Abstract
Expression of the three bithorax complex homeotic genes is orchestrated by nine parasegment-specific regulatory domains. Autonomy of each domain is conferred by boundary elements (insulators). Here, we have used an in situ replacement strategy to reanalyze the sequences required for the functioning of one of the best-characterized fly boundaries, Fab-7. It was initially identified by a deletion, Fab-71, that transformed parasegment (PS) 11 into a duplicate copy of PS12. Fab-71 deleted four nuclease hypersensitive sites, HS*, HS1, HS2, and HS3, located between the iab-6 and iab-7 regulatory domains. Transgenic and P-element excision experiments mapped the boundary to HS*+HS1+HS2, while HS3 was shown to be the iab-7 Polycomb response element (PRE). Recent replacement experiments showed that HS1 is both necessary and sufficient for boundary activity when HS3 is also present in the replacement construct. Surprisingly, while HS1+HS3 combination has full boundary activity, we discovered that HS1 alone has only minimal function. Moreover, when combined with HS3, only the distal half of HS1, dHS1, is needed. A ~1,000 kD multiprotein complex containing the GAF protein, called the LBC, binds to the dHS1 sequence and we show that mutations in dHS1, that disrupt LBC binding in nuclear extracts, eliminate boundary activity and GAF binding in vivo. HS3 has binding sites for GAF and Pho proteins that are required for PRE silencing. In contrast, HS3 boundary activity only requires the GAF binding sites. LBC binding with HS3 in nuclear extracts, and GAF association in vivo, depend upon the HS3 GAF sites, but not the Pho sites. Consistent with a role for the LBC in HS3 boundary activity, the boundary function of the dHS1+HS3mPho combination is lost when the flies are heterozygous for a mutation in the GAF gene. Taken together, these results reveal a novel function for the iab-7 PREs in chromosome architecture.
Author summary
Polycomb group proteins (PcG) are important epigenetic regulators of developmental genes in all higher eukaryotes. In Drosophila, these proteins are bound to specific regulatory DNA elements called Polycomb group Response Elements (PREs). Drosophila PREs are made up of binding sites for a complex array of DNA binding proteins, including GAF and Pho. In the regulatory region of the bithorax complex (BX-C), the boundary/insulator elements organize the autonomous regulatory domains, and their active or repressed states are regulated by PREs. Here, we studied functional properties of sequences that constitute the Fab-7 boundary and the adjacent iab-7 PRE. It was previously thought that the sole function of the iab-7 PRE is to recruit PcG proteins in parasegments anterior to PS12 and silence the iab-7 domain. However, we found that the iab-7 PRE also functions as a component of the Fab-7 boundary. The boundary activity of the iab-7 PRE sequence depends upon a large complex called the LBC. We show that it is possible to reconstitute a fully functional boundary by combining the LBC binding sequences in HS1 with the iab-7 PRE. Moreover, its boundary function is independent of its PcG silencing activity.
Introduction
Chromosomes in multicellular organisms are subdivided into a series of independent topologically associating domains (or TADs) [1,2]. The average length of these domains in humans is 180 kb, while they are only on the order of 5–20 kb in flies [3–5]. In mammals, TADs are frequently defined by binding sites for the conserved zinc finger protein CTCF [6,7]. While a single CTCF is thought to be necessary and sufficient for boundary function in mammals, this is not true in flies. More than a dozen DNA binding proteins in flies that function as architectural factors have been identified and it is likely that many more remain to be discovered [8–10]. Because of extensive redundancy, any one of these individual recognition sequences for these factors might be dispensable for boundary function.
One of the best examples of functional redundancy is the Fab-7 boundary in the Drosophila bithorax complex (BX-C). BX-C contains three homeotic genes, Ultrabithorax (Ubx), abdominal-A (abd-A), and Abdominal-B (Abd-B), which are responsible for specifying the parasegments (PS5 to PS13) that make up the posterior two-thirds of the fly segments [11–14]. Expression of the homeotic genes in the appropriate parasegment-specific pattern is orchestrated by a series of nine cis-regulatory domains, abx/bx, bxd/pbx, iab-2—iab-9 (Fig 1A). For example, the iab-5, iab-6, iab-7, and iab-8 cis-regulatory domains direct Abd-B expression in PS10-PS13 [15,16]. BX-C regulation is divided into two phases, initiation and maintenance [11,17]. During the initiation phase, a combination of gap and pair-rule proteins interact with initiation elements in each regulatory domain, setting it in the on or off state. In PS10, for example, initiators in iab-5 activate the iab-5 domain, while iab-6, iab-7, and iab-8 are set in the off state. In PS11, iab-6 is activated, while iab-7 and iab-8 are off. Once the gap and pair-rule gene proteins disappear during gastrulation, the on and off states of the regulatory domains are maintained by Trithorax (Trx) and Polycomb (PcG) group proteins, respectively [18–21]. These maintenance factors are recruited to the domains by special cis-acting elements called Trithorax Response Elements (TREs) and Polycomb Response Elements (PREs) [22–26]. In addition to elements that establish and maintain the on/off state, each domain has a series of tissue and cell type specific enhancers that direct the expression of the target homeotic gene in an appropriate pattern [11,16]. For example, the tissue/cell type enhancers in iab-6 drive Abd-B expression in a pattern that orchestrates the proper differentiation of cells with a PS11 identity. This pattern of expression is distinct from that in PS12, where Abd-B is regulated by enhancers in iab-7.
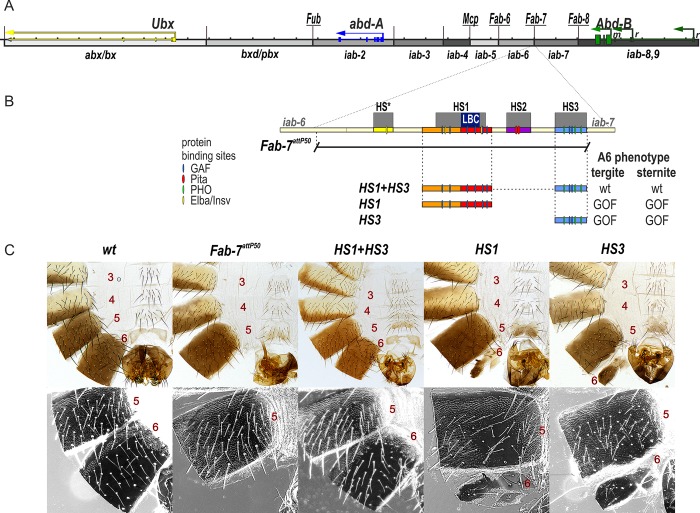
Fig 1. HS1 alone is not sufficient for boundary function.
(A) Map of the bithorax complex showing the location of the three homeotic genes and the parasegment-specific regulatory domains. (B) Map of Fab-7 region showing the four hypersensitive sites, HS*, HS1, HS2, and HS3. The locations of recognition motifs for proteins known to be associated with Fab-7 are indicated. Replacement fragments are shown below the map, with a summary of their cuticle (tergite and sternite) phenotypes. (C) Bright field (top) and dark field (bottom) images of cuticles prepared from wild type (wt), Fab-7attP50 (replacement platform), HS1+HS3, HS1, and HS3 male flies. In wild type adult males, segment A7 (PS12) is absent. Segments A6 (PS11) and A5 (PS10) can be distinguished from each other by the characteristic morphological features of their sternites and tergites. The A6 sternite has a banana shape and is devoid of bristles. By contrast, the A5 sternite has a quadrilateral shape and is covered in bristles. The A6 tergite only has trichomes along the anterior and ventral edges, while the entire A5 tergite is typically covered in trichomes (though there can be some small internal patches that lack trichomes or have a reduced number of trichomes). In Fab7attP50 males, A6 (PS11) is transformed into a duplicate copy of A7 (PS12), and in adults, both segment A7 and segment A6 are absent. It is thought that in the absence of both the Fab-7 boundary (HS*+HS1+HS2) and the HS3 iab-7 PRE, the iab-6 initiator ectopically activates iab-7 in all PS11 cells. As noted in the text, this complete GOF transformation is not observed in Fab-7 boundary deletions that retain the HS3 iab-7 PRE. In these mutants, iab-6 is sometime silenced by iab-7. In this case, iab-5 drives Abd-B expression in PS11. LOF phenotypes can also be observed when Fab-7 is replaced by an “incompatible” boundary like scs or su(Hw) [35] that does not support bypass. The HS1+HS3 replacement resembles wild type. In contrast, the HS1 and HS3 replacements have a strong GOF transformation. The sternite is absent, while the tergite is markedly reduced in size. In the case of HS1, the residual tergite seems to be properly specified in that extraneous trichomes do not appear to be present, while extraneous trichomes are observed for the HS3 replacement, indicating a LOF transformation. Coordinates of the various replacements are listed in Materials and Methods.
The Fab-7 boundary, like other boundary elements in BX-C, is required to ensure that the flanking regulatory domains, iab-6 and iab-7, are able to function autonomously [27–29]. During the initiation phase it blocks crosstalk between initiators in iab-6 and iab-7, while during the maintenance phase it keeps the domains in an on or off state by preventing interactions between their PREs and TREs. In addition, like most other BX-C boundaries, Fab-7 must also facilitate “bypass”, enabling the distal regulatory domains iab-5 and iab-6 to “jump over” and contact Abd-B in PS10 and PS11, respectively. Like blocking, bypass activity is essential for proper Abd-B regulation.
Fab-7 was initially defined by a 4 kb X-ray induced deletion that had an unusual dominant gain-of-function (GOF) phenotype, transforming PS11 into a duplicate copy of PS12 [27]. The Fab-71 deletion spanned four nuclease hypersensitive regions, HS*, HS1, HS2, and HS3 [30]. Subsequent transgene studies showed that a 1.2 kb fragment spanning HS*+HS1+HS2 had enhancer blocking activity in embryos and in adults [31,32]. The fourth hypersensitive region, HS3, had no detectable boundary activity; however, when included in a white transgene, it induced pairing sensitive silencing, which is a characteristic activity of PREs [33]. This separation of functions was confirmed by Mihaly et al [29] who generated a series of new deletions that removed either HS*+HS1+HS2 or HS3. Unlike Fab-71, deletions that removed only HS*+HS1+HS2 have a mixed GOF and LOF (loss-of-function phenotype) (PS11→PS12 and PS11→P10, respectively). Mutations in PcG genes enhance the GOF phenotypes, while mutations in Trx enhance the LOF phenotypes. By contrast, PcG and Trx mutations have no effect on the GOF phenotypes of deletions, like Fab-71, that remove all four hypersensitive regions. Finally, flies carrying HS3 deletions are wild type as heterozygotes. As homozygotes, they are either fully wild type or display only very weak GOF phenotypes [29,34]. It is thought that other PRE-like elements within the iab-7 domain are able to sustain the silenced state in PS11 in the absence of the HS3 iab-7 PRE. Boundary mediated pairing of the iab-7 domains in trans also appears to help compensate for the loss of the HS3 iab-7 PRE.
While the LOF phenotypes in deletions that remove HS*+HS1+HS2 require silencing of the iab-6 domain by the iab-7 PRE, this is not the only mechanism that can give rise to LOF transformations of PS11 (and PS10). LOF phenotypes are also observed when Fab-7 is replaced by heterologous boundaries such scs, su(Hw), or the BX-C boundary Mcp [35–37]. Like Fab-7, these boundaries prevent crosstalk between iab-6 and iab-7; however, they fail to support bypass, and instead block the iab-6 domain from regulating Abd-B. Thus far, the only heterologous boundary that recapitulates both the blocking and bypass activity of Fab-7 is the neighboring boundary Fab-8 [36,38]. Remarkably, the bypass activity of Fab-8 is orientation dependent. When the orientation of the Fab-8 replacement is reversed, it is still able to block crosstalk between iab-6 and iab-7; however, it prevents iab-6 from regulating Abd-B. As a consequence, the morphology of A6 resembles A5.
Previous studies indicate that Fab-7 (HS*+HS1+HS2) boundary activity is generated by a combination of ubiquitously expressed factors and stage/cell type specific factors. One ubiquitously expressed factor is the zinc finger protein Pita, which binds to sites in HS2 [37,39]. The known developmentally regulated factors are Elba, Insensitive (Insv), and the LBC (Fig 1B) [40]. The LBC is a multiprotein complex that contains at least three distinct DNA binding proteins, the GAGA factor (GAF), Clamp, and Mod(mdg4) [41]. In the case of Fab-7, three contiguous sequences spanning GAGA sites 3, 4, and 5 generate LBC shifts [34,42].
While transgene experiments indicated that sequences spanning HS*+HS1+HS2 are required for blocking activity [43,44], boundary replacement using an attP platform that deletes HS*+HS1+HS2+HS3 suggested that the requirements for boundary function out of context are more demanding than those in BX-C. These replacement experiments indicated that HS* and HS2 are not required for boundary activity, while the largest hypersensitive region, HS1, appeared to be both necessary and sufficient [34]. However, there was a confounding factor in these experiments: because the HS3 iab-7 PRE has Polycomb silencing activity and as such is an important regulatory component of the iab-7 regulatory domain, it was retained in these replacement experiments. Here we have asked whether HS1 alone is sufficient in the absence of HS3 iab-7 PRE. Surprisingly, it is not. Instead, our studies indicate that HS3 not only has PRE activity, but also that it contributes to the boundary function of Fab-7. Moreover, it appears that like HS1, the LBC is important for the boundary and PRE functions of HS3.
Results
HS3 rescues boundary activity of HS1
The experiments of Wolle et al [34] analyzing the boundary activity of HS1 were done with replacements that also contained the HS3 iab-7 PRE. For this reason we wondered whether HS1 would be sufficient in the absence of HS3. Fig 1B shows the HS1 replacement, and as controls, the starting platform Fab-7attP50, HS1+HS3, and HS3 alone. The Fab-7attP50 platform deletes HS*+HS1+HS2+HS3, and as observed for the Fab-71 deletion, the A6 segment is completely transformed into a duplicate copy of A7 (Fig 1C). As reported previously, we found that flies carrying the HS1+HS3 replacement are indistinguishable from wild type, while for the HS3 replacement, there is a strong GOF transformation, and the A6 tergite is greatly reduced in size or absent, while the sternite is missing (Fig 1C and S1 Fig). Unexpectedly, like HS3, HS1 alone also has a strong GOF phenotype. The sternite is typically missing, while the tergite is typically greatly reduced in size (Fig 1C and S1 Fig).
The fact that HS1 is insufficient for boundary function in the absence of HS3 prompted us to reassess the role of HS3 in Fab-7 boundary function. To address this question, we examined the functional properties of different combinations of the HS*, HS1, and HS2 regions either in the presence or absence of the iab-7 PRE, HS3. We first attempted to rebuild the boundary using combinations of HS*, HS1, and HS2. As previously reported in studies on the Pita sites in HS2, HS1+HS2+HS3, or HS1+HS2ΔPita +HS3 replacements are fully functional [37]. In contrast, the boundary function of the HS1+HS2 replacement is tissue-specific (Fig 2). In HS1+HS2 flies, the A6 tergite is fully wild type. In contrast, the sternite is absent indicative of a GOF transformation of PS11→PS12 in the cells that give rise to the ventral cuticle. In this context, the Pita sites in HS2 are essential for boundary function in the cells that give rise to the tergite [37].
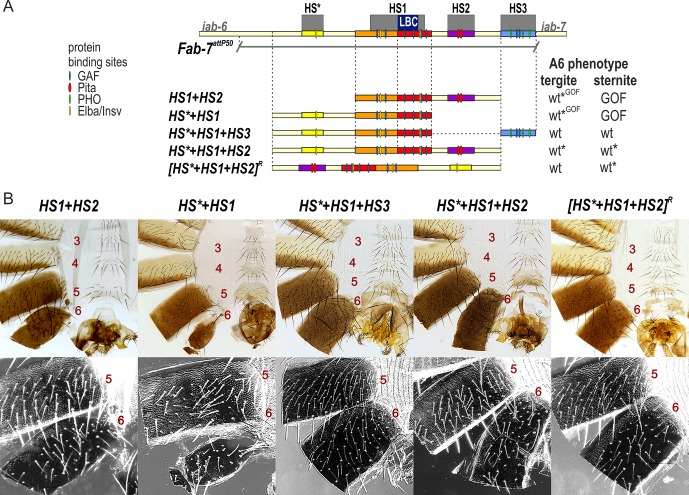
Fig 2. HS1 combinations with either HS2 or HS* are not fully functional.
(A) Map of Fab-7 region showing the four hypersensitive sites, HS*, HS1, HS2, and HS3 and the locations of recognition motifs for proteins known to be associated with Fab-7. Replacement fragments are shown below the map with a summary of their cuticle (tergite and sternite) phenotypes. (B) Bright field (top) and dark field images (bottom) of cuticles prepared from HS1+HS2, HS*+HS1+HS3, HS*+HS1, HS*+HS1+HS2, and [HS*+HS1+HS2]R (reverse) male flies. As detailed in the text, the HS1+HS2 replacement lacks a sternite (GOF), but has a nearly normal tergite size with wild type morphology. The HS*+HS1+HS3 looks like wild type. The HS*+HS1 replacement lacks a sternite (GOF), while there is a variable reduction in the size of tergite. The morphology of the residual tergite suggests that it has the appropriate A6 (PS11) identity. HS*+HS1+HS2 males frequently show some A6 cuticle defects indicative of a weak A6(PS11)→A7(PS12) (also see S2 Fig). [HS*+HS1+HS2]R flies have a normal A6 tergite; however, the sternite shows evidence of a weak LOF transformation (bristles). wt*—minor deviations in phenotype. wt*GOF–variable phenotype between wt and GOF.
We next tested the HS*+HS1 combination with or without HS3. While the HS*+HS1+H3 combination is fully functional, HS*+HS1 retains only limited tissue-specific boundary activity (Fig 2). HS*+HS1 appears to have no boundary activity in the histoblasts giving rise to the ventral cuticle, and in all male flies the A6 sternite is completely absent. In about 80% of the males, the A6 tergite is greatly reduced in size and has an irregular shape, as expected for an A6→A7 (or PS11→PS12) transformation in segment identity (Fig 2 and S2 Fig). In the remaining 20%, the boundary appears to be at least partially functional and there is only a slight reduction in the size of the A6 tergite.
The experiments described above indicate that HS3 complements the blocking defects of boundaries composed of just HS1 or HS1 plus either HS2 or HS*. We wondered whether HS3 might also contribute to the bypass activity. To test this possibility, we reinvestigated the effects of inverting the Fab-7 boundary. In a previous study, we showed that the blocking and bypass activity of HS*+HS1+HS2 is orientation independent [36]. However, this experiment was done in the presence of HS3. To determine if HS3 contributes to orientation independence, we generated forward and reverse HS*+HS1+HS2 replacements that lacked HS3 (Fig 2). The properties of the HS*+HS1+HS2 replacement resemble Class III deletions described by Mihaly et al [29]. While the size of A6 tergite is normal in all but about 5% males, the sternites are typically thinner and slightly malformed. This phenotype is indicative of a very weak GOF transformation of the A6 sternite (Fig 2 and S2 Fig). A different result was obtained for the inverted [HS*+HS1+HS2]R replacement (Fig 2). All flies had a fully wild type tergite, while the sternites had bristles and are sometimes were misshapen. The presence of bristles on the A6 sternite is indicative of a weak LOF transformation of A6 into A5. This weak LOF phenotype can’t be due to the PcG silencing activity of the HS3 iab-7 PRE because this element is absent. Instead, the LOF phenotype is expected to arise because bypass activity of the inverted [HS*+HS1+HS2]R replacement is partially compromised in cells giving rise to the ventral adult cuticle.
Functional dissection of HS1
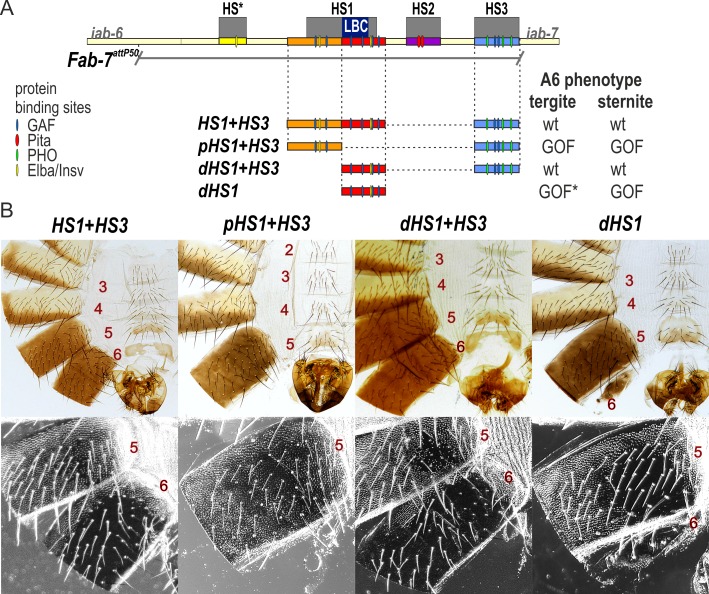
In contrast to HS1 alone, the HS1+HS3 combination has full boundary function. To further probe the requirements for HS1 boundary activity when combined with HS3, we subdivided HS1 into proximal and distal parts, pHS1 and dHS1 (Fig 3). Previous experiments have implicated the LBC in the late blocking activity of dHS1, while an Elba/Insv recognition sequence is likely to contribute to early blocking activity of pHS1 [40]. Fig 3 and S3 Fig show that the pHS1+HS3 combination gives a strong GOF transformation of A6, indicating that the pHS1 sequence is not able to reconstitute boundary activity. In contrast, the dHS1+HS3 combination has a fully wild type A6 segment, just like the HS1+HS3 combination.
Fig 3. dHS1 but not pHS1 can function as a boundary together with HS3.
(A) Map of Fab-7 region showing the four hypersensitive sites, HS*, HS1, HS2, and HS3 and the location of recognition motifs for proteins known to be associated with Fab-7. Replacement fragments are shown below the map with a summary of their cuticle (tergite and sternite) phenotypes. (B) Bright field (top) and dark field images of cuticles prepared from HS1+HS3, pHS1+HS3, dHS1+HS3, and dHS1 male flies. As described in text, the HS1+HS3 and dHS1+HS3 replacements resemble wild type males, while pHS1+HS3 and dHS1 flies have strong GOF phenotypes. In flies that have residual A6 cuticle (typically, a tergite), there are LOF transformations. GOF*—incomplete GOF phenotype in most males.
Wolle et al [34] showed that the LBC can bind independently to ~65–80 bp probes spanning the GAGA3, GAGA4, and GAGA5 sequences in dHS1, and that binding to the 65 bp GAGA3 and GAGA4 probes requires the GAGAG motif. However, we subsequently found that optimal LBC binding is to larger DNA probes that span GAGA3-4, GAGA3-5, or even GAGA3-6. The experiments shown in S4A and S4B Fig compare LBC binding to the 65 bp GAGA3 and GAGA4 probes with binding to a larger GAGA3+4 probe. In both binding and competition experiments we found that there is a 6–12 fold differential in LBC binding to the larger GAGA3+4 probe. This difference in relative affinity isn’t due to just probe length. The competition experiment in S4C Fig shows that a hybrid GAGA3+LacZ probe of the same length as GAGA3+4 is a poor competitor for LBC binding compared to GAGA3+4. Even larger differences in relative affinity are observed for probes spanning GAGA3-5 or GAGA3-6.
While LBC binding to the 65 bp GAGA3 and GAGA4 probes requires the GAGAG motif, how mutations in this motif affect LBC binding to larger dHS1 probes hasn’t been investigated. For this purpose, we compared LBC binding to dHS1 probes that are wild type or have mutations in GAGA3-6. Fig 4A shows that LBC binding to the dHS1 probe is largely abrogated when all four GAGA motifs are mutant (GAGAmut3-6).
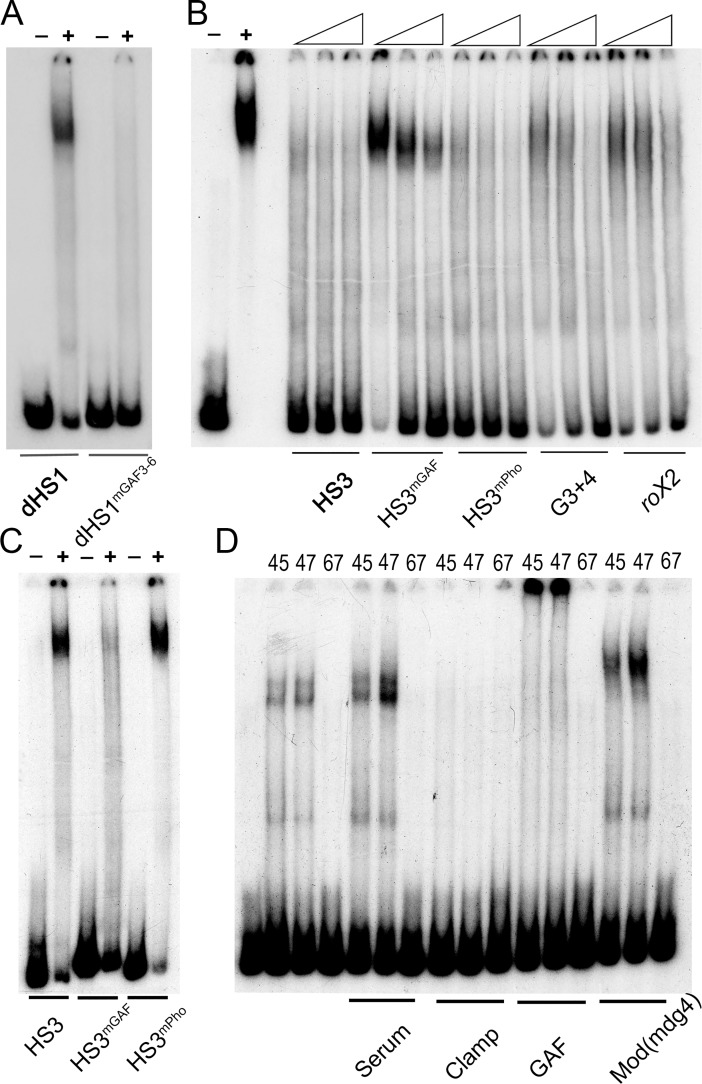
Fig 4. LBC binding to dHS1 and HS3 requires the GAGAG motifs.
Nuclear extracts prepared from 6–18 hr embryos were used for EMSA experiments: (-) no extract, (+) with extract. (A) EMSA experiments with a wild type and GAGAG mutant (as indicated) dHS1 probe. (B) EMSA competition experiments with a probe spanning HS3. Control lanes on the left show the LBC shift of HS3 in the absence of cold competitors. As illustrated by the triangles, increasing concentrations (25x, 50x, 100x) of cold competitor were added. The cold competitor used in each set of three lanes is indicated below. (C) EMSA of wild type and mutant HS3 probes. The two HS3 GAGAG sites are mutant in the HS3mGAF probe. The three HS3 Pho sites are mutant in the HS3mPho probe. (D) Antibody supershift experiments using fractions from a gel filtration column. Fraction numbers (45, 47, and 67) are indicated above each lane. 45 and 47 are two of LBC peak fractions while fraction 67 doesn’t have LBC activity. The antibody used for each set of supershift experiments is indicated below. Note: after fractionation by gel filtration, the LBC shift is typically slightly stimulated by the inclusion of non-specific serum.
If the LBC binding to dHS1 is important for boundary function in the dHS1+HS3 combination, then it should be disrupted when the dHS1mGAF fragment is used for the replacement instead of the wild type dHS1 fragment. Fig 5 shows that this is the case. Like the HS3 replacement alone, the dHS1mGAF+HS3 combination exhibited a strong GOF phenotype.
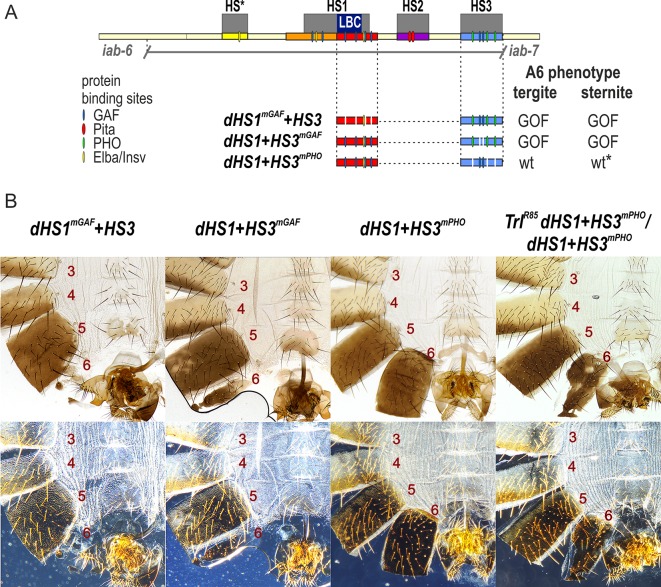
Fig 5. HS3 boundary activity requires the two GAGAG motifs but not the three Pho recognition sequences.
(A) Map of Fab-7 region showing the four hypersensitive sites, HS*, HS1, HS2, and HS3 and the locations of recognition motifs for proteins known to be associated with Fab-7. Replacement fragments are shown below the map with a summary of their cuticle (tergite and sternite) phenotypes. (B) Bright field (top) and dark field (bottom) images of cuticles prepared from dHS1mGAF+HS3, dHS1+HS3mGAF, dHS1+HS3mPho, and TrlR85 dHS1+HS3mPho/ dHS1+HS3mPho male flies. As described in text, most dHS1+HS3mPho flies have a wild type phenotype, while the other mutant replacements typically exhibit strong GOF transformations. While nearly all dHS1+HS3mPho males are wild type, reducing the dose of the Trl gene in half induces a GOF transformation. The sternite is usually absent while the tergite had an irregular shape and is reduced in size. wt*—minor deviations in phenotype.
The GAGA motifs in HS3 are required for boundary function while the Pho motifs are dispensable
A number of different mechanisms could potentially explain how HS3 contributes to the boundary functions of the Fab-7 hypersensitive regions HS*, HS1, and HS2. One intriguing possibility is that the PcG dependent silencing activity of the HS3 iab-7 PRE is needed for the boundary activity of replacements that contain only HS1/dHS1 (or HS1 plus either HS* or HS2). The HS3 iab-7 PRE has two GAF recognition sequences (GAGAG) and three recognition sequences for the zinc finger protein Pleiohomeotic (Pho) [45]. Like many other Drosophila PREs [45,46], these DNA binding motifs are important for the PcG dependent silencing activity of the iab-7 PRE. Mutations in either the GAGAG or Pho sequences compromise the silencing activity of the iab-7 PRE in mini-white transgene assays [47]. Moreover, consistent with a role for the GAF (Trl) and Pho proteins in silencing, mutations in the Trl and pho genes suppress the silencing activity of the HS3 iab-7 PRE [33,48].
If the PcG dependent silencing activity of the iab-7 PRE is required to complement the boundary defects of HS1, then mutations in either the HS3 GAGA or Pho sequences should abrogate the boundary activity of the dHS1+HS3 combination. To test this prediction, we generated dHS1+HS3mGAF and dHS1+HS3mPho replacements. As expected, dHS1+HS3mGAF lacks boundary activity, and flies carrying this replacement exhibit a strong GOF transformation of A6 (PS11) (Fig 5). However, contrary to our predictions, most dHS1+HS3mPho flies are fully wild type, and exhibit no evidence of either GOF or LOF transformation. As was reported by Mihaly et al [29] for HS3 deletions, a small percentage (~2–5%) of the male dHS1+HS3mPho flies have a weak GOF transformation. In these flies the size of the tergite is reduced and/or the sternite is misshapen.
The LBC binds to HS3 and binding depends upon two GAGA motifs
The fact that mutations in the GAGA motifs disrupt the boundary activity of HS3, while those in the Pho sites do not would argue that the PcG dependent silencing activity of HS3 is probably not responsible for its ability to complement HS1. Instead, it would appear that HS3 boundary function is separable from PRE activity. With aim of identifying factors contributing to HS3 boundary activity, we used three overlapping probes spanning HS3 (227 bp) for EMSA experiments with embryonic nuclear extracts. The EMSA experiment in S5 Fig shows that each probe generates several shifts. Though the identity of most of these shifts is unknown, the prominent slowly migrating shift observed with probe 2 resembles the shift generated by the LBC.
To determine if this slowly migrating shift corresponds to the LBC, we used a 200 bp fragment containing most of HS3, rather than the shorter probe 2. Like probe 2, the larger probe generates an LBC-like shift (Fig 4B). Two experiments indicate that this HS3 shift corresponds to the LBC. The first is a competition experiment with two different DNA fragments known to bind the LBC, Fab-7 G3+4 and CES roX2. As could be predicted, the HS3 shift is competed by itself and also by excess unlabeled G3+4 and roX2. In the second experiment, we used peak LBC fractions from a gel filtration column, plus a control fraction that lacks LBC activity for antibody “supershift” experiments. Previous studies showed that the LBC shift is sensitive to antibodies direct against Clamp, GAF, and Mod(mdg4). Fig 4D shows that LBC binding to HS3 in the peak gel filtration fractions 45 and 47 is inhibited by Clamp antibody, while GAF and Mod(mdg4) antibodies generate a supershift.
These experiments indicate that like dHS1, the LBC binds to the full length HS3 sequence in vitro. This finding suggests a plausible mechanistic explanation for why HS3 contributes to Fab-7 boundary and is able to reconstitute boundary activity when combined with dHS1. If this explanation were correct, we would expect that LBC binding to HS3 should require the GAGA motifs but not the Pho binding sequences. This is indeed the case. Fig 4C shows that mutations in the HS3 GAGA motifs (HS3mGAF) abrogate the LBC shift, while mutations in the Pho binding sequence (HS3mPho) have no effect. The requirement for the GAGA motifs, but not the Pho binding sequences is confirmed by competition experiments (Fig 4B) with mutant HS3 DNAs. HS3mPho competes as well as the wild type HS3 for LBC binding, while HS3mGAF is a poor competitor.
Protein occupancy is altered by mutations that impair function
To extend this analysis, we used chromatin immunoprecipitation (ChIP) experiments to compare GAF association with wild type and mutant versions of dHS1+HS3 in embryos and pupae. In the wild type dHS1+HS3 replacement, GAF is found associated with both dHS1 and HS3 in embryos and pupae (Fig 6). As would be predicted from the loss of LBC binding to dHS1mGAF DNA in nuclear extracts, GAF association with dHS1 in the dHS1mGAF +HS3 replacement is substantially reduced. Interestingly, GAF association with HS3 is also reduced in dHS1mGAF+HS3 compared to the wild type dHS1+HS3 replacement. This secondary effect is seen not only in embryos, but also in pupae (Fig 6).
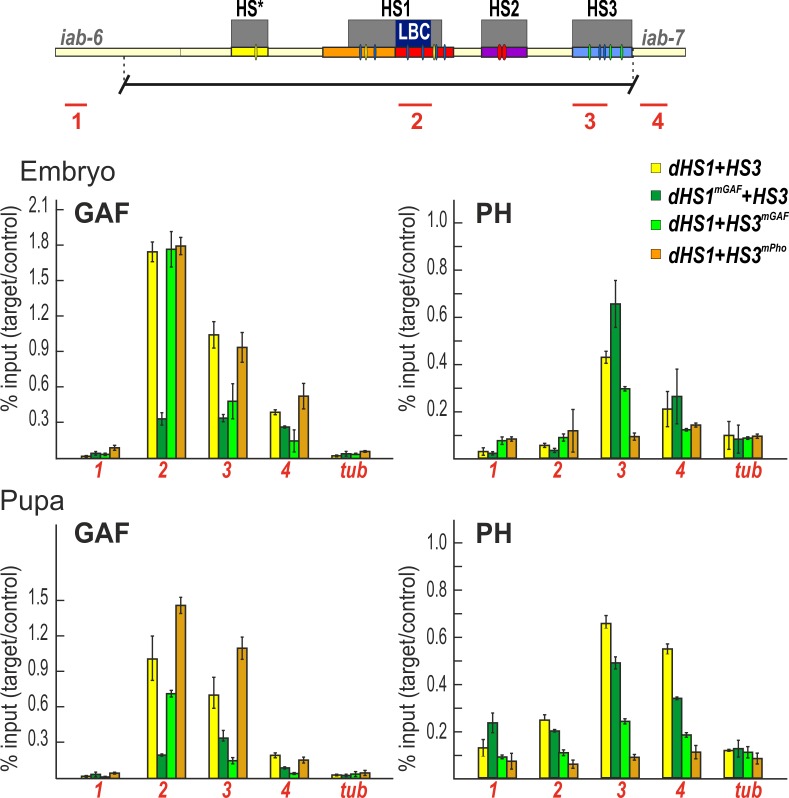
Fig 6. ChIPs with GAF and Ph antibodies at sites across the Fab-7 region.
Binding of GAF and Ph across the Fab-7 region (corresponding PCR products 1,2,3,4 marked as red lines under map of Fab-7 region) in different Fab-7 replacements in embryos and pupae. The results of ChIPs are presented as a percentage of the input DNA normalized to a positive genomic site, Hsp70 region–for GAF binding, PRE of engrailed—for Ph binding. Negative control is the γTub37C (tub) gene. Error bars indicate standard deviations of triplicate PCR measurements from three independent biological samples of chromatin.
Our EMSA experiments predict that GAF association with HS3 will be disrupted by mutations in the GAGA sequences, but not the Pho sequences. This expectation is correct. GAF association with HS3 is reduced in the dHS1+HS3mGAF replacement while mutations in the Pho site (dHS1+HS3mPho) have no effect (Fig 4C and Fig 6).
We also compared the dHS1/HS3 association of the Polycomb Repressive Complex 1 (PRC1) protein Polyhomeotic (Ph) in the different dHS1+HS3 replacements. As would be predicted from our previous studies on the silencing activity of HS3 in transgene assays, mutations in either the GAGA or Pho sequences reduce Ph-HS3 association (Fig 6). Ph also binds to a nearby sequence in the iab-7 cis-regulatory domain and, to a lesser extent, to HS1. In both cases, this association is reduced by mutations in the HS3 GAGA and Pho sequences. By contrast, mutations in the dHS1 GAGA sequences (dHS1mGAF) have limited effects on Ph association in HS3 or iab-7.
Our mutant replacement experiments showed that dHS1+HS3mPho has nearly full boundary activity, while boundary activity is compromised in dHS1+HS3mGAF. These findings, together with our in vitro experiments on the LBC, would predict that the boundary activity of the dHS1+HS3mPho replacement should depend on the GAF protein. If this is case, boundary activity might be sensitive to the dose of the gene encoding the GAF protein, Trl. To test this possibility we recombined the dHS1+HS3mPho replacement with a Trl null mutation, TrlR85. The GOF transformations evident in dHS1+HS3mPho/TrlR85 dHS1+HS3mPho flies show that the boundary activity of this replacement is compromised by a reduction in the dose of the Trl gene (Fig 5B).
Discussion
Previous functional studies indicated that the Fab-7 region of BX-C is subdivided into two seemingly distinct elements [29]. One of these elements spans the distal nuclease hypersensitive site HS3 and corresponds to a PRE for the iab-7 regulatory domain. The other element spans the three proximal nuclease hypersensitive sites, HS*, HS1, and HS2. This element corresponds to the Fab-7 boundary. In each case, the functional assignment was based on a combination of transgene assays and analysis of deletions in the Fab-7 region generated by excision of a P-element insertion located between HS2 and HS3. This functional analysis has recently been extended with an attP replacement platform in which the entire region has been deleted [34]. This attP platform makes it possible to systematically test the functional properties of different Fab-7 sequences in their native context. Using this platform, we recently found that hypersensitive site HS1 was both necessary and sufficient for wild type Fab-7 boundary activity in a context in which the iab-7 PRE, HS3, was present.
Since boundary activity in transgene assays required a sequence spanning HS*, HS1, and HS2, this finding suggested that the demands for full activity are considerably less stringent in the native context than in enhancer blocking assays. To confirm this conclusion, we tested the HS1 by itself. Unexpectedly, it is not sufficient for wild type boundary activity. Likewise, combinations of HS1 with either HS* or HS2 have only partial, tissue-specific boundary activity. In both combinations, the A6 sternite in males is absent, indicative of a PS11→PS12 GOF transformation. For the HS1+HS2 combination, the A6 tergite is wild type, while, for the HS*+HS1 combination, the tergite displays weak to moderate GOF phenotypes. The only combination of these Fab-7 sequences that has nearly complete boundary activity in the native context is HS*+HS1+HS2.
Like HS1 alone, the impaired boundary function of HS*+HS1 and HS1+HS2 can be rescued by the addition of HS3. These findings argue that HS3 must be able to contribute to Fab-7 boundary function. Two different models could potentially explain the boundary activity of HS3. In the first, its boundary function would depend on the ability of HS3 to recruit PcG proteins and induce silencing. In the second, boundary function would reflect a PRE associated activity that is independent of PcG recruitment and silencing. For example, since PcG silencing is facilitated by pairing interactions between PRE containing transgene inserts on each homolog, the iab-7 PRE, HS3, might have a chromosome architectural activity just like the classical boundaries [48–52]. A number of lines of evidence are consistent with this second model.
We compared the factors binding to HS1 and HS3 sequences using EMSA experiments with embryonic nuclear extracts. While probes spanning HS3 gave multiple shifts, the most prominent HS3 shift corresponds to a HS1 boundary factor, the LBC. This conclusion is supported by several observations. First, the HS3 LBC shift is competed by two DNA sequences, GAGA3+4 (Fab-7) and roX2, which are known to bind the LBC. Second, as observed for other LBC recognition sequences, antibodies against GAF, Clamp, and Mod(mdg4) either generate a supershift or interfere with binding to the HS3 sequence. Third, the peak LBC fractions after gel filtration of embryonic nuclear extracts have an apparent molecular weight of ~1,000 kD. These peak LBC fractions generate a shift with the HS3 probe and were used for the antibody “supershift” experiments.
LBC binding to the Fab-7 probes GAGA3, GAGA4, and dHS1 and to three X-linked CES depends upon GAGAG motifs (or GA rich sequences). This is also true for HS3. Mutations in the two HS3 GAGA motifs substantially reduce the yield of the HS3 LBC shift. Consistent with this finding, competition experiments indicate that the HS3mGAF is a poor competitor for LBC binding to the wild type HS3 probe. While the HS3 GAGAG motifs are required for LBC binding, the two Pho sites are not.
Previous studies on the HS3 iab-7 PRE indicate that like other fly PREs, it requires the GAF and Pho proteins for its silencing (and pairing-sensitive) activity [33,47,48]. Mutations in the two GAGAG motifs and in the two Pho recognition sequences disrupt silencing activity. Pho interacts with Sfmbt and is directly involved in the recruitment of PRC1 to PREs [53–56]. In vitro, GAF facilitates Pho binding to a chromatinized template [57].
If PсG silencing activity is critical for HS3 boundary activity, then boundary activity should be eliminated by mutations in either the GAGAG motifs or the Pho binding sites. In contrast, if LBC binding to HS3 is important, then mutations in the GAGAG motifs should disrupt the boundary function of the dHS1+HS3 replacement, while mutations in the HS3 Pho binding sequences should not. Consistent with the expectations of the second model, we found that the HS3 GAGAG motifs are important for boundary function, while the Pho binding sequences are not.
This distinction is also reflected in the protein occupancy of wild type and mutant HS1+HS3 replacements. In the wild type replacement, both GAF and Pho are associated with HS3. As would be predicted from the effects of GAGAG mutations on the PcG silencing activity of HS3 in transgene assays, the levels of both GAF and the Polycomb protein Ph are reduced in the HS1+HS3mGAF replacement. In contrast, mutations in the HS3 Pho sites have no effect on GAF occupancy, while they reduce Ph occupancy. A prediction that follows from these findings is that the GAF protein is important for the boundary activity of dHS1+HS3mPho replacement. Consistent with this prediction, dHS1+HS3mPho boundary function is compromised when the flies are heterozygous for a mutation in Trl.
While the findings reported here support the idea that the boundary activity of both dHS1 and HS3 is mediated, at least in part, by the LBC, many questions remain. For example, why does HS3 iab-7 PRE have PcG silencing activity while HS1 doesn’t? Likewise, why are the X-chromosome CES able to recruit the Msl dosage compensation complexes? One idea is that the silencing activities of the HS3 iab-7 PRE and the dosage compensation functions of the CES depend upon the association of functionally specialized ancillary factors with a platform that is provided by LBC binding. This idea would be consistent with our findings. Both the silencing and boundary activities of the HS3 iab-7 PRE depend upon GAF, while only the silencing activity depends upon Pho (which in other PREs is thought to function in the recruitment of PRC1). That functionally specialized factors might associate with different LBC recognition elements would also fit with our gel filtration experiments. We found that the LBC shifts in nuclear extracts (with Fab-7 and several CES probes) migrate more slowly than the shifts observed after gel filtration. This difference in mobility suggests that there are factors that associate with the LBC:DNA complex in nuclear extracts that are not integral components of the LBC, and thus don’t co-fractionate with the LBC during gel filtration. These factors could contribute not only to the PcG silencing or MSL recruitment activities of individual LBC recognition elements, but also to the boundary activity of elements like Fab-7 dHS1.
Materials and methods
Generation of the Fab-7attP50 replacement lines
The strategy of the creation of the Fab-7attP50 landing platform and generation of the Fab-7 replacement lines is described in detail in [34,36]. DNA fragments used for the replacement experiments were generated by PCR amplification and verified by sequencing. The sequences of the used fragments are shown in the Supporting S1 Table.
Cuticle preparations
Adult abdominal cuticles of homozygous eclosed 3–4 day old flies were prepared essentially as described in [36] and mounted in 100% glycerol. Photographs in the bright or dark field were taken on the Nikon SMZ18 stereomicroscope using Nikon DS-Ri2 digital camera, processed with ImageJ 1.50c4 and Fiji bundle 2.0.0-rc-46, and assembled using Impress of LibreOffice 5.3.7.2.
Nuclear extracts
Nuclear extracts from 6- to 18-h embryos were prepared as described previously [58] with small modifications. Embryos from Oregon R were collected from apple juice plates and aged 10 h at room temperature. The extraction was completed with the final concentration of KCl at 360 mM. Fractionation of the nuclear extracts derived from 6- to 22-h embryos was performed by size exclusion chromatography using Superose 6 10/330 GL column (GE Healthcare). Molecular mass markers ranging from 1,350 to 670,000 Da (Bio-Rad) were used as gel filtration standards.
Electrophoretic mobility shift assay (EMSA)
Electrophoretic mobility shift assays were performed using γ-32P-labeled DNA probes under conditions described previously [34]. Probes for EMSA were obtained by PCR, purified on agarose–1XTris-acetate-EDTA (TAE) gel followed by phenol/chloroform extraction. Probe sequences are listed in Supporting S1 Table. Purified DNA probes (1 pmol) were 5’ end labeled with [γ-32P]ATP (MP Biomedicals/ Perkin Elmer) using T4 polynucleotide kinase (New England Biolabs) in a 50 μl total reaction volume at 37°C for 1 h. Samples were run through columns packed with Sephadex G-50 fine gel (Amersham Biosciences) to separate free ATP from the labeled probes. The volume of the sample eluted from the column was adjusted to 100 μl using deionized water so that the final concentration of the probe was 10 fmol/μl.
Binding reactions were performed in a 20 μl volume using the conditions described previously [34] except for the concentration of the non-specific competitor poly(dA-dT):poly(dA-dT) in the binding reaction. The final concentration of poly(dA-dT):poly(dA-dT) was varied between 0.1 and 0.25 mg/ml depending on the DNA probe used. 1 μl of nuclear extract (corresponding to about 20 ng of protein) or an equal volume of 360 mM nuclear extraction buffer (for negative control) was used. 2 or 3 μl of nuclear extract was used when indicated. In some reactions, unlabeled competitor DNA was included so that the final concentration of the competitor would be in 25- to 100-fold excess. The reaction mixtures containing the γ-32P-labeled DNA probes were incubated for 30 min at room temperature.
For supershift experiments, pre-immune rabbit serum or antibodies against different proteins were pre-incubated in the reaction mixtures described above with the nuclear extract or gel column fractions for 30 min at room temperature to allow the protein-antibody association, followed by incubation with 32P- labeled DNA probes for 30 min at room temperature. Either 4 μl of rabbit polyclonal anti-CLAMP antibody [59], 1 μl of rabbit polyclonal antibodies against GAF and Mod(mdg4) was used.
Binding reactions were electrophoresed using the conditions described previously [34]. The gels were run at 180 V for 3 to 4 h at 4°C, dried, and imaged using a Typhoon 9410 scanner and Image Gauge software or X-ray film.
Antibodies
ChIP antibodies against GAF (full length) were raised in rats and purified from the sera by ammonium sulfate fractionation followed by affinity purification on the CNBr-activated Sepharose (GE Healthcare, United States) according to standard protocols. Anti-Ph rabbit antibodies used in ChIP experiments were a gift from Maxim Erokhin. EMSA antibodies against GAF were obtained as gift from Carl Wu and David Gilmour, against Mod(mdg4)–from Anton Golovnin and Elissa Lei.
Chromatin immunoprecipitation
Chromatin for the subsequent immunoprecipitations was prepared from 12–24 h embryos and mid-late pupae as described in [39,60]. Aliquots of chromatin were incubated with antibodies against GAF (1:200), and Ph (1:500), or with nonspecific rat or rabbit IgG (control). At least three independent biological replicates were made for each chromatin sample. The results of the ChIP experiments are measured as a percentage of the input chromatin DNA after triplicate PCR measurements. To compare the obtained values for different transgenic lines the results were normalized to positive control genomic sites for GAF and Ph. Therefore all values were presented as the ratio of the % input of the experimental genomic site (from Fab7 region) to the positive control genomic site for corresponding protein. The γTub37C coding region (devoid of binding sites for the tested proteins) was used as negative control; Hsp70 region was used as positive control for GAF binding, PRE of engrailed was used as positive control for Ph binding. The sequences of used primers are presented in Supporting S1 Table.
Replacement constructs
Coordinates for the replacement constructs are from Martin et al [61]:
HS1+HS3: 83647–84504; HS1: 84115–84504; pHS1: 84334–84504; dHS1: 84115–84356; HS*+HS1: 84115–85118; HS*+HS1+HS2: 83647–85118; HS1+HS2: 83647–84504; HS3: 83398–83665. The HS3 iab-7 PRE replacement corresponds closely to the PRE fragment used in the studies of Mishra et al [47]: 83404–83664.
Supporting information
Brightfield and darkfield images of male cuticles, as indicated. dHS1: Three classes, I, II, and III, are observed. These classes differ in the size of the tergite. Class II is the most frequent. HS3: Two different classes of phenotypes are observed. The first class (I) resembles the GOF transformation of the starting Fab-7attP50 replacement platform. The second class (II) has a small residual tergite that (based on trichome hairs) appears to have an appropriate A6 (PS11) identity. Sternites are not observed in either class. Fab-7attP50: A6 is absent, indicating that PS11 is transformed into a duplicate copy of PS12.
(TIF)
Brightfield and darkfield images of male cuticles, as indicated. HS*+HS1: The cuticular phenotypes fall into three different classes depending on the size of the A6 tergite. In the most frequent class, class I, the tergite is significantly reduced in size and misshapen. There is a modest reduction in the size of the tergite in class II, while in class III, which is the least frequent, there is only a slight reduction in the size of the tergite compared to wild type. In these flies, trichome pattern in the A6 tergite resembles that in wild type, suggesting that surviving histoblasts that give rise to the dorsal cuticle are properly specified. In all HS*+HS1 male flies the A6 sternite is missing. HS*+HS1+HS2: Three classes of cuticular phenotypes are observed. In the most frequent class, class III, the size of tergite is close to that in wild type, though sometimes the edges of the tergite are irregular. The sternite is present, but typically misshapen. Flies in the next most frequent class, class II, lack a sternite, while their tergite resembles that of class I. Finally, in class III, the tergite is noticeably reduced in size, while the sternite is misshapen.
(TIF)
Two roughly equal classes of phenotypes are observed for the pHS1+HS3 replacement. In class I, A6 is transformed into a duplicate copy of A7, and is absent. In class II, the transformation is not complete, and a small residual A6 tergite is observed.
(TIF)
Nuclear extracts prepared from 6–18 hr embryos were used for EMSA experiments: (-) no extract, (+) with extract. Comparison of LBC binding to probes spanning just GAGA3 (G3) or GAGA4 (G4) to probes spanning both GAGA3 and GAGA4 (G3+4). (A) EMSAs of G3, G4, and G3+4. (B) EMSAs of G3 and G3+4 with increasing amount of extract (1 μl, 2 μl, 3 μl). (C) Competition experiments with probe G3+G4 and excess cold G3+G4 or G3+LacZ (left to right: 100x, 75x, 50x, 25x, and10x).
(TIF)
Nuclear extracts prepared from 6–18 hr embryos were used for EMSA experiments with three overlapping HS3 probes: Probe #1, 100 bp from proximal side of HS3. Probe #2, 100 bp probe from center of HS3. Probe #3, 88 bp probe from distal side of HS3. *–unique shifts; arrow–shifts observed with two or more probes.
(TIF)
(PDF)
Acknowledgments
We thank Farhod Hasanov and Aleksander Parshikov for fly injections. We would especially like to thank François Karch for the use of the Fab-7 attP replacement platform. This study was performed using the equipment of the IGB RAS facilities supported by the Ministry of Science and Education of the Russian Federation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The salaries for OK, MS, OM, VM and resources for all genetics manipulations, cuticle preparations and ChIPs were provided by Russian Science Foundation 14-24-00166 (to PG). Also this grant covers all publication charges. Resources for EMSA experiments (AK, TA, DW) were covered by NIH R35GM126975 (to PS). The section about functional dissection of the Fab-7 boundary was supported by Russian Foundation for Basic Research 16-04-00387-a (to OK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Szabo Q, Jost D, Chang JM, Cattoni DI, Papadopoulos GL, et al. (2018) TADs are 3D structural units of higher-order chromosome organization in Drosophila. Sci Adv 4: eaar8082 10.1126/sciadv.aar8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, et al. (2014) A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159: 1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez F, Bhardwaj V, Arrigoni L, Lam KC, Gruning BA, et al. (2018) High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat Commun 9: 189 10.1038/s41467-017-02525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Sun Q, Czajkowsky DM, Shao Z (2018) Sub-kb Hi-C in D. melanogaster reveals conserved characteristics of TADs between insect and mammalian cells. Nat Commun 9: 188 10.1038/s41467-017-02526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker J, Mirny L (2016) The 3D Genome as Moderator of Chromosomal Communication. Cell 164: 1110–1121. 10.1016/j.cell.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nora EP, Goloborodko A, Valton AL, Gibcus JH, Uebersohn A, et al. (2017) Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization. Cell 169: 930–944 e922. 10.1016/j.cell.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SSP, Huang SC, Glenn St Hilaire B, Engreitz JM, Perez EM, et al. (2017) Cohesin Loss Eliminates All Loop Domains. Cell 171: 305–320 e324. 10.1016/j.cell.2017.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedotova AA, Bonchuk AN, Mogila VA, Georgiev PG (2017) C2H2 Zinc Finger Proteins: The Largest but Poorly Explored Family of Higher Eukaryotic Transcription Factors. Acta naturae 9: 47–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Chetverina D, Fujioka M, Erokhin M, Georgiev P, Jaynes JB, et al. (2017) Boundaries of loop domains (insulators): Determinants of chromosome form and function in multicellular eukaryotes. Bioessays 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz YB, Cavalli G (2017) Three-Dimensional Genome Organization and Function in Drosophila. Genetics 205: 5–24. 10.1534/genetics.115.185132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda RK, Karch F (2015) The open for business model of the bithorax complex in Drosophila. Chromosoma 124: 293–307. 10.1007/s00412-015-0522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis EB (1978) A gene complex controlling segmentation in Drosophila. Nature 276: 565–570. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Herrero E, Vernos I, Marco R, Morata G (1985) Genetic organization of Drosophila bithorax complex. Nature 313: 108–113. [DOI] [PubMed] [Google Scholar]
- 14.Kyrchanova O, Mogila V, Wolle D, Magbanua JP, White R, et al. (2015) The boundary paradox in the Bithorax complex. Mech Dev 138 Pt 2: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihaly J, Barges S, Sipos L, Maeda R, Cleard F, et al. (2006) Dissecting the regulatory landscape of the Abd-B gene of the bithorax complex. Development 133: 2983–2993. 10.1242/dev.02451 [DOI] [PubMed] [Google Scholar]
- 16.Iampietro C, Gummalla M, Mutero A, Karch F, Maeda RK (2010) Initiator elements function to determine the activity state of BX-C enhancers. PLoS Genet 6: e1001260 10.1371/journal.pgen.1001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casares F, Sanchez-Herrero E (1995) Regulation of the infraabdominal regions of the bithorax complex of Drosophila by gap genes. Development 121: 1855–1866. [DOI] [PubMed] [Google Scholar]
- 18.Kassis JA, Kennison JA, Tamkun JW (2017) Polycomb and Trithorax Group Genes in Drosophila. Genetics 206: 1699–1725. 10.1534/genetics.115.185116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassis JA, Brown JL (2013) Polycomb group response elements in Drosophila and vertebrates. Adv Genet 81: 83–118. 10.1016/B978-0-12-407677-8.00003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman SK, Deaton AM, Domingues H, Wang PI, Sadreyev RI, et al. (2014) H3K27 modifications define segmental regulatory domains in the Drosophila bithorax complex. Elife 3: e02833 10.7554/eLife.02833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang A, O'Connor MB, Paro R, Simon J, Bender W (1995) Discrete Polycomb-binding sites in each parasegmental domain of the bithorax complex. Development 121: 1681–1689. [DOI] [PubMed] [Google Scholar]
- 22.Chan CS, Rastelli L, Pirrotta V (1994) A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J 13: 2553–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon J, Chiang A, Bender W, Shimell MJ, O'Connor M (1993) Elements of the Drosophila bithorax complex that mediate repression by Polycomb group products. Dev Biol 158: 131–144. 10.1006/dbio.1993.1174 [DOI] [PubMed] [Google Scholar]
- 24.Muller J, Bienz M (1991) Long range repression conferring boundaries of Ultrabithorax expression in the Drosophila embryo. EMBO J 10: 3147–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringrose L, Rehmsmeier M, Dura JM, Paro R (2003) Genome-wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster. Dev Cell 5: 759–771. [DOI] [PubMed] [Google Scholar]
- 26.Laprell F, Finkl K, Muller J (2017) Propagation of Polycomb-repressed chromatin requires sequence-specific recruitment to DNA. Science 356: 85–88. 10.1126/science.aai8266 [DOI] [PubMed] [Google Scholar]
- 27.Gyurkovics H, Gausz J, Kummer J, Karch F (1990) A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J 9: 2579–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karch F, Galloni M, Sipos L, Gausz J, Gyurkovics H, et al. (1994) Mcp and Fab-7: molecular analysis of putative boundaries of cis-regulatory domains in the bithorax complex of Drosophila melanogaster. Nucleic Acids Res 22: 3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihaly J, Hogga I, Gausz J, Gyurkovics H, Karch F (1997) In situ dissection of the Fab-7 region of the bithorax complex into a chromatin domain boundary and a Polycomb-response element. Development 124: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 30.Galloni M, Gyurkovics H, Schedl P, Karch F (1993) The bluetail transposon: evidence for independent cis-regulatory domains and domain boundaries in the bithorax complex. EMBO J 12: 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagstrom K, Muller M, Schedl P (1996) Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev 10: 3202–3215. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Barolo S, Szymanski P, Levine M (1996) The Fab-7 element of the bithorax complex attenuates enhancer-promoter interactions in the Drosophila embryo. Genes Dev 10: 3195–3201. [DOI] [PubMed] [Google Scholar]
- 33.Hagstrom K, Muller M, Schedl P (1997) A Polycomb and GAGA dependent silencer adjoins the Fab-7 boundary in the Drosophila bithorax complex. Genetics 146: 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolle D, Cleard F, Aoki T, Deshpande G, Schedl P, et al. (2015) Functional Requirements for Fab-7 Boundary Activity in the Bithorax Complex. Mol Cell Biol 35: 3739–3752. 10.1128/MCB.00456-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogga I, Mihaly J, Barges S, Karch F (2001) Replacement of Fab-7 by the gypsy or scs insulator disrupts long-distance regulatory interactions in the Abd-B gene of the bithorax complex. Mol Cell 8: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 36.Kyrchanova O, Mogila V, Wolle D, Deshpande G, Parshikov A, et al. (2016) Functional Dissection of the Blocking and Bypass Activities of the Fab-8 Boundary in the Drosophila Bithorax Complex. PLoS Genet 12: e1006188 10.1371/journal.pgen.1006188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyrchanova O, Zolotarev N, Mogila V, Maksimenko O, Schedl P, et al. (2017) Architectural protein Pita cooperates with dCTCF in organization of functional boundaries in Bithorax complex. Development 144: 2663–2672. 10.1242/dev.149815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iampietro C, Cleard F, Gyurkovics H, Maeda RK, Karch F (2008) Boundary swapping in the Drosophila Bithorax complex. Development 135: 3983–3987. 10.1242/dev.025700 [DOI] [PubMed] [Google Scholar]
- 39.Maksimenko O, Bartkuhn M, Stakhov V, Herold M, Zolotarev N, et al. (2015) Two new insulator proteins, Pita and ZIPIC, target CP190 to chromatin. Genome Res 25: 89–99. 10.1101/gr.174169.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki T, Sarkeshik A, Yates J, Schedl P (2012) Elba, a novel developmentally regulated chromatin boundary factor is a hetero-tripartite DNA binding complex. Elife 1: e00171 10.7554/eLife.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaye EG, Kurbidaeva A, Wolle D, Aoki T, Schedl P, et al. (2017) Drosophila Dosage Compensation Loci Associate with a Boundary-Forming Insulator Complex. Mol Cell Biol 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cleard F, Wolle D, Taverner AM, Aoki T, Deshpande G, et al. (2017) Different Evolutionary Strategies To Conserve Chromatin Boundary Function in the Bithorax Complex. Genetics 205: 589–603. 10.1534/genetics.116.195586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schweinsberg S, Hagstrom K, Gohl D, Schedl P, Kumar RP, et al. (2004) The enhancer-blocking activity of the Fab-7 boundary from the Drosophila bithorax complex requires GAGA-factor-binding sites. Genetics 168: 1371–1384. 10.1534/genetics.104.029561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweinsberg SE, Schedl P (2004) Developmental modulation of Fab-7 boundary function. Development 131: 4743–4749. 10.1242/dev.01343 [DOI] [PubMed] [Google Scholar]
- 45.Brown JL, Mucci D, Whiteley M, Dirksen ML, Kassis JA (1998) The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell 1: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 46.Busturia A, Lloyd A, Bejarano F, Zavortink M, Xin H, et al. (2001) The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development 128: 2163–2173. [DOI] [PubMed] [Google Scholar]
- 47.Mishra RK, Mihaly J, Barges S, Spierer A, Karch F, et al. (2001) The iab-7 polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol Cell Biol 21: 1311–1318. 10.1128/MCB.21.4.1311-1318.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dejardin J, Cavalli G (2004) Chromatin inheritance upon Zeste-mediated Brahma recruitment at a minimal cellular memory module. EMBO J 23: 857–868. 10.1038/sj.emboj.7600108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kassis JA (1994) Unusual properties of regulatory DNA from the Drosophila engrailed gene: three "pairing-sensitive" sites within a 1.6-kb region. Genetics 136: 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuettengruber B, Bourbon HM, Di Croce L, Cavalli G (2017) Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 171: 34–57. 10.1016/j.cell.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 51.Ciabrelli F, Comoglio F, Fellous S, Bonev B, Ninova M, et al. (2017) Stable Polycomb-dependent transgenerational inheritance of chromatin states in Drosophila. Nat Genet 49: 876–886. 10.1038/ng.3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonev B, Cavalli G (2016) Organization and function of the 3D genome. Nat Rev Genet 17: 772 10.1038/nrg.2016.147 [DOI] [PubMed] [Google Scholar]
- 53.Alfieri C, Gambetta MC, Matos R, Glatt S, Sehr P, et al. (2013) Structural basis for targeting the chromatin repressor Sfmbt to Polycomb response elements. Genes Dev 27: 2367–2379. 10.1101/gad.226621.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frey F, Sheahan T, Finkl K, Stoehr G, Mann M, et al. (2016) Molecular basis of PRC1 targeting to Polycomb response elements by PhoRC. Genes Dev 30: 1116–1127. 10.1101/gad.279141.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahn TG, Stenberg P, Pirrotta V, Schwartz YB (2014) Combinatorial interactions are required for the efficient recruitment of pho repressive complex (PhoRC) to polycomb response elements. PLoS Genet 10: e1004495 10.1371/journal.pgen.1004495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kahn TG, Dorafshan E, Schultheis D, Zare A, Stenberg P, et al. (2016) Interdependence of PRC1 and PRC2 for recruitment to Polycomb Response Elements. Nucleic Acids Res 44: 10132–10149. 10.1093/nar/gkw701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmoudi T, Zuijderduijn LM, Mohd-Sarip A, Verrijzer CP (2003) GAGA facilitates binding of Pleiohomeotic to a chromatinized Polycomb response element. Nucleic Acids Res 31: 4147–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aoki T, Schweinsberg S, Manasson J, Schedl P (2008) A stage-specific factor confers Fab-7 boundary activity during early embryogenesis in Drosophila. Mol Cell Biol 28: 1047–1060. 10.1128/MCB.01622-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urban JA, Doherty CA, Jordan WT 3rd, Bliss JE, Feng J, et al. (2017) The essential Drosophila CLAMP protein differentially regulates non-coding roX RNAs in male and females. Chromosome Res 25: 101–113. 10.1007/s10577-016-9541-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zolotarev N, Maksimenko O, Kyrchanova O, Sokolinskaya E, Osadchiy I, et al. (2017) Opbp is a new architectural/insulator protein required for ribosomal gene expression. Nucleic Acids Res 45: 12285–12300. 10.1093/nar/gkx840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin CH, Mayeda CA, Davis CA, Ericsson CL, Knafels JD, et al. (1995) Complete sequence of the bithorax complex of Drosophila. Proc Natl Acad Sci U S A 92: 8398–8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brightfield and darkfield images of male cuticles, as indicated. dHS1: Three classes, I, II, and III, are observed. These classes differ in the size of the tergite. Class II is the most frequent. HS3: Two different classes of phenotypes are observed. The first class (I) resembles the GOF transformation of the starting Fab-7attP50 replacement platform. The second class (II) has a small residual tergite that (based on trichome hairs) appears to have an appropriate A6 (PS11) identity. Sternites are not observed in either class. Fab-7attP50: A6 is absent, indicating that PS11 is transformed into a duplicate copy of PS12.
(TIF)
Brightfield and darkfield images of male cuticles, as indicated. HS*+HS1: The cuticular phenotypes fall into three different classes depending on the size of the A6 tergite. In the most frequent class, class I, the tergite is significantly reduced in size and misshapen. There is a modest reduction in the size of the tergite in class II, while in class III, which is the least frequent, there is only a slight reduction in the size of the tergite compared to wild type. In these flies, trichome pattern in the A6 tergite resembles that in wild type, suggesting that surviving histoblasts that give rise to the dorsal cuticle are properly specified. In all HS*+HS1 male flies the A6 sternite is missing. HS*+HS1+HS2: Three classes of cuticular phenotypes are observed. In the most frequent class, class III, the size of tergite is close to that in wild type, though sometimes the edges of the tergite are irregular. The sternite is present, but typically misshapen. Flies in the next most frequent class, class II, lack a sternite, while their tergite resembles that of class I. Finally, in class III, the tergite is noticeably reduced in size, while the sternite is misshapen.
(TIF)
Two roughly equal classes of phenotypes are observed for the pHS1+HS3 replacement. In class I, A6 is transformed into a duplicate copy of A7, and is absent. In class II, the transformation is not complete, and a small residual A6 tergite is observed.
(TIF)
Nuclear extracts prepared from 6–18 hr embryos were used for EMSA experiments: (-) no extract, (+) with extract. Comparison of LBC binding to probes spanning just GAGA3 (G3) or GAGA4 (G4) to probes spanning both GAGA3 and GAGA4 (G3+4). (A) EMSAs of G3, G4, and G3+4. (B) EMSAs of G3 and G3+4 with increasing amount of extract (1 μl, 2 μl, 3 μl). (C) Competition experiments with probe G3+G4 and excess cold G3+G4 or G3+LacZ (left to right: 100x, 75x, 50x, 25x, and10x).
(TIF)
Nuclear extracts prepared from 6–18 hr embryos were used for EMSA experiments with three overlapping HS3 probes: Probe #1, 100 bp from proximal side of HS3. Probe #2, 100 bp probe from center of HS3. Probe #3, 88 bp probe from distal side of HS3. *–unique shifts; arrow–shifts observed with two or more probes.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.