Abstract
Objective
The aim of this study was to develop pH sensitive nanoparticles of budesonide for the treatment of ulcerative colitis.
Methods
The NPs system was characterized by the transmission electron microscopy (TEM), particle size, drug loading and encapsulation efficiency. In addition, in vitro drug release prop-erties and pharmacokinetics were also investigated in detail. The optimized formulation was examined for its in-vivo targeting potential using 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis in a rat model.
Results
Dynamic light-scattering results showed that the particle size of budesonide-Eudragit S100/poly(lactic-co-glycolic acid) nanoparticles was around 110.5 nm, with a polydispersity index of 0.098. Transmission electron microscopy images showed that BUD-ES100/PLGA NPs were spherical with uniform size and relatively smooth surfaces. In vitro release showed that BUD-ES100/PLGA NPs required minimal release of drugs during its transit in the stomach and the upper small intestine to ensure that a maximum dose reached the colon. After the pharma-codynamic treatment, the myeloperoxidase value of BUD-ES100/PLGA NPs was close to the normal group. The histopathological examination of rectum showed that no sign of damages such as epithelial necrosis and sloughing epithelial cells was detected.
Conclusion
Our findings suggested that BUD-ES100/PLGA NPs were a promising alternative to single pH-dependent systems for colitis therapy.
Keywords: budesonide, nanoparticles, ES100/PLGA, in vitro release, pharmacodynamic
Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) associated with exten-sive mucosal inflammation, including the length of the rectum and colon. Ulcerative colitis is a chronic inflammation that is prone to relapse. Systemic corticosteroids are one of the preferred methods for the treatment of UC. Clinical trials from 1950s to 1960s demonstrated the effectiveness of induction remission with the clinical response rate in some studies close to 80%.1
Over the past half century, these results have been further supported by clinical experience.2–4 The effect of glucocorticoids on the immune response is achieved by their interaction with the cytoplasmic glucocorticoid receptor. This interaction leads to downregulation of several inflammatory cytokines and inhibits proliferation and recruitment of inflammatory cells.5
Budesonide (BUD) is a synthetic glucocorticoid with no halogen and is structurally related to the 16-alpha hydroxyprednisolone. This chemical structure explains the good characteristics of BUD, including its increased affinity with the hormone receptor and its local potency, which is nearly 5 times that of prednisone.6,7 It also allows rapid removal of the drug through extensive pri-mary metabolism in the liver, resulting in low systemic bioavail-ability and minimizing its systemic effects.7 Because of these characteristics, BUD is considered to be the prototype of locally acting corticosteroids and the most widely studied form in IBD. It is recommended as a first-line therapy for the remission of mild or moderate symptoms of Crohn’s disease, especially those involving distal ileum and/or right colon disease distribution.8,9
Previous studies did not provide sufficient evidence to support the use of oral BUD in UC patients, and traditionally only the rectal BUD preparation is considered a potential treatment option.3 Several recent trials using BUD sustained-release preparations have shown encouraging results that may lead to the use of this compound in patients with UC.10–12 However, the application of BUD sustained-release preparation for colonic delivery also suffers from problems such as unpredictable gastric emptying, gastrointestinal (GI) transit variations resulting from intersubject variability in transit patterns, and incomplete drug delivery in GI tract due to the risk of not dissolving the polymer coat on the large, low surface area-coated tablets. Therefore, how to directly send appropriate drugs through a smart delivery system has always been a hot topic for researchers. The pH-sensitive drug delivery system is a classic UC-targeting method due to the different pH along the GI tract.13 Eudragit S100 (ES100), the most commonly used biocompatible polymer for colon-specific drug delivery, has been approved by authorities in the USA, Europe, and Japan for oral administration.14 The poly-mer could selectively dissolve in aqueous media of pH 6–7 and release encapsulated drug to the colon. It is reported that with the change of intestinal pH, ES100-loaded sustained-release preparations can release the largest amount of encapsulated drugs immediately.15 Previously, our team used guar gum to prepare BUD microspheres. However, this approach had some shortcomings such as the cumbersome preparation process and poor reproducibility. Meanwhile, no pharmaco-dynamic evaluation was performed at that time.16
The main objective of this study was to develop, optimize, and evaluate an effective colon-targeted oral nanoparticle (NP) of BUD for the treatment of UC (BUD-ES100/poly(lactic-co-glycolic acid) [PLGA] NP). The NP system was charac-terized by transmission electron microscopy (TEM), particle size (PS), drug loading (DL%), and encapsulation efficiency (EE%). In addition, in vitro drug release properties and phar-macokinetics were also investigated in detail. The optimized formulation was examined for its in vivo targeting potential using 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis in a rat model. To the best of our knowledge, this is the first study examining the therapeutic efficacy of BUD-ES100/PLGA NPs against an experimental mice model of UC.
Materials and methods
Materials
BUD was obtained from Hangzhou Moon Fine Chemical Co. (purity 99.6%, batch number 05126); PLGA (average molecular weight [MW] 41,000 Da, lactic acid:glycolic acid 50:50) was pursued from Shandong Medical Instrument Research Institute (Shandong, People’s Republic of China). Polyvinyl alcohol (PVA) was supplied by Shanghai Colorcon Coating Technology Co. Ltd. Other reagents were of analytical grade. Purified water from Milli-Q system (Millipore, Bed-ford, MA, USA) was used throughout the experiment.
Preparation
BUD-ES100/PLGA NPs were formulated by a modified emulsion-solvent evaporation method.17,18 In total, 120 mg polymeric mixtures of ES100 and PLGA with a weight ratio of 1–2 and 20 mg BUD were dissolved in a solvent system consisting of 5 mL dichloromethane. The polymeric solution was dripped into 5 mL of 2% (w/v) acidic PVA solution under vortex. This emulsion was immediately poured into 100 mL of 0.2% (w/v) acidic PVA solution. After that, the organic solvent was evaporated under low vacuum conditions and the mixture was further homogenized using a microfluidizer to obtain fine BUD-ES100/PLGA NPs. The obtained NPs were stored at −20°C in an airtight container. As a control, blank NPs with ES100/PLGA=1:2 were prepared.
Characterization
The surface morphology of the NPs was observed under TEM. The mean PS, polydispersity index (PDI), and zeta potential (ZP) of the developed NPs were measured at 25°C using a ZP ana-lyzer (Zetasizer Nano ZS; Malvern Instruments, Malvern, UK). Before the measurements, all samples were diluted with double distilled water. All measurements were made in three copies.
To determine EE% and DL%, 50 mg of NPs was accurately weighed and triturated with 10 mL of methanol and kept for 12 h. After filtration, appropriate dilutions were made with methanol and the BUD concentration was measured with high performance liquid chromatography at 245 nm. EE% and DL% were calculated using the following formula:
Stability study
The proposal of stability study was mainly revised in accor-dance with the guidelines of the Chinese Pharmacopoeia. The NPs were placed in a stable box at room temperature and saturated with sodium chloride solution with a relative humidity of 75%±5%. Then, at 0, 1, 2, 3, and 6 months of testing, they were assessed to determine whether the appearance, PS, PDI, ZP, EE%, and DL% had changed.
In vitro release
In vitro release characteristics of BUD from the formulations were investigated in a release medium with gradually changing pH by the dialysis bag method.19 Briefly, 100 mg of NPs was introduced into a dialysis bag (MW 8–10 kDa). The closed bag was then immersed in 20 mL release medium with pH of 1.2 (0–2 h), 4.5 (2–4 h), and 7.4 (4–24 h) at 37°C. Each time the releasing medium was replaced. The release medium, consisting of hydrochloric acid/potassium chloride (pH 1.2), acetic acid/sodium acetate (pH 4.5), and PBS (pH 7.4), was then stirred at the speed of 100 rpm for 24 h. In total, 1 mL of sample was withdrawn at different time intervals and replaced with the same amount of fresh release medium. The concentration of BUD in the samples was determined by the high performance liquid chromatography method. In addition, in the release study, we selected two pH conditions (pH=1.2 and 7.4 for 24 h) as control groups.
GI distribution
All animal experiments were performed and approved in accor-dance with the guidelines on animal studies provided by the ethics committee of Soochow University. As described in other reports, Kunming mice (20 g) were used for this study. A total of 90 mice were randomly divided into three groups and fasted with access to drinking water for 24 h before administration. Free BUD, BUD-ES100/PLGA NPs, and BUD foams (10 mg/kg) were orally or rectally administered followed by the intake of a sufficient volume of drinking water. After 2, 6, 8, 10, and 24 h, the rats were killed by deep ether anesthesia. Immediately after death, the entire GI tract was removed, and blood vessels and fatty tissues were separated. The GI tract was segmented into stomach, small intestine, and colon. The contents of the lumen were removed by gentle pressure. The organs were washed with normal saline to remove the remaining luminal contents and collected to determine the BUD tissue concentrations.
Treatment efficiency
TNBS-induced colitis rat model was selected to evaluate the new formulation of BUD on colonic damage. Male SD rats (weighing 220–250 g, 10–12 weeks old) were fasted for food 24 h prior to the experiment, allowing food and water to be fed after the administration of TNBS. Colitis was induced according to the procedure described by Morris et al.20
In short, after light ether anesthesia, the rats were cath-eterized by 8 cm intrarectally and TNBS dissolved in ethanol (40% v/v) was infused into the colon (100 mg/kg) slowly in a total volume of 0.5 mL. Then the rats were kept at a head down position for 1–2 min to prevent leakage of the intracolonic instillate before being returned to their cages. Rats with instillate leakage through the anus were excluded from the study. The same procedure was applied to the nor-mal control group rats, which were administered with normal saline instead of TNBS.
Grouping and treatment
Animals were randomly divided into five groups, six in each. Group A (untreated group) received 0.5 mL saline instead of TNBS. Rats in this group were treated orally once daily with normal saline 24 h after colitis induction and the treatment was continued for 7 days. In other four groups, colitis was induced by TNBS and treatments were made orally or rectally with one of the drugs similar to control groups. Group B was treated with the BUD suspension formulation (300 mg/kg/day), group C with BUD-ES100/PLGA NPs (300 mg/kg/day), and group D with BUD foams (100 mg/kg/day, rectally). Group E was the normal group without colitis induction.
On the 8th day, all the animals were sacrificed. The colon was removed, and a 10 cm segment of the distal colon proximal to rectum was resected and rinsed in ice-cold saline. The histology and myeloperoxidase (MPO) activity were evaluated.21,22
Rectal irritation
Immediately after the pharmacodynamic study, three animals were collected from the BUD suspension; NPs group and untreated group, respectively. The rectum was separated and washed with physiological saline, fixed in 10% neutral carbonate-buffered formalin, paraffin embedded, and sliced. The tissue was stained with H&E and observed under light microscopy.
Statistical analysis
Data were statistically described in terms of mean±SD. Student’s t-test was used to evaluate associations between the two groups’ data. P⩽0.05 was considered statistically significant. All statistical calculations were done using the computer program SPSS (SPSS Inc., Chicago, IL, USA) version 21 for Microsoft Windows.
Results and discussion
Characterization
In this study, BUD-ES100/PLGA NPs were formulated by the emulsion-solvent evaporation method for the treatment of UC. During the preparation process, the organic phase (containing polymers and drug) was added to the aqueous phase (containing an emulsifier [PVA]) rapidly, and then an oil/water emulsion was immediately formed. Then according to the principle of “similar phase dissolving”, the evaporation of the organic solvents under reduced pressure transferred BUD to the polymer-based hydrophobic core through hydro-phobic interactions, and then further solidified the particles to form compacted NPs. In addition, the presence of emulsifier on the interface helped to separate oil and water phases as well as to prevent the aggregation of NPs.21
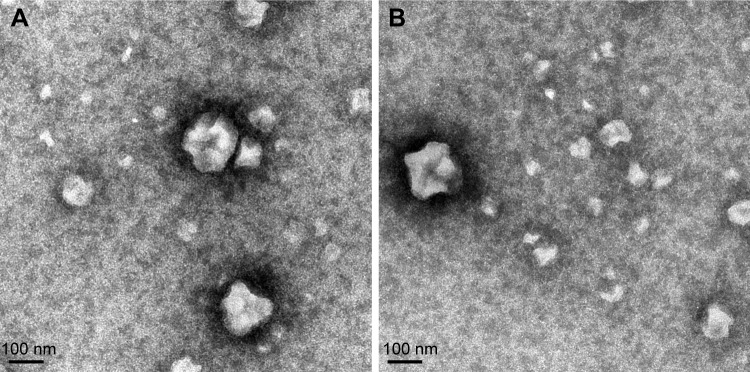
Dynamic light-scattering results showed that the PS of BUD-ES100/PLGA NPs was around 110.5 nm, with a PDI of 0.098 (Table 1). TEM images showed that BUD-ES100/PLGA NPs were spherical with a uniform size and relatively smooth surfaces (Figure 1). In our study, BUD-ES100/PLGA NPs had EE% of 82.3%±4.6% and DL% of 8.1%±0.3% (n=3). In the process of prescription optimization, it was observed that as the concentration of polymers increased, the drug EE% increased. This might be due to the enhanced viscosity. In addition, the drug EE% increased with a rise in the amount of the solvent but then decreased with further increases in the solvent amount. This might be caused by the decrease in the system viscosity and matrix density at higher solvent levels (data not shown).
Table 1.
Characteristics and stability data at room temperature of BUD-ES100/PLGA NPs
| Time | PS (nm) | PDI | ZP (mV) | DL% | EE% |
|---|---|---|---|---|---|
| 0 month | 110.5±1.4 | 0.098±0.01 | −11.4±1.1 | 8.1±0.4 | 82.3±2.6 |
| 1 month | 109.6±1.1 | 0.102±0.02 | −12.6±1.1 | 7.9±0.6 | 81.9±2.3 |
| 3 months | 112.7±1.3 | 0.104±0.02 | −11.7±1.2 | 8.0±0.7 | 81.8±1.8 |
| 6 months | 111.3±1.6 | 0.101±0.03 | −13.3±1.3 | 7.9±0.3 | 81.6±1.9 |
Note: Data represent the mean±SD (n=3).
Abbreviations: BUD, budesonide; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles; PS, particle size; PDI, polydispersity index; ZP, zeta potential; DL%, drug loading; EE%, encapsulation efficiency.
Figure 1.
Transmission electron microscope of BUD-ES100/PLGA NPs.
Note: (A) Newly prepared sample and (B) sample stored for 6 months (·75,000).
Abbreviations: BUD, budesonide; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles.
The stability studies of the BUD-ES100/PLGA NPs were observed over a period of 6 months. Under close observation, BUD-ES100/PLGA NPs were remarkably stable for the period of study (6 months) at test temperature. Appearance, PS, PDI, ZP, EE%, and DL% of the NPs were found to be consistent and no signs of separation or deterioration were observed (Table 1). This revealed the reproducibility of physical and chemical parameters, thereby ensuring the consistency of NP formulations over a long period of time. The good stability was due to two factors. One was the principle of same-charge repulsion. The second was that our prepared method did not cause the aggregation of NPs. This could be seen from the results of TEM.
In vitro release
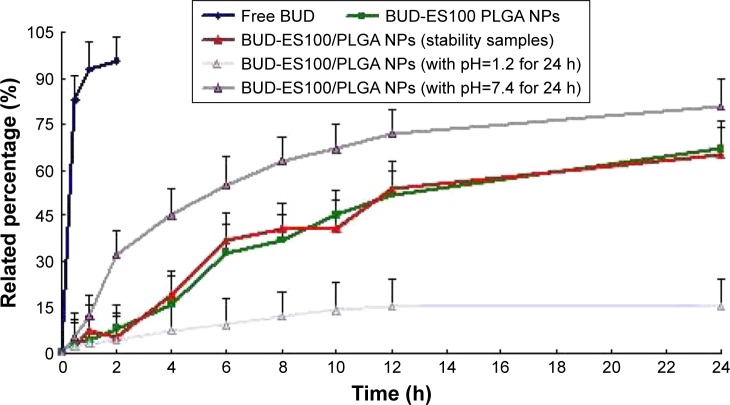
In vitro drug release behavior of BUD-ES100/PLGA NPs was studied using the dialysis bag method. The release profiles of free BUD, BUD-ES100/PLGA NPs, and BUD-ES100/PLGA NPs (6 months stability samples) are shown in Figure 2. A very fast release behavior of free BUD was observed, whereas the cumulative release rate of BUD NPs was much slower followed by a sustained release. In the free BUD group, 95% of BUD was released in the first 2 h. By contrast, only 10% was released from NPs in the first 2 h (p⩽0.05). With the increase of the pH value, the release of BUD also gradually increased. During the observation period (24 h), the total release amount was almost 70%. Moreover, there was no significant difference in the release pattern after 6 months of storage.
Figure 2.
In vitro release profiles of BUD-ES100/PLGA NPs and BUD-ES100/PLGA NPs (6 months stability samples) from three batches.
Notes: Release experiments were carried out in 20 mL release medium (pH 1.2 [0–2 h], 4.5 [2–4 h], and 7.4 [4–24 h]) at 37°C. Each point represents the mean value of three different mean±SD.
Abbreviations: BUD, budesonide; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles.
In the study of in vitro release, the pH condition was set to simulate the GI condition without enzyme. pH values of 1.2 and 4.5 were used for 2 h (stomach) and 2 h (duodenum), respectively, followed by a pH value of 7.4 (distal ileum and colon) for the remaining period of the study.22 The optimized colon-targeted preparation required minimal release of drug during its transit in the stomach and the upper small intestine to ensure that a maximum dose reached the colon. In this study, the release characteristics of BUD-ES100/PLGA NPs showed this phenomenon. In addition, ES100 is a free carboxyl ester group of methacrylic acid and methyl meth-acrylate copolymer with a ratio of 1:2. It is almost insoluble in water, but dissolves in intestinal fluid with a pH value of more than 7.0.23 Due to the pH-dependent solubility of this polymer, it could help BUD NPs pass through the gastric cavity and the upper small intestine without being rapidly dissolved. At the same time, it could be observed from the release study that the BUD-ES100/PLGA NPs released the drug very slowly under acidic conditions (pH=1.2). On the contrary, in the alkaline release medium (pH=7.4), the drug was released quickly. This to some extent also reflected the sensitivity of BUD-ES100/PLGA NPs to pH f.
The release kinetics in vitro was analyzed by zero-order and first-order kinetics, as well as diffusion controlled release mechanisms. A higher correlation coefficient obtained from the analysis of the amount of the drug released versus the square root of time indicated that the release followed the Higuchi (Higuchi, 1962) kinetic model (Table 2).24
Table 2.
Correlation coefficients for kinetic analysis of release data for BUD-ES100/PLGA NPs
| Formulation | Correlation coefficient (r)
|
||
|---|---|---|---|
| Zero order | First order | Higuchi | |
| BUD-ES100/PLGA NPs | 0.9723 | 0.9542 | 0.9987 |
Abbreviations: BUD, budesonide; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles.
GI distribution
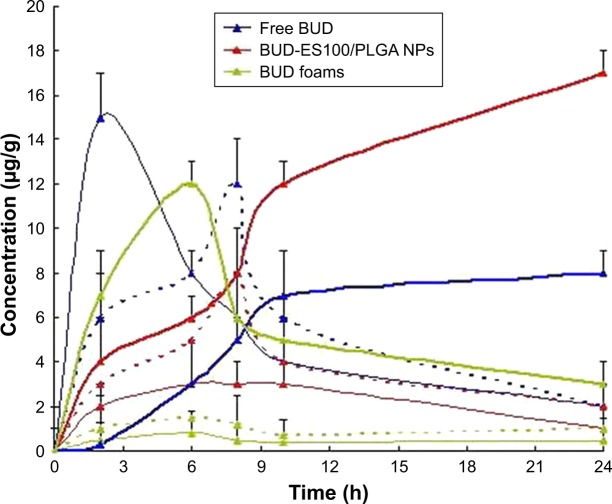
The in vivo biodistribution behavior of different BUD for-mulations in mice was investigated (Figure 3). The amounts of drug distributed in unit mass of stomach, small intestine, and colon were measured at various times. The results showed that the maximum concentration of BUD (15 μg/g) was observed in the stomach 2 h after the intragastric admin-istration of free BUD. A small amount reached the small intestine, but no drug was found in the colon. However, in the group of NPs, only a 5.2 μg/g concentration of drug was measured in the colon after 8 h. The BUD-ES100/PLGA NPs were found to be intact in the upper part of the GI tract. After 8 h of administration, the largest proportion of drug was observed in the colon, and a small amount was found in the small intestine and a negligible amount in the stomach.
Figure 3.
Distribution in tissue in mice by following different administration of a single 10 mg/kg dose of free BUD (blue line); BUD-ES100/PLGA NPs (red line) and BUD foams (green line) (each point represents the mean±SD of six mice).
Note: Stomach (fine line), small intestine (dotted line), and colon (thickening line).
Abbreviations: BUD, budesonide; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles.
There was a significant difference in drug concentration between NPs and suspensions in the stomach, small intestine, and colon. In addition, in the group of BUD foams, the drug concentration could reach a higher level at the initial stage due to local administration, but dropped rapidly later. There were few BUD concentrations in the stomach and small intestine. Hence, BUD-ES100/PLGA NPs are considered to have the potential to maintain the BUD concentration for a long period of time, reducing adverse effects caused by concentration fluctuations, ensuring the efficiency of treatment, and improving the compliance of the patients.25,26
Treatment efficiency
MPO is a specific marker of neutrophils. It is released in vitro or in vivo after the activation of neutrophils. Extracellular MPO is an indicator of neutrophil activation under various clinical conditions, including IBD, rheumatoid arthritis, and acute respiratory distress. MPO assay could be used to evaluate the average inflammatory level of the colon in either surgical or biopsy specimens. This technology is more useful in evaluating the efficacy of drugs and the site-specific dosage forms in animal models of inflammation.27
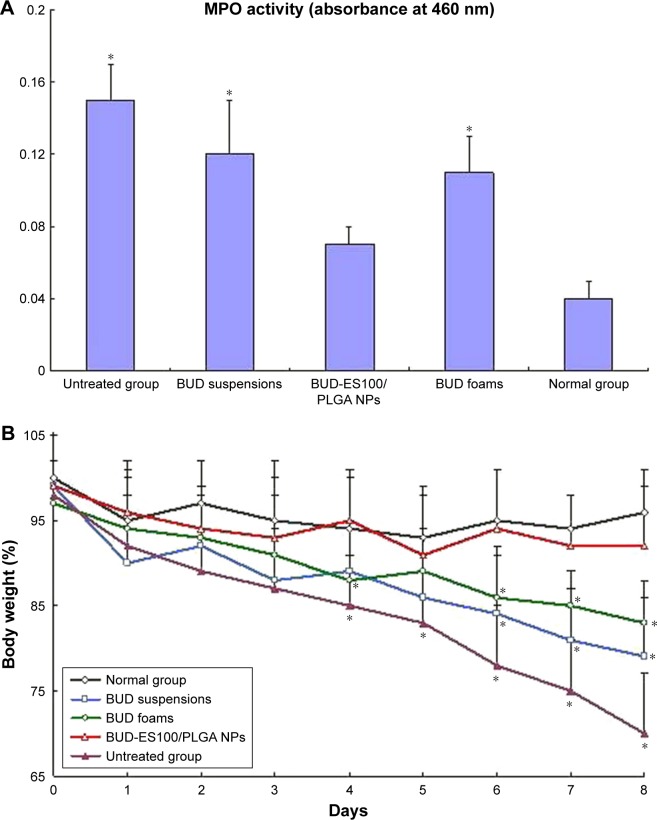
The present study was conducted to test the efficacy of different BUD formulations for TNBS-induced colitis rat model (Figure 4A). In the rats that were administered TNBS for a period of 7 days after administration, there were almost full symptoms of colitis (MPO=0.15). The MPO activity mea-surement showed significant inflammation in BUD-ES100/PLGA NPs group compared with the other groups (p⩽0.05), and the degrees of inflammation in the suspensions and foams groups were similar (p⩾0.05). After treatment, the MPO value of BUD-ES100/PLGA group was close to 0.07.
Figure 4.
Effect of different BUD formulations on TNBS-induced colitis rat model.
Notes: (A) MPO activity, (B) body weight (n=6). *p⩽0.05 vs BUD-ES100/PLGA NPs.
Abbreviations: BUD, budesonide; TNBS, 2,4,6-trinitrobenzenesulfonic acid; MPO, myeloperoxidase; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles.
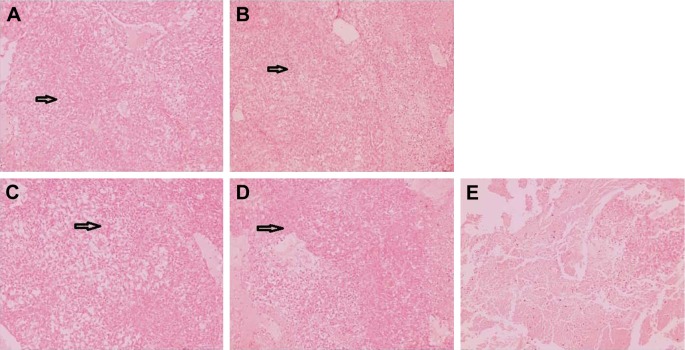
As shown in Figure 4B, the body weight in the normal group remained relatively stable throughout the study. Rats in the untreated group demonstrated decreased body weight bŷ30% after the 7-day TNBS treatment, which waŝ7-fold greater than the weight loss seen in the normal group. Of all the groups, the smallest weight loss (~7.5%) was observed in the BUD-ES100/PLGA NP group (21% in the suspensions group and 17% in the foams group). The observed decreases in body weight were correlated with substantial increases in MPO activity. The histopathological examination of rectum was carried out to identify any damage done to the tissue (Figure 5). No sign of damages such as epithelial necrosis and sloughing epithelial cells was detected.
Figure 5.
The morphology of rectal tissues after treatment of BUD different formulations.
Notes: (A) Normal group, (B) BUD suspensions, (C) BUD-ES100/PLGA NPs, (D) BUD foams, and (E) untreated group. Black arrows indicate inflammation in the mucosa of the rectal tissues (scale bars=50 μm).
Abbreviations: BUD, budesonide; ES100, Eudragit S100; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles.
Now nanoparticulate drug delivery systems are suffering from low mass fraction of therapeutics under the controlled drug release premise. The microfluidic technique has created unique opportunities toward the full control over the production processes of drug delivery carriers, owing to the miniaturization of the fluidic environment. In comparison to the conventional methods, the microfluidic setup provides higher DL%, smaller volume for administration, reduced amount of excipient, and, ultimately, decreased cost. There-fore, in the follow-up study, our research team will introduce microfluidic technique to prepare new nanoparticulate drug delivery systems.28,29
Conclusion
In this study, we developed novel pH-sensitive NPs of BUD for the treatment of UC. The systems could minimize pre-mature drug release in the stomach and small intestine and deliver sustained release in the colon. In vivo, distribution and pharmacodynamic studies exhibited obvious colon-specific drug release and brought the level of MPO close to the normal group value. Our findings suggested that BUD-ES100/PLGA NPs were a promising alternative to single pH-dependent systems for colitis therapy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Truelove SC, Watkinson G, Draper G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br Med J. 1962;2(5321):1708–1711. doi: 10.1136/bmj.2.5321.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JA. Treatment of inflammatory bowel disease with corticosteroids. Gastroenterol Clin North Am. 2004;33(2):171–189. vii. doi: 10.1016/j.gtc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–523. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–599. doi: 10.1038/ajg.2011.70. quiz 600. [DOI] [PubMed] [Google Scholar]
- 5.Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178(3):339–346. doi: 10.1677/joe.0.1780339. [DOI] [PubMed] [Google Scholar]
- 6.Dahlberg E, Thalén A, Brattsand R, et al. Correlation between chemi-cal structure, receptor binding, and biological activity of some novel, highly active, 16 alpha, 17 alpha-acetal-substituted glucocorticoids. Mol Pharmacol. 1984;25(1):70–78. [PubMed] [Google Scholar]
- 7.Hamedani R, Feldman RD, Feagan BG. Review article: drug development in inflammatory bowel disease: budesonide–a model of targeted therapy. Aliment Pharmacol Ther. 1997;11(Suppl 3):98–107. doi: 10.1111/j.1365-2036.1997.tb00814.x. discussion 107–108. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein GR, Hanauer SB, Sandborn WJ, Practice Parameters Com-mittee of American College of Gastroenterology Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104(2):465–483. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 9.Rezaie A, Kuenzig ME, Benchimol EI, et al. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;6:CD000296. doi: 10.1002/14651858.CD000296.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012;143(5):1218.e2–1226.e2. doi: 10.1053/j.gastro.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2014;63(3):433–441. doi: 10.1136/gutjnl-2012-304258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin D, Russell C, William S, et al. O-001 budesonide MMX(R) 9 mg for inducing remission in patients with mild-to-moderate ulcerative colitis not adequately controlled with oral 5-ASAs. Inflamm Bowel Dis. 2014;20(Suppl 1):S1. [Google Scholar]
- 13.Mura C, Nácher A, Merino V, et al. Design, characterization and in vitro evaluation of 5-aminosalicylic acid loaded N-succinyl-chitosan microparticles for colon specific delivery. Colloids Surf B Biointerfaces. 2012;94:199–205. doi: 10.1016/j.colsurfb.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Seremeta KP, Chiappetta DA, Sosnik A. Poly(ε-caprolactone), Eudragit® RS 100 and poly(ε-caprolactone)/Eudragit® RS 100 blend submicron particles for the sustained release of the antiretroviral efavirenz. Colloids Surf B Biointerfaces. 2013;102:441–449. doi: 10.1016/j.colsurfb.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Makhlof A, Tozuka Y, Takeuchi H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm. 2009;72(1):1–8. doi: 10.1016/j.ejpb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Zhou H. Budesonide-loaded guar gum microspheres for colon delivery: preparation, characterization and in vitro/in vivo evaluation. Int J Mol Sci. 2015;16(2):2693–2704. doi: 10.3390/ijms16022693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao B, Zhang M, Viennois E, et al. Inhibition of MDR1 gene expres-sion and enhancing cellular uptake for effective colon cancer treatment using dual-surface-functionalized nanoparticles. Biomaterials. 2015;48:147–160. doi: 10.1016/j.biomaterials.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao B, Yang Y, Viennois E, et al. Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle. J Mater Chem B. 2014;2(11):1499–1508. doi: 10.1039/C3TB21564D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daheb K, Lecours JP, Lipman ML, Hildgen P, Roy JJ. Prediction of in vivo atenolol removal by high-permeability hemodialysis based on an in vitro model. J Pharm Pharm Sci. 2013;16(5):657–664. doi: 10.18433/j3930s. [DOI] [PubMed] [Google Scholar]
- 20.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. [PubMed] [Google Scholar]
- 21.Mu L, Feng SS. PLGA/TPGS nanoparticles for controlled release of paclitaxel: effects of the emulsifier and drug loading ratio. Pharm Res. 2003;20(11):1864–1872. doi: 10.1023/b:pham.0000003387.15428.42. [DOI] [PubMed] [Google Scholar]
- 22.Chandran S, Sanjay KS, Ali Asghar LF. Microspheres with pH modulated release: design and characterization of formulation variables for colonic delivery. J Microencapsul. 2009;26(5):420–431. doi: 10.1080/02652040802424021. [DOI] [PubMed] [Google Scholar]
- 23.Yoo JW, Giri N, Lee CH. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403(1–2):262–267. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi WI, Lau PK. Experimental method for studying growth rates of particles in aqueous media. J Pharm Sci. 1962;51:1081–1084. doi: 10.1002/jps.2600511116. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Zhang H, Herranz-Blanco B, et al. Microfluidic assembly of monodisperse multistage pH-responsive polymer/porous silicon composites for precisely controlled multi-drug delivery. Small. 2014;10(10):2029–2038. doi: 10.1002/smll.201303740. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Liu D, Zhang H, et al. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017;48:238–246. doi: 10.1016/j.actbio.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Fedorak RN, Haeberlin B, Empey LR, et al. Colonic delivery of dexamethasone from a prodrug accelerates healing of colitis in rats without adrenal suppression. Gastroenterology. 1995;108(6) doi: 10.1016/0016-5085(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Zhang H, Cito S, et al. Core/shell nanocomposites produced by superfast sequential microfluidic nanoprecipitation. Nano Lett. 2017;17(2):606–614. doi: 10.1021/acs.nanolett.6b03251. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Zhang H, Fontana F, Hirvonen JT, Santos HA. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip. 2017;17(11):1856–1883. doi: 10.1039/c7lc00242d. [DOI] [PubMed] [Google Scholar]