVO techniques among patients with advanced HF undergoing CRT implantation.20 This trial was approved by an institutional review committee at each institution, and all subjects gave informed consent. Details of the RV-LV analysis of SMART-AV have been published previously.25 There were 275 patients included in the RV-LV substudy, which included subjects randomized to a nominal AV delay or AVO using the SmartDelay (SD) algorithm. The sensed and paced AV delay was 120 ms in the nominal group, whereas the SD algorithm was used to program sensed and paced AV delays separately in the AVO group. Simultaneous RV and LV pacing (ie, LV offset, 0 ms) was used in both groups. The RV and LV leads were positioned at the discretion of the implanting physician with no guidance to maximize RV-LV duration. At the final lead positions, surface lead II, RV and LV electrograms were recorded simultaneously on paper strips at a sweep speed of 100 mm/s. RV-LV interval was measured by a blinded core laboratory with no knowledge of lead position or clinical outcomes. The RV-LV interval was measured in sinus rhythm and in the absence of pacing as the interval from the first major peaks of the RV and LV electrograms during a cardiac cycle with the resolution of 5 ms (Figure 1). The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because this was a study using a proprietary algorithm.
Figure 1.
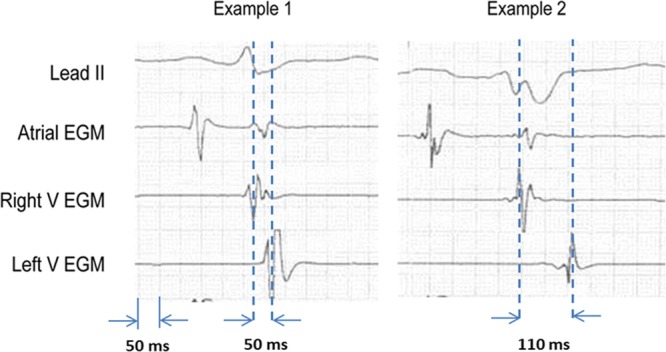
Two examples of right ventricular (RV)-left ventricular (LV) duration measurements in study patients. The calipers are aligned with the peaks of the RV and LV electrogram (EGM).
The primary end point of the SMART-AV trial was LV end-systolic volume (LVESV). The CRT response rate for the present analysis was defined prospectively as a >15% reduction in LVESV.18,20,24,25 The echocardiographic end points were analyzed blindly by a core laboratory. Off-line software (Pro-Solv, version 3.0, or GE Echo Pac, version 6.0) was used for measurements. Two-dimensional–derived LV volumes were determined in the apical 4- and 2-chamber views by the biplane method of discs. In 84% of images, the apical 2-chamber view image quality was deemed excellent or good with respect to visualization of the anterior wall. All echocardiographic measures were performed at baseline and after 6 months of CRT.
Baseline clinical parameter data were compared across quartiles of RV-LV duration. Continuous variables were summarized as means and standard deviations, unless otherwise noted, and compared across RV-LV quartiles with a Cuzick test for trend. Binary variables were compared across quartiles with a Cochran-Armitage test for trend. Categorical variables with >2 response levels were compared across quartiles with a Cochran-Mantel-Haenszel test.
The effects of RV-LV on changes in relative and absolute LVESV were evaluated using general linear models (for continuous RV-LV) and 1-way ANOVA F tests for trend (for quartile of RV-LV). Responses to CRT were compared by RV-LV durations grouped in quartiles. Potential interactions between RV-LV timing and AVO were assessed via general linear modeling (for continuous RV-LV) and logistic regression (for RV-LV quartile).
Changes in relative LVESV between the 2 AVO groups were compared within each quartile of RV-LV using a 2-sample t test. The effects of AVO on CRT response within various RV-LV and clinical characteristic subgroups were evaluated using multivariable logistic regression modeling, adjusted for the clinical characteristics of baseline ejection fraction (EF), LVESV, pathogenesis of HF, LBBB, sex, New York Heart Association classification (NYHA class), QRS duration, and age.
Two group comparisons were made using 2-sample t tests for continuous variables and χ2 tests for categorical variables. An α =0.05 threshold was used to demonstrate statistical significance, with no adjustments made for multiple comparisons. SAS, version 9.4, was used for statistical analysis.
Results
Patient Population
This substudy included 275 patients and was representative of those included in the main SMART-AV study.20,25 The mean age was 65±11 years, and 68% were men. The LVEF was 28±8%, the baseline LVESV was 130±61 mL, and 94% of subjects had NYHA class III functional status at enrollment. An LBBB morphology was present in 77% of subjects with a QRS duration of 149±25 ms and PR interval of 197±46 ms. The clinical characteristics were well matched between the SD and nominal programming groups (P>0.05).
The sensed and paced programmed AV delays were 120 ms for all subjects in the nominal group by protocol, and 123±34 and 171±35 ms, respectively, in the SD group. The percentage of atrial pacing was 21.7±25.1% versus 22.8±26.7% (P=0.74), and the percentage biventricular pacing was 95.8±7.8% versus 95.7±6.6% for the 2 groups, respectively.
RV-LV Analyses
The RV-LV duration quartiles were at 40, 65, and 100 ms for the full cohort. As expected, the RV-LV duration was longer for patients with LBBB compared with non-LBBB (77±38 versus 40±37 ms; P<0.001). Of note, 97% of LBBB and 81% of non-LBBB subjects had a positive RV-LV duration, indicating earlier activation of the RV electrode than the LV electrode in sinus rhythm. This is consistent with LV electrical delay even in the presence of non-LBBB in a vast majority of subjects.
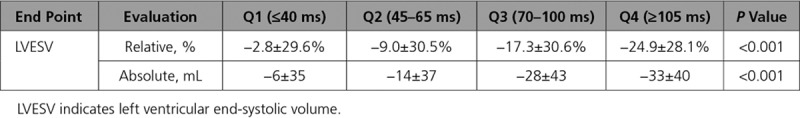
The magnitudes of relative and absolute changes in LVESV from baseline to 6 months are shown in Table 1 for each RV-LV quartile. Both end points were strongly associated with RV-LV duration (both P<0.001). To ensure that these findings were not an artifact of grouping into quartiles, further analysis was performed with RV-LV duration assessed as a continuous variable. Again, there was a strong relationship between interventricular delay and the change in LVESV with a 2.0% relative decrease and 2.5 mL absolute decrease in LVESV for every 10-mL prolongation of RV-LV duration (both P<0.001).
Table 1.
Relationship of Right Ventricular-Left Ventricular Duration Quartile With LVESV Changes
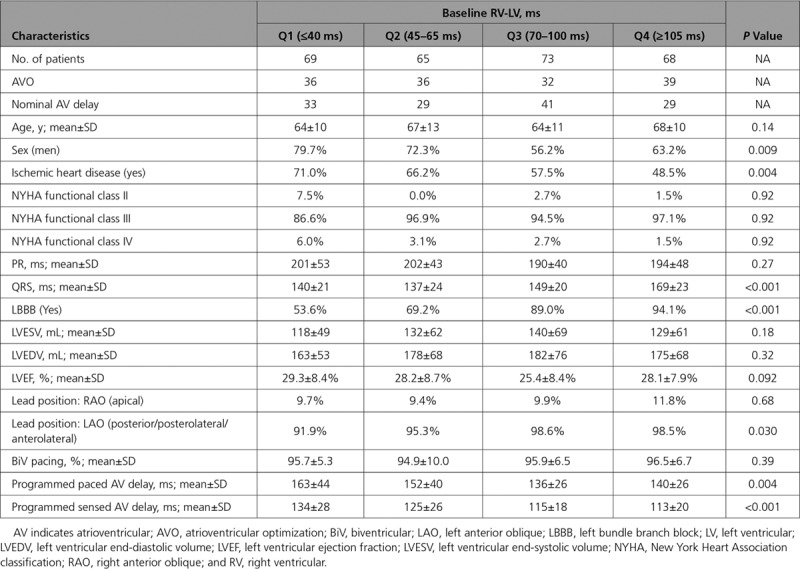
There were important clinical differences between subjects in the various RV-LV duration quartiles as shown in Table 2. Specifically, at longer interventricular delays, subjects were more likely to be women, have nonischemic cardiomyopathy, LBBB, longer QRS duration, and lateral or posterior LV lead position—all markers of an improved response with CRT. To explore this relationship further, multivariable analysis was performed on response rates (>15% reduction of LVESV). Baseline EF, LVESV, pathogenesis of HF, LBBB, sex, NYHA class, QRS duration, and age were included as main effects in these analyses. RV-LV duration remained a strong independent predictor of the reverse remodeling response for the entire cohort, with an 11% increased odds of response per 10-ms increase in RV-LV duration (P=0.005).
Table 2.
Comparison of Baseline Clinical Parameters in RV-LV Quartiles
AVO Analyses
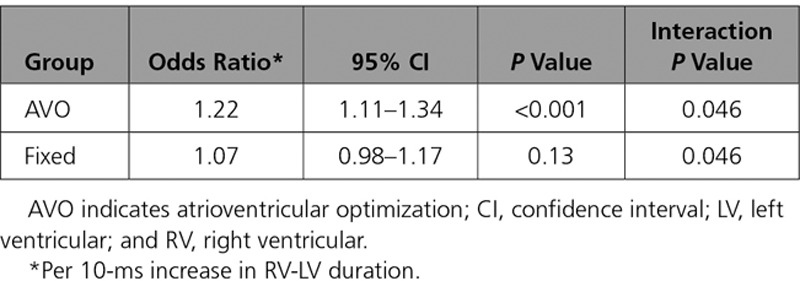
To evaluate the impact of AVO on this effect, the 2 groups (nominal and AVO) were analyzed separately. Figure 2 provides relative changes in LVESV from implant to 6 months by RV-LV quartile for each group. The magnitude of LVESV reduction for the AVO group was greater than the control group as RV-LV duration prolonged, approaching clinical significance in the longest quartile (P=0.051), although a significant interaction between AVO and RV-LV quartile was not observed (P=0.66). However, a significant interaction between AVO group and continuous RV-LV duration was observed (Table 3). For every 10-ms increase in RV-LV duration, patients in the AVO group had a 22% increase in the odds of response compared with a 7% increase in the odds of response for the control group (interaction P value, 0.046).
Figure 2.
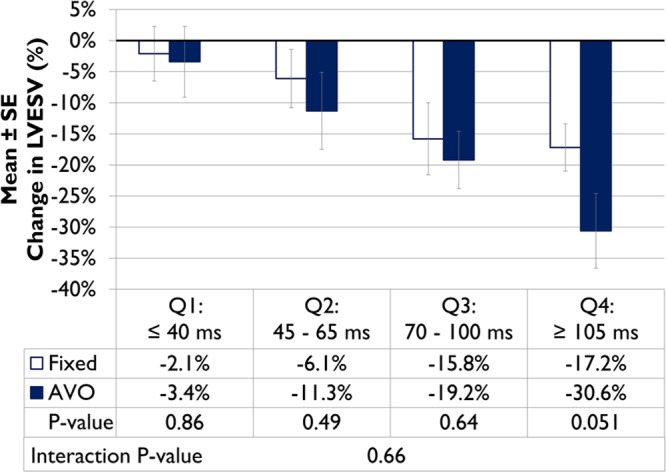
The relationship of atrioventricular optimization (AVO) subgroups and right ventricular-left ventricular duration on the percentage change in left ventricular end-systolic volume (LVESV).
Table 3.
Interaction of AVO With RV-LV on Cardiac Resynchronization Therapy Response: Continuous RV-LV
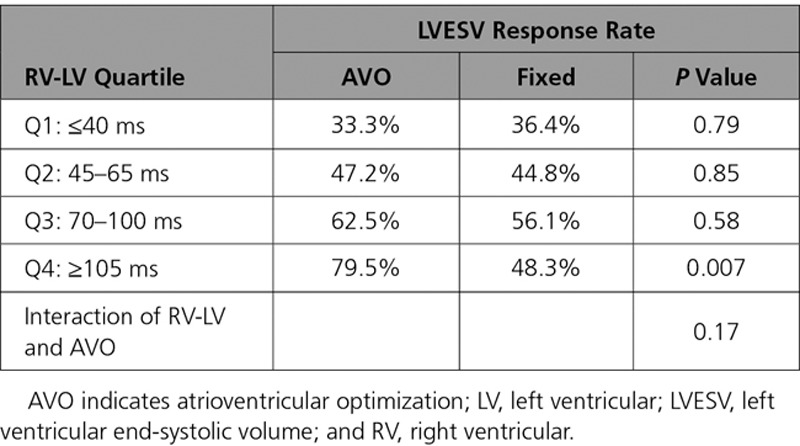
A similar finding was observed using CRT response rate—the primary end point of this study. As RV-LV duration prolongs, there was a progressive separation between the 2 groups with the significance reached for the longest quartile (Table 4). The overall response rate in this cohort was 52%. Patients with long RV-LV times (≥70 ms) had significantly greater response rates than those with short RV-LV (<70 ms; 62% versus short 40%, respectively; P<0.001).
Table 4.
Interaction of AVO With RV-LV on Cardiac Resynchronization Therapy Response: Quartile of RV-LV
To explore this relationship further, multivariable analysis was performed on response rates. These results are shown in Figure 3, where it can be observed that the magnitude of response increased monotonically as interventricular delay prolonged. No benefit of AVO was noted at the shortest quartile of RV-LV intervals (≤40 ms). However, at the longest 1 (≥105 ms), 2 (≥70 ms) or 3 (≥45 ms) quartiles, there was a significantly higher response rate with AVO. AVO improved the odds of an LVESV response ≈2-fold compared with the nominal AV delay in patients with RV-LV ≥45 ms (second to fourth quartiles). At the longest RV-LV durations (fourth quartile, ≥105 ms), there was a 4.26× greater odds of an LVESV response with AVO compared with nominal AV delay programming (P=0.010).
Figure 3.
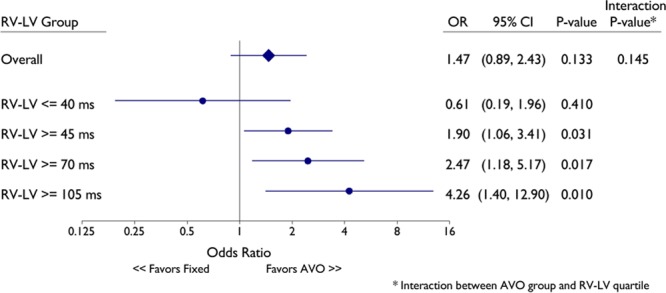
Multivariable logistic regression model of the impact of SD on cardiac resynchronization therapy response as defined by >15% decrease in left ventricular end-systolic volume (LVESV). The LVESV responses of AVO (SD) vs fixed AV delay are shown at different right ventricular-left ventricular cutoffs after adjusting for baseline ejection fraction, LVESV, pathogenesis of heart failure, left bundle branch block (LBBB), sex, NYHA (New York Heart Association classification), QRS, and age. AVO indicates atrioventricular optimization; CI, confidence interval; and OR, odds ratio.
Additional analyses were performed to assess the impact of AVO in prespecified subgroups. These results are summarized in Figure 4. Among patients with RV-LV duration below the median (<70 ms), there was no indication for a benefit of AVO overall or in any subgroup (Figure 4A). In contrast, the Forest plots (Figure 4B) show a beneficial effect of AVO programming among patients with longer RV-LV (≥70 ms) durations for the whole cohort and for several subgroups. Of note, none of the interaction P values were statistically significant for any of the other subgroups. The estimated effect of AVO in subgroups with lower responses to CRT (eg, men and non-LBBB morphology) were similar to that in women and those with LBBB morphology who are more likely to benefit from CRT.
Figure 4.
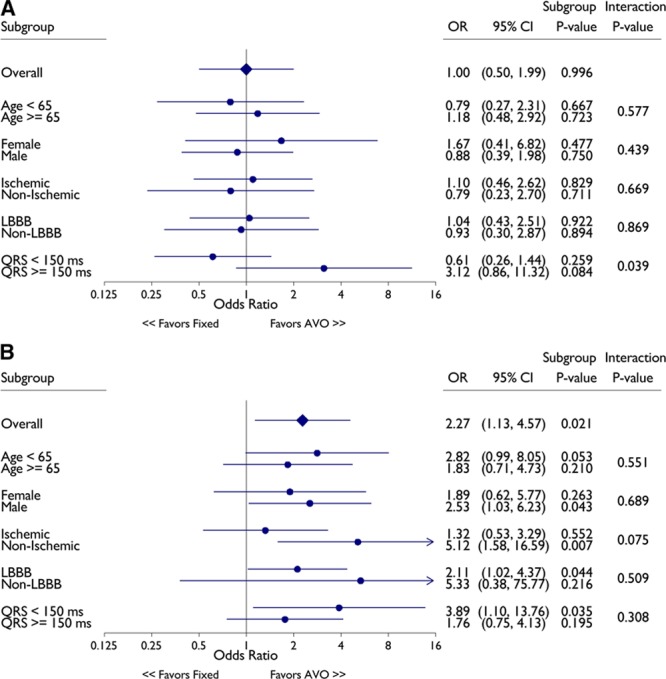
Univariable logistic regression results for cardiac resynchronization therapy (CRT) response at right ventricular (RV)-left ventricular (LV) duration < 70 ms (A) and RV-LV ≥ 70 ms (B) by subgroups. CRT response as defined by >15% decrease in left ventricular end-systolic volume. AVO indicates atrioventricular optimization; CI, confidence interval; and OR, odds ratio.
Discussion
The present study is an analysis of the interaction between interventricular electrical delay and AVO with an electrogram-based algorithm. The primary finding is that reverse remodeling with CRT, as assessed by changes in LVESV, is strongly dependent on the RV-LV interval with increased response as this interval prolongs for both the nominal and AVO subgroups. Moreover, the impact of AVO is also influenced by interventricular delay with greater changes in remodeling parameters at longer RV-LV intervals compared with nominal AV delay programming.
The SD algorithm is designed to maximize the acute hemodynamic response (LV dP/dtmax) with CRT.26 The algorithm recommends AV delays based on the sensed and paced intrinsic AV interval, as well as QRS duration and morphology. Previous studies of biventricular or LV-only pacing suggest that optimal fusion of intrinsic conduction down the right bundle branch with LV pacing maximizes the hemodynamic response.27–31 Thus, as the interventricular conduction delay increases, the greater the electrical resynchronization that can occur with an optimally timed stimulus. This would explain the relationship between RV-LV duration and the incremental benefit of this electrogram-based AVO method.
As noted above, even in non-LBBB subjects, most patients have earlier activation of the RV than LV at the implanted electrode sites, which suggests the presence of LV electrical delay. This also helps explain the similar benefit of AVO compared with patients with LBBB when the RV-LV duration is prolonged.
Several factors impact the magnitude of reverse remodeling and the clinical response to CRT in addition to QRS morphology. In general, better response rates are noted in women, nonischemic cardiomyopathy, and patients with longer unpaced QRS duration.20,32–35 These factors were all associated with improved volumetric remodeling in the SMART-AV trial. Although these factors predict response, there is still incremental predictive value of interventricular conduction delay within these subgroups. It is noteworthy that there may also be a benefit of AVO in many subgroups that are less likely to respond to CRT if the RV-LV duration was prolonged.
Previously, we showed that the QLV duration was also a predictor of CRT response10 and identified a subgroup that responded to AVO in the SMART-AV trial.24 QLV is a measure of LV delay and is independent of RV lead position in contrast to RV-LV duration. When both RV-LV and QLV were included in a multivariable analysis of response in this trial, the resulting model excluded QLV and retained RV-LV, indicating that RV-LV is the better predictor of the remodeling response.25 As further evidence that there are differences between the 2 measures, only 47 of the 68 patients (69%) in the fourth quartile of RV-LV were also in the fourth quartile of QLV. Overall, a net of 11.3% of responders were reclassified correctly when using RV-LV compared with QLV. Futhermore, implanted CRT devices can automatically measure RV-LV times, and in fact, this feature is now in multiple manufacturers’ devices, whereas QLV cannot be measured directly from intracardiac signals. Thus, RV-LV is likely to become the standard measure for assessing electrical delay.
Clinical Implications
Maximizing the magnitude of response continues to be a goal for CRT, despite optimized device programming. Recent studies suggest that a purely anatomic approach to lead position will be of limited value other than avoiding apical positions.7,8 However, placing leads in areas of late mechanical or electrical delay is associated with better echocardiographic response. The incremental benefit of AVO with physiological selection of pacing sites could have significant impact on reducing nonresponder rates. Thus, it seems reasonable to place LV leads or pace from electrodes of multipolar leads with long RV-LV durations and utilize AVO, such as the algorithm evaluated in this study (ie, SmartDelay) that maximizes electrical resynchronization, during implant and follow-ups. Of note, these algorithms optimize AV delay and either use simultaneous biventricular pacing or pace the LV only because multicenter studies of LV offset (ie, V-V timing) have been disappointing.36,37 Although the emphasis on CRT lead placement has been primarily on the LV lead, RV-LV duration is also affected by the RV lead position. Further study is warranted to assess whether a strategy of placing RV leads to maximize interventricular delay would improve outcomes. Our results indicate that AVO is unlikely to be of benefit in the absence of significant interventricular conduction delay, which may explain the neutral results from previous multicenter studies of such algorithms in which the lead implantation strategy was largely anatomic and the lead positioning was not targeted to maximize RV-LV delay.19–21
Limitations
This study should be interpreted in light of certain methodological limitations. RV-LV duration was measured at the final lead position, so there was only 1 interval associated with each patient. Therefore, the impact of lead repositioning on interventricular delay cannot be determined from this study. In addition, echocardiographic end points were used rather than more long-term follow-ups with clinical end points, such as HF hospitalizations or mortality. However, it is well documented that reverse remodeling is a strong predictor of outcomes in CRT.38 Finally, only 1 AVO algorithm (ie, SmartDelay) and no VV optimization was performed in this study. This algorithm was chosen because it was designed to promote electrical resynchronization and has been used in the present or similar forms in long-term studies, including COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure) and MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy).2,5 However, these results may not extrapolate to other optimization methods.
In summary, interventricular electrical dyssynchrony, as measured by RV-LV duration, was strongly associated with reverse remodeling with CRT. The incremental benefit of AVO was observed at the longest RV-LV durations. Further study is warranted to assess the value of guiding placement of leads for CRT by RV-LV durations with AVO to improve response rates with CRT.
Sources of Funding
This study was funded by Boston Scientific Corporation.
Disclosures
Dr Gold reports consulting fees and research grants from Boston Scientific and Medtronic. Dr Singh discloses research grants from St. Jude, Medtronic, Boston Scientific, and Biotronik, as well as Advisory Board, Steering Committee, and Consulting with Boston Scientific, Biotronik, St. Jude, Medtronic, Impulse Dynamics, Respicardia, Inc, and EBR, Inc. He also receives honoraria/speaker fees from Medtronic, Biotronik, Boston Scientific, St. Jude, and Liva Nova, Inc. Dr Birgersdotter-Green reports speakers bureau and research grants from Medtronic and St. Jude, as well as honorarium with Biotronik. Y. Yu, Dr Stein, N. Wold, and Dr Meyer are Boston Scientific employees. Dr Ellenbogen reports consulting fees and honoraria from Medtronic and Boston Scientific; speakers bureau fees from Medtroinic, Boston Scientific, St. Jude, Biotronik, and Sanofi; research grants from Medtronic, Boston Scientific, Biosense Webster, and Sanofi; and fellowship support from Medtronic, Boston Scientific, and Biosense Webster.
Supplementary Material
Footnotes
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 6.Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C, Schöndube F, Wolfhard U, Böcker D, Krahnefeld O, Kirkels H Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–2033. doi: 10.1016/s0735-1097(02)01895-8. doi: 10.1016/S0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 7.Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP, Zareba W, Moss AJ. Left ventricular lead position and clinical outcome in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 8.Thébault C, Donal E, Meunier C, Gervais R, Gerritse B, Gold MR, Abraham WT, Linde C, Daubert JC REVERSE Study Group. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J. 2012;33:2662–2671. doi: 10.1093/eurheartj/ehr505. doi: 10.1093/eurheartj/ehr505. [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Fan D, Heist EK, Alabiad CR, Taub C, Reddy V, Mansour M, Picard MH, Ruskin JN, Mela T. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm. 2006;3:1285–1292. doi: 10.1016/j.hrthm.2006.07.034. doi: 10.1016/j.hrthm.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Gold MR, Birgersdotter-Green U, Singh JP, Ellenbogen KA, Yu Y, Meyer TE, Seth M, Tchou PJ. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J. 2011;32:2516–2524. doi: 10.1093/eurheartj/ehr329. doi: 10.1093/eurheartj/ehr329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansalone G, Giannantoni P, Ricci R, Trambaiolo P, Fedele F, Santini M. Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. J Am Coll Cardiol. 2002;39:489–499. doi: 10.1016/s0735-1097(01)01772-7. [DOI] [PubMed] [Google Scholar]
- 12.Becker M, Kramann R, Franke A, Breithardt OA, Heussen N, Knackstedt C, Stellbrink C, Schauerte P, Kelm M, Hoffmann R. Impact of left ventricular lead position in cardiac resynchronization therapy on left ventricular remodelling. A circumferential strain analysis based on 2D echocardiography. Eur Heart J. 2007;28:1211–1220. doi: 10.1093/eurheartj/ehm034. doi: 10.1093/eurheartj/ehm034. [DOI] [PubMed] [Google Scholar]
- 13.Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52:1402–1409. doi: 10.1016/j.jacc.2008.06.046. doi: 10.1016/j.jacc.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Delgado V, van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR, Ypenburg C, Boersma E, Holman ER, Schalij MJ, Bax JJ. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78. doi: 10.1161/CIRCULATIONAHA.110.945345. doi: 10.1161/CIRCULATIONAHA.110.945345. [DOI] [PubMed] [Google Scholar]
- 15.Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 16.D’Onofrio A, Botto G, Mantica M, La Rosa C, Occhetta E, Verlato R, Molon G, Ammendola E, Villani GQ, Bongiorni MG, Gelmini GP, Ciardiello C, Dicandia CD. The interventricular conduction time is associated with response to cardiac resynchronization therapy: interventricular electrical delay. Int J Cardiol. 2013;168:5067–5068. doi: 10.1016/j.ijcard.2013.07.201. doi: 10.1016/j.ijcard.2013.07.201. [DOI] [PubMed] [Google Scholar]
- 17.D’Onofrio A, Botto G, Mantica M, LA Rosa C, Occhetta E, Verlato R, Molon G, Ammendola E, Villani GQ, Bongiorni MG, Bianchi V, Gelmini GP, Valsecchi S, Ciardiello C. Incremental value of larger interventricular conduction time in improving cardiac resynchronization therapy outcome in patients with different QRS duration. J Cardiovasc Electrophysiol. 2014;25:500–506. doi: 10.1111/jce.12381. doi: 10.1111/jce.12381. [DOI] [PubMed] [Google Scholar]
- 18.Gold MR, Yu Y, Wold N, Day JD. The role of interventricular conduction delay to predict clinical response with cardiac resynchronization therapy. Heart Rhythm. 2017;14:1748–1755. doi: 10.1016/j.hrthm.2017.10.016. doi: 10.1016/j.hrthm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Abraham WT, Gras D, Yu CM, Guzzo L, Gupta MS FREEDOM Steering Committee. Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J. 2010;159:944.e1–948.e1. doi: 10.1016/j.ahj.2010.02.034. doi: 10.1016/j.ahj.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 21.Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, Gasparini M, Starling RC, Milasinovic G, Rogers T, Sambelashvili A, Gorcsan J, III, Houmsse M Adaptive CRT Study Investigators. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9:1807–1814. doi: 10.1016/j.hrthm.2012.07.009. doi: 10.1016/j.hrthm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, Borri-Brunetto A, Silvestre J. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. Europace. 2012;14:1324–1333. doi: 10.1093/europace/eus059. doi: 10.1093/europace/eus059. [DOI] [PubMed] [Google Scholar]
- 23.Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, Starling RC, Milasinovic G, Gorcsan J, III, Houmsse M, Abeyratne A, Sambelashvili A, Martin DO. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm. 2013;10:1368–1374. doi: 10.1016/j.hrthm.2013.07.007. doi: 10.1016/j.hrthm.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR, Yu Y, Singh JP, Stein KM, Birgersdotter-Green U, Meyer TE, Seth M, Ellenbogen KA. The effect of left ventricular electrical delay on AV optimization for cardiac resynchronization therapy. Heart Rhythm. 2013;10:988–993. doi: 10.1016/j.hrthm.2013.03.009. doi: 10.1016/j.hrthm.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Gold MR, Singh JP, Ellenbogen KA, Yu Y, Wold N, Meyer TE, Birgersdoter-Green U. Interventricular electrical delay is predictive of response to cardiac resynchronization therapy. JACC Clin Electrophysiol. 2016;2:438–447. doi: 10.1016/j.jacep.2016.02.018. doi: 10.1016/j.jacep.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant JL, Kim MH, Yu Y, Ding J, Waggoner AD. A prospective comparison of AV delay programming methods for hemodynamic optimization during cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2007;18:490–496. doi: 10.1111/j.1540-8167.2007.00770.x. doi: 10.1111/j.1540-8167.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 27.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Kramer A, Ding J, Salo R, Tockman B, Pochet T, Spinelli J. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Kramer A, Spinelli J, Ding J, Hoersch W, Auricchio A. Biventricular mechanical asynchrony predicts hemodynamic effect of uni- and biventricular pacing. Am J Physiol Heart Circ Physiol. 2003;285:H2788–H2796. doi: 10.1152/ajpheart.00119.2003. doi: 10.1152/ajpheart.00119.2003. [DOI] [PubMed] [Google Scholar]
- 29.van Gelder BM, Bracke FA, Meijer A, Pijls NH. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol. 2005;46:2305–2310. doi: 10.1016/j.jacc.2005.02.098. doi: 10.1016/j.jacc.2005.02.098. [DOI] [PubMed] [Google Scholar]
- 30.Lee KL, Burnes JE, Mullen TJ, Hettrick DA, Tse HF, Lau CP. Avoidance of right ventricular pacing in cardiac resynchronization therapy improves right ventricular hemodynamics in heart failure patients. J Cardiovasc Electrophysiol. 2007;18:497–504. doi: 10.1111/j.1540-8167.2007.00788.x. doi: 10.1111/j.1540-8167.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 31.Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant JL, Kim MH, Yu Y. A prospective, randomized comparison of the acute hemodynamic effects of biventricular and left ventricular pacing with cardiac resynchronization therapy. Heart Rhythm. 2011;8:685–691. doi: 10.1016/j.hrthm.2010.12.039. doi: 10.1016/j.hrthm.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, Freemantle N, Cleland JG, Tavazzi L, Daubert C CARE-HF Investigators. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11:699–705. doi: 10.1093/eurjhf/hfp074. doi: 10.1093/eurjhf/hfp074. [DOI] [PubMed] [Google Scholar]
- 33.Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ MADIT-CRT Investigators. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 34.Gold MR, Thébault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert JC. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126:822–829. doi: 10.1161/CIRCULATIONAHA.112.097709. doi: 10.1161/CIRCULATIONAHA.112.097709. [DOI] [PubMed] [Google Scholar]
- 35.Cheng A, Gold MR, Waggoner AD, Meyer TE, Seth M, Rapkin J, Stein KM, Ellenbogen KA. Potential mechanisms underlying the effect of gender on response to cardiac resynchronization therapy: insights from the SMART-AV multicenter trial. Heart Rhythm. 2012;9:736–741. doi: 10.1016/j.hrthm.2011.12.013. doi: 10.1016/j.hrthm.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Christenson SD, Chareonthaitawee P, Burnes JE, Hill MR, Kemp BJ, Khandheria BK, Hayes DL, Gibbons RJ. Effects of simultaneous and optimized sequential cardiac resynchronization therapy on myocardial oxidative metabolism and efficiency. J Cardiovasc Electrophysiol. 2008;19:125–132. doi: 10.1111/j.1540-8167.2007.00996.x. doi: 10.1111/j.1540-8167.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 37.Boriani G, Muller CP, Seidl KH, Grove R, Vogt J, Danschel W, Schuchert A, Djiane P, Biffi M, Becker T, Bailleul C, Trappe HJ. Randomized comparison of simultaneous biventricular stimulation versus optimized interventricular delay in cardiac resynchronization therapy. Resynchronization for HemodYnamic Treatment for Heart Failure Management II (RHYTHM II) investigators. Pacing Clin Electrophysiol. 2009;32:S120–S125. [Google Scholar]
- 38.Gold MR, Daubert C, Abraham WT, Ghio S, St John Sutton M, Hudnall JH, Cerkvenik J, Linde C. The effect of reverse remodeling on long-term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: results of the REVERSE study. Heart Rhythm. 2015;12:524–530. doi: 10.1016/j.hrthm.2014.11.014. doi: 10.1016/j.hrthm.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


