Abstract
Background
The long noncoding RNA X-inactive specific transcript (XIST) was reported to play vital roles in tumor progression. In the present study, we determined the regulatory function of XIST in papillary thyroid carcinoma (PTC).
Materials and methods
XIST expression was determined in PTC tissues and cell lines by quantitative real-time polymerase chain reaction (PCR) (qRT-PCR). Cellular proliferation, migration, and invasion were measured using the Cell Counting Kit-8 (CCK-8) assay, wound-healing assay, and transwell invasion assay, respectively. Western blotting was used to determine protein expression. The downstream target miRNAs for XIST were identified by luciferase reporter assay and qRT-PCR.
Results
Relative expression of XIST was upregulated in PTC tissues and cell lines. High XIST expression was positively correlated with TNM stage and lymph node metastasis. Function assay demonstrated that knockdown of XIST significantly decreased cell proliferation, migration, and invasion in PTC cells. Moreover, we showed that the effects of XIST on PTC cell progression were mediated by miR-141.
Conclusion
Our results demonstrated that XIST functioned as an oncogene in PTC progression by regulating miR-141, suggesting that XIST might be a promising therapeutic target for PTC treatment.
Keywords: papillary thyroid carcinoma, XIST, miR-141, proliferation
Introduction
Thyroid cancer is the most common malignancy of the endocrine system, and it has shown a steadily increasing incidence over the past 40 years.1 Papillary thyroid carcinoma (PTC) is the most common histotype of thyroid cancer, accounting for 85%–90% of all thyroid cancers.1,2 Most patients with PTC can be effectively treated by surgical removal, followed by adjuvant radioactive iodine (RAI) therapy. However, 10%–15% of patients with PTC do not respond to RAI therapy or they progress to metastatic disease, which has poor prognosis.3 Therefore, it is urgent to identify potential biomarkers and therapeutic targets that correlate with tumorigenesis and progression in PTC.
Long noncoding RNAs (lncRNAs) constitute a newly identified class of RNAs, whose transcripts are >200 nucleotides in length without protein-encoding capabilities.4 As a new class of modulators, lncRNAs have been shown to be involved in regulating various biological processes and diseases in humans.5 Increasing evidence has suggested that lncRNAs play vital roles in progression of cancers, including PTC.6 A number of lncRNAs have been identified as oncogenes or tumor suppressors in PTC,7,8 suggesting that lncRNAs could serve as diagnostic markers and therapeutic agents for PTC.
Recently, researchers have paid more attention to the lncRNA X-inactive specific transcript (XIST). XIST, a product of the XIST gene, has been reported to participate in the differentiation, proliferation, and genome maintenance of human cells.9 XIST has been found to be upregulated and to function as an oncogene in multiple cancers, such as breast cancer,10 non-small-cell lung cancer,11 pancreatic cancer,12 bladder cancer,13 hepatocellular carcinoma,14 gastric cancer,15 and colorectal cancer.16 However, the expression levels and exact role of XIST in PTC remain unclear. In this study, we investigated the expression level, biological function, and the underlying mechanisms of XIST action in PTC using a series of experiments.
Materials and methods
Clinical specimens and ethics
A total of 36 patients with PTC who had undergone thyroidectomy and node dissection without chemotherapy or radiotherapy at the China–Japan Union Hospital of Jilin University (Changchun, China) were enrolled in this study. After surgery, PTC samples and paired adjacent noncancerous samples were harvested and confirmed by two independent pathologists. All specimens were immediately preserved in liquid nitrogen until RNA extraction. Before the tissue samples were collected, written informed consent was obtained from all patients. This study was approved by the ethics review board of the China–Japan Union Hospital of Jilin University.
Cell lines and transfection
Human thyroid follicular epithelial cell line Nthy-ori 3-1 and human thyroid cancer cell lines (TPC-1, HTH83, 8505C, and SW1736) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U/mL penicillin (Sigma-Aldrich, St Louis, MO, USA), and 100 μg/mL streptomycin (Sigma-Aldrich) at 37°C in a humidified incubator with 5% CO2.
miR-141 mimic (miR-141), mimic negative control (miR-NC), miR-141 inhibitor (anti-miR-141), inhibitor scrambled control (anti-miR-NC), siRNA specifically targeting XIST (si-XIST), and scrambled negative control siRNA (si-NC) were chemically synthesized by GenePharma Co, Ltd (Shanghai, China). TPC-1 cells in logarithmic phase were transfected with these molecular products using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Further experiments were performed at 24 h posttransfection.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were isolated from tissues or cultured cells using Trizol reagent (Invitrogen) and subsequently reverse-transcribed into cDNAs using the PrimeScript RT reagent Kit (Promega, Madison, WI, USA) according to the protocol of the manufacturer. Relative gene expression levels of miR-141 and XIST were measured using TaqMan miRNA assay (Applied Biosystems, Foster City, CA, USA) and SYBR Green PCR Kit (Takara Biochemicals, Kyoto, Japan) under the ABI 7900 Fast Real-Time PCR system (Applied Biosystems), respectively. U6 snRNA and GAPDH were used as the internal control for miR-141 and XIST, respectively. The relative quantification of XIST and miR-141 expression levels was achieved by the 2−ΔΔCt method. The primers for qRT-PCR were as follows: XIST – forward, 5′-CTC TCC ATT GGG TTC AC-3′, reverse, 5′-GCG GCA GGT CTT AAG AGA TGA G-3′; GAPDH – forward, 5′-CAC CCACTCCTCCACCTTTG-3′, reverse, 5′-CCACCACCC TGTTGCTGTAG-3′; miR-141 – forward, 5′-AGACCTCACCTGGCCTGTGGCC-3′, reverse 5′-GAACCCACCCGGGAGCCATCTT-3′; U6 – forward, 5′-CTC GCT TCG GCAGCA CA-3′, reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′.
Cell proliferation, cell cycle, migration, and invasion assays
Cell proliferation was determined using the Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the manufacturer’s protocol. Briefly, logarithmically growing cells (5×103 cells per well) were inoculated into 96-well culture plates. At the indicated time points (24 h, 48 h, and 72 h), a volume of 10 μL of CCK-8 solution was added into each well and cultured for 4 h at 37°C. The absorbance at 450 nm was measured using enzyme immunoassay analyzer (Thermo Fisher Scientific, Inc, Waltham, MA, USA).
Flow cytometric analysis was conducted to analyze the cell cycle. Forty-eight hours after transfection, the cells were collected and fixed in 70% ethanol overnight. Then, cells were incubated with RNase (50 μg/mL) and propidium iodide (PI) (50 μg/mL, Sigma) for 30 min in the dark. Cell cycle analysis was conducted using a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA).
Cell migration capacity was determined by the scratch assay. In brief, 2×105 transfected cells were plated into six-well plates and grown to reach 100% confluence. The cells were then wounded by scraping with a 100 μL tip, followed by three washes in PBS and incubation in serum-free medium. Wounds were observed at 0 h and 24 h with a light microscope (IX71; Olympus, Tokyo, Japan) at 200× magnification. The cell migration distances were calculated by subtracting the wound width at each time point from the wound width at the 0 h time point.
Cell invasion was measured using transwell chambers (8 μm pore size; EMD Millipore, Billerica, MA, USA). The transfected cells (5×104) suspended in 150 μL serum-free medium were plated into the upper chamber coated with Matrigel (BD, Franklin Lakes, NJ, USA), whereas the lower chamber was filled with culture medium containing 20% (v/v) FBS as a chemoattractant. After incubation for 48 h, the noninvading cells were scraped off with a cotton swab, and the invaded cells on the lower chamber were fixed with 4% formaldehyde for 10 min and stained with 0.1% crystal violet for 5 min. The cells were counted in five independent fields using an inverted fluorescence microscope (Olympus Corp) at 200× magnification.
Dual luciferase reporter assay
XIST was predicted to be a directly regulated target of miR-141 by Starbase2.0 bioinformatics tools (http://starbase.sysu.edu.cn). XIST 3′-untranslated region (UTR) fragment was amplified and cloned into psiCHECK-2 vectors, and the product was named as WT-XIST. The XIST-mutant construct was obtained using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA), named as MUT-XIST. For reporter assays, TPC-1 cells were co-transfected with 100 nM miR-141 mimic or miR-NC mimic (GenePharma, Suzhou, China) and 100 ng of WT-XIST or MUT-XIST reporter plasmid using Lipofectamine 2000. After 48 h transfection, luciferase activity was measured using the Dual Luciferase Reporter Assay Kit (Promega).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc, Chicago, IL, USA). All data are represented as mean ± SD. Significant differences in the continuous data between groups were compared using two-tailed Student’s t-test and one-way analysis of variance (ANOVA). The relationship between XIST and miR-141 expressions was tested using Pearson’s correlation assay. A value of P<0.05 was considered statistically significant.
Results
XIST expression is upregulated in PTC tissues and cell lines
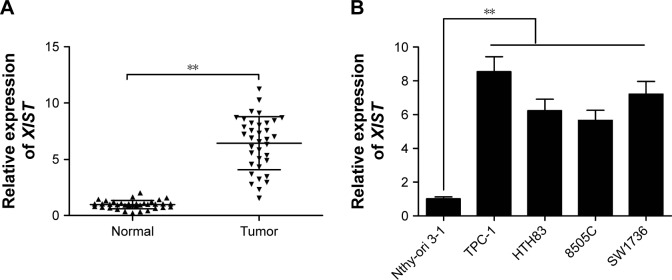
To determine the role of XIST in PTC progression, we first determined its expression levels in 36 pairs of PTC tissues and adjacent normal tissues by qRT-PCR. We found that XIST expression was upregulated in PTC tissues compared with the expression in adjacent normal tissues (Figure 1A). In addition, we verified the expression of XIST in four PTC cell lines, namely, TPC-1, HTH83, 8505C, and SW1736, and in human thyroid follicular epithelial cell line Nthy-ori 3-1. XIST was clearly elevated in the four PTC cell lines compared to Nthy-ori 3-1 (Figure 1B). We also investigated the potential associations between XIST expression and the clinicopathological features of patients with PTC (Table 1). High XIST expression was significantly positively correlated with a late TNM stage and lymph node metastasis (Table 1). These results revealed that XIST has potential oncogenicity in PTC.
Figure 1.
XIST was upregulated in PTC tissues and cell lines.
Notes: (A) qRT-PCR was performed to examine the expression levels of XIST in 36 PTC tissues and adjacent normal tissues. (B) qRT-PCR was applied to estimate the expression levels of XIST in four human thyroid cancer cell lines (TPC-1, HTH83, 8505C, and SW1736) and a thyroid follicular epithelial cell line Nthy-ori 3-1. **P<0.01. Data are expressed as the mean ± SD of at least three independent experiments.
Abbreviations: XIST, X-inactive specific transcript; PTC, papillary thyroid carcinoma; qRT-PCR, quantitative real-time polymerase chain reaction.
Table 1.
Correlation between clinicopathological features and XIST expression in 36 patients with PTC
| Variables | No. of cases |
XIST expression
|
P-value | |
|---|---|---|---|---|
| High, n (%) | Low, n (%) | |||
| Age, years | P>0.05 | |||
| <60 | 15 | 9 (60.0) | 6 (40.0) | – |
| ≥60 | 21 | 11 (52.3) | 10 (47.7) | – |
| Gender | P>0.05 | |||
| Male | 13 | 7 (53.8) | 6 (46.2) | – |
| Female | 23 | 13 (56.5) | 10 (42.5) | – |
| TNM stage | P<0.01 | |||
| I–II | 28 | 12 (42.9) | 16 (57.1) | – |
| III–IV | 8 | 8 (100) | 0 (0) | – |
| Tumor size | P>0.05 | |||
| <5 cm | 26 | 14 (53.8) | 12 (46.2) | – |
| ≥5 cm | 10 | 6 (60.0) | 4 (40.0) | – |
| Lymph node metastasis | P<0.01 | |||
| No | 27 | 12 (44.4) | 15 (55.6) | – |
| Yes | 9 | 8 (88.9) | 1 (10.1) | – |
Abbreviations: PTC, papillary thyroid carcinoma; XIST, X-inactive specific transcript.
Knockdown of XIST inhibits cell proliferation of PTC cells
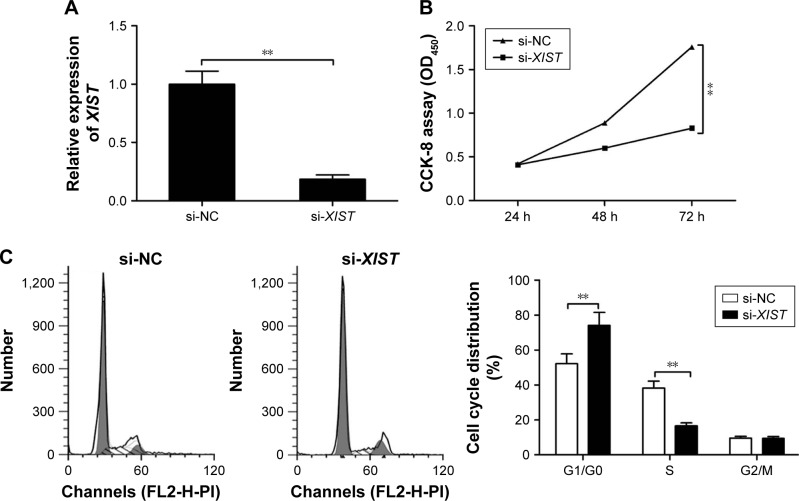
To test the biological function of XIST in PTC, we knocked down the expression of the lncRNA XIST using a specific siRNA (si-XIST), while using a nonsilencing siRNA (si-NC) as the negative control. We found that transfection of si-XIST significantly decreased XIST expression in TPC-1 cells compared with cells transfected with si-NC (Figure 2A). CCK-8 assay revealed that knockdown of XIST significantly decreased cell proliferation in TPC-1 cells (Figure 2B). Moreover, flow cytometry was utilized to test the effect of XIST on the cell cycle. The results showed that knockdown of XIST in TPC-1 cells induced a significant increase in the percentage of cells in the G1/G0 phase and a decrease in the percentage of cells in the S phase (Figure 2C). These results suggested that downregulation of XIST suppressed PTC cell proliferation in vitro.
Figure 2.
Knockdown of XIST inhibits cell proliferation of PTC cells.
Notes: (A) qRT-PCR was performed to examine the expression levels of XIST in TPC-1 cells transfected with si-XIST or si-NC. (B) CCK-8 assay was performed to examine cell proliferation in TPC-1 cells transfected with si-XIST or si-NC. (C) Flow cytometric analysis was conducted to analyze the cell cycle arrest in TPC-1 cells transfected with si-XIST or si-NC. **P<0.01. Data are expressed as the mean ± SD of at least three independent experiments.
Abbreviations: XIST, X-inactive specific transcript; PTC, papillary thyroid carcinoma; qRT-PCR, quantitative real-time polymerase chain reaction; NC, negative control; CCK, Cell Counting Kit; OD, optical density.
Knockdown of XIST inhibits cell migration and invasion of PTC cells
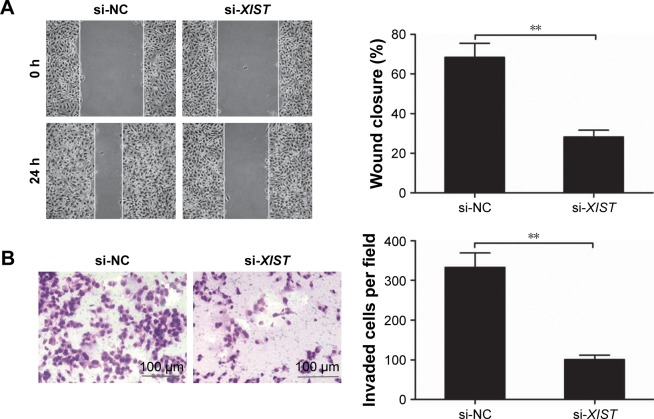
Furthermore, we explored whether XIST was involved in cell migration and invasion in TPC-1 cells. Scratch assay showed that downregulation of XIST remarkably decreased migration ability in TPC-1 cells (Figure 3A). The transwell assay showed that knockdown of XIST in TPC-1 cells resulted in much weaker abilities to invade through Matrigel than for cells transfected with si-NC (Figure 3B). These results suggested that downregulation of XIST suppressed cell migration and invasion of PTC cells in vitro.
Figure 3.
Knockdown of XIST inhibits cell migration and invasion of PTC cells.
Notes: (A) Wound-healing assay to examine cell migration in TPC-1 cells transfected with si-XIST or si-NC. (B) Transwell invasion assay was performed to examine cell invasion in TPC-1 cells transfected with si-XIST or si-NC. **P<0.01. Data are expressed as the mean ± SD of at least three independent experiments.
Abbreviations: XIST, X-inactive specific transcript; PTC, papillary thyroid carcinoma; NC, negative control.
miR-141 expression is directly regulated by XIST
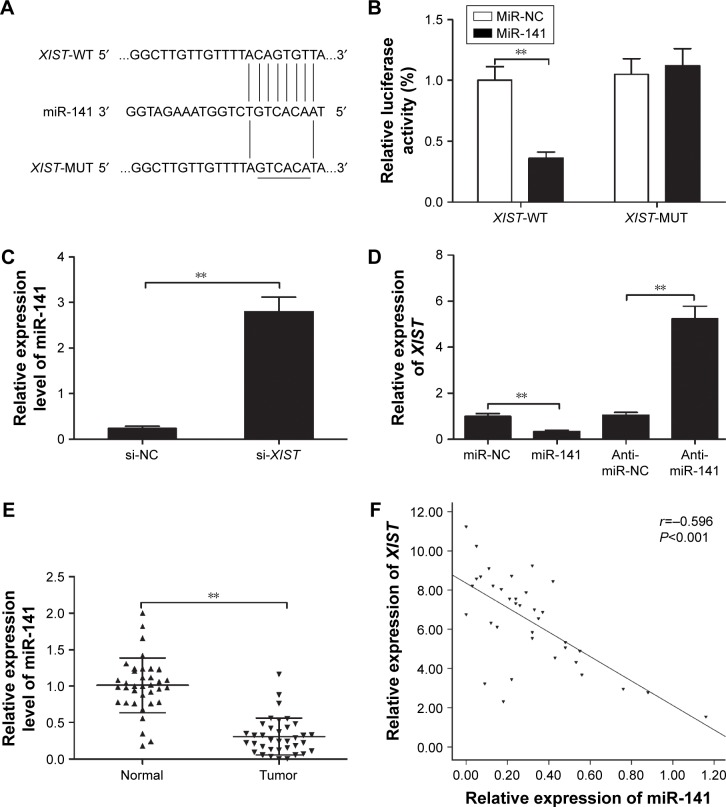
It has been elucidated that lncRNA can competitively bind to miRNAs and function as a competing endogenous RNA (ceRNA), thus participating in the pathogenesis and development of cancers.17 To better reveal the underlying molecular mechanism by which lncRNA XIST exerts its biological function in PTC progression, we used the online bioinformatics tool Starbase2.0 (http://starbase.sysu.edu.cn) to predict the downstream target of XIST. miR-141 was predicted to be a target of XIST with a potential binding site (Figure 4A). To verify that XIST can bind to miR-141 directly, the luciferase reporter assay was performed. Our result demonstrated that luciferase activity was remarkably decreased in TPC-1 cells co-transfected with WT-XIST and miR-141 mimics (Figure 4B; P<0.05). Moreover, our results also showed that knockdown of XIST clearly increased miR-141 expression level in TPC-1 cells (Figure 4C), whereas transfection of miR-141 mimic significantly inhibited the expression of XIST in TPC-1 cells, and transfection with miR-141 inhibitor (anti-miR-141) increased XIST expression in TPC-1 cells (Figure 4D). We also found that miR-141 expression was downregulated in PTC tissues (Figure 4E) and was inversely correlated with XIST expression (Figure 4F). These results suggested that XIST directly binds to miR-141 in PTC cells.
Figure 4.
miR-141 expression is directly regulated by XIST.
Notes: (A) Bioinformatics analysis showed that miR-141 could directly target the 3′-UTR of the XIST wild-type (WT-XIST) sequence. XIST-mutant (MUT-XIST) indicates the mutation of the binding sites in the 3′-UTR of XIST. (B) Luciferase activity was determined in TPC-1 cells co-transfected with the miR-141 mimic or miR-NC and WT-XIST or MUT-XIST reporter plasmid. (C) qRT-PCR was performed to examine the expression levels of miR-141 in TPC-1 cells transfected with si-XIST or si-NC. (D) qRT-PCR was performed to examine the expression levels of XIST in TPC-1 cells transfected with miR-141 mimic, miR-NC, miR-141 inhibitor (anti-miR-141), and anti-miR-NC. (E) qRT-PCR was performed to examine the expression levels of miR-141 in 36 PTC tissues and adjacent normal tissues. (F) The negative association between XIST and miR-141 expression levels was analyzed in PTC tissues (n=36). **P<0.01. Data are expressed as the mean ± SD of at least three independent experiments.
Abbreviations: XIST, X-inactive specific transcript; UTR, untranslated region; NC, negative control; qRT-PCR, quantitative real-time polymerase chain reaction.
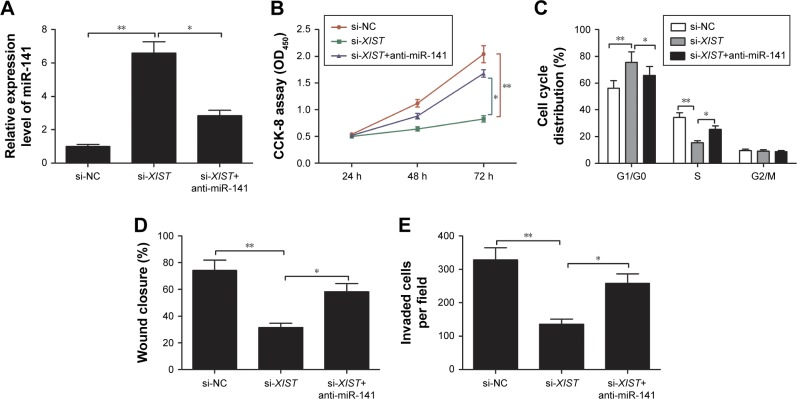
miR-141 inhibitor reversed the inhibitory effect of XIST knockdown on PTC cell proliferation, migration, and invasion
To determine whether XIST exerts its biological functions through miR-141, we performed rescue experiments by inhibiting miR-141 expression using miR-141 inhibitor in XIST-knockdown cells (Figure 5A). CCK-8 assay showed that cell proliferation was decreased in XIST-knockdown TPC-1 cells, whereas miR-141 inhibitor partially reversed the reduction of proliferation (Figure 5B; P<0.05). Furthermore, our result also revealed that miR-141 inhibitor reversed the cell cycle arrest, migration, and invasion in TPC-1 cells induced by XIST knockdown (Figure 5C–E).
Figure 5.
miR-141 inhibitor reverses the inhibitory effect of XIST knockdown on PTC cell proliferation, cycle, migration, and invasion.
Notes: (A) qRT-PCR assay showed that XIST knockdown increased miR-141 expression, whereas miR-141 inhibition reversed it in the meantime. (B) CCK-8 assay showed that XIST knockdown inhibited TPC-1 cell proliferation, whereas miR-141 inhibition reversed it. (C–E) XIST knockdown increased cell cycle arrest at G1/G0 stage and decreased cell migration and invasion, whereas miR-141 inhibition in the meantime reversed these trends. *P<0.05, **P<0.01. Data are expressed as the mean ± SD of at least three independent experiments.
Abbreviations: XIST, X-inactive specific transcript; PTC, papillary thyroid carcinoma; qRT-PCR, quantitative real-time polymerase chain reaction; CCK, Cell Counting Kit; NC, negative control.
Discussion
Accumulating evidence has revealed that lncRNAs play crucial critical roles in regulating many physiological and pathological processes of various cancers, including PTC.6–8 For example, Yuan et al reported that lncRNA HOTTIP knockdown downregulated Akt1 expression and suppressed cell proliferation, invasion, and migration in PTC cells by regulating miR-637.18 Chen et al demonstrated that lncRNA CNALPTC1 promotes PTC progression via sponging miR-30 family.19 Wang et al showed that lncRNA H19 inhibited cell viability, migration, and invasion via downregulation of insulin receptor substrate (IRS)-1 in thyroid cancer cells.20 Zhang et al found that lncRNA NEAT1 regulates PTC progression by modulating miR-129-5p/KLK7 expression.21 Here, we found that XIST expression is upregulated in PTC tissues and cell lines, and its expression was associated with advanced clinical stage and lymph node metastasis. Knockdown of XIST inhibited cell proliferation, migration, and invasion of PTC cells. Mechanistically, we demonstrated that XIST acted as a ceRNA to sponge miR-141 in PTC. These findings suggested that XIST might act as a novel target for PTC treatment.
Recently, the ceRNA hypothesis showed that lncRNAs implicated in the pathogenesis and development of cancers act as miRNA sponges to modulate the expression of miRNA target genes.17 To explore how XIST regulates PTC development and progression, we proposed to search the target microRNAs of XIST with an online tool (Starbase2.0). There are potential binding sites of miR-141 in XIST; thus, miR-141 was predicted as a potential target of XIST. Moreover, miR-141 was identified to act as a downstream target of XIST in non-small-cell lung cancer.22 Luciferase reporter assays demonstrated that luciferase activity was remarkably decreased in TPC-1 cells co-transfected with XIST-WT and miR-141 mimic, suggesting that XIST can bind to miR-141. Moreover, we also found that knockdown of XIST clearly increased miR-141 expression level in TPC-1 cells, whereas transfection of miR-141 mimic significantly inhibited the expression of XIST in TPC-1 cells. These results suggested that XIST directly binds to miR-141 in PTC cells.
miR-141, a member of the miR-200 family, has attracted much attention because it is aberrantly downregulated and acts as an important tumor suppressor in multiple types of cancer, such as nasopharyngeal carcinoma,23 prostate cancer,24 breast cancer,25 non-small-cell lung cancer,26 colorectal cancer,27 and hepatocellular carcinoma.28 Interestingly, miR-141 was reported to be significantly downregulated in PTC patients,29 and overexpression of miR-141 could inhibit cell proliferation, migration, and invasion in PTC cells by targeting IRS-2.30 The present study convincingly demonstrated that miR-141 expression was downregulated in PTC tissues compared to adjacent normal tissues. We also found that miR-141 expression was inversely correlated with XIST expression in PTC tissues. In addition, rescue experiments revealed that miR-141 inhibitor could reverse the cell proliferation, migration, and invasion ability of XIST-knockdown TPC-1 cells. These studies implied that XIST exerted its oncogene role in PTC via sponging miR-141.
Some limitations were present in this study. First, the biological function of XIST was evaluated in a loss-of-function model in PTC; gain-of-function studies via upregulation of XIST in PTC are needed to further confirm our findings. Second, to investigate the clinical significance of XIST, large enough samples are needed. Third, two or more PTC cell lines should be adopted to test the biological function of XIST in PTC.
Conclusion
We found that XIST expression level was upregulated in PTC tissues and cell lines. Knockdown of lncRNA XIST inhibits PTC cell proliferation, migration, and invasion through regulation of miR-141. Our findings elucidated the potential mechanism underlying the oncogenic role of XIST in PTC and suggested that XIST might be a potential target for PTC.
Acknowledgments
This study was funded by The First Affiliated Hospital of Jilin University Foundation (No 2016B05) and the National Natural Science Foundation of China (No 81402062).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331(2):139–146. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology. 2018;72(1):40–52. doi: 10.1111/his.13348. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi D, Accorona R, Paderno A, Cappelli C, Nicolai P. Morbidity of central neck dissection for papillary thyroid cancer. Gland Surg. 2017;6(5):492–500. doi: 10.21037/gs.2017.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gloss BS, Dinger ME. The specificity of long noncoding RNA expression. Biochim Biophys Acta. 2016;1859(1):16–22. doi: 10.1016/j.bbagrm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Wang H, Wu C, et al. Construction and investigation of lncRNA-associated ceRNA regulatory network in papillary thyroid cancer. Oncol Rep. 2018;39(3):1197–1206. doi: 10.3892/or.2018.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo YH, Liang L, He RQ, et al. RNA-sequencing investigation identifies an effective risk score generated by three novel lncRNAs for the survival of papillary thyroid cancer patients. Oncotarget. 2017;8(43):74139–74158. doi: 10.18632/oncotarget.18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Li H, Zhang L, Zhang C, Yan W, Wang C. Identification of novel long non-coding RNA biomarkers for prognosis prediction of papillary thyroid cancer. Oncotarget. 2017;8(28):46136–46144. doi: 10.18632/oncotarget.17556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pintacuda G, Young AN, Cerase A. Function by structure: spotlights on Xist long non-coding RNA. Front Mol Biosci. 2017;4:90. doi: 10.3389/fmolb.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng R, Lin S, Guan L, et al. Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem Biophys Res Commun. 2018;498(4):1002–1008. doi: 10.1016/j.bbrc.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H, Zhang H, Hu X, Li W. Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Int J Biol Macromol. 2018;111:623–631. doi: 10.1016/j.ijbiomac.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Zhang B, Cui T. Long non-coding RNA XIST exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncol Rep. 2018;39(4):1591–1600. doi: 10.3892/or.2018.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y, Deng C, Zhang H, Zhang J, Peng B, Hu C. Long non-coding RNA XIST promotes cell growth and metastasis through regulating miR-139-5p mediated Wnt/beta-catenin signaling pathway in bladder cancer. Oncotarget. 2017;8(55):94554–94568. doi: 10.18632/oncotarget.21791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Mo Y, Lu Y, Wang P, et al. Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumour Biol. 2017;39(2) doi: 10.1177/1010428317690999. 1010428317690999. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Zhou Y, Luo X, Gao H, Deng X, Jiang Y. Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancer. Oncotarget. 2017;8(3):4125–4135. doi: 10.18632/oncotarget.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen DL, Chen LZ, Lu YX, et al. Long noncoding RNA XIST expedites metastasis and modulates epithelial-mesenchymal transition in colorectal cancer. Cell Death Dis. 2017;8(8):e3011. doi: 10.1038/cddis.2017.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Q, Liu Y, Fan Y, et al. LncRNA HOTTIP promotes papillary thyroid carcinoma cell proliferation, invasion and migration by regulating miR-637. Int J Biochem Cell Biol. 2018;98:1–9. doi: 10.1016/j.biocel.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Zhou L, Wang H, et al. Long noncoding RNA CNALPTC1 promotes cell proliferation and migration of papillary thyroid cancer via sponging miR-30 family. Am J Cancer Res. 2018;8(1):192–206. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Liu G, Xu W, Liu H, Bu Q, Sun D. Long noncoding RNA H19 inhibits cell viability, migration, and invasion via downregulation of IRS-1 in thyroid cancer cells. Technol Cancer Res Treat. 2017;16(6):1102–1112. doi: 10.1177/1533034617733904. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Zhang H, Cai Y, Zheng L, Zhang Z, Lin X, Jiang L. Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. J Cell Physiol. 2018 doi: 10.1002/jcp.26425. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Wan L, Liu Z, et al. Long non-coding RNA XIST promotes TGF-beta-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018;418:185–195. doi: 10.1016/j.canlet.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Zhao R, Wei Y, et al. BRD7 expression and c-Myc activation forms a double-negative feedback loop that controls the cell proliferation and tumor growth of nasopharyngeal carcinoma by targeting oncogenic miR-141. J Exp Clin Cancer Res. 2018;37(1):64. doi: 10.1186/s13046-018-0734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S, Wa Q, Pan J, et al. Downregulation of miR-141-3p promotes bone metastasis via activating NF-kappaB signaling in prostate cancer. J Exp Clin Cancer Res. 2017;36(1):173. doi: 10.1186/s13046-017-0645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Zhang W, Li B, et al. MicroRNA-200c and microRNA-141 are regulated by a FOXP3-KAT2B axis and associated with tumor metastasis in breast cancer. Breast Cancer Res. 2017;19(1):73. doi: 10.1186/s13058-017-0858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu WF, Chen WB, Dai L, et al. Inhibition of miR-141 reverses cisplatin resistance in non-small cell lung cancer cells via upregulation of programmed cell death protein 4. Eur Rev Med Pharmacol Sci. 2016;20(12):2565–2572. [PubMed] [Google Scholar]
- 27.Wu PP, Zhu HY, Sun XF, Chen LX, Zhou Q, Chen J. MicroRNA-141 regulates the tumour suppressor DLC1 in colorectal cancer. Neoplasma. 2015;62(5):705–712. doi: 10.4149/neo_2015_084. [DOI] [PubMed] [Google Scholar]
- 28.Lou G, Dong X, Xia C, et al. Direct targeting sperm-associated antigen 9 by miR-141 influences hepatocellular carcinoma cell growth and metastasis via JNK pathway. J Exp Clin Cancer Res. 2016;35:14. doi: 10.1186/s13046-016-0289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorris ER, Smyth P, O’Leary JJ, Sheils O. MIR141 expression differentiates Hashimoto thyroiditis from PTC and benign thyrocytes in Irish archival thyroid tissues. Front Endocrinol (Lausanne) 2012;3:102. doi: 10.3389/fendo.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong S, Meng X, Xue S, Yan Z, Ren P, Liu J. microRNA-141 inhibits thyroid cancer cell growth and metastasis by targeting insulin receptor substrate 2. Am J Transl Res. 2016;8(3):1471–1481. [PMC free article] [PubMed] [Google Scholar]