Intravitreal injection of bevacizumab or aflibercept significantly reduced plasma vascular endothelial growth factor level for up to 1 month, whereas intravitreal ranibizumab produced no such effect. Plasma vascular endothelial growth factor showed no correlation with the severity of diabetic macular edema or diabetic retinopathy.
Key words: aflibercept, anti–vascular endothelial growth factor agents, arterial thromboembolic events, bevacizumab, intravitreal injection, diabetic macular edema, diabetic retinopathy, plasma vascular endothelial growth factor level, ranibizumab, vascular endothelial growth factor
Abstract
Purpose:
The aim of this study was to investigate the changes in plasma vascular endothelial growth factor (VEGF) level depending on the severity of diabetic retinopathy (DR) or diabetic macular edema (DME) and after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for treatment of DME.
Methods:
Plasma VEGF level was evaluated in 72 patients with DR and changes were measured in 42 patients with DME receiving intravitreal injections of bevacizumab, aflibercept, or ranibizumab at the initial injection.
Results:
There were no correlations between plasma VEGF level and the severity of DME or DR. Baseline plasma VEGF level (51.9 pg/mL) was significantly reduced using bevacizumab to 11.9 pg/mL after 1 week and 24.1 pg/mL after 4 weeks (P = 0.0130 and 0.0201, respectively). In aflibercept-treated eyes, plasma VEGF decreased from 52.2 pg/mL to 7.8 pg/mL and 12.6 pg/mL, respectively, at the same time points (both P < 0.001). No such reductions were observed in patients receiving ranibizumab.
Conclusion:
Baseline plasma VEGF level showed no correlations with DR or DME severity, whereas intravitreal injection of bevacizumab or aflibercept significantly reduced plasma VEGF for up to 4 weeks and ranibizumab produced no such effects. Changes in plasma VEGF level seemed not to be critical in progression or treatment of DME and DR.
In the progression of diabetic retinopathy (DR), vascular endothelial growth factor (VEGF) overexpression contributes in a dual pathway involving proliferative changes using retinal neovascularization and the induction of diabetic macular edema (DME) inducing hyperpermeability of retinal vasculature.1–4 After extensive basic and clinical research attributing DME to VEGF,1–9 regimens of intravitreal anti-VEGF agent injections were developed and investigation of their efficacy and safety in large-scale clinical trials have established these drugs as the new first line of DME treatment.5–9 In addition to the vision improvement achieved in DME therapy, excellent results in halting disease progression by suppressing retinal neovascularization in the advancement of nonproliferative diabetic retinopathy (NPDR) to proliferative diabetic retinopathy (PDR) by anti-VEGF agents have been reported.10,11 In eyes with PDR, treatment using ranibizumab produced visual outcomes that were comparable with those of panretinal photocoagulation treatment at 2 years.10 Overexpressed VEGF may cause DME even in eyes with NPDR before inducing retinal neovascularization that is the hallmark of progression to PDR. Because DME may persist throughout such DR progression, anti-VEGF agents can be adopted for the treatment of both NPDR and PDR.
Vascular endothelial growth factor may reach retinal vessels from the abluminal side through intraocular fluid or from the intraluminal side through systemic circulation as plasma VEGF. Intraocular VEGF level was found to correlate with DME12,13 and DR severity,14–16 whereas plasma VEGF showed no association with DME status.15 As far as we know, there have been no reports investigating the correlation between plasma VEGF level and DR severity. Because elevated plasma VEGF was correlated with tumor angiogenesis and subsequent progression and metastasis, intravenously administered bevacizumab became the first antiangiogenic treatment approved by the American Food and Drug Administration in the first-line treatment of metastatic colorectal cancer.17 Thus, we considered the possibility that circulating plasma VEGF was related to retinal angiogenesis and resultant DR severity. One aim of the current study was to investigate for correlations between plasma VEGF level, which may promote retinal neovascularization and retinal vessel hyperpermeability, and DR and DME status accordingly.
Intravitreal injections of anti-VEGF agents have been associated with detectable levels of the drugs in the systemic circulation and suppression of plasma VEGF.18 Although the safety of intravitreal anti-VEGF agents has been confirmed in large clinical trials, longer term administration of anti-VEGF drugs may be associated with systemic adverse events.19,20 When intravenous bevacizumab was used in the treatment of solid tumors, adverse events related to VEGF activity abrogation, such as arterial thromboembolic events, myocardial infarction, and stroke, were observed.19,21 Consequently, there are concerns regarding the potential adverse events caused by intravitreal injections of anti-VEGF agents because they lead to systemic VEGF suppression to below the lower limit of quantitation.22 After intravitreal injection, VEGF suppression in the systemic circulation has been investigated mainly in patients with age-related macular degeneration (AMD).23–27 Although the much smaller injection doses than those in intravenous cancer treatment were determined as safe in multicenter trials,5,28 these series excluded patients with the highest risk of systemic adverse effects under anti-VEGF agents, that is, those with recent cerebrovascular events or myocardial infarction. In a population-based study of 57,919 patients with AMD aged 65 years or older in Ontario, Canada, comparisons of emergency visits for thromboembolic events before intravitreal anti-VEGF injections and those observed at 1 year of treatment revealed an absolute change in risk from 10.7 to 18.6 per 1,000 patients annually.29 When treating patients with DME, clinicians must be especially vigilant because diabetic patients with retinopathy often have underlying systemic comorbidities that can put them at risk for cardiovascular or cerebrovascular events.30
The objective of the current study was to evaluate the role of plasma VEGF level in the development of DR and DME according to disease severity. In patients with DME treated with intravitreal injections of bevacizumab, aflibercept, or ranibizumab, we also investigated the changes in plasma VEGF concentration before and after therapy.
Materials and Methods
This prospective study was planned using the tenets set forth by the Declaration of Helsinki and was performed after approval from the institutional review committee of the Shinshu University School of Medicine, Japan (No. 2256 and 2287). Written informed consent was obtained from all enrolled patients.
Diabetic retinopathy was diagnosed using ophthalmoscopy and contact lens slit-lamp biomicroscopy after mydriasis independently by two ophthalmologists (T.H. and T.M.) who were masked to HbA1c findings. Diabetic retinopathy and DME severity were determined according to respective international clinical DR and DME severity scales.31 Briefly, DR was categorized as no DR, mild, moderate, or severe NPDR, or PDR. Diabetic macular edema was classified as mild, moderate, or severe. Data on age, sex, earlier HbA1C (National Glycohemoglobin Standardization Program) level, and duration of DM were collected from medical records.
Eligible participants were at least 20 years of age and afflicted with Type 2 diabetes mellitus. Other inclusion criteria were: 1) visual impairment due to DME; 2) fovea-involving macular edema defined as central subfield macular thickness (CMT) ≥300 μm measured as mean retinal thickness in the central 1-mm diameter circle by spectral domain optical coherence tomography (Cirrus OCT; Carl Zeiss Meditec, Inc, Dublin, CA); and 3) no history or presence of other ocular diseases causing vision deterioration, such as AMD or severe PDR. The exclusion criteria were: 1) history of previous injections of anti-VEGF agents; 2) any retinal photocoagulation treatment in the study eye within the 3 months preceding the initial injection of anti-VEGF agents; 3) history of local steroid treatment; 4) history of cerebrovascular accident, myocardial infarction, or other systemic disease requiring medications that could affect the results of the study; and 5) poorly controlled diabetes mellitus (i.e., HbA1c > 12.0%); Patients who were judged as ineligible for any other reason by the investigators were excluded. The control group consisted of 17 patients without DR or DME who underwent cataract surgery. Seventy-five patients with DME satisfied the inclusion criteria between June 2013 and August 2016 and were randomly assigned bevacizumab, aflibercept, or ranibizumab treatment. Loses to follow-up were due to a failure to meet medical appointments (i.e., no show) in all cases.
Vision and Central Subfield Macular Thickness Assessment
Best-corrected visual acuity was measured on a decimal vision scale and converted to logarithm of the minimum angle of resolution (logMAR) vision scores for comparison and statistical analysis. Central subfield macular thickness was determined as the mean retinal thickness in the central 1-mm diameter circle using spectral domain optical coherence tomography.
Blood Sample Collection
Blood samples were obtained before the intravitreal injection of anti-VEGF agents and at 1 and 4 weeks afterward. For the VEGF assay, blood samples were collected in citrate, theophylline, adenosine, and dipyridamole tubes to preserve platelets and prevent activation, thus minimizing the release of VEGF and other cytokines and growth factors derived from platelets. Centrifugation was performed at 3,000 rpm for 20 minutes within 1 hour after sampling. Plasma was stored at −20°C until testing.
Vascular Endothelial Growth Factor Assay
Plasma VEGF levels were determined using ELISA (Quantikine VEGF ELISA Kit; R&D Systems, Minneapolis, MN) as described by the manufacturer. All samples were analyzed together in duplicate.
Statistical Analysis
Statistical analysis was performed using Prism version 5 software (GraphPad software, La Jolla, CA). Categorical variables were presented as number and percentage and analyzed using the chi-square or Fisher's exact tests. Paired t-tests or the Mann–Whitney U test were used for continuous variables. The Friedman repeated-measures analysis of variance on ranks was used to compare three groups or more. Spearman's correlation coefficient was adopted to identify correlations between plasma VEGF and DR or DME severity. A P value of less than 0.05 was judged as statistically significant. For analytic purposes, a VEGF concentration of less than the lower limit of quantitation (15.6 pg/mL) was considered to be 7.8 pg/mL.
Results
The baseline characteristics of the participants are presented in Table 1. There were no apparent differences among the three test groups in terms of age, sex, duration of diabetes, HbA1c, or severity according to DR and DME disease severity scales. The patients with DME and control group were age-matched at 64 ± 10 and 62 ± 13 years, respectively (P = 0.5324). Only the patients who met all medical appointments were included. No ocular or systemic adverse events were observed during the 1-month study period.
Table 1.
Key Baseline Characteristics and Patient Demographics
Plasma Vascular Endothelial Growth Factor Levels at Baseline
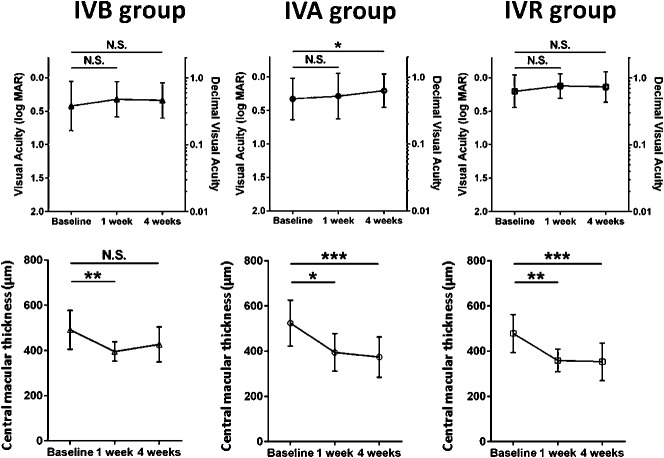
Despite considerable variability among individual VEGF levels, there was no significant difference between mean values in patients with DME (56.5 ± 47.7 pg/mL) and age-matched controls (51.7 ± 31.1 pg/mL) (P = 0.9238) (Figure 1).
Fig. 1.
Plasma VEGF level in patients with DME and nondiabetic age-matched control subjects. There was no significant difference between mean values in patients with DME (56.5 ± 47.7 pg/mL) and controls (51.7 ± 31.1 pg/mL) (P = 0.9238). N.S., not significant.
Relationship Between Plasma Vascular Endothelial Growth Factor and Diabetic Retinopathy Severity
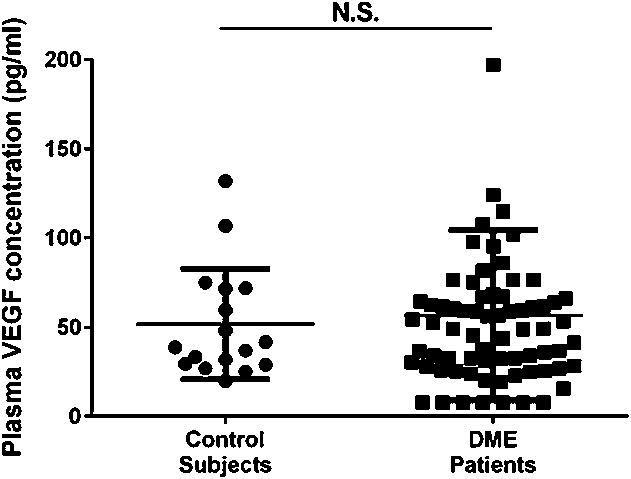
Plasma VEGF level showed no statistically significant correlations with DR stage advancement as follows: mild NPDR (66.3 ± 36.9 pg/mL), moderate NPDR (52.3 ± 45.2 pg/mL), severe NPDR (51.7 ± 47.4 pg/mL), and PDR (72.0 ± 55.0 pg/mL) (r = 0.1200, P = 0.3152) (Figure 2).
Fig. 2.
Correlation between the severity of DR and plasma VEGF concentration. Plasma VEGF showed no statistically significant correlation with DR stage advancement for mild NPDR (66.3 ± 36.9 pg/mL), moderate NPDR (52.3 ± 45.2 pg/mL), severe NPDR (51.7 ± 47.4 pg/mL), or PDR (72.0 ± 55.0 pg/mL) (r = 0.1200, P = 0.3152).
Relationship Between Plasma Vascular Endothelial Growth Factor and Diabetic Macular Edema Severity
Plasma VEGF level showed no statistically significant correlations with DME severity as follows: mild DME (46.9 ± 29.7 pg/mL), moderate DME (44.3 ± 25.3 pg/mL), and severe DME (62.8 ± 55.2 pg/mL) (r = 0.1401, P = 0.2405) (Figure 3).
Fig. 3.
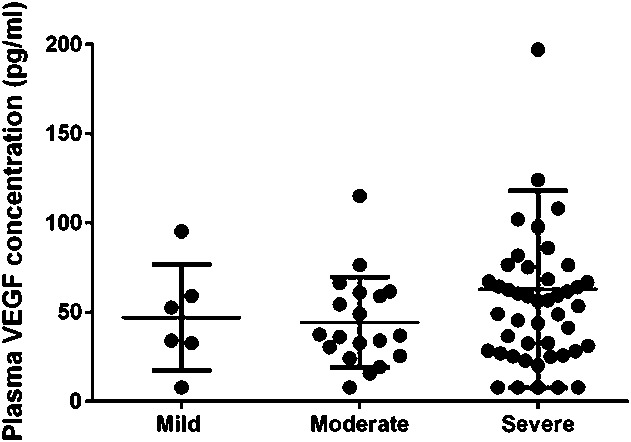
Correlation between the severity of DME and plasma VEGF concentration. Plasma VEGF level did not show any statistically significant correlation with DME severity for mild DME (46.9 ± 29.7 pg/mL), moderate DME (44.3 ± 25.3 pg/mL), or severe DME (62.8 ± 55.2 pg/mL) (r = 0.1401, P = 0.2405).
Changes in Best-Corrected Visual Acuity and Central Subfield Macular Thickness After the Initial Anti–Vascular Endothelial Growth Factor Injection
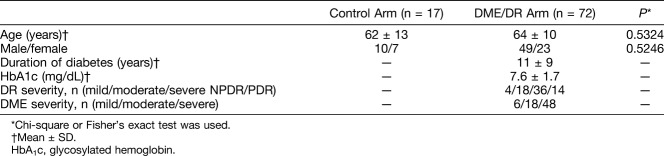
In the intravitreal bevacizumab (IVB), intravitreal aflibercept (IVA), and intravitreal ranibizumab (IVR) groups, best-corrected visual acuity tended to improve from baseline values of 0.42 ± 0.37 logMAR (20/53), 0.33 ± 0.31 logMAR (20/43), and 0.20 ± 0.24 logMAR (20/32), respectively, to 0.32 ± 0.26 logMAR (20/42), 0.28 ± 0.34 logMAR (20/38), and 0.12 ± 0.18 logMAR (20/27), respectively, at 1 week. At 4 weeks, only the IVA group achieved statistically significant vision improvement (0.24 ± 0.26 logMAR [20/34]) (Figure 4).
Fig. 4.
Changes in vision and CMT after a single intravitreal anti-VEGF agent injection. In the IVB, IVA, and IVR groups, vision was not improved significantly at 1 week and reached statistically significant amelioration in the IVA group only at 4 weeks. In all test groups, CMT showed a significant reduction at 1 week. The IVA and IVR groups retained this reduction at 4 weeks. *P < 0.05, **P < 0.01, ***P < 0.001. N.S., not significant.
Central subfield macular thickness showed a significant reduction from baseline (491.3 ± 86.4 μm, 524.1 ± 68.6 μm, and 478.3 ± 83.8 μm, respectively) to 1 week (395.3 ± 42.5 μm, 394.3 ± 196.6 μm, and 358.8 ± 50.5 μm, respectively) in all test groups. The IVA and IVR groups (374.3 ± 214.2 μm and 353.5 ± 82.8 μm, respectively), but not the IVB group (426.8 ± 77.5 μm), maintained significant CMT reductions at 4 weeks.
Changes in Plasma Vascular Endothelial Growth Factor Level After the Initial Anti–Vascular Endothelial Growth Factor Injection
The mean baseline plasma VEGF level in patients with DME was 51.9 ± 37.8 pg/mL, 52.2 ± 25.4 pg/mL, and 65.8 ± 43.4 pg/mL for the bevacizumab, aflibercept, and ranibizumab groups, respectively. Bevacizumab and aflibercept produced significant suppression of plasma VEGF level after intravitreal injection, with most plasma samples displaying VEGF levels below detectable limits at 1 and 4 weeks after injection. Individual patient values for bevacizumab showed marked suppression of VEGF of 11.9 ± 8.7 pg/mL at 1 week and 24.1 ± 11.8 pg/mL at 4 weeks compared with the baseline of 51.9 ± 37.8 pg/mL. Patient treated with aflibercept also exhibited prominent suppression of plasma VEGF at 1 week, with most samples showing suppression to the lower limit of quantitation, and mean VEGF level remained suppressed at 12.6 ± 8.6 pg/mL at 4 weeks compared with the baseline of 52.2 ± 25.4 pg/mL. However, mean plasma VEGF level after intravitreal injection of ranibizumab was largely unchanged at 50.1 ± 30.5 pg/mL at 1 week and 61.9 ± 43.4 pg/mL at 4 weeks versus the baseline of 65.8 ± 43.4 pg/mL (Figure 5).
Fig. 5.
Plasma VEGF levels after a single injection of IVB, IVA, or IVR in patients with DME. Intravitreal bevacizumab and IVA induced significant suppression of plasma VEGF at 1 and 4 weeks after injection. Mean plasma VEGF after IVR was largely unchanged. *P < 0.05, ***P < 0.001. N.S., not significant.
Discussion
Despite numerous reports on the effects of intravitreal anti-VEGF agents on plasma VEGF level in patients with AMD, limited data are available on those in patients with DME.27,32 At the conception of this study, there existed only one report investigating the influence of anti-VEGF drugs on circulating plasma VEGF in patients with DME that compared the relative effects of bevacizumab, ranibizumab, and pegaptanib.27 However, the three most common anti-VEGF agents are currently bevacizumab, aflibercept, and ranibizumab.32 Very recently, Avery et al described the changes in systemic VEGF level after intravitreal injection of these drugs in patients with DME and reported similar suppression patterns to those in patients with AMD, that is, bevacizumab and aflibercept suppressed plasma VEGF level below the lower limit of quantitation for at least 1 month, whereas ranibizumab had no significant effect.32 We believe that the study of plasma VEGF level after intravitreal anti-VEGF agent injection in patients with DME remains important because even patients with NPDR are at risk of cardiovascular or cerebrovascular events,30 and the probability of thromboembolic events may increase by the suppression of VEGF-induced angiogenesis in blood supply to ischemic lesions.21
In the current study, bevacizumab and aflibercept significantly suppressed plasma VEGF level at 1 and 4 weeks after a single intravitreal injection, whereas ranibizumab exerted no remarkable effects. These results were consistent with the above-mentioned studies on patients with DME27,32 and comparable with those observed in patients with AMD. Wang et al26 reported that in patients with AMD, IVR did not significantly alter plasma VEGF concentration. However, patients receiving an intravitreal injection of bevacizumab or aflibercept showed dramatic reductions in plasma VEGF from baseline at 1 and 4 weeks after injection.22,27
Bevacizumab is a full-length monoclonal antibody (149 kDa) against VEGF containing an Fc region and is likely subjected to neonatal Fc receptor recycling to confer a long systemic half-life of approximately 20 days after intravenous infusion.33
Aflibercept is a soluble fusion protein (115 kDa) that combines ligand-binding elements taken from the extracellular components of VEGF receptor-1 and VEGF receptor-2 and the Fc portion of IgG.34 Because aflibercept has an intact Fc region, it is recycled through binding to the neonatal Fc receptor in endothelial cells and salvaged from proteolytic catabolism. The reported half-life of aflibercept in the plasma is 5 to 6 days after intravenous administration.32 By contrast, ranibizumab is an anti–VEGF-A affinity-matured monovalent monoclonal antibody fragment that lacks the Fc antibody region. Consequently, it is cleared from the circulation more rapidly, with a short systemic elimination half-life of 2 hours.35
Baseline plasma VEGF level did not differ between the DR group and controls. We also observed no correlation between plasma VEGF level and DR severity, in which retinal neovascularization is the hallmark of progression from NPDR to PDR according to international clinical DR and DME severity scales.31 Moreover, no significant relationship between DME severity and plasma VEGF was found.
In the treatment of DME, the efficacy of vision improvement tends to be the primary factor taken into consideration when selecting an anti-VEGF agent. The DRCR.net protocol T randomized 660 patients with DME with an Early Treatment Diabetic Retinopathy Study letter score of 78 (approximate Snellen equivalent: 20/32) to 24 (approximate Snellen equivalent: 20/320) and were examined about aflibercept, bevacizumab, or ranibizumab. When the initial visual acuity loss was mild (78–69 Early Treatment Diabetic Retinopathy Study letters; equivalent to 20/32–20/40), there were no apparent differences in vision recovery among the anti-VEGF agents. However, at diminished initial visual acuity levels of less than 69 Early Treatment Diabetic Retinopathy Study letters (equivalent to <20/40), aflibercept was more effective in improving vision than the others.28 This advantage of aflibercept over ranibizumab noted at 1 year decreased and was no longer statistically significant at 2 years, whereas aflibercept remained superior to bevacizumab.36 In the current study as well, the patients in the aflibercept group whose initial vision (20/42) corresponded to the worse level vision group in protocol T showed statistically significant vision improvement at 4 weeks, in contrast to no remarkable improvement for bevacizumab or ranibizumab. However, all drugs produced a significant reduction in CMT that indicated successful resolution of DME.
Intraocular VEGF level, but not plasma VEGF level, is correlated with both DME resolution and retinal neovascularization.15,37,38 The results of this study also showed that plasma VEGF was unrelated to DME and DR severity, implying that suppression of plasma VEGF was not indispensable in the treatment of DME or DR using intravitreal anti-VEGF injections. However, sustained suppression of plasma VEGF over years may be caused by prolonged regimens of bevacizumab or aflibercept.
Provided that vision improvement does not greatly differ among the most commonly used anti-VEGF agents in patients with an Early Treatment Diabetic Retinopathy Study initial letter score of 69 (approximately 20/40) or better as reported in DRCR.net protocol T,28,36 ranibizumab that exerts a minimal reductive effect on plasma VEGF may be the treatment modality of choice in diabetic patients who are prone to thromboembolic events.30 Despite this, Avery and Gordon20 reported that even ranibizumab showed a possible increased risk of cerebrovascular accidents when used monthly for 2 years. These studies together with our own suggest that minimizing the total exposure to anti-VEGF agents is important to avoid sustained suppression of plasma VEGF level and potentially resultant thromboembolic events in patients with DME or DR.
However, each patient's baseline VEGF level is different, and it is uncertain whether these VEGF level changes after intravitreal injections may have a true clinical impact on such morbidity and mortality of thromboembolic events. This investigation has several limitations. It was a single-center study containing a limited sample size, and changes in plasma VEGF level was evaluated just once per patient, all of which might have affected the results. Further investigation with increased number of patients is warranted.
Footnotes
Supported by JSPS KAKENHI Grant Number JP 16K11283.
None of the authors has any financial/conflicting interests to disclose.
References
- 1.Murata T, Ishibashi T, Khalil A, et al. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res 1995;27:48–52. [DOI] [PubMed] [Google Scholar]
- 2.Murata T, Nakagawa K, Khalil A, et al. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest 1996;74:819–825. [PubMed] [Google Scholar]
- 3.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994;118:445–450. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122:2044–2052. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801. [DOI] [PubMed] [Google Scholar]
- 7.Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064–1077.e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010;33:2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang GE, Berta A, Eldem BM, et al. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology 2013;120:2004–2012. [DOI] [PubMed] [Google Scholar]
- 10.Writing Committee for the Diabetic Retinopathy Clinical Research N, Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015;314:2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross JG, Glassman AR. A novel treatment for proliferative diabetic retinopathy: anti-vascular endothelial growth factor therapy. JAMA Ophthalmol 2016;134:13–14. [DOI] [PubMed] [Google Scholar]
- 12.Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina 2012;32:2150–2157. [DOI] [PubMed] [Google Scholar]
- 13.Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002;133:70–77. [DOI] [PubMed] [Google Scholar]
- 14.Endo M, Yanagisawa K, Tsuchida K, et al. Increased levels of vascular endothelial growth factor and advanced glycation end products in aqueous humor of patients with diabetic retinopathy. Horm Metab Res 2001;33:317–322. [DOI] [PubMed] [Google Scholar]
- 15.Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol 2005;243:3–8. [DOI] [PubMed] [Google Scholar]
- 16.Santhan Gopal KS. Increased levels of vascular endothelial growth factor in aqueous humor of patients with diabetic retinopathy: is it the whole truth? Indian J Ophthalmol 2011;59:405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranieri G, Patruno R, Ruggieri E, et al. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem 2006;13:1845–1857. [DOI] [PubMed] [Google Scholar]
- 18.Csaky K, Do DV. Safety implications of vascular endothelial growth factor blockade for subjects receiving intravitreal anti-vascular endothelial growth factor therapies. Am J Ophthalmol 2009;148:647–656. [DOI] [PubMed] [Google Scholar]
- 19.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol 2016;134:21–29. [DOI] [PubMed] [Google Scholar]
- 21.Schutz FA, Je Y, Azzi GR, et al. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol 2011;22:1404–1412. [DOI] [PubMed] [Google Scholar]
- 22.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;98:1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida I, Shiba T, Taniguchi H, et al. Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2014;252:1483–1489. [DOI] [PubMed] [Google Scholar]
- 24.Enders P, Muether PS, Hermann M, et al. Long-term alterations of systemic vascular endothelial growth factor levels in patients treated with ranibizumab for age-related macular degeneration. Retina 2015;35:454–458. [DOI] [PubMed] [Google Scholar]
- 25.Jin E, Bai Y, Luo L, et al. Serum levels of vascular endothelial growth factor before and after intravitreal injection of ranibizumab or conbercept for neovascular age-related macular degeneration. Retina 2017;37:971–977. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Sawada T, Sawada O, et al. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol 2014;158:738–744. [DOI] [PubMed] [Google Scholar]
- 27.Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol 2013;97:454–459. [DOI] [PubMed] [Google Scholar]
- 28.Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlenker MB, Thiruchelvam D, Redelmeier DA. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am J Ophthalmol 2015;160:569–580.e565. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki R, Tanaka S, Tanaka S, et al. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology 2013;120:574–582. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson CP, Ferris FL, III, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682. [DOI] [PubMed] [Google Scholar]
- 32.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017;37:1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol 2008;146:508–512. [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis 2012;15:171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci 2013;54:1616–1624. [DOI] [PubMed] [Google Scholar]
- 36.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funatsu H, Yamashita H, Mimura T, et al. Risk evaluation of outcome of vitreous surgery based on vitreous levels of cytokines. Eye (Lond) 2007;21:377–382. [DOI] [PubMed] [Google Scholar]
- 38.Dong N, Xu B, Chu L, Tang X. Study of 27 aqueous humor cytokines in type 2 diabetic patients with or without macular edema. PLoS One 2015;10:e0125329. [DOI] [PMC free article] [PubMed] [Google Scholar]