ABSTRACT
LIMKs (LIMK1 and LIMK2) are serine/threonine protein kinases that involve in various cellular activities such as cell migration, morphogenesis and cytokinesis. However, its roles during mammalian early embryo development are still unclear. In the present study, we disrupted LIMK1/2 activity to explore the functions of LIMK1/2 during mouse early embryo development. We found that p-LIMK1/2 mainly located at the cortex of each blastomeres from 2-cell to 8-cell stage, and p-LIMK1/2 also expressed at morula and blastocyst stage in mouse embryos. Inhibition of LIMK1/2 activity by LIMKi 3 (BMS-5) at the zygote stage caused the failure of embryo early cleavage, and the disruption of LIMK1/2 activity at 8-cell stage caused the defects of embryo compaction and blastocyst formation. Fluorescence staining and intensity analysis results demonstrated that the inhibition of LIMK1/2 activity caused aberrant cortex actin expression and the decrease of phosphorylated cofilin in mouse embryos. Taken together, we identified LIMK1/2 as an important regulator for cofilin phosphorylation and actin assembly during mouse early embryo development.
KEYWORDS: LIMK1/2, actin, embryo development, blastocyst
Introduction
Following fertilization, mammalian oocyte completes its second meiosis and forms zygote, which is characteristic with female pronucleus and male pronucleus formation [1]. Subsequently, embryo undergoes successive cleavage and develops to 2-cell, 4-cell, 8-cell, morula stage, and finally forms blastocyst, showing with the presence of a fluid-filled cavity and an inner cell mass (ICM) surrounded by trophectoderm (TE). After 8-cell stages, embryo undergoes two processes: compaction and cavitation. During embryo compaction, blastomeres increase intercellular flattening, form tight junction, gap junctions and cytoskeletal connections that finally develop to polarized intracellular structures [2–4]. Failure of compaction could lead to embryonic death [5–7]. After morula formation, one or more small cavities form between blastomeres. These cavities are derived from intracellular vesicles which are secreted by the exocytosis of external blastomeres [8]. Once cavities form, cavities continually expand and fuse with each other to form a blastocyst. During the morula to blastocyst transition, Na/K-ATPase regulates fluid movement across the trophectoderm, resulting in the formation of the fluid-filled blastocoelic cavity. Meanwhile, the transcription factors are essential to generate TE and ICM in mouse blastocyst such as Oct4, Cdx2 and Tead4 [9].
Actin filaments are important for embryo cleavage, while Rho GTPase RhoA and ROCK are actin-related proteins that play critical roles in actin organization and cell polarity. Our recent studies demonstrated RhoA and ROCK were important for pre-implantation embryos development [10]. Disruption of their activities with specific inhibitors impaired embryo polarization and blastocyst formation [11,12]. Besides the GTPases, actin nucleators such as Arp2/3 complex also regulated actin filaments in mammalian embryos [13]. The inhibition of Arp2/3 by CK666 caused the failure of embryo cleavage and blastocyst formation [14]. In addition, the upstream regulators of Arp2/3, actin nucleation-promoting factors JMY and WAVE2 were also involved in mouse early embryo cleavage through mediating actin assembly [15]. Although several molecules were shown to play critical roles in embryo compaction and polarity establishment during early embryo development, the underlying molecular mechanism and signaling pathway for regulating actin dynamics in early embryo development still need to be explored.
LIMK1 and LIMK2 form the LIMK family of serine/threonine kinases that regulate actin cytoskeletal organization for multiple cellular functions such as cell migration, morphogenesis, cytokinesis, differentiation and oncogenesis. Previous work showed that LIMK1/2 phosphorylated cofilin for actin assembly, and the phosphorylated-cofilin could inhibit actin depolymerization and maintained actin dynamics [16,17]. Recently, LIMK1/2 was shown to participate in mammalian oocyte meiosis by mediating cytoskeleton organization [18–20]. However, whether LIMK1/2 plays roles in mouse early embryo development is still unknown.
In the present study, we inhibited LIMK1/2 activity by LIMK kinase inhibitor LIMKi 3 (also called BMS-5) which could inhibit both LIMK1 and LIMK2 to investigate the functions of LIMK1/2 in mouse early embryo development. Our results showed LIMK1/2 might regulate actin assembly through mediating cofilin phosphorylation, which was essential for embryo cleavage and blastocyst formation.
Materials and methods
Antibodies and chemicals
Rabbit polyclonal anti-p-LIMK1/2 antibody was purchased from Santa Cruz (Santa Cruz, CA, USA). Phalloidin-TRITC and Alexa Fluor 488 antibodies were purchased from Invitrogen (Carlsbad, CA, USA). Rabbit monoclonal anti-p-cofilin antibody was purchased from Cell Signal Technology. LIMKi 3 was from Calbiochem (Darmstadt, Germany).
In vitro fertilization (IVF) and embryo culture
Animal manipulations were in accordance with the Animal Research Institute Committee guidelines of Nanjing Agriculture University, China. Female ICR mice (6–8 week) were super-ovulated by intraperitoneal injection of 5 IU pregnant mare serum gonadotrophin (PMSG); after 48h, the mice were injected with 5 IU human chorionic gonadotrophin (HCG). Ovulated metaphase II-stage (MII) oocytes were collected from the ampullae of oviducts and placed in human tubal fluid (HTF) after 14-15h [21]. Spermatozoa were collected from adult ICR males epididymides and pre-incubated in HTF for 1h in mineral oil at 37°C with 5% CO2. after insemination, fertilized oocytes were washed and cultured in KSOM (Chemicon, Billerica, MA, USA) medium under paraffin oil at 37°C in a 5% CO2 atmosphere.
LIMKi 3 treatment
A solution of LIMKi 3 in DMSO (50mM) was diluted in KSOM medium (Chemicon, Billerica, MA, USA) to a concentration of 200 μM and 400 μM. Then embryos were cultured in KSOM medium for different times and were used for immunofluorescent staining. The control group embryos were exposed to the same concentration of DMSO.
Immunofluorescent analysis and confocal microscopy
Embryos were fixed in 4% paraformaldehyde in PBS for 30 mins at room temperature and transferred to a membrane permeabilization solution (0.5% Triton X-100) for 20 mins. Then the embryos were blocked in 1% BSA-supplemented PBS for 1h and incubated at 4°C overnight with a rabbit anti-p-LIMK1/2 (1:50) or anti-p-cofilin (1:100). After washing three times (three mins each) with PBS containing 0.1% Tween 20 and 0.01% Triton X-100; embryos were labeled with Alexa Fluor 488 goat-anti-rabbit IgG (1:100) at room temperature for 1 h. The embryos were incubated in 10 μg/ml of Phalloidin-TRITC at room temperature for 1 h, and then were co-stained with Hoechst 33342 for 10 mins. Embryos were mounted on glass slides and examined with a confocal laser-scanning microscope (Zeiss LSM 700 META). At least 30 embryos were examined for each experimental condition. Different group embryos were used the same parameters for confocal microscope.
Fluorescence intensity analysis
Fluorescence intensity was assessed using Image J software (NIH) according to our previous study [22]. For fluorescence intensity analysis, samples of control and treated embryos were mounted on the same glass slide. In addition, we used the same parameters to normalize across replicates. After immunofluorescent staining, the average fluorescence intensity per unit area (for the cortical region) within the region of interest (ROI) of immunofluorescence images was examined. Independent measurements using identically sized ROIs were taken for the cell cytoplasm. Average values of all measurements were used to determine the final average intensities for control and treated embryos. The fluorescence intensity of control embryos was standardized as 1. For actin fluorescence intensity analysis, we measured one single panel of the central oocytes.
Statistical analysis
At least three biological replicates were used for each treatment. All statistic results were given as means ± SEM. Statistical comparisons were made by analysis of variance (ANOVA), followed by Duncan’s multiple comparisons test. A p < 0.05 was considered as significant difference.
Results
The distribution of p-LIMK1/2 during early embryo development
To determine the function of LIMK1/2 during mouse early embryo development, we first examined p-LIMK1/2 localization during the different stages of early embryo development since the phosphorylated LIMK1/2 was the active form. As shown in Figure 1, we found that p-LIMK1/2 was mainly located at the cortical region of each blastomeres at 2-cell, 4-cell and 8-cell stages. And p-LIMK1/2 also expressed at morula and blastocyst stage in mouse embryos. We also co-stained p-LIMK1/2 with actin, and p-LIMK1/2 showed similar localization pattern with actin at the cortical region of embryos, which indicated that the functional role of LIMK1/2 in embryo development might be associated with actin filaments.
Figure 1.
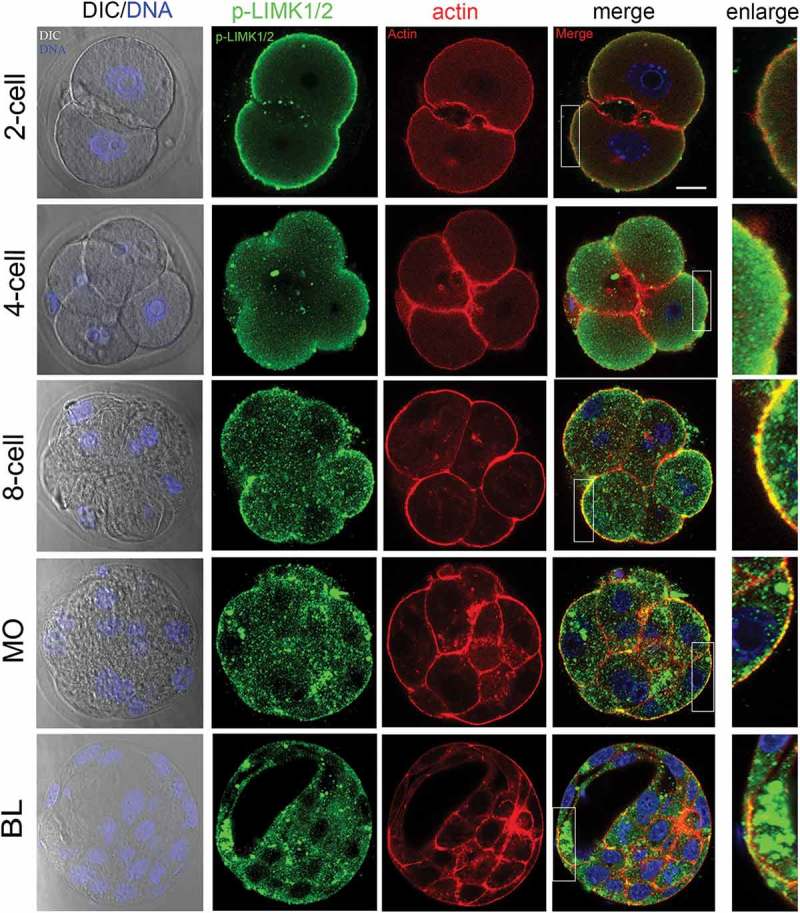
The localization of p-LIMK1/2 during mouse embryo development. From 2-cell to 8-cell stages, p-LIMK1/2 was primarily localized at cortical region of blastomeres, where it co-localized with actin. And p-LIMK1/2 localized at the apical morula and blastocyst embryos. Green, p-LIMK1/2; red: actin; blue, DNA. Bar = 20μm.
Inhibition of LIMK1/2 activity causes the failure of embryo cleavage and blastocyst formation
We next added LIMK1/2 specific inhibitor LIMKi 3 at zygote stage to disrupt LIMK1/2 activity. After culture for 96 h, we found that few embryos developed to the morula stage under the 200μM LIMKi 3 treatment, while the embryos all failed to d2/2 inhibition under 400μM LIMKi 3 treatment (Figure 2(a)). Statistics analysis data showed that the rate of 2-cell embryos in the LIMKi 3 treatment group was significantly decreased compared with that of control group (Control: 96.1 ± 2.2%, n = 150; 200μM: 85.7 ± 6.1%, p > 0.05, n = 127; 400μM: 46.1 ± 5.4%, p < 0.05, n = 106). After culture for 48h, the rate of embryo developing to 4-cell stage was significantly reduced with the increase of LIMKi 3 concentration (Control: 94.9 ± 1.6%, n = 150; 200μM: 72.36 ± 6.9, n = 127, p < 0.05; 400μM: 9.44 ± 0.56, n = 106, p < 0.05). In addition, the rate of 8-cell embryo was also significantly decreased after LIMKi 3 treatment (Control: 89.6 ± 1.9%, n = 150; 200μM: 63.3 ± 5.8%, n = 127, p < 0.05; 400μM: 0.84 ± 0.8%, n = 106, p < 0.05). After culture for 96h, most embryos developed to morula/blastocyst in control group (82.6 ± 6.6%), however, in LIMKi 3 treatment group, embryos failed to develop to morula/blastocyst, and the percentage of morula/blastocyst was significantly reduced (200μM: 52.0 ± 4.38%, n = 127, p < 0.05; 400μM: 0, n = 106, p < 0.05) (Figure 2(b)). Thus, the results revealed 2 involved in mous/2 involved in mouse embryo early cleavage. 400μM was used for the following experiments.
Figure 2.
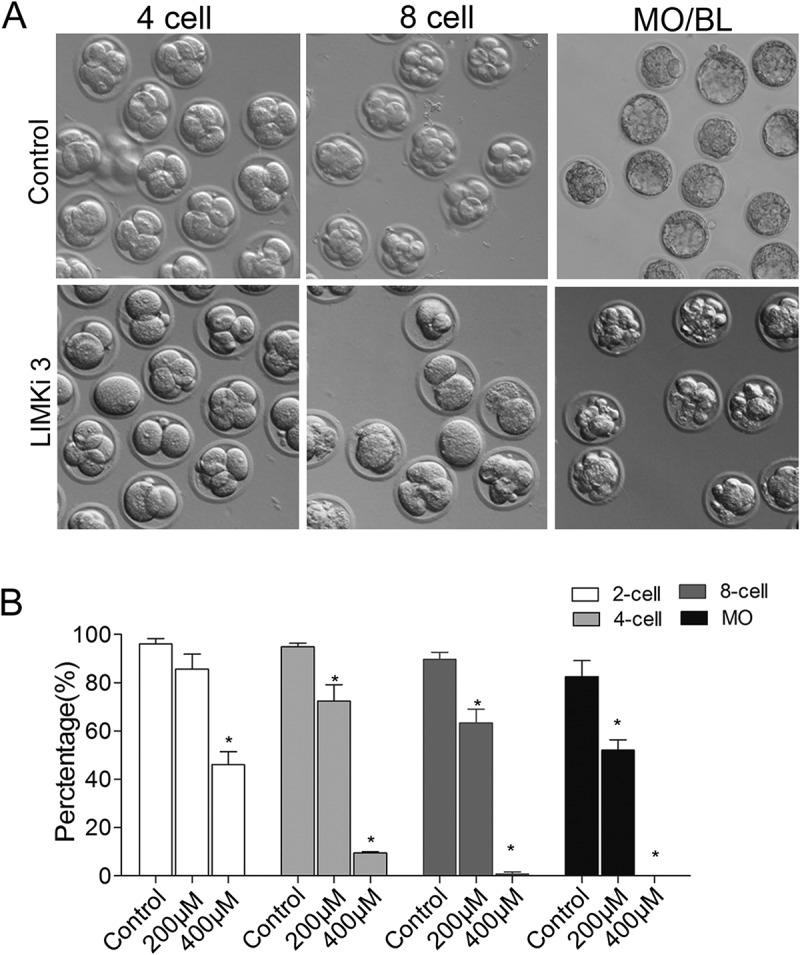
Inhibition of LIMK1/2 caused the failure of early embryo cleavage and blastocyst formation. (a) LIMKi 3 treatment caused the failure of blastocyst formation after culture for 96h. (b) Rate of 2-cell, 4-cell, 8-cell, morula and blastocyst after differential concentration of LIMKi 3-treated. *, significantly different (P < 0.05).
After 8-cell stage, embryo undergoes morphogenetic change to prepare for implantation. To explore whether LIMK1/2 was participated in this process, we added LIMKi 3 after embryo developing to 8-cell stage. As shown in Figure 3(a), most embryos failed to develop to morula stage and there was no blastocyst formation after LIMKi 3 treatment. Meanwhile, the rate of morula embryo was significantly decreased compare with that of control group (Control: 98.2 ± 0.92%, n = 104; LIMKi 3: 46.5 ± 13.9%, n = 92, p < 0.05). In addition, none of embryos in the LIMKi 3-treated group developed to blastocyst stage, but there was 76.5 ± 10.7% embryos developing to blastocyst in the control group (Figure 3(b)).
Figure 3.
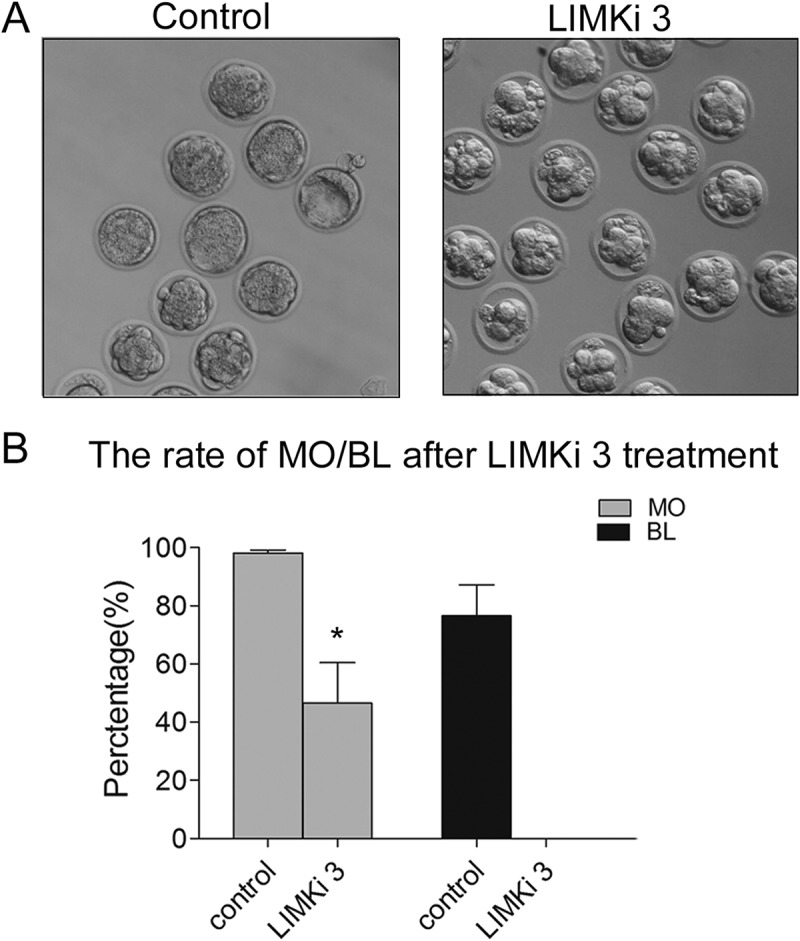
Inhibition of LIMK1/2 caused the failure of embryo compaction and blastocyst formation. (a) Embryo failed to compaction and blastocyst formation after LIMKi 3 treatment. (b) The percentage of morula and blastocyst were significantly decreased after LIMKi 3-treated at 8-cell stage. *, significantly different (P < 0.05).
Inhibition of LIMK1/2 activity affects cortex actin amount in mouse embryos
Previous study showed that LIMK1/2 involved in actin organization, we next explored whether the effect of LIMK1/2 on embryo cleavage was related with actin. Our result showed that after cultured for 48h, the actin fluorescence signal at cortex of blastomeres was significantly decreased after LIMKi 3 treatment (Figure 4(a)). Furthermore, the fluorescent intensity of curve showed in the typical picture also confirmed this (Figure 4(b)). The quantification results by Image J also showed that the amount of actin in LIMKi 3 treatment group was significantly decreased compared with the control group (1 vs 0.67 ± 0.1, n = 21, p < 0.05).
Figure 4.
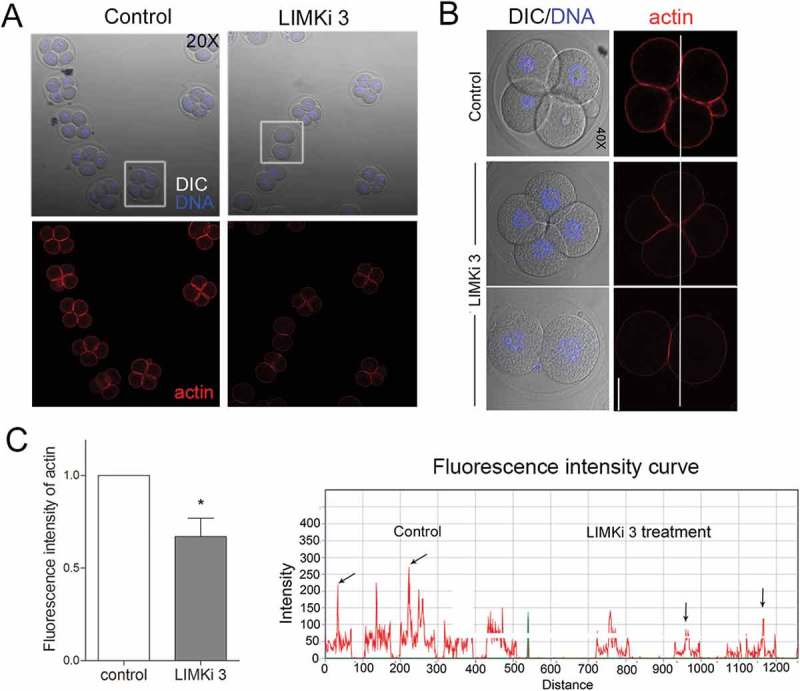
Inhibition of LIMK1/2 disrupted actin assembly during early embryo development. (a) After culture for 48h, the control embryo developed to 4-cell stage, while in a proportion of LIMKi 3 treated embryos were arrested at 2-cell stage, and cortex actin signal was significantly reduced. In addition, although some embryos developed to 4-cell, the actin signal decreased compared with the control group. (b) The fluorescence intensity curve analysis showed a comparison of the actin fluorescence intensity of representative embryos (white line). (c) The fluorescent signal of actin was significantly decreased after LIMKi 3 treatment. Red: actin; blue, DNA. Bar = 20μm; *, significantly different (P < 0.05).
LIMK1/2 phosphorylates cofilin during mouse embryo development
To further explore the regulatory mechanism of LIMK1/2 in actin assembly during mouse embryo development, we used immunofluorescent staining to examine cofilin phosphorylation levels after LIMKi 3 treatment, since the phosphorylated cofilin was the active form. The result showed that in LIMKi 3-treated embryos, the fluorescence signal of p-cofilin decreased, indicating that cofilin phosphorylation was altered in the LIMK1/2 inhibition embryos (Figure 5(a)). The fluorescent intensity of curve analysis and the quantification of fluorescent intensity results further confirmed this (1 vs 0.72 ± 0.08, n = 26, p < 0.05) (Figure 5(b)). This indicated that LIMK1/2 inhibition affected the phosphorylation level of cofilin in mouse embryos.
Figure 5.
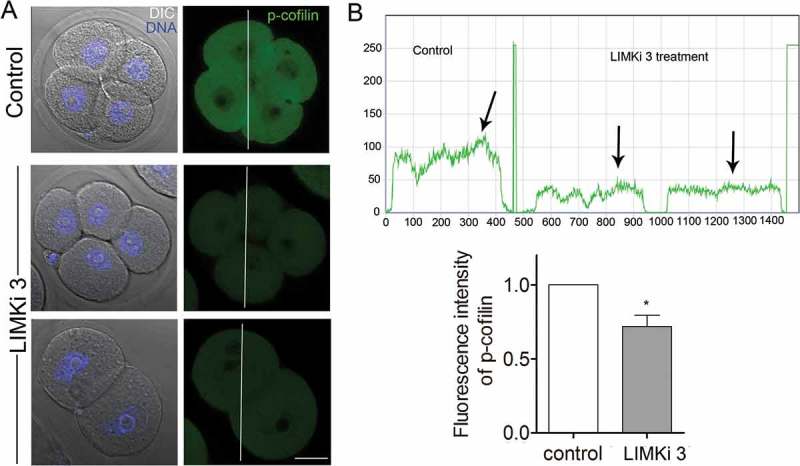
Inhibition of LIMK1/2 reduced the phosphorylation of cofilin during mouse early embryo development. (a) After culture for 48h, the immunofluorescent staining result demonstrated that the phosphorylation of cofilin was decreased. (b) The fluorescence intensity curve showed a comparison of the p-cofilin intensity of representative embryos in Figure 5(a) (white line). The histogram result suggested that the phosphorylation level of cofilin was significantly decreased compare with control embryos. Bar = 20μm; *, significantly different (P < 0.05).
Discussion
In the present study, we investigated the possible roles of LIMK1/2 during mouse early embryo development. Our results showed that LIMK1/2 might regulate cofilin phosphorylation for cortex actin assembly during mouse embryo early cleavage and blastocyst formation.
We first found that p-LIMK1/2 expressed during all stages of early mouse embryo development. And the embryos at zygote stage failed to cleavage and reach to blastocyst stage after LIMK1/2 inhibition by LIMKi 3. Moreover, inhibition of LIMK1/2 from 8-cell stage caused the failure of embryo compaction. These results indicated the critical roles of LIMK1/2 in mammalian embryos. From 2-cell to blastocyst stages, p-LIMK1/2 was mainly located at cortical area of blastomeres, and this localization pattern was similar with actin during embryo development. Actin was localized at the cortical region of blastomeres and cleavage furrow during embryo development for embryonic cleavage [23]. And actin was not only essential for spindle rotation and pronuclear fusion, but also involved in polarity establishment during embryo compaction [24]. After 8-cell stage, embryo began to undergo compaction which exhibited blastomeres adherent and polarized [25]. Meanwhile, the cell surface and cortical change of blastomeres were mediated by the proteins that regulated actin filament polymerization [26]. Together with the facts that LIMK1/2 was involved in actin polymerization in different models [18,27–29], we speculated that the effects of LIMK1/2 on embryo cleavage and compaction might be associated with actin-mediated cytokinesis.
In our study, although we did not examine the intracellular actin network due to its hard judgment in one single blastomere, inhibition of LIMK1/2 by LIMKi 3 caused the aberrant cortex actin amount, which further demonstrated our hypothesis. Our result was similar with the effect of actin nucleation factors Arp2/3 complex on embryo development showing with that CK666 treatment disrupted embryo cleavage and blastocyst formation [14,30]; in addition, recent study showed that Rho family GTPases Rho, Rac1 and Cdc42 were critical for embryo polarity establishment and compaction during early embryo development [11,31,32]. Rho and Cdc42 could induce actin cytoskeletal reorganization by mediating LIMK2 activity, and LIMK1 participated in the regulation of Rac1 on actin dynamics [33]. These facts suggested that LIMK1/2 might be an essential for actin reorganization during early embryo development.
Cofilin was an actin-severing protein belonging to ADF/cofilin family which promoted actin dynamics by depolymerizing and severing preexisting filaments [34]. The activity of cofilin was directly suppressed by LIMK1/2 through phosphorylation on the N terminal serine 3 residue [35]. And cofilin was essential for cell adherence and polarization during embryo compaction: the depletion of cofilin could promote embryo compaction after 8-cell, which indicated that cofilin involved in mouse early embryo compaction and blastocyst formation by mediating actin assembly [36]. Our results showed that inhibition of LIMK1/2 by LIMKi 3 decreased cofilin phosphorylation level in 2-cell stage, which was consisted with previous work showing that the level of phosphorylation of cofilin was severely impaired in the LIMK1/2 double knockout mice [37]. In addition, this work showed that LIMK1/2 double knockout mice were fertile, indicating that LIMK1/2 might partly affect oocyte maturation for the litter size. Recently another work showed that LIMK1 inhibitor BSM3 could disrupt spindle formation and chromosome alignment in mouse oocyte [19]; therefore, a compensation mechanism for oocyte meiosis and embryo development may exist in LIMK1/2 double knockout mice.
In summary, our study provided direct evidence of the involvement of LIMK1/2 in mouse early embryo development; meanwhile, the function of LIMK1/2 might be through the regulation of cofilin phosphorylation level for actin assembly.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31622055, 31571547); the National Basic Research Program of China (2014CB138503); the Fundamental Research Funds for the Central Universities (KYTZ201602, KJYQ201701), China.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Schatten G, Simerly C, Schatten H.. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci U S A. 1985;82:4152–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fleming TP, Hay MJ.. Tissue-specific control of expression of the tight junction polypeptide ZO-1 in the mouse early embryo. Development. 1991;113:295–304. [DOI] [PubMed] [Google Scholar]
- [3].Goodall H, Johnson MH. The nature of intercellular coupling within the preimplantation mouse embryo. J Embryol Exp Morphol. 1984;79:53–76. [PubMed] [Google Scholar]
- [4].Yamanaka Y, Ralston A, Stephenson RO, et al. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006;235:2301–2314. [DOI] [PubMed] [Google Scholar]
- [5].Landry DW, Zucker HA, Sauer MV, et al. Hypocellularity and absence of compaction as criteria for embryonic death. Regen Med. 2006;1:367–371. [DOI] [PubMed] [Google Scholar]
- [6].Le Cruguel S, Ferre-L’Hotellier V, Moriniere C, et al. Early compaction at day 3 may be a useful additional criterion for embryo transfer. J Assist Reprod Genet. 2013;30:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Skiadas CC, Jackson KV, Racowsky C. Early compaction on day 3 may be associated with increased implantation potential. Fertil Steril. 2006;86:1386–1391. [DOI] [PubMed] [Google Scholar]
- [8].Aziz M, Alexandre H. The origin of the nascent blastocoele in preimplantation mouse embryos ultrastructural cytochemistry and effect of chloroquine. Roux Arch Dev Biol. 1991;200:77–85. [DOI] [PubMed] [Google Scholar]
- [9].Marikawa Y, Alarcon VB. Establishment of trophectoderm and inner cell mass lineages in the mouse embryo. Mol Reprod Dev. 2009;76:1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Duan X, Liu J, Zhu CC, et al. RhoA-mediated MLC2 regulates actin dynamics for cytokinesis in meiosis. Cell Cycle. 2016;15:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Y, Duan X, Cao R, et al. Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development. Cell Cycle. 2014;13:3390–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Duan X, Chen KL, Zhang Y, et al. ROCK inhibition prevents early mouse embryo development. Histochem Cell Biol. 2014;142:227–233. [DOI] [PubMed] [Google Scholar]
- [13].Sun SC, Wang ZB, Xu YN, et al. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PloS One. 2011;6:e18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun SC, Wang QL, Gao WW, et al. Actin nucleator Arp2/3 complex is essential for mouse preimplantation embryo development. Reprod Fertil Dev. 2013;25:617–623. [DOI] [PubMed] [Google Scholar]
- [15].Wang QC, Liu J, Wang F, et al. Role of nucleation-promoting factors in mouse early embryo development. Microsc Microanal. 2013;19:559–564. [DOI] [PubMed] [Google Scholar]
- [16].Manetti F. LIM kinases are attractive targets with many macromolecular partners and only a few small molecule regulators. Med Res Rev. 2012;32:968–998. [DOI] [PubMed] [Google Scholar]
- [17].Takahashi H, Funakoshi H, Nakamura T. LIM-kinase as a regulator of actin dynamics in spermatogenesis. Cytogenet Genome Res. 2003;103:290–298. [DOI] [PubMed] [Google Scholar]
- [18].Jia RX, Duan X, Song SJ, et al. LIMK1/2 inhibitor LIMKi 3 suppresses porcine oocyte maturation. PeerJ. 2016;4:e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li X, Zhu Y, Cao Y, et al. LIM kinase activity is required for microtubule organising centre positioning in mouse oocyte meiosis. Reprod Fertil Dev. 2017;29:791–804. [DOI] [PubMed] [Google Scholar]
- [20].Lee SR, Xu YN, Jo YJ, et al. The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol Reprod Dev. 2015;82:849–858. [DOI] [PubMed] [Google Scholar]
- [21].Inoue A, Aoki F. Role of the nucleoplasmin 2 C-terminal domain in the formation of nucleolus-like bodies in mouse oocytes. FASEB J. 2010;24:485–494. [DOI] [PubMed] [Google Scholar]
- [22].Lu Y, Zhang Y, Pan MH, et al. Daam1 regulates fascin for actin assembly in mouse oocyte meiosis. Cell Cycle. 2017;16:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gumus E, Bulut HE, Kaloglu C. Cytoskeletal changes in oocytes and early embryos during in vitro fertilization process in mice. Anat Histol Embryol. 2010;39:51–58. [DOI] [PubMed] [Google Scholar]
- [24].Chew TG, Lorthongpanich C, Ang WX, et al. Symmetric cell division of the mouse zygote requires an actin network. Cytoskeleton (Hoboken). 2012;69:1040–1046. [DOI] [PubMed] [Google Scholar]
- [25].Saiz N, Plusa B. Early cell fate decisions in the mouse embryo. Reproduction. 2013;145:R65–80. [DOI] [PubMed] [Google Scholar]
- [26].Ducibella T, Ukena T, Karnovsky M, et al. Changes in cell surface and cortical cytoplasmic organization during early embryogenesis in the preimplantation mouse embryo. J Cell Biol. 1977;74:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vorster PJ, Guo J, Yoder A, et al. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J Biol Chem. 2011;286:12554–12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mashiach-Farkash E, Rak R, Elad-Sfadia G, et al. Computer-based identification of a novel LIMK1/2 inhibitor that synergizes with salirasib to destabilize the actin cytoskeleton. Oncotarget. 2012;3:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Y, Hu F, Chen HJ, et al. LIMK-dependent actin polymerization in primary sensory neurons promotes the development of inflammatory heat hyperalgesia in rats. Sci Signal. 2014;7:ra61. [DOI] [PubMed] [Google Scholar]
- [30].Vauti F, Prochnow BR, Freese E, et al. Arp3 is required during preimplantation development of the mouse embryo. FEBS Lett. 2007;581:5691–5697. [DOI] [PubMed] [Google Scholar]
- [31].Cui XS, Li XY, Kim NH. Cdc42 is implicated in polarity during meiotic resumption and blastocyst formation in the mouse. Mol Reprod Dev. 2007;74:785–794. [DOI] [PubMed] [Google Scholar]
- [32].Song SJ, Wang QC, Jia RX, et al. Inhibition of Rac1 GTPase activity affects porcine oocyte maturation and early embryo development. Sci Rep. 2016;6:34415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sumi T, Matsumoto K, Takai Y, et al. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol. 1999;147:1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ohashi K. Roles of cofilin in development and its mechanisms of regulation. Dev Growth Differ. 2015;57:275–290. [DOI] [PubMed] [Google Scholar]
- [35].Tanaka K, Okubo Y, Abe H. Involvement of slingshot in the Rho-mediated dephosphorylation of ADF/cofilin during Xenopus cleavage. Zoolog Sci. 2005;22:971–984. [DOI] [PubMed] [Google Scholar]
- [36].Ma M, Zhou L, Guo X, et al. Decreased cofilin1 expression is important for compaction during early mouse embryo development. Biochim Biophys Acta. 2009;1793:1804–1810. [DOI] [PubMed] [Google Scholar]
- [37].Meng Y, Takahashi H, Meng J, et al. Regulation of ADF/cofilin phosphorylation and synaptic function by LIM-kinase. Neuropharmacology. 2004;47:746–754. [DOI] [PubMed] [Google Scholar]


