ABSTRACT
Circular RNAs (circRNAs) are a novel class of noncoding RNAs (ncRNAs), which have been shown to participate in intracellular RNA regulatory networks and play vital roles in many pathological processes. Recently, circular RNA_PRKCI (circ-PRKCI) has been reported to regulate cell proliferation, migration and invasion in several human cancers. Hirschsprung disease (HSCR) is a well-known congenital gut motility disorder which roots in the aberrance of cranial-caudal neural crest cell migration. In this study, we investigated whether circ-PRKCI may affect cell migration and proliferation in HSCR. Quantitative reverse transcription PCR (qRT-PCR) was performed to detect the expression of circ-PRKCI in 48 HSCR aganglionic tissues and 48 normal bowel tissues. Luciferase reporter assay and RNA immunoprecipitation (RIP) assay verified the direct interaction between miR-1324 and PLCB1 or circ-PRKCI. Cell counting Kit-8 (CCK-8) and Ethynyldeoxyuridine (EdU) assays were employed to appraise the effects of miR-1324 or circ-PRKCI on cell proliferative potential, while transwell was performed to detect the migration in vitro. We found that circ-PRKCI was significantly down-regulated in HSCR aganglionic tissues. Morever, knockdown of circ-PRKCI suppressed cell proliferation and migration in vitro. Mechanistically, we confirmed that circ-PRKCI functioned as a molecular sponge for miR-1324 to upregulate the expression of PLCB1. In conclusion, our present study revealed the important role of circ-PRKCI-miR-1324-PLCB1 regulatory network in HSCR, providing a novel insight for the pathogenesis of HSCR.
KEYWORDS: Hirschsprung disease, circular RNA_PRKCI, miR-1324, proliferation, migration
Introduction
Hirschsprung disease (HSCR) is a common congenital digestive tract malformation characterized by the disruption of ganglion cells colonizing the distal gut, which causes bowel obstruction and megacolon[1]. The prevalence of HSCR is approximately 1 in 5000 newborns and the incidence rate in males is 4 times more than females[2]. It has been confirmed that some protein coding genes participate in the pathogenesis of HSCR, including RET, EDNRB, PHOX2B, SOX10, etc [3,4]. To date, some noncoding RNAs have also been proven to take part in the biological processes of HSCR, such as microRNA-939, lncRNA HOTTIP and LOC100507600 [5–7]. However, the underlying pathogenesis of HSCR occurrence still remains unclear.
Circular RNAs (circRNAs) are a naturally occurring family of noncoding RNAs, which are characterized by covalent closed-loop structures via joining 3ʹ and 5ʹterminals [8,9]. Therefore, circRNAs have higher stability and conservation compared to linear RNAs[10]. Recent studies have revealed that circRNAs play essential roles in multiple pathological processes, such as cardiovascular system disease, nervous system disorders, cancer diseases, etc [11–14]. Concretely, circRNAs function as regulators at the transcription or post-transcription level to affect the gene expression. Moreover, emerging researches demonstrate that natural endogenous circRNAs resist inherently to exonucleolytic RNA decay and harbor multiple electively conserved microRNA (miRNA) binding sites[15]. So, circRNAs can work as efficient miRNA sponges to regulate gene expression[16]. For example, Han D et al revealed that circMTO1 inhibited human hepatocellular carcinoma (HCC) cell proliferation and invasion via miR-9/p21 axis[17]. CircLARP4 could function as a sponge for miR-424 to suppress proliferation and invasion of gastric cancer cells[18].
Circular RNA PRKCI (ID: hsa_circ_0067934, www.circbase.org/) is located at chr3:170,013,698–170,015,181. Recent studies have indicated that circ_0067934 promoted cell proliferation and migration capacity in esophageal squamous cell carcinoma[19]. CircPRKCI could act as competing endogenous RNAs to promote the progression of lung adenocarcinoma (LAC) through increasing the expression of both miR-545 and miR-589 target E2F7[20]. However, whether circ-PRKCI exerts effect on cell migration and proliferation in HSCR is still unknown.
In this study, we investigated the underlying regulation of circ-PRKCI on cell proliferation and migration in HSCR via sponging miR-1324 via target PLCB1. Also, it may provide us a new perspective to go deep into the diagnosis of HSCR.
Results
Clinical information analysis
A total of 48 HSCR cases and 48 normal controls without HSCR and other congenital malformation were enrolled in this study. The patients’ demographic and clinical characteristics were listed in Table 1, ranging over age, weight and sex. There were no statistically significant differences between the HSCR patients and the control group, including age (112.35 ± 5.39 and 105.79 ± 3.50 days), gender (Male/Female) and body weight (5.7 5 ± 0.14 and 5.71 ± 0.11 kg).
Table 1.
Clinical characteristics of study population.
| Variable | Control(n = 48) | HSCR(n = 48) | P |
|---|---|---|---|
| Age(days, mean, SE) | 112.35(5.39) | 105.79(3.50) | 0.31a |
| Weight(kg, mean, SE) | 5.75(0.14) | 5.71(0.11) | 0.82a |
| Sex(%) | |||
| Male | 31(64.58) | 34(70.83) | 0.51b |
| Female | 17(35.42) | 14(29.17) |
aStudent’s t-test.
bTwo-sided chi-squared test.
Characteristics and expression of circ-PRKCI in HSCR
To validate the circular nature of circ-PRKCI, we treated total RNA with RNaseR, which can deplete linear RNAs characterized by a free 3′terminus but have no effect on circRNAs. This analysis showed that circular RNAs were resistant to RNaseR digestion, whereas the linear ones were not (Figure 1(a)). qRT-PCR analysis was then performed to confirm the expression of circ-PRKCI in HSCR samples and matched controls. Circ-PRKCI was dramatically decreased in HSCR patients as depicted in Figure 1(b), which suggested that circ-PRKCI may be implicated in the appearance of HSCR.
Figure 1.
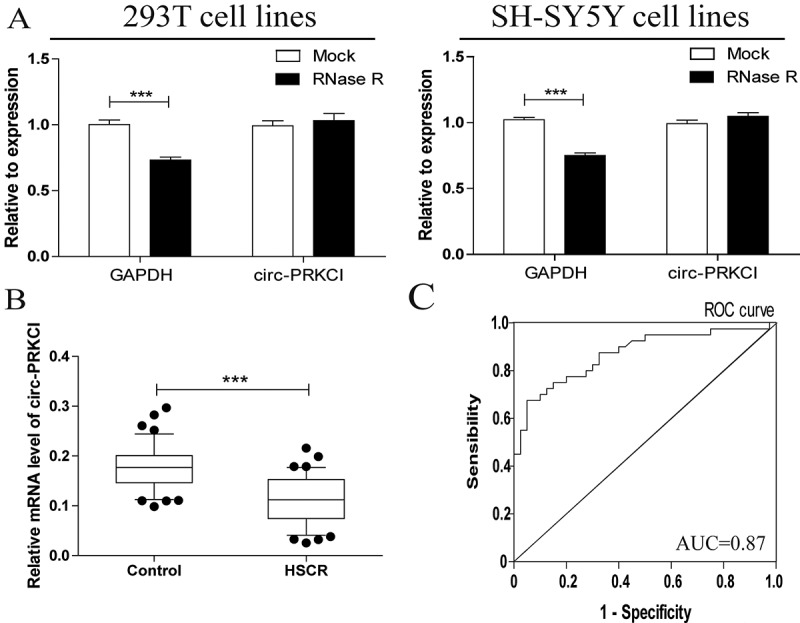
Characteristics and expression of circ-PRKCI in HSCR.
(a)Circular RNAs were resistant to RNaseR digestion in 293T cell lines and SH-SY5Y cell lines. (b) Circ-PRKCI expression was validated in 48 pairs of HSCR tissue and matched samples using RT-PCR. Circ-PRKCI expression was significantly decreased in HSCR tissue compared to matched samples. (c) Receiver Operating Characteristic (ROC) curve for the circ-PRKCI to distinguish HSCR cases from controls.
Regarding high stability and tissue specificity of circRNAs, they are taken as promising novel biomarkers in numerous diseases[21]. To further identify whether circ-PRKCI can serve as a biomarker in HSCR, the receiver operating characteristic (ROC) curves analysis was then evaluated. The area under the ROC curve (AUC) was 0.869 (Figure 1(c)); the cutoff value, sensitivity, and specificity were 0.1506, 83%, and 70%, respectively. These data showed the potential diagnostic value of circ-PRKCI in HSCR comparing with the sensitivity (88%) and specificity (89%) of the anorectal manometry, which is a common method for clinical auxiliary diagnosis of HSCR[22].
Circ-PRKCI and miR-1324 expressions were negatively correlated in HSCR tissues
To further investigate the molecular mechanism of circ-PRKCI involvement in HSCR progression, we identified the subcellular location of circ-PRKCI. Semi-quantitative PCR of nuclear and cytoplasmic fractions verified that circ-PRKCI was mostly localized in the cytoplasm of 293T and SH-SY5Y cells (Figure 2(a)). In view of the fact that circRNAs can act as miRNAs sponges[23], we then forecasted the potential targets of circ-PRKCI (RegRNA, Circinteractome, miRDB), and miR-1324 might be the most potentially complementary miRNA to bind with circ-PRKCI. To explore the relationship between circ-PRKCI and the predicted miRNA in tissues, the expression of miR-1324 in HSCR and matched groups was examined. RT-PCR results showed that miR-1324 was observably up-regulated in HSCR aganglionic tissues compared to normal tissues (Figure 2(b)), which indicated the inverse correlation between circ-PRKCI and miR-1324 expression levels in HSCR.
Figure 2.
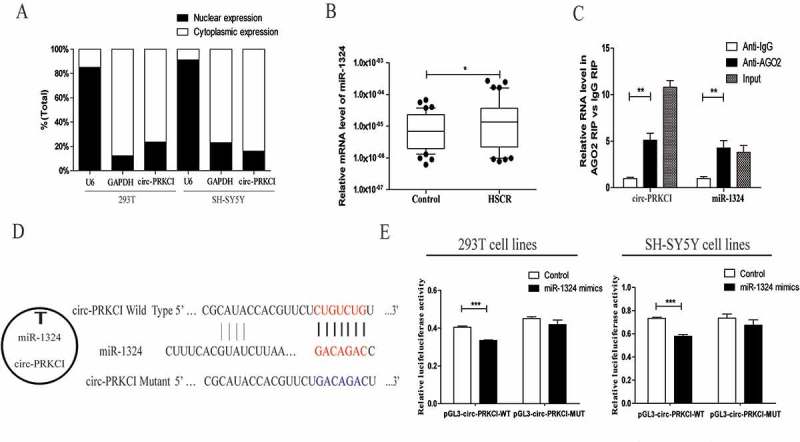
Bioinformatics analysis and experiments results manifested that circ-PRKCI sponged miR-1324.
(a)The levels of circ-PRKCI were assessed by qRT-PCR in nuclear and cytoplasmic fractions. GAPDH and U6 were used as cytoplasmic and nuclear markers, respectively. (b) The differential expression of miR-1324 between HSCR tissues and control tissues were examined by qRT-PCR analysis. MiR-1324 was significantly over-expressed in patients’ tissues compared with control tissues. (c) RNA immunoprecipitation (RIP) experiments were performed using extracts of 293T cells. Ago2 and IgG antibody were used to immunoprecipitate and qRT-PCR was applied to detect relative RNA levels of circ-PRKCI and miR-1324. (d) The seed sequences of circ-PRKCI, miR-1324 and circ-PRKCI mutant. (e) Dual-luciferase reporter assays were performed to detect the correlation between miR-1324 and circ-PRKCI in 293T and SH-SY5Y cells.
Circ-PRKCI was targeted by miR-1324
Since bioinformatics analysis has shown that circ-PRKCI contains mutual miRNA response elements (MREs) to miR-1324, we then attempted to validate the hypothesis that miR-1324 directly targeted circ-PRKCI. First, we conducted RIP assay for AGO2 on 293T cells and observed that endogenous circ-PRKCI was specifically enriched in Ago2-containing beads by qRT-PCR analysis compared to control immunoglobulin G (IgG) immunoprecipitates (Figure 2(c)). We further constructed a fragment of the mutant or wild-type circ-PRKCI sequence and the predicted miR-1324 recognition site (Figure 2(d)), and then inserted them into the downstream of the luciferase reporter gene (LUC+circ-PRKCI-Wild; LUC+circ-PRKCI-Mut). Dual-Luciferase Reporter Assay System was then adopted in 293T and SH-SY5Y cells. Relative luciferase activity was significantly reduced in cells co-transfected with the circ-PRKCI segment and miR-1324 mimics compared to controls. By comparison, the luciferase activity between the control group and cells co-transfected with miR-1324 mimics and the circ-PRKCI-Mut was explored, which showed no obvious difference (Figure 2(e)). Taken together, these results indicated the direct binding of miR-1324 targeting circ-PRKCI.
Silencing of circ-PRKCI suppressed the proliferative and migrative potential of cells in vitro
We observed that circ-PRKCI was downregulated in HSCR tissues compared with matched normal tissues by previous results, which prompted us to investigate the functional performance of circ-PRKCI in vitro. We used siRNAs targeting circ-PRKCI to silence its expression and designed the circ-PRKCI overexpression by plasmid. After circ-PRKCI overexpression and siRNA vector were respectively transfected into 293T and SH-SY5Y cells, their transfection efficiency was calculated (Supplementary Figure 1(a) and 1(b)). Succedent cell proliferation and migration assays clarified that silencing of circ-PRKCI inhibited the proliferative and migrative potential of cells, while overexpression of circ-PRKCI reversed these effects. CCK8 and EdU assays demonstrated that the proliferation of cells was significantly impaired by inhibition of circ-PRKCI and intensified by transfection with circ-PRKCI overexpression vector (Figure 3(a)). Transwell assay revealed that the down-regulation of circ-PRKCI exerted a repressive effect on cell migration. Meanwhile cell migration rate was enhanced by circ-PRKCI overexpression (Figure 3(b)). Collectively, these findings suggested that down-regulated circ-PRKCI suppressed cell migration and proliferation. In addition, we further investigated whether silencing of circ-PRKCI may affect cell cycle and apoptosis by flow cytometry analysis. The cell cycle and apoptosis process showed not obvious statistical difference between cells transfected with circ-PRKCI inhibitor and the negative control (Supplementary Figure 1(c) and 1(d)).
Figure 3.
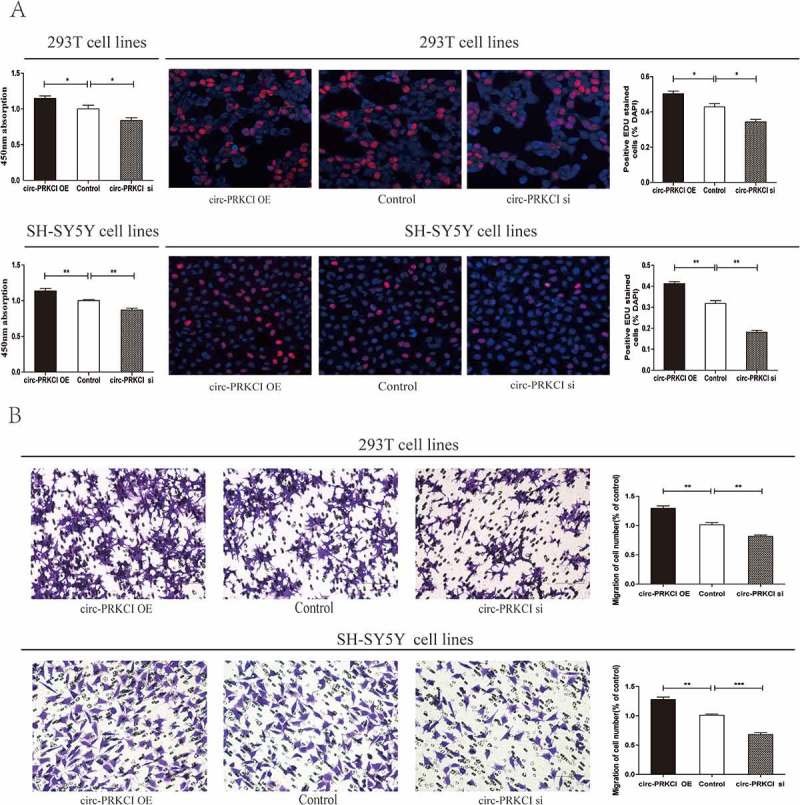
Cytobiology change of human 293T and SH-SY5Y cell lines transfected with circ-PRKCI siRNA.
(a) Knockdown of circ-PRKCI suppressed cell proliferation, while overexpression of circ-PRKCI promoted the cell proliferation. Absorbance at 450 nm measured by CCK8 was expressed as Mean ± SE (left panel). The EDU assay showed the representative images of proliferated cells (middle panel). Quantification of cell proliferation were presented as percentage proliferated cell numbers (right panel). (b) Transwell assay was performed as described in materials and methods. The quantifications of cell migration were presented as percentage migrated cell numbers, which indicated that down-regulation of circ-PRKCI inhibited cell migration and overexpression of circ-PRKCI had the opposite effect.
Circ-PRKCI upregulated expression of the miR-1324 target gene PLCB1 by sequestering mir-1324
The potential target gene of miR-1324 was searched by bioinformatic analysis (DIANA, miRanda, targetscan), and Phospholipase C,β1(PLCB1) was predicted. To further determine the interaction between miR-1324 and PLCB1, we constructed the putative binding site and mutant type as exhibited in Figure 4(a). Then we utilized plasmid pGL3-PLCB1-Wild and pGL3-PLCB1-Mut to transfect with 293T and SH-SY5Y cells. The luciferase activity was obviously reduced in cells co-transfected with the pGL3-PLCB1-Wild and miR-1324 mimics, while there were no distinct differences in cells co-transfected with mutated vectors and miR-1324 mimics or controls (Figure 4(b)). We then detected the mRNA level of PLCB1 in HSCR tissues and matched groups. PLCB1 was markedly down-regulated in HSCR cases compared with control cases as shown in Figure 4(c). Analogously, the protein expression of PLCB1 showed lower level in HSCR patients (Figure 4(d)). Furthermore, bivariate correlation analysis was carried out to assess the correlation between circ-PRKCI, miR-1324 and PLCB1 expression levels in HSCR and control colon tissues (Figure 4(eg)). These graphs and data indicated that PLCB1 was positively related to the expression of circ-PRKCI, while the level of miR-1324 had negative correlation with that of circ-PRKCI and PLCB1 in HSCR samples and controls. Above all, these findings corroborated that PLCB1 was targeted by miR-1324.
Figure 4.
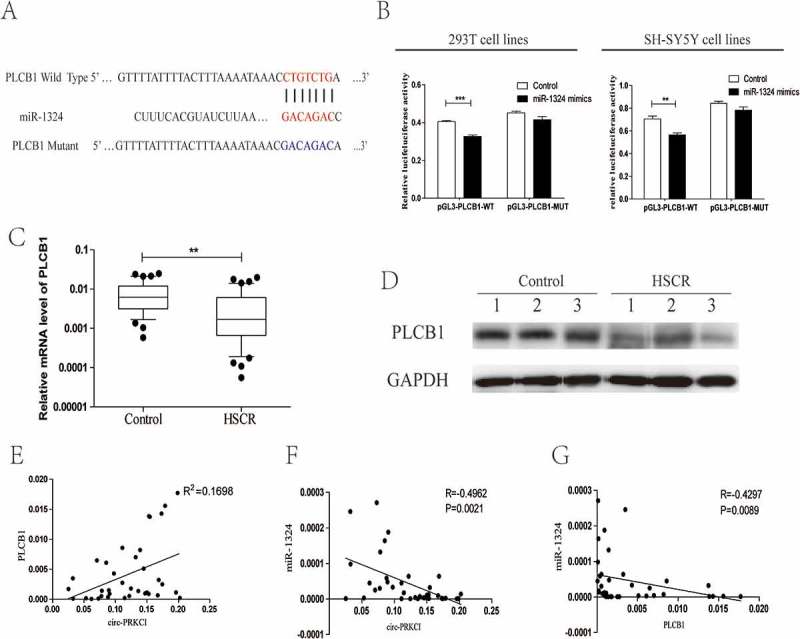
Circ-PRKCI regulated PLCB1 by sequestering miR-1324.
(a)Putative miR-1324 binding sites in the 3′-UTR of PLCB1 mRNA was showed. The sequence in the PLCB1 3′-UTR at the complementary sites of miR-1324 was mutated. (b) Dual luciferase reporter showed significant decrease of luciferase activity of the PLCB1 wild-type and luciferase activity was restored by the PLCB1 mutant sequence. (c) The expression of PLCB1 in HSCR tissues and control tissues. PLCB1 was distinctly reduced in patient tissues compared with control tissues. (d) Western blot analysis showed that the protein level of PLCB1 was down-regulated in 3 pairs of HSCR tissues compared to normal control samples. (e) Bivariate correlation analysis of the relationship between circ-PRKCI and PLCB1 expression level. (f) Circ-PRKCI was inversely correlated with miR-1324 expression in the same paired intestinal tissues (P = 0.0021, Spearman). (g) PLCB1 was negatively correlated with the expression level of miR-1324 in the same paired intestinal tissues (P = 0.0089, Spearman).
Having confirmed that PLCB1 was a direct target of miR-1324, we were curious about whether circ-PRKCI could mediate the expression of PLCB1 by combining with miR-1324. The mRNA and protein levels of PLCB1 were detected in normal cells and other two cell groups which were treated with miR-1324 inhibitor or silencing circ-PRKCI. As was depicted in Figure 5(a,b), the mRNA and protein level of PLCB1 were reduced in cells treated with inhibiting circ-PRKCI. Nevertheless, the inhibition of miR-1324 resulted in the elevation of PLCB1 in both mRNA and protein levels. We then analyzed the expression of PLCB1 in control cells and cells transfected with miR-1324 mimics, and the result showed that the mRNA and protein level of PLCB1 was inhibited by miR-1324 mimics. Meanwhile, circ-PRKCI plasmid could neutralize the inhibition function caused by miR-1324 mimics (Figure 5(c,d)). Briefly, these data implied that circ-PRKCI acted as a decoy by binding miR-1324 to mitigate the inhibiting effect of miR-1324 during the expression of PLCB1.
Figure 5.
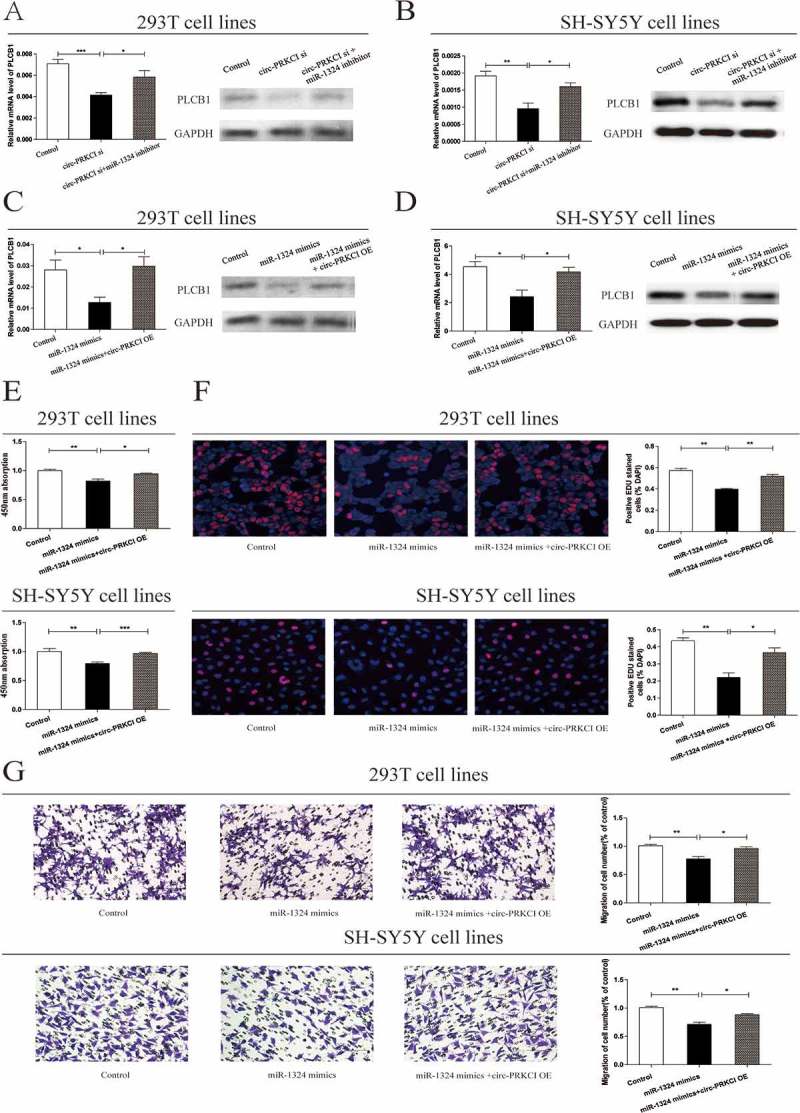
Circ-PRKCI regulated cell proliferation and migration via the miR-1324-PLCB1 axis.
(a, b) qRT-PCR and western blot analysis of PLCB1 were conducted in human 293T and SH-SY5Y cell lines after transfection with circ-PRKCI siRNA with or without miR-1324 inhibitor. GAPDH was used as control. (c, d) Two types of cells were transfected with miR-1324 mimics with or without circ-PRKCI overexpress plasmid. The mRNA level of PLCB1 and relative PLCB1 protein level were detected. (e, f) CCK8 assay and EDU assay were performed to determine the proliferation of cells transfected with miR-1324 mimics and cells treated with miR-1324 mimics in combination with circ-PRKCI overexpress plasmid. (g) Transwell analysis of 293T and SH-SY5Y cells transfected with miR-1324 with or without circ-PRKCI overexpress plasmid (left panel); Migrated cells stained with crystal violet was shown (right panel).
Circ-PRKCI exerted its regulatory effects on cell function by targeting miR-1324/PLCB1 pathway
We next inspected the correlation between miR-1324 and proliferation, migration function in cell lines. qRT-PCR analysis was carried out to evaluate miR-1324 expression levels in cells dealing with miRNA mimics or miRNA inhibitor (Supplementary Figure 1(e) and 1(f)). Compared to the normal control, proliferative and migrative potentials of cells were restricted by miR-1324 mimics, which could be partly redeemed by circ-PRKCI overexpression (Figures 5(eg)). Altogether, the above graphs and data further elaborated on the regulatory effects on cell function by circ-PRKCI/miR-1324/PLCB1 pathway.
Discussion
Recently, large numbers of circRNAs have been discovered in the mammalian transcriptome via bioinformatics and experimental analysis[24]. In view of their diversity and abundance, increasingly research focus on the function of circRNAs in human diseases[25]. It is noteworthy that circRNAs are extraordinarily abundant in neural tissues and are overall in an uptrend during neuronal differentiation and development [26,27]. Recent findings support that circRNAs are unequally distributed in the neuronal compartments, but largely enriched in the synapses for brain development or synaptic plasticity. These circRNAs species possess potential value as non-invasive clinical biomarkers in complex disorders of the central nervous system (CNS)[28]. However, few researches pay attention to the role of circRNAs in disorders of enteric nervous system (ENS), which has been likened to a second brain because of the similar microstructure of enteric ganglia with central nervous system (CNS) [29,30]. Regarded as a prototypical hypogenesis of ENS, HSCR is a potentially fatal birth defect which resulted from the lack of the enteric ganglia along a variable length of the gut [31,32]. Hence, dysregulation of circRNAs in HSCR pathology deserve further investigation. Given that circ-PRKCI has been found to promote tumor cell proliferation and migration, such as hepatocellular carcinoma, lung adenocarcinoma, we try to investigate whether circ- PRKCI affect enteric neural crest cells (ENCCs) migration and proliferation in HSCR [20,33].
In our study, we verified that circ-PRKCI showed lower levels in HSCR aganglionic tissues compared to normal controls. Besides, we further elucidated that downregulation of circ-PRKCI significantly suppressed the migration and proliferation of cells via a series of functional experiments. Contrarily, the circ-PRKCI plasmid had a prominent role in facilitating cell migrative and proliferative potential. These lines of findings indicated that circ-PRKCI may be a circRNA involved in the occurrence of HSCR.
CircRNAs have been confirmed to regulate gene expression through multiple mechanisms[34]. By using semi-quantitative PCR, we verified that the subcellular fractionation location of circ-PRKCI was mostly in the cytoplasm, indicating the probability that circ-PRKCI may take part in the translation process. Recent studies have identified that the circRNAs-miRNAs-mRNA regulatory network is involved in the occurrence and progression of a variety of diseases, such as hepatocellular carcinoma, diabetes mellitus [35,36]. Thus, we probed whether circ-PRKCI may interact with miRNAs via a miRNA sponge to eliminate the inhibiting effect on the miRNA target. By bioinformatic analysis and subsequent luciferase reporter assay, circ-PRKCI was verified to serve as a molecular sponge for miR-1324 to promote the expression of PLCB1. To date, few researches focus on the function of miR-1324. We proved that miR-1324 was upregulated in HSCR aganglionic tissues and miR-1324 mimics could restrict cell migration and proliferation. Furthermore, PLCB1 showed lower levels in HSCR aganglionic tissues compared with normal controls. Knockdown of circ-PRKCI reduced the expresssion of PLCB1, which was consistent with the repressive action of miR-1324 mimics on the PLCB1 levels. All these results identified the correlations among circ-PRKCI, miR-1324 and PLCB1 in HSCR.
Phospholipase C,β1 (PLCB1) is encoded by the PLCB1 gene which is located at chromosome 20p12[37]. It belongs to the family of PLC enzymes, which hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG)[38]. Subsequently, a series of signaling pathways were triggered to regulate cellular processes [39–41]. For example, PLCB1 was proved to positively target cyclin D3 and regulate cell cycle and cell proliferation via PKCα-mediated pathways[42]. PLCB1 and PTPRN2 acted as coregulators of PI (4,5) P2 in the plasma membrane of breast cancer cells. After depleting PI (4,5) P2, PLCB1 and PTPRN2 increased cell metastatic migration via promotion of active cytoplasmic cofilin levels[43]. Accordingly, we deduce that downregulated circ-PRKCI results in the low expression of miR-1324 target PLCB1, which may induce the abnormality of the ENCCs proliferation and migration in the distal gut. This hypothesis may be involved in the occurrence of HSCR, which needs further validation at a deeper level.
Altogether, our research team manifests that circ-PRKCI makes sense in the pathogenesis of HSCR via inhibition of miR-1324 targeting PLCB1. However, further study should be put into effect in view of substantial unidentified circRNAs which may be associated with the occurrence of HSCR. In conclusion, further research on circRNAs may obtain clinical significance on the diagnosis and therapy of HSCR and other diseases in the future.
Materials and methods
Patients and tissue specimens
Clinical samples were obtained with the consent of patients and with approval from the Institutional Ethics Committee of Nanjing Medical University. Tissue samples were collected from 48 patients with HSCR and 48 matched controls between 2010 and 2017 (NJMU Birth Cohort). The diagnosis of HSCR was confirmed by pathological examination through available biopsy samples. The matched controls were obtained from patients’ intestinal without HSCR or other congenital anomalies. All tissue samples were freshly frozen and stored at −80°C.
RNA extraction and RNase R digestion
Total RNA was isolated from tissues and cultured cells by using Trizol reagent in accordance with the manufacturer’s recommendations (Life Technologies, CA, US). Extracted RNA was subsequently purified by phenol-chloroform and re-precipitated in three volumes of ethanol. The amount and purity of RNA was detected using NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) according to the criterion of total RNA.
Total RNA (5 ug) was kept at 37°C for 15 min with 3 units of RNase R (Epicentre Biotechnologies) per 1μg RNA to remove the linear RNAs. After treatment with RNase R, circ-PRKCI expression was detected by qRT-PCR.
Quantitative real-time PCR (qRT-PCR)
Extracted RNA (500 ng) was reverse transcribed to cDNA by virtue of the Reverse Transcription Kit (Takara, Tokyo, Japan). Subsequently, the qRT- PCR was performed to estimate circRNA, mRNA and miRNA expression. Each sample was analyzed three times. The expression levels were normalized to the levels of GAPDH using 2−ΔCt method. Relevant primers were depicted in Table 2.
Table 2.
Sequences of primers for qRT-PCR and siRNA related sequence.
| Circ-PRKCI | F: 5ʹ-TAGCAGTTCCCCAATCCTTG-3ʹ R: 5ʹ-CACAAATTCCCATCATTCCC-3’ |
| PLCB1 | F: 5ʹ-CCACCTGATGTGTGTGCTTTG-3ʹ R: 5ʹ-TTCAGTAGTGGTCTGGTCTTGT-3’ |
| GAPDH | F: 5ʹ-GCACCGTCAAGGCTGAGAAC-3′ R: 5ʹ-GGATCTCGCTCCTGGAAGATG-3′ |
| U6 | F: 5ʹ-CTCGCTTCGGCAGCACA-3′ R: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ′ |
| miR-1324 | F: 5ʹ-ACACTCCAGCTGGGCCAGACAGAATTCTATGC-3ʹ R: 5ʹ-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGAAAGTGC-3’ |
| Circ-PRKCI siRNA | Sense: 5ʹ-UGUUGAUUGGGAUAUGUUAUU-3ʹ Antisense: 5ʹ- UAACAUAUCCCAAUCAACAUU-3’ |
| miR-1324 mimics | Sense: 5ʹ- CCAGACAGAAUUCUAUGCACUUUC −3ʹ Antisense: 5ʹ- AAGUGCAUAGAAUUCUGUCUGGUU −3’ |
| miR-1324 inhibitor | Sense: 5ʹ- GAAAGUGCAUAGAAUUCUGUCUGG −3’ |
Cell culture and transfection
Human 293T cell and SH-SY5Y cell lines were purchased from American Type Culture Collection (ATCC, Manassas VA, USA). We mixed 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml of penicillin, and 100μg/ml of streptomycin into Dulbecco’s Modified Eagle medium (DMEM) (Hyclone, UT, USA). Then, the above two cell lines were cultured in this medium and kept in an incubator under 5% CO2 at 37°C. Circ-PRKCI overexpression vector, small interfering RNA (siRNA) targeting circ-PRKCI, miR-1324 mimics and miR-1324 inhibitor were synthesized by GenePharma Co (Shanghai, China). Cells were respectively transfected with the above vector and microRNAs using Lipofectamine 2000 Reagent (Invitrogen, CA, USA). Detailed primer sequences were listed as shown in Table 2.
Western blot analysis
RIPA buffer were mixed with protease inhibitors to extract proteins from colon tissues and cultured cells. Then bicinchoninic acid (BCA) solution (Beyotime, Nantong, China) was opted for quantifying the protein concentrations. Equal amounts of proteins loaded on 10% SDS-PAGE gels were subsequently transferred into polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After kept with blocking buffer at room temperature for 1 h, separated protein bands were respectively incubated with the primary anti-PLCB1 (1:1000, ab182368, Abcam, Cambridge, MA, USA) and anti-GAPDH (1:1000, sc-25,778, Santa Cruz, CA, USA) overnight at 4°C. The protein bands were throughly washed with TBST for three times after the incubation with the secondary antibodies. Chemiluminescence detection relied on ECL-PLUS/Kit (GE Healthcare, Piscataway, NJ, USA).
Luciferase activity assay
We inserted the binding site of circ-PRKCI and PLCB1 into the KpnI and SacI sites of pGL3 promoter vector (Realgene, Nanjing, China) to construct the following fragments: pGL3-circ-PRKCI-Wild, pGL3-circ-PRKCI-Mut, pGL3-PLCB1-Wild and pGL3-PLCB1-Mut. Cells were plated into 24-well plates and cultured to 50–70% density. Then we co-transfected 80 ng plasmid, 5 ng renilla luciferase vector pRL-SV40, 50 nM miR-1324 mimics and negative control into cells using lipofectamine 2000 (Invitrogen, Shanghai, China). After incubation for 48 h, changes in luciferase activity were analyzed through the dual Glo luciferase assay system (Promega, Madison, WI, USA) in accordance with the manufacturer’s protocol. Each assay was repeated three times independently.
Evaluation of cell migration
For the evaluation of migration assay, transfected cells were plated on 24-well transwell chambers (8 um pore size, Millipore Corporation, Billerica, MA). After transfected for 36 h, cells were fixed with methanol and stained with crystal violet solution (Beyotime, Nantong, China). Then the bottom of the chamber was washed with PBS for three times. Images and quantifications of cell migration were conducted under 20×magnification. Five randomly selected views per well were counted. Each assay was repeated triplicate independently.
Evaluation of cell proliferation
Cells were seeded on 96-well plates with serum-free DMEM medium. After incubation for indicated time, 10μl of CCK-8 reagent (Beyotime, Nantong, China) was added to each well. After incubation 1h, the absorbance at 450 nm was detected by the TECAN infinite M200 Multimode microplate reader (Tecan, Mechelen, Belgium). In addition, the proliferative ability of cells was evaluated by EdU assay. Each assay was repeated a minimum of three times.
Subcellular fractionation location
Cytoplasmic and nuclear RNA was isolated with the PARIS Kit (Life Technologies, USA) according to the protocol. qRT-RCR was applied as described above to determine total RNA isolated from each fraction. GAPDH was regarded as cytoplasmic marker, while U6 was taken as nuclear control transcript.
RNA immunoprecipitation (RIP)
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA) was applied for RIP assay. Experimental procedure was performed in accordance to the manufacturer’s instructions. Anti-AGO2 used for RIP assay were from Abcam (ab32381, Shanghai, China).
Statistical analysis
Statistical analysis was performed using Chisquare tests and Student’s t-test. The correlation between HSCR occurrence and the expression level of circ-PRKCI was detected by Receiver operating characteristic (ROC) curves analysis. P value <0.05 was considered as significant difference. Each assay was repeated a minimum of three times.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81701493]; National Natural Science Foundation of China [81700449]; National Natural Science Foundation of China [81570467].
Acknowledgments
We thank Dr. Jie Zhang, Huan Chen, Weiwei Jiang, Xiaofeng Lv, Wei Li and Changgui Lu (Children’s Hospital of Nanjing Medical University) for sample collection. This study was supported by the Natural Science Foundation of China (NSFC 81701493, NSFC 81700449, NSFC 81570467).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- [1].Villalba-Benito L, Torroglosa A, Fernandez RM, et al. Overexpression of DNMT3b target genes during enteric nervous system development contribute to the onset of Hirschsprung disease. Sci Rep. 2017;7:6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lai FP, Lau ST, Wong JK, et al. Correction of Hirschsprung-associated mutations in human induced pluripotent stem cells via clustered regularly interspaced short palindromic repeats/cas9, restores neural crest cell function. Gastroenterology. 2017;153:139–53 e8. [DOI] [PubMed] [Google Scholar]
- [3].Borrego S, Fernandez RM, Dziema H, et al. Investigation of germline GFRA4 mutations and evaluation of the involvement of GFRA1, GFRA2, GFRA3, and GFRA4 sequence variants in Hirschsprung disease. J Med Genet. 2003;40:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chatterjee S, Kapoor A, Akiyama JA, et al. Enhancer variants synergistically drive dysfunction of a gene regulatory network in hirschsprung disease. Cell. 2016;167:355–68 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xie H, Zhu D, Xu C, et al. Long none coding RNA HOTTIP/HOXA13 act as synergistic role by decreasing cell migration and proliferation in Hirschsprung disease. Biochem Biophys Res Commun. 2015;463:569–574. [DOI] [PubMed] [Google Scholar]
- [6].Su Y, Wen Z, Shen Q, et al. Long non-coding RNA LOC100507600 functions as a competitive endogenous RNA to regulate BMI1 expression by sponging miR128-1-3p in Hirschsprung’s disease. Cell Cycle. 2018;17:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen G, Du C, Shen Z, et al. MicroRNA-939 inhibits cell proliferation via targeting LRSAM1 in Hirschsprung’s disease. Aging (Albany NY). 2017;9:2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. [DOI] [PubMed] [Google Scholar]
- [9].Greene J, Baird AM, Brady L, et al. Circular RNAs: Biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ebbesen KK, Hansen TB, Kjems J.. Insights into circular RNA biology. RNA Biol. 2017;14:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li CY, Ma L, Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother. 2017;95:1514–1519. [DOI] [PubMed] [Google Scholar]
- [12].Rybak-Wolf A, Stottmeister C, Glazar P, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–885. [DOI] [PubMed] [Google Scholar]
- [13].Piwecka M, Glazar P, Hernandez-Miranda LR, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357:6357. [DOI] [PubMed] [Google Scholar]
- [14].Guarnerio J, Bezzi M, Jeong JC, et al. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. [DOI] [PubMed] [Google Scholar]
- [15].Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. [DOI] [PubMed] [Google Scholar]
- [16].Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. [DOI] [PubMed] [Google Scholar]
- [17].Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. [DOI] [PubMed] [Google Scholar]
- [18].Zhang J, Liu H, Hou L, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xia W, Qiu M, Chen R, et al. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci Rep. 2016;6:35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qiu M, Xia W, Chen R, et al. The circular RNA circPRKCI promotes tumor growth in lung adenocarcinoma. Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- [21].Yang Z, Xie L, Han L, et al. Circular RNAs: regulators of cancer-related signaling pathways and potential diagnostic biomarkers for human cancers. Theranostics. 2017;7:3106–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].De Lorijn F, Kremer LC, Reitsma JB, et al. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006;42:496–505. [DOI] [PubMed] [Google Scholar]
- [23].Qu S, Yang X, Li X, et al. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. [DOI] [PubMed] [Google Scholar]
- [24].Salzman J, Gawad C, Wang PL, et al. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205–211. [DOI] [PubMed] [Google Scholar]
- [26].Chen W, Schuman E. Circular RNAs in brain and other tissues: a functional enigma. Trends Neurosci. 2016;39:597–604. [DOI] [PubMed] [Google Scholar]
- [27].Cortes-Lopez M, Miura P. Emerging functions of circular RNAs. Yale J Biol Med. 2016;89:527–537. [PMC free article] [PubMed] [Google Scholar]
- [28].Lu D, Xu AD. Mini review: circular RNAs as potential clinical biomarkers for disorders in the central nervous system. Front Genet. 2016;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Furness JB, Stebbing MJ. The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil. 2018;30:2. [DOI] [PubMed] [Google Scholar]
- [31].Heuckeroth RO. Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15:152–167. [DOI] [PubMed] [Google Scholar]
- [32].Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. [DOI] [PubMed] [Google Scholar]
- [33].Zhu Q, Lu G, Luo Z, et al. CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR-1324/FZD5/Wnt/beta-catenin axis. Biochem Biophys Res Commun. 2018;497:626–632. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Liang W, Zhang P, et al. Circular RNAs: emerging cancer biomarkers and targets. J Exp Clin Cancer Res. 2017;36:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shi L, Yan P, Liang Y, et al. RNA expression is suppressed by androgen receptor (AR)-regulated adenosine deaminase that acts on RNA (ADAR1) in human hepatocellular carcinoma. Cell Death Dis. 2017;8:e3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang G, Ma Y, An T, et al. Relationships of circular RNA with diabetes and depression. Sci Rep. 2017;7:7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li J, Zhao X, Wang D, et al. Up-regulated expression of phospholipase C, beta1 is associated with tumor cell proliferation and poor prognosis in hepatocellular carcinoma. Onco Targets Ther. 2016;9:1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lattanzio R, Piantelli M, Falasca M. Role of phospholipase C in cell invasion and metastasis. Adv Biol Regul. 2013;53:309–318. [DOI] [PubMed] [Google Scholar]
- [40].Tomas NM, Masur K, Piecha JC, et al. Akt and phospholipase Cgamma are involved in the regulation of growth and migration of MDA-MB-468 breast cancer and SW480 colon cancer cells when cultured with diabetogenic levels of glucose and insulin. BMC Res Notes. 2012;5:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang B, Wang F, Dai L, et al. Lentivirus-mediated PLCgamma1 gene short-hairpin RNA suppresses tumor growth and metastasis of human gastric adenocarcinoma. Oncotarget. 2016;7:8043–8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Faenza I, Fiume R, Piazzi M, et al. Nuclear inositide specific phospholipase C signalling - interactions and activity. FEBS J. 2013;280:6311–6321. [DOI] [PubMed] [Google Scholar]
- [43].Sengelaub CA, Navrazhina K, Ross JB, et al. PTPRN2 and PLCbeta1 promote metastatic breast cancer cell migration through PI(4,5)P2-dependent actin remodeling. EMBO J. 2016;35:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


