Abstract
Purpose
To determine the risk of primary open angle glaucoma (POAG) following vitreoretinal surgery.
Design
Retrospective, population-based cohort study.
Methods
All residents of Olmsted County, Minnesota, undergoing scleral buckle and/or vitrectomy between 2004 and 2015 were included in the operative cohort. The fellow non-operative eyes were included in the comparison cohort. The study and comparison cohorts consisted of 344 and 277 eyes respectively. The main outcome measure was the development of POAG. Secondary glaucomas were excluded. The probability of glaucoma in operative eyes and non-operative fellow eyes was compared. The observed rate of POAG in the operative eyes was also compared to the rate of POAG in the population of Olmsted County.
Results
The mean age was 64.7 years and the median follow up period was 4.9 years. There were 58, 57, and 229 study eyes in the scleral buckle, scleral buckle with vitrectomy, and vitrectomy only cohorts respectively. The 10 year cumulative probability of developing glaucoma was significantly greater in the operative group (8.9%, 95% CI 3.8–14%) compared to the non-operative group (1.0%, 95% CI 0–2.4%; p=0.02). None of the eyes in the scleral buckle group developed glaucoma. The 10 year probability of POAG was 17.5 % (95% CI 0–34.9%) and 10.0 % (95% CI 3.0–17.0%) in the scleral buckle with vitrectomy and vitrectomy alone cohorts respectively. The rates of POAG in operative eyes undergoing scleral buckle with vitrectomy and vitrectomy alone was significantly greater than the rate of POAG for the Olmsted County general population (1.0 %, p<0.001).
Conclusion
The risk of POAG is increased after vitrectomy.
Introduction
Vitreoretinal surgeries performed include scleral buckle, scleral buckle with vitrectomy, and vitrectomy alone. The acute effect of these surgeries on the elevation of intraocular pressure (IOP) has been studied,1,2but the long term effect on the development of glaucoma is not well understood. Chang, at the LXII Edward Jackson Memorial Lecture in 2006, presented evidence demonstrating an increased risk of open angle glaucoma (OAG) following vitrectomy.3 He put forth the hypothesis that following vitrectomy there is increased oxidation of the trabecular meshwork leading to elevated IOP and OAG. Subsequent studies also reported associated risk of OAG following vitrectomy, but they did not have a comparison cohort.4,5 Additionally, there have been other studies that reported contradictory results and thus there is no clear consensus.6,7
The effect of scleral buckles, which may compress the episcleral veins, is also unclear. Animal models for glaucoma report IOP elevation with ligature of the episcleral veins.8 Contrary to this, one study investigating late onset OAG after scleral buckle surgery in humans reported no associated risk.9 However, the available data is very limited. Some studies have included patients with vitrectomy and concurrent scleral buckle surgery,3,5 but others have excluded patients with scleral buckle,7 and thus the long term effect of scleral buckles on IOP and risk of OAG is poorly understood.
With overall rates of vitrectomy surgery on the rise,10the potential for an increase in long term risk of OAG is of particular concern. This study was undertaken to determine the risk of OAG following vitreoretinal surgery in comparison with the non- operative fellow eyes for separate cohorts of patients who have undergone scleral buckle, scleral buckle with vitrectomy, and vitrectomy alone.
Methods
Data Collection
This is a population- based retrospective cohort study of all residents of Olmsted County, Minnesota who underwent vitreoretinal surgery between January 1, 2004 and December 31, 2015. The study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center, and conformed to HIPAA regulations. We ascertained patients undergoing scleral buckle, scleral buckle with vitrectomy, or vitrectomy without scleral buckle procedures through the records-linkage system of the Rochester Epidemiology Project. This is a unique resource that links and indexes demographic information and medical charts of the population of Olmsted County such that longitudinal population-based studies can be reliably conducted.11 The database includes records from Mayo Clinic and its affiliated hospitals, Olmsted Medical Center and its hospitals, and several private practitioners, capturing virtually all of the health information of a population that is relatively isolated from other urban centers.11 The Rochester Epidemiology Project database was searched for relevant ICD-9 procedural codes (14.41 scleral buckling with implant, 14.49 other scleral buckling, 14.72/1 removal of vitreous by posterior sclerotomy, 14.72/9 other or unspecified removal of vitreous with or without replacement, 14.74 mechanical vitrectomy, 14.74/1 mechanical vitrectomy posterior, 14.74/9 other mechanical vitrectomy). We reviewed the medical records of patients identified and constructed a study cohort by applying inclusion and exclusion criteria (Table 1). Records were reviewed for all available ophthalmology visits prior to the surgery to exclude patients with a diagnosis of glaucoma or glaucoma suspect prior to the vitreoretinal surgery. We then divided the cohort of operative eyes into three groups based on the type of surgery (scleral buckle, scleral buckle with vitrectomy, or vitrectomy without scleral buckle). Eyes that underwent scleral buckle and vitrectomy as separate surgeries but within 3 months of each other were included in the ‘scleral buckle with vitrectomy’ group. If the interval was longer than 3 months, the eye was excluded. Demographic and pre-operative data were recorded, including visual acuity, refractive error, IOP, cup-to-disc ratio, lens status and family history of glaucoma. Operative reports were reviewed for indication, date and type of surgery, details of vitrectomy (gauge, use of indocyanine green, heavy liquid, gas, intravitreal steroid, lens extraction), details of the scleral buckle (type of buckle element, band and sleeve, extent of buckling, orientation, gas injection), and use of intra- or periocular steroids. We noted post-surgical data including early IOP spikes, visual acuity, and details of additional surgeries if any. Each patient’s record was reviewed until the development POAG (primary endpoint), death, or end of the study period. Patients with a follow up period of less than 1 year were excluded. We also evaluated the eyes for development of suspicion of glaucoma (POAG suspect), and the development of ‘POAG or POAG suspect’ was taken as a secondary endpoint. The development of ocular hypertension was also taken as a secondary endpoint. The fellow non-operative eyes were included in the unexposed cohort for comparison and were followed for the development of POAG or POAG suspect. Relevant details were recorded in the same manner as for the operative eyes.
Table 1.
Inclusion and exclusion criteria
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| Age>40 years Either gender |
Glaucoma or glaucoma suspect prior to the surgery Uveitis or endophthalmitis at any point during the study period Proliferative diabetic retinopathy with neovascularization of the iris or angle |
|
For operative cohort Eyes undergoing scleral buckle, scleral buckle with vitrectomy, or vitrectomy alone |
Multiple intravitreal injections (more than 3) Use of silicon oil during surgery Severe penetrating trauma with corneal opacification or ciliary body loss Chronic use of oral or inhalational steroids (longer than 2 months) Chronic use of topical steroids (longer than 2 months) |
|
For non-operative cohort Fellow eyes of those included in the operative cohort |
Multiple periocular steroid injections (more than 2) Follow up of less than 1 year Previous surgeries like penetrating keratoplasties, previous posterior segment surgeries outside the study period Secondary glaucomas |
|
For operative cohort Patients with more than three vitrectomies Complicated posterior segment surgery like suprachoroidal hemorrhage intraoperatively or during the post-operative period Vitrectomies performed for amyloidosis Limited vitrectomies like vitreous biopsies Anterior segment vitrectomies |
|
|
For non-operative cohort Vitreoretinal surgery |
Diagnostic Criteria
Primary open angle glaucoma was defined according to the American Academy of Ophthalmology Preferred Practice Pattern criteria as the presence of an open angle along with optic nerve damage seen by the presence of one or both of the following characteristics: (1) optic nerve damage consistent with glaucoma (diffuse thinning, focal narrowing, or notching of the optic disc rim; documented progression of cupping of the optic disc; diffuse or localized abnormalities of the peripapillary retinal nerve fiber layer, disc rim, or peripapillary retinal nerve fiber layer hemorrhages; or optic disc neural rim asymmetry of the 2 eyes); (2) visual field (VF) damage consistent with retinal nerve fiber layer damage(nasal step, arcuate field defect, or paracentral depression in clusters of test sites).12 Visual fields (Humphrey Field Analyzer with SITA standard 24-2 test strategy) were considered reliable if fixation losses were less than 20% and false positive and negative rates were less than 33%. Normal tension glaucoma was defined as IOP ≤ 21 mmHg and included under the definition of POAG. Primary open angle glaucoma suspect was defined according to the American Academy of Ophthalmology Preferred Practice Pattern criteria as the presence of an open angle along any of the following: (1) an appearance of the optic disc or retinal nerve fiber layer that is suspicious for glaucomatous damage; (2) a visual field suspicious for glaucomatous damage in the absence of clinical signs of other optic neuropathies; or (3) consistently elevated IOP associated with normal appearance of the optic disc, RNFL, and visual field.13 The above definitions exclude the secondary causes of open-angle glaucoma such as pseudoexfoliation, pigment dispersion, and traumatic angle recession. An outcome of ocular hypertension was considered when the IOP was consistently more than 21 mmHg and 4 mmHg above baseline pressure (at least 2 visits). This included patients from both the POAG and POAG suspect group that fit the raised IOP criteria.
Data Analysis
Probability of POAG after vitreoretinal surgery and comparison with fellow eyes
We estimated the cumulative risk of developing POAG after vitreoretinal surgery using Kaplan-Meier methods.14 The risk was also evaluated in the fellow non-operative eyes as the unexposed cohort. Because some patients had bilateral surgery, we compared the operative and non-operative cohorts’ survival experience using the Cox proportional hazards models with sandwich estimates of the variance to attempt to account for potential correlation among right and left eyes from the same patient. We also estimated cumulative risk of POAG within the three types of surgery groups (scleral buckle, scleral buckle with vitrectomy, and vitrectomy without scleral buckle) and used a similar analysis to compare the survival experience between the groups. We evaluated for potential risk factors for POAG using Cox proportional hazards models, again using the sandwich estimates to account for potential correlations. We repeated the same statistical tests with ‘POAG or POAG suspect’ and ‘ocular hypertension’ as an endpoint. Multivariate models for these endpoints were constructed using the Cox proportional hazards models with the sandwich estimates for the standard errors. Overall comparisons of baseline characteristics between operative and non-operative eyes as well as among the 3 main surgery groups were performed using generalized estimating equation (GEE) models, to account for potential correlation between eyes from the same patients. For all analyses comparing more than 2 groups, the pairwise comparisons among the groups were adjusted for multiple comparisons using the Bonferroni method. Bonferroni correction was not applied for the total number of statistical tests performed in this study since the variables are highly interdependent (e.g., risk of POAG is highly dependent on the risk of POAG or POAG suspect), and could unnecessarily increase the risk of a Type II error.
Comparison between probability of POAG in operative eyes and general population of Olmsted County
We compared the observed probability of POAG in the operative eyes to the probability of POAG in the population of Olmsted County, Minnesota. We utilized data collected from a previous study, which reported the long-term trends in glaucoma-related blindness in Olmsted County.15 Expected probability of POAG was estimated using the age and sex specific incidence rates from that previous study. Our observed probability of POAG in operative eyes was compared to the expected probability for the general population by using a 1-sample log rank test.16 We also compared the probability of POAG in the cohort of non-operative eyes with the probability for the general population.
Dealing with confounding factors
Because of the varied indications for vitreoretinal surgery, there was concern that the comorbidities associated with the indications could be potential confounders. We scrutinized the potential confounders and made all attempts to minimize their impact. For all patients, every visit to the ophthalmologist was reviewed to detect any suspicion for secondary glaucoma. If IOP was raised in any of the visits leading up to surgery, that patient was excluded. For patients with vitreous hemorrhage, mention of red blood cells in the anterior chamber led to exclusion. Gonioscopy findings were reviewed as well and mention of cells in the angle or abnormal angle findings like angle recession, neovascularization or closed angle led to exclusion. Use of inhalational and oral steroids more than 2 months was an exclusion criterion. Also, those receiving topical steroids longer than 2 months after vitreoretinal surgery were excluded.
To deal with other potential confounders, including indication for surgery, use of intraocular gas, myopia, early IOP rise following surgery, and pre-operative IOP level, we performed a multivariate analysis to observe if any of these factors affected the development of glaucoma. We performed uni- and multivariate analysis to ensure age was not associated with the increase in risk of glaucoma.
Despite these measures, there remained a few potential confounding factors that could not be determined from the clinical records. These included undetected pseudoexfoliation syndrome in dislocated intraocular lens patients, history of trauma in retinal detachment and vitreous hemorrhage patients, and undetected abnormal angles. For this reason we chose to perform a sub-analysis in patients operated for epiretinal membrane, a very well-characterized cohort with very few confounding factors. These patients did not receive intraocular gas during surgery, and there was less concern for unidentified secondary causes of glaucoma such as trauma or pseudoexfoliation. Also, these patients typically had multiple visits prior to surgery, with reliable preoperative records of IOP. In contrast, retinal detachment patients were often seen for the first time on the day of or a day prior to surgery.
The study was limited for some of the endpoints given the total number of events, but every attempt was made to adjust for the factors that might have some effect on the relationships.
Results
A total of 688 operative eyes were identified based on the procedure codes for posterior segment surgery and 344 eyes fulfilled the inclusion and exclusion criteria (Figure 1). There were 160 male and 184 female eyes with a mean age of 64.7 ± 11.1 years (mean ± S.D.) at the time of surgery. The median follow-up time was 58.4 months (Q1 26.0 months, Q3 90.6 months) and the mean follow-up time was 62.1 ± 38.4 months. There were 58, 57, and 229 patients in the scleral buckle (SB), scleral buckle with vitrectomy (SBV), and vitrectomy only (PPV) cohorts, respectively. There were 277 non-operative fellow eyes in the comparison cohort. Table 2 summarizes the characteristics of the operative and non-operative cohorts, which were similar in age, sex, presence of hypertension, diabetes, family history of glaucoma, baseline IOP, history of retinal laser treatment, and myopia. The two cohorts differed in baseline visual acuity (p<0.001) and lens status, with the operative cohort more likely to be pseudophakic at baseline (p<0.001).
Figure 1.
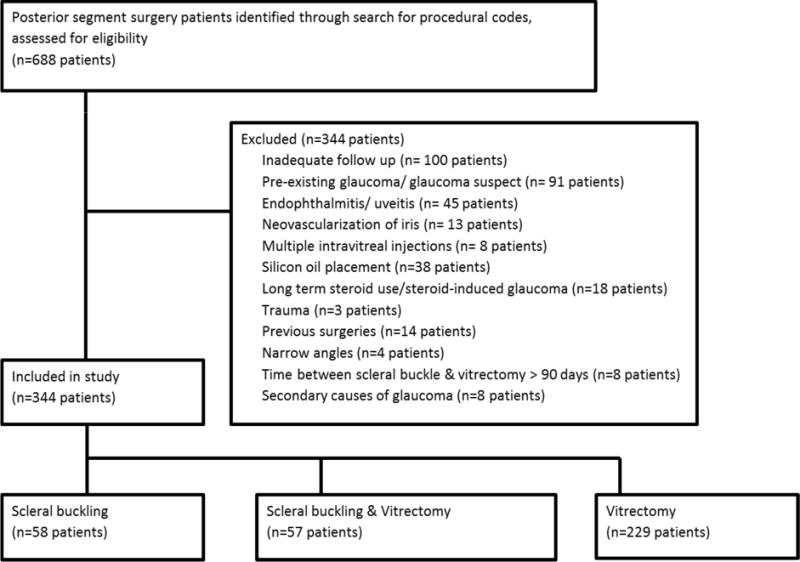
Flow chart of study patients
Table 2.
Baseline Characteristics of Operative & Non-operative Fellow Eyes
| Operative eyes (N=344) | Non-operative eyes (N=277) | Total (N=621) | p value | |
|---|---|---|---|---|
| Age | 0.13 | |||
| Mean (SD) | 64.7 (11.1) | 65.2 (11.1) | 64.9 (11.1) | |
| Range | (40.0–92.0) | (40.0–92.0) | (40.0–92.0) | |
| Sex | 0.90 | |||
| F | 184 (53.5%) | 147 (53.1%) | 331 (53.3%) | |
| M | 160 (46.5%) | 130 (46.9%) | 290 (46.7%) | |
| Hypertension | 0.17 | |||
| No | 150 (43.6%) | 127 (43.6%) | 277 (44.6%) | |
| Yes | 194 (56.4%) | 150 (54.2%) | 344 (55.4%) | |
| Diabetes mellitus | 0.73 | |||
| No | 251 (73.0%) | 203 (73.3%) | 454 (73.1%) | |
| Yes | 93 (27.0%) | 74 (26.7%) | 167 (26.9%) | |
| Family History of | 0.84 | |||
| Glaucoma | ||||
| No | 284 (82.3%) | 228 (82.3%) | 512 (82.4%) | |
| Yes | 60 (17.7%) | 49 (17.2%) | 109 (17.1%) | |
| Baseline IOP | 0.76 | |||
| N | 286 | 224 | 525 | |
| Mean (SD) | 15.2(3.1) | 15.2(2.7) | 15.2(2.9) | |
| Retinal laser | 0.53 | |||
| No | 291 (84.6%) | 232 (83.8%) | 523 (84.2%) | |
| Yes | 53 (15.4%) | 45 (16.2%) | 98 (15.8%) | |
| Myopia | 0.33 | |||
| Missing | 4 | 2 | 6 | |
| No | 214 (62.9%) | 171 (62.2%) | 385 (62.6%) | |
| Yes | 126 (37.1%) | 104 (37.8%) | 230 (37.4%) | |
| Lens status | <0.001 | |||
| Pseudophakia | 112 (32.6%) | 46 (16.6%) | 158 (25.4%) | |
| Decimal visual acuity | <0.001 | |||
| Mean (SD) | 0.3 (0.3) | 0.8 (0.3) | 0.5 (0.3) | |
| Range | (0.0–1.3) | (0.0–1.3) | (0.0–1.3) |
The indications for surgery in the operative cohort are summarized in Table 3. Epiretinal membrane (37.1%), macular hole (33.2%), and vitreous hemorrhage (13.1%) were the most common indications for the vitrectomy group, while rhegmatogenous retinal detachment was the predominant indication for the scleral buckle and scleral buckle with vitrectomy groups. The baseline characteristics comparison between scleral buckle, scleral buckle with vitrectomy, and vitrectomy alone patients are summarized in Table 4. The three groups were similar in sex distribution, presence of hypertension or diabetes, and family history of glaucoma. The vitrectomy group had a mean age of 67.2 years which was significantly higher than the mean age of the scleral buckle with vitrectomy (61.1 years) and the scleral buckle groups (58.5 years), with p<0.001, after adjusting for multiple comparisons. Also the 3 groups differed in lens status with the scleral buckle eyes less likely to be pseudophakic than the scleral buckle with vitrectomy (p=0.009) or vitrectomy (p=0.006) alone eyes, after adjusting for multiple comparisons.
Table 3.
Indications for surgery in the operative cohort
| Indication | Scleral Buckle (SB) (N=58) | Scleral Buckle with Vitrectomy (SBV) (N=57) | Vitrectomy (PPV) (N=229) | Total (N=344) |
|---|---|---|---|---|
| Rheg RD | 58 (100.0%) | 53 (93.0%) | 23 (10.0%) | 134 (39.0%) |
| Tract RD | 0 (0.0%) | 0 (0.0%) | 2 (0.9%) | 2 (0.6%) |
| ERM | 0 (0.0%) | 1 (1.8%)* | 85 (37.1%) | 86 (25.0%) |
| Mac Hole | 0 (0.0%) | 1 (1.8%)* | 76 (33.2%) | 77 (22.4%) |
| Dislocated lens | 0 (0.0%) | 0 (0.0%) | 4 (1.7%) | 4 (1.2%) |
| Dislocated IOL | 0 (0.0%) | 0 (0.0%) | 3 (1.3%) | 3 (0.9%) |
| Submacular Hem | 0 (0.0%) | 0 (0.0%) | 2 (0.9%) | 2 (0.6%) |
| Vitreous Hem | 0 (0.0%) | 2 (3.5%)* | 30 (13.1%) | 32 (9.3%) |
| VMT | 0 (0.0%) | 0 (0.0%) | 4 (1.7%) | 4 (1.2%) |
Rheg RD- Rhegmatogenous retinal detachment, Tract RD- Tractional retinal detachment, ERM- Epiretinal membrane, Mac Hole- Macular hole, IOL- Intraocular lens, Hem - Hemorrhage, VMT- Vitreomacular traction
Scleral buckles were placed in these cases at the discretion of the attending surgeon due to the presence of high risk peripheral degenerations noted intra-operatively and the need to provide additional support to the vitreous base
Table 4.
Baseline Characteristics of Scleral Buckle, Scleral Buckle with Vitrectomy & Vitrectomy groups
| Scleral Buckle (SB) (N=58) | Scleral Buckle with Vitrectomy (SBV) (N=57) | Vitrectomy (PPV) (N=229) | Total (N=344) | p value | |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 58.5 (11.6) | 61.1 (10.3) | 67.2 (10.2) | 64.7 (11.1) | PPV vs. SBV p*<0.001 |
| Range | (41.0–86.0) | (40.0–85.0) | (41.0–92.0) | (40.0–92.0) | PPV vs. SB p*<0.001 SBV vs. SB p*=0.54 |
| Sex | |||||
| F | 27 (46.6%) | 18 (31.6%) | 139 (60.8%) | 184 (53.5%) | NS |
| M | 31 (53.4%) | 39 (68.4%) | 90(39.3%) | 160 (46.5%) | |
| Hypertension | |||||
| No | 29 (50.0%) | 31 (54.4%) | 90 (39.3%) | 150 (43.6%) | NS |
| Yes | 29 (50.0%) | 26 (45.6%) | 139 (60.7%) | 194 (56.4%) | |
| Diabetes mellitus | |||||
| No | 48 (82.8%) | 44 (77.2%) | 159 (69.4%) | 251 (73.0%) | NS |
| Yes | 10 (17.2%) | 13 (22.8%) | 70 (30.6%) | 93 (27.0%) | |
| Family History of Glaucoma | |||||
| No | 45 (77.6%) | 51 (89.5%) | 188 (82.1%) | 284 (82.6%) | NS |
| Yes | 13 (22.4%) | 6 (10.5%) | 41 (17.9%) | 60 (17.4%) | |
| Myopia | |||||
| Missing | 1 | 1 | 2 | 4 | PPV vs. SBV p*=0.003 |
| No | 22 (38.6%) | 27 (48.2%) | 165 (72.7%) | 214 (62.9%) | PPV vs. SB p*<0.001 |
| Yes | 35 (61.4%) | 29 (45.8%) | 62 (27.3%) | 128 (37.1%) | SBV vs. SB p*=0.66 |
| Lens status | |||||
| PPV vs. SBV p*=1.0 | |||||
| Pseudophakia | 8 (13.8%) | 22 (38.6%) | 82 (35.8%) | 112 (32.6%) | PPV vs. SB p*=0.006 SBV vs. SB p*=0.009 |
| Follow up time (months) | |||||
| Median | 75.6 | 67.8 | 58.3 | 62.6 | NS |
| Q1, Q3 | 45.7, 105.0 | 31.0, 84.5 | 24.4, 86.8 | 26.2, 91.4 | |
| Number of visits | |||||
| Median | 5.0 | 6.0 | 5.0 | 5.0 | NS |
| Q1, Q3 | 4.0, 9.0 | 3.0, 8.0 | 3.0, 8.0 | 3.0, 8.0 |
NS- Not significant, p*-adjusted p-value for multiple comparisons
Probability of POAG after vitreoretinal surgery and comparison with fellow eyes
Among the operative eyes, 15 eyes developed POAG. The 10 year cumulative probability of developing glaucoma in the operative eyes was 8.9% (95% CI 3.8–14.0%). Among the non-operative eyes, 2 eyes developed POAG and the 10 year cumulative probability of developing POAG in the non-operative eyes was 1.0% (95% CI 0–2.4%). The survival rates of the operative and non-operative eyes was significantly different (Figure 2; p=0.02). We repeated the analysis after excluding eyes with bilateral disease, and obtained the same level of significance (p=0.02). Of the 15 operative eyes that developed POAG, 11 were from the vitrectomy group and 4 were from the scleral buckle with vitrectomy group. The 10 year probability of POAG was 17.5 % (95% CI 0–34.9%) and 10.0 % (95% CI 3.0–17.0%) in the scleral buckle with vitrectomy and vitrectomy alone cohorts, respectively. No glaucoma developed in the scleral buckle only patients. The median time to the diagnosis of POAG was 40.2 months (mean ± S.D. 46.1 ±28.3 months, range 10.7–102.7 months) after surgery and the mean IOP at the time of diagnosis was 23.4 mmHg (range 14–41 mmHg). The mean cup-disk ratio was 0.7 (range 0.4–0.9) at diagnosis. Four of 15 patients (26.7%) with POAG had normal tension glaucoma. Fourteen of 15 (26.7%) were started on IOP lowering medication, while 2 of 15 (13.3%) received selective laser trabeculoplasty following diagnosis. There was no significant difference in the rate of glaucoma between the vitrectomy alone and scleral buckle with vitrectomy groups (p=0.64). (Figure 3)
Figure 2.
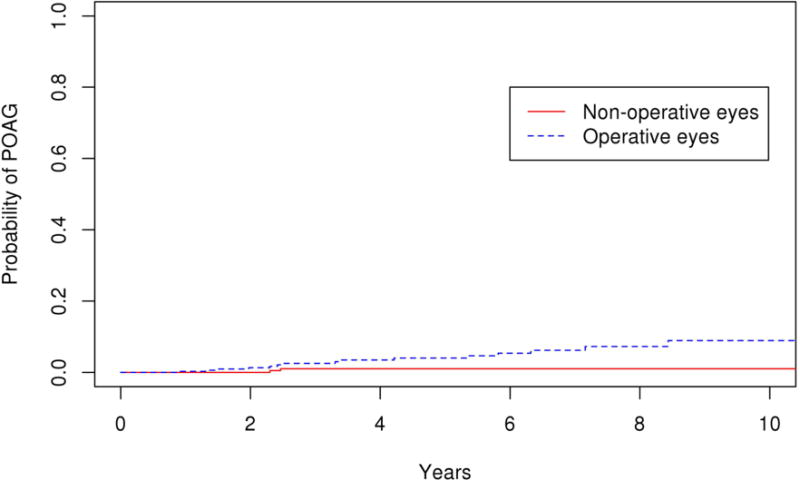
Survival curves for operative and non-operative cohorts for primary endpoint of primary open angle glaucoma
Figure 3.
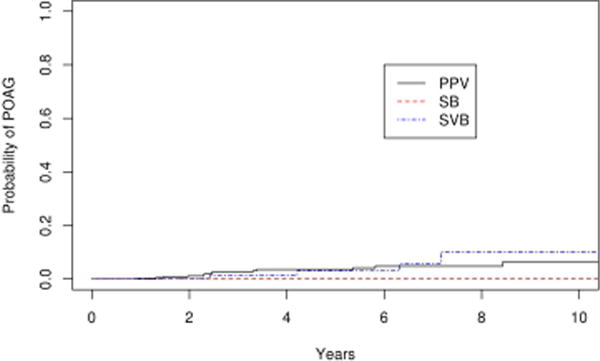
Survival curves for scleral buckle (SB), scleral buckle with vitrectomy (SBV) and vitrectomy (PPV) for primary endpoint of primary open angle glaucoma
Probability of POAG or POAG suspect after vitreoretinal surgery and comparison with fellow eyes
Suspicion of POAG developed in 23 eyes in the operative group and 10 eyes in the non-operative group. Therefore, the combined secondary endpoint of suspicion of POAG or POAG developed in 38 eyes in the operative group and 12 eyes in the nonoperative group. The 10 year cumulative probability of this endpoint was 27.7 % (95% CI 17.6–37.8%) in the operative group, which was significantly greater than the nonoperative group (15.5 %; 95% CI 5.3–25.7%; p=0.004; Figure 4). Similar results were obtained with repeating the analysis after excluding eyes with bilateral disease (p=0.005). Of the 38 operative eyes that were POAG suspects or had POAG, 21 met the criteria for ocular hypertension with a mean maximum IOP of 24.6 mmHg (range 22–41 mmHg), and 17 had normal IOP but the disc was suspicious for glaucomatous damage with a mean cup-disc ratio of 0.7 at the time of diagnosis. The probability of POAG or POAG suspect was significantly greater in eyes that had vitrectomy (SBV and PPV cohorts) compared with eyes that had scleral buckle only (SB cohort) with a hazard ratio of 6.0 after adjusting for age. (p=0.01) Comparing the survival rate among the three surgical groups, there was a trend towards a difference between the vitrectomy and scleral buckle groups (PPV vs SB, p=0.06) and scleral buckle with vitrectomy and scleral buckle alone groups (SBV vs SB, p=0.12) after adjusting for multiple comparisons. There was no difference in survival rate between the vitrectomy alone and vitrectomy with scleral buckle groups (PPV vs SBV, p=1.0; Figure 5). The rate of ocular hypertension was greater in operative eyes (8.9%) than non-operative eyes (1.0%) (p=0.02; Figure 6).
Figure 4.
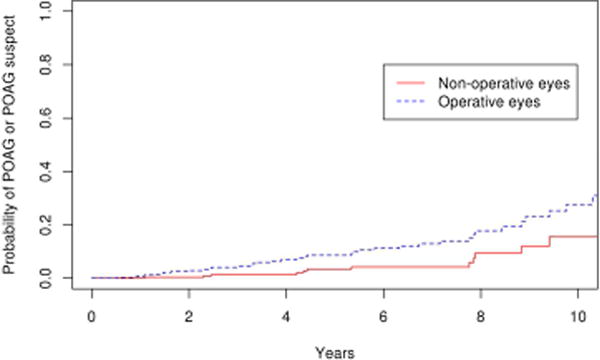
Survival curves for operative and non-operative cohorts for secondary endpoint of primary open angle glaucoma or primary open angle glaucoma suspect
Figure 5.
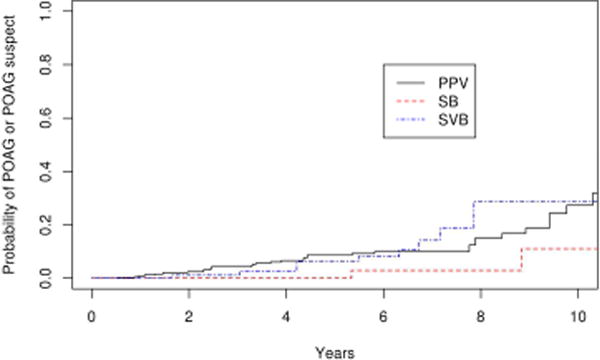
Survival curves for scleral buckle (SB), scleral buckle with vitrectomy (SBV) and vitrectomy (PPV) for secondary endpoint of primary open angle glaucoma or primary open angle glaucoma suspect
Figure 6.
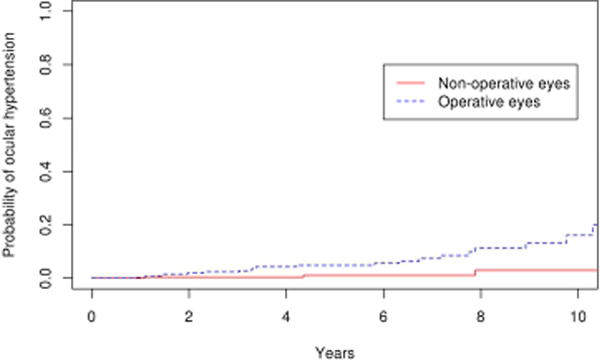
Survival curves for operative and non-operative cohorts for secondary endpoint of ocular hypertension
Probability of POAG or POAG suspect after vitrectomy surgery in the epiretinal membrane subgroup
Comparing the 85 eyes with epiretinal membrane undergoing vitrectomy to the 179 fellow eyes in the control group, there was a significantly higher number of operative eyes that developed POAG or POAG suspect (p=0.03). (Figure 7) The 10 year cumulative probability of developing POAG or POAG suspect in the operative eyes and non-operative was 31% and 19% respectively. For the outcome of POAG alone, the difference was not statistically significant (p=0.1; Figure 8), with the 10 year cumulative probability computed to be 15% and 2% for operative and non-operative eyes, respectively. As a secondary analysis, we included only the fellow eyes of the epiretinal membrane patients, to ensure that the age was the same in the operative and nonoperative groups. In this comparison also we found there was a significantly higher number of operative eyes that developed POAG or POAG suspect (p=0.05; Figure 9) The 10 year cumulative probability for developing POAG or POAG suspect in the operative versus non-operative cohorts was 31% and 3% respectively. The difference was not significant when the POAG suspects were excluded. (p=0.26; Figure 10)
Figure 7.
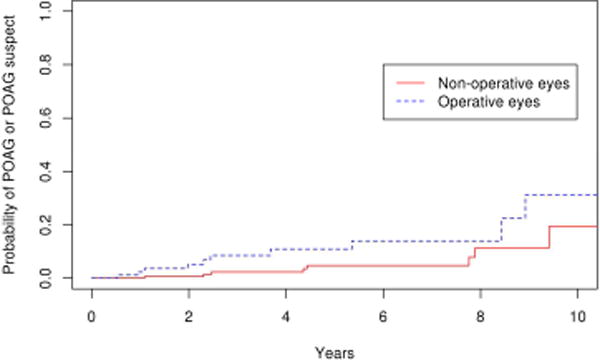
Survival curves for epiretinal membrane operative and non-operative cohorts for endpoint of primary open angle glaucoma or primary open angle glaucoma suspect
Figure 8.
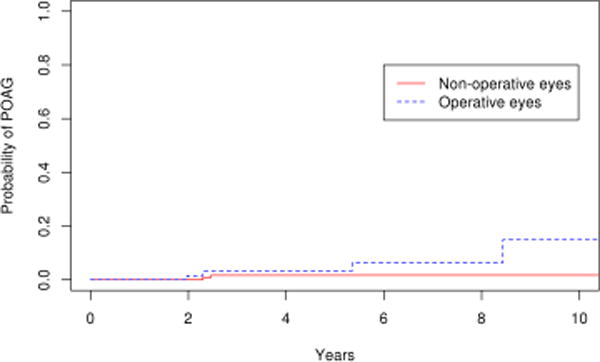
Survival curves for epiretinal membrane operative and non-operative cohorts for endpoint of primary open angle glaucoma
Figure 9.
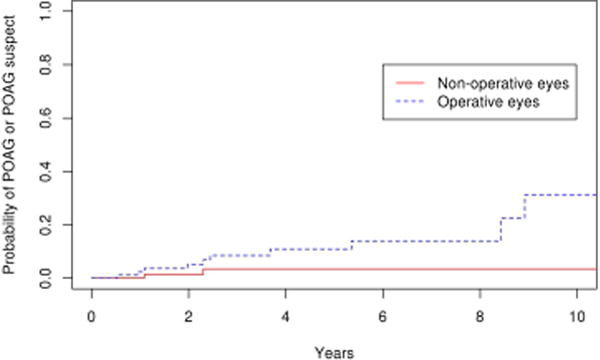
Survival curves for epiretinal membrane operative cohort and fellow eyes of epiretinal membrane non-operative cohorts for endpoint of primary open angle glaucoma or primary open angle glaucoma suspect
Figure 10.
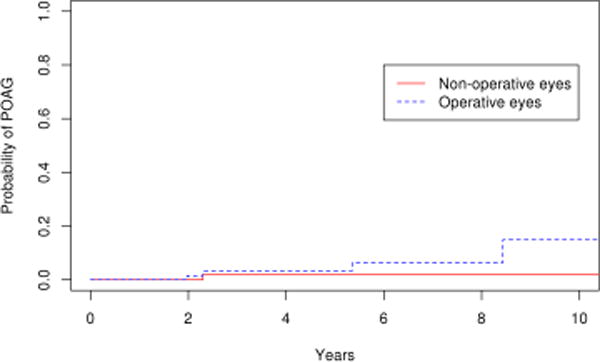
Survival curves for epiretinal membrane operative cohort and fellow eyes of epiretinal membrane non-operative cohorts for endpoint of primary open angle glaucoma or primary open angle glaucoma suspect
Risk factors for POAG and POAG suspect
Multivariate analysis for possible risk factors amongst operative eyes showed that higher baseline IOP increased the risk of developing POAG with a hazard ratio of 1.17 (p=0.046). Baseline IOP tended towards significance as a risk factor for the outcome of POAG or POAG suspect, with a hazard ratio of 1.10 (p=0.08). Cataract surgery performed before, along with, or after vitreoretinal surgery was not significant as a risk factor. There was no difference in risk associated with sex, age, retinal laser photocoagulation, myopia, pseudophakia, hypertension, diabetes mellitus, family history of glaucoma, baseline visual acuity, early post-operative IOP spike, indication for vitreoretinal surgery (rhegmatogenous retinal detachment, epiretinal membrane, or macular hole), use of indocyanine green, or injection of gas during surgery (Table 5).
Table 5.
Multivariate analysis of possible risk factors for development of POAG and POAG or POAG suspect
| Factors | Development of POAG | Development of POAG or POAG suspect | ||
|---|---|---|---|---|
|
| ||||
| HR | p-value | HR | p-value | |
|
|
|
|||
| Sex (M:F) | 1.40 | 0.52 | 1.63 | 0.14 |
| Age | 1.00 | 0.86 | 1.02 | 0.21 |
| Hypertension | 0.73 | 0.54 | 0.89 | 0.71 |
| Diabetes melllitus | 0.88 | 0.053 | 1.02 | 0.95 |
| High myopia | 1.72 | 0.29 | 1.29 | 0.43 |
| Retinal laser | 1.48 | 0.55 | 1.90 | 0.11 |
| Family history of glaucoma | 1.82 | 0.30 | 0.81 | 0.61 |
| Baseline intraocular pressure | 1.17 | 0.0461 | 1.097 | 0.09 |
| Pseudophakia | 0.86 | 0.07 | 1.37 | 0.36 |
| Early IOP rise | 2.02 | 0.18 | 1.44 | 0.26 |
| Indication of surgery | ||||
| Rheg RD | 0.49 | 0.21 | 0.65 | 0.19 |
| ERM | 1.27 | 0.68 | 1.52 | 0.24 |
| Mac Hole | 1.23 | 0.73 | 1.06 | 0.87 |
| Use of gas | 1.27 | 0.69 | 1.51 | 0.24 |
| Use of ICG | 0.98 | 0.97 | 1.86 | 0.11 |
Vitrectomy vs scleral buckle as risk factors for POAG or POAG suspect after retinal detachment
As described above, the probability of POAG or POAG suspect was significantly higher in eyes that had vitrectomy (SBV and PPV cohorts) compared with scleral buckle alone (SB cohort), with a hazard ratio of 6.0 (p=0.01) after adjusting for age. However this analysis included varied indications for vitrectomy, while the indication for scleral buckle surgery was only rhegmatogenous retinal detachment. Therefore we performed a secondary analysis including only patients undergoing vitreoretinal surgery for rhegmatogenous retinal detachment. Out of the 134 patients, 58 underwent scleral buckle, 53 scleral buckle with vitrectomy, and 23 underwent vitrectomy alone. Survival analysis demonstrated that the probability POAG or POAG suspect was significantly higher in the PPV cohort when compared to the SB cohort (p=0.006) after adjusting for multiple comparisons. There was a trend towards a difference in the rates of POAG or POAG suspect when comparing the SBV and SB cohorts (p=0.06), but difference between the PPV and SBV cohorts was not significant. (p=0.69; Figure 11).
Figure 11.
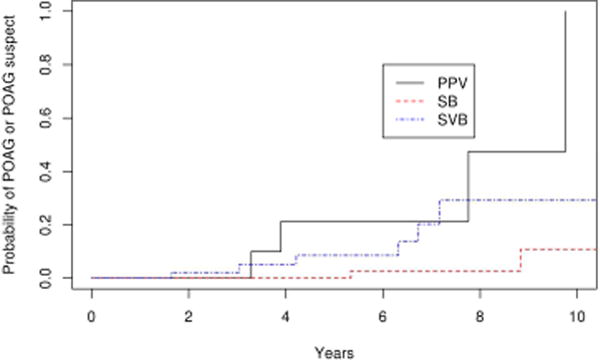
Survival curves in retinal detachment patients for scleral buckle (SB), scleral buckle with vitrectomy (SBV) and vitrectomy (PPV) for endpoint of primary open angle glaucoma or primary open angle glaucoma suspect
Comparison of POAG risk between operative eyes and the Olmsted County general population
The rate of POAG in operative eyes undergoing scleral buckle with vitrectomy or vitrectomy alone (10 year rate is 17.5% in the SBV group, 10.0% in the PPV group) was significantly greater (p<0.001) than the rate of POAG for the Olmsted County general population (1.0 %) (Figure 12 & 13). There was no significant difference in the rate of glaucoma in the fellow non-operative eyes compared with general population (p=0.17).
Figure 12.
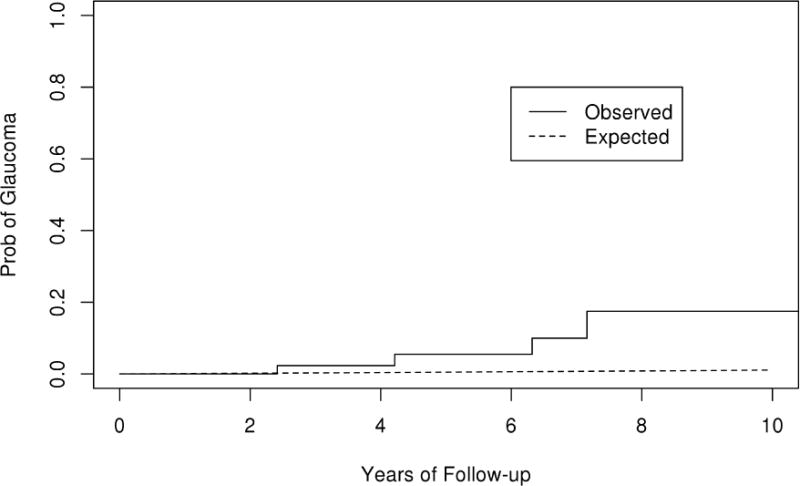
Observed and expected curves of primary open angle glaucoma (POAG) in the scleral buckle with vitrectomy cohort
Figure 13.
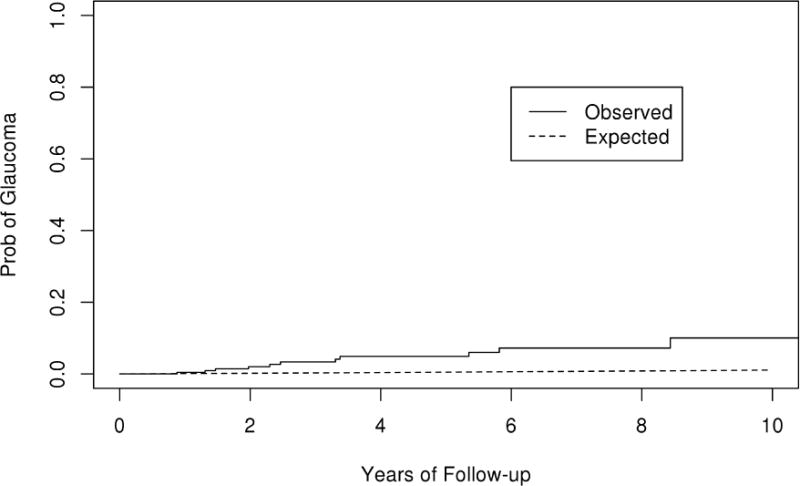
Observed and expected curves of primary open angle glaucoma (POAG) in the vitrectomy cohort
Discussion
Close monitoring of IOP in the early post-operative period following vitreoretinal surgery is common clinical practice. However, the need for long term monitoring for glaucoma after vitreoretinal surgery is less clear. Identifying the risk of late onset open angle glaucoma after scleral buckle and vitrectomy, along with baseline risk factors, can help facilitate earlier detection and treatment.
We found that there was a 10 to 17 fold increased risk of POAG in eyes following vitrectomy or scleral buckle with vitrectomy surgery when compared with fellow nonoperative eyes. This was very similar to the relative risk of glaucoma in eyes with vitrectomy or scleral buckle with vitrectomy when compared with the expected rate of POAG in the age and sex-matched general population of Olmsted County. We also compared the observed rate of glaucoma in the non-operative cohort with the expected rates of POAG in the age and sex-matched general population and found no difference. This suggests that glaucoma after vitreoretinal surgery is not occurring in patients with a predisposition to development of the disease, but due to risks related to the surgery itself.
Previous studies have also reported associated risk of open angle glaucoma after vitrectomy. Chang et al estimated that 15–20% of eyes may be at risk for OAG after vitrectomy, with a majority being unilateral in the eye of surgery.3 Luk et al and Koreen et al reported that 7.9% and 11.6% of vitrectomized eyes develop OAG respectively.4,5However, these studies did not compare the rates to unexposed cohorts. Other studies have reported contradictory results concerning the risk of open angle glaucoma after vitrectomy. Yu et al reported no difference in the proportion of OAG in vitrectomized and fellow eyes (4.31% and 2.49% respectively).17 However they did not report the incidence rate of OAG. As well, it is likely that the study lacked sufficient power, since the combined outcome of OAG and ocular hypertension came close to statistically significant difference compared with fellow eyes (p= 0.06), as observed by Chang et al in a letter to the editor.18It is also difficult to generalize their results as all the surgeries were performed by a single surgeon. Lalezary et al reported that vitrectomy does not appear to increase risk of OAG.6 However, their main focus was increased IOP, arguing that abnormal optic nerve findings and visual field defects may be present pre-operatively or may occur secondary to laser and use of ICG. Therefore they may not have captured those with low tension glaucoma. Among our OAG patients, 26.7% had IOP ≤21mmHg with glaucomatous visual field defects and optic nerve damage. We excluded patients who underwent pan retinal photocoagulation and may have had optic nerve changes that mimic glaucomatous optic neuropathy. As well, the multivariate analysis showed no association between glaucoma risk and use of ICG and retinal laser.
The mean and median time interval for development of POAG after surgery was 40.2 and 46.1 months, respectively, in our study. This was similar to the mean time interval of 45.95 months reported by Chang et al in their phakic patients.3 They reported a shorter mean interval of 18.39 months in their pseudophakic patients following vitrectomy. Luk et al and Koreen et al also reported a shorter mean time interval of 28.1 months and 20.5 months to development of POAG.4,5However, this could be explained by the longer follow-up duration of our study (mean 61.2 months) in comparison with Luk et al (mean 51.0 months) and Koreen et al (mean 31.2 months).
As a secondary end point, we assessed the risk of POAG or POAG suspect and found an increased incidence in operative compared with fellow non-operative eyes. We also assessed ocular hypertension as a secondary outcome, and found an increased incidence in operative compared with fellow non-operative eyes (8.9% versus 1.0%). While some previous studies have also reported an increased risk of development of suspicion for glaucoma, others have found no difference between operative and control eyes. Wu et al reported that 19.2 % of operated eyes developed sustained elevation of IOP compared to 4.5% in the comparison cohort followed over a mean of 47.3 months.19 Fujikawa et al reported that patients who underwent surgery for macular hole but not epiretinal membrane had an increased risk of ocular hypertension.7 Lalezary et al in a prospective study reported a decreased peripapillary retinal nerve fiber layer thickness a year after vitrectomy.20 In contrast, in a retrospective study Lalezary et al found no significant difference in the risk of ocular hypertension between operated and fellow eyes.6Yu et al reported that 4.31% of operated and 2.95% of fellow eyes developed ocular hypertension which was not statistically significant.17 Lee et al found a significant difference in IOP fluctuation in vitrectomized compared to matched control eyes, but no difference in the mean IOP.21
While numerous risk factors have been associated with POAG, those that have been reported to be associated with development of POAG after vitreoretinal surgery are pseudophakia and family history of glaucoma.3–5,19 However, in our study, only higher baseline IOP was associated with increased risk of POAG with a hazard ratio of 1.17. This result, along with the lack of a difference between the risk of glaucoma in fellow non-operative eyes and the general population, suggests that pre-existing risk factors for glaucoma may not be significant contributors to the increased risk of glaucoma after vitreoretinal surgery.
Previous studies concerning glaucoma risk after vitreoretinal surgery have focused on Chang’s hypothesis of increased oxidation of the trabecular meshwork after vitrectomy and the potential for IOP elevation.4,6 However, we found that 27% of the glaucoma patients had normal tension glaucoma. Also out of our POAG suspect patients, 60.9% (14 out of 23) had normal pressures, but optic disc or visual field findings that were suspicious for glaucoma. This indicates that multiple mechanisms may be involved in the pathogenesis POAG after vitrectomy, instead of just elevated IOP. Oxidative stress to the optic nerve head has been previously implicated in the pathogenesis of glaucoma, particularly normal tension glaucoma.22 Holekamp et al demonstrated that oxygen tension significantly increases during vitrectomy throughout the vitreous cavity and remains elevated even when measured many months after initial surgery (range 3–20 months).23 They suggested that the excess oxidative stress in the lens was responsible for post vitrectomy cataracts. If increased oxygen tension after vitrectomy is a contributor to the higher post-operative risk of glaucoma, then our study results suggest that the mechanism of action may include direct effects on the retinal ganglion cells, and not just trabecular meshwork damage. Supportive evidence comes from a study performed on rat models of glaucoma that reported that injecting a reducing substance into the vitreous cavity can increase the survival of retinal ganglion cells following axotomy or induced ocular hypertension.24 However, it is unclear whether or not oxygen tension changes within the vitreous cavity can influence the oxygen tension of the retinal ganglion cell microenvironment.
In addition to the altered intraocular environment after vitrectomy, it is necessary to consider intraoperative procedures that can contribute to optic nerve or retinal ganglion cell damage. In particular, air-fluid exchange (during which the cannula often lies in close proximity to the optic disc), peeling of the internal limiting membrane, and insertion of intraocular gas, are all steps that may contribute to retinal ganglion cell injury and glaucoma. To assess the impact of these procedures we performed a sub-analysis of the epiretinal membrane group (which was our largest subgroup of indications for surgery), since the surgery in this group tended to be the simplest and was least likely to have additional steps. We found that an increased risk of POAG or POAG suspect existed in this subgroup of patients as well, and the 10 year probability was similar (31%) when compared to the rate of the vitreoretinal surgery group as a whole (27.7%). We also compared the risk of glaucoma among patients in first half of the study period versus the second half, to assess possible effects of changes in surgical techniques. There was no statistically significant difference among the groups (endpoint POAG; p=0.14 and endpoint POAG or POAG suspect; p=0.06), but the 6 year period for each half is fairly short for the development of glaucoma, and a negative result may not necessarily indicate that there is no difference. Although our study does not suggest an increased risk of glaucoma from specific types of surgery, the potential risk of specific intraoperative steps is an area that requires further research.
Of note, we have classified the patients who developed glaucoma as ‘POAG’ to highlight that the etiology could not be attributed to a cause of secondary glaucoma based on our current knowledge. With further research into the etiopathogenesis, ‘glaucoma following vitrectomy’ may become a secondary cause in its own right. As well, it is possible that the management and prognosis of vitrectomy-associated glaucoma may differ from POAG. However, additional research is required to elucidate the clinical course of glaucoma following vitrectomy.
Our study did not find an increased risk of POAG among scleral buckle patients. The risk of POAG or POAG suspect was significantly less when compared with vitrectomy and vitrectomy with scleral buckle. This finding is consistent with Pinninti et al who studied 68 patients undergoing scleral buckle surgery and reported no increased risk of open angle glaucoma over a ten year follow up period when compared to the fellow eyes.9 A potential difference between patients with rhegmatogenous retinal detachments is that those undergoing vitrectomy with or without scleral buckle likely have more complicated or chronic detachments. While it is feasible that more complex retinal disease and the underlying etiologies increase the risk of glaucoma, as indicated above, patients undergoing epiretinal membrane surgery had a similarly increased risk of glaucoma compared with patients with other indications for surgery. The lack of increased glaucoma risk after scleral buckle is consistent with the hypothesis of increased oxidative stress after vitrectomy, but somewhat surprising since an encircling band has the potential to compress episcleral veins. Indeed, animal models for glaucoma include the use of episcleral vein ligature to cause IOP elevation.8 However, our results suggest that scleral buckles have minimal or only transient effects on IOP.
Significant advantages of our study compared with previous studies include its population-based nature and large sample size. This enabled calculation of incidence rates for POAG in various cohorts and comparison to an unexposed cohort as well as expected rates for the general population. We were able to focus on open angle glaucoma as our primary endpoint and not just ocular hypertension due to our long follow up time and the availability of linked records through the Rochester Epidemiology Project. Furthermore, glaucoma suspects could be more fully evaluated since diagnosis was based on findings recorded over all of the follow up visits. Selection bias was also likely very low in our study, since it included all patients of the Olmsted County population undergoing vitreoretinal surgery.
The main limitation of the current study was its retrospective nature. Optic disc findings for the primary outcome were based on findings documented by examining physician, and not by masked data abstractors. However, all patients with glaucoma were evaluated by glaucoma specialists, and thus we presume good reliability of the recorded findings. Pre-operative baseline visual fields and/or optic disc photographs were not available to confirm the absence of visual field defects and optic disc damage. However baseline IOP and clinical optic disc examinations were available for all patients and all pre-operative ophthalmic records were reviewed to exclude patients who had findings suspicious for glaucoma and raised IOP on prior visits. Another potential limitation is the number of patients that were excluded (100 patients, including 33 who had surgery less than 2 years from the end of the study period) due to less than one year of follow up, corresponding to a follow-up rate of 90.3%. Postoperative visits were not at regular, standardized time periods, but a median of 5 follow-up visits over a median time period of 5.1 years was available. We also may not have been able to exclude all secondary causes of glaucoma. For example, pseudoexfoliation in patients with dislocated intraocular lenses may have been missed on initial slit lamp examination. However, if these patients subsequently developed glaucoma, they would likely have been diagnosed with pseudoexfoliation based on the presence of exfoliative deposits on the lens capsule and pupil margin. Another potential limitation is that some of our patients did not have extensive pre-operative records and early stage glaucoma may have been missed. However, if there was no increase in glaucoma risk after vitreoretinal surgery, we would expect that the observed rate of glaucoma would have been similar to the population incidence. Finally, Olmsted County has a predominantly white population and therefore findings may not be generalizable to other regions of the United States (90.3% versus 75.1% white).25
Future studies will be required to better understand the mechanism of POAG after vitreoretinal surgery, and any potential changes in surgical technique or post-operative care that may mitigate the risk. The risk of progression from ocular hypertension to glaucoma also needs to be investigated. The risk and rate of disease progression in those diagnosed with glaucoma will need to be investigated to understand if the glaucoma after vitreoretinal surgery behaves similarly to glaucoma with no history of vitreoretinal surgery. Finally, investigation of possible changes to the aqueous outflow pathway and episcleral veins following scleral buckle surgery is required to understand the lack of increased risk for ocular hypertension or glaucoma, even in eyes that have an encircling element.
In summary, this the first population-based study to investigate late-onset POAG after uncomplicated vitreoretinal surgery. The results provide strong evidence for an increased risk of POAG after vitrectomy or vitrectomy with scleral buckle, but not scleral buckle alone. This is of particular concern since the rates of vitrectomy are increasing and there is a trend towards vitrectomy replacing scleral buckle for management of retinal detachment.10 Our study suggests a need for more frequent monitoring for glaucoma after vitrectomy and the discussions of glaucoma risk with patients prior to vitreoretinal surgery.
Acknowledgments
Financial Support: Unrestricted departmental grant from Research to Prevent Blindness, New York, and the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Other acknowledgements: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented as a poster to the American Academy of Ophthalmology conference 2017
Disclosure
Financial Disclosures:
Sasha A Mansukhani: No financial disclosures
Andrew J. Barkmeier: No financial disclosures
Sophie J. Bakri: Dr. Bakri has served as a consultant for Allergan, Genentech, Novartis, Zeiss
Raymond Iezzi: Dr. Iezzi has served as a consultant for Alcon Surgical in the past
Jose S. Pulido: Dr Pulido has equity interest in Lagen Laboratories that makes iPSC-RPE for in vitro (laboratory use).
Cheryl L. Khanna: No financial disclosures
Jeffrey R. Bennett: No financial disclosures
David O. Hodge: No financial disclosures
Arthur J. Sit: No financial disclosures
Contributor Information
Dr. Sasha A. Mansukhani, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
Dr. Andrew J. Barkmeier, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
Dr. Sophie J. Bakri, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
Dr. Raymond Iezzi, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
Dr. Jose S. Pulido, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
Dr. Cheryl L. Khanna, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
Dr. Jeffrey R. Bennett, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
David O. Hodge, Department of Health Sciences Research, Mayo Clinic, Jacksonville, Florida
Dr. Arthur J. Sit, Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota
References
- 1.Hasegawa Y, Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Intraocular pressure elevation in the early postoperative period after vitrectomy for rhegmatogenous retinal detachment. Jpn J Ophthalmol. 2012;56(1):46–51. doi: 10.1007/s10384-011-0094-3. [DOI] [PubMed] [Google Scholar]
- 2.Anderson NG, Fineman MS, Brown GC. Incidence of intraocular pressure spike and other adverse events after vitreoretinal surgery. Ophthalmology. 2006;113(1):42–47. doi: 10.1016/j.ophtha.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Chang S. LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006;141(6):1033–1043. doi: 10.1016/j.ajo.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Luk FOJ, Kwok AKH, Lai TYY, Lam DSC. Presence of crystalline lens as a protective factor for the late development of open angle glaucoma after vitrectomy. Retina. 2009;29(2):218–224. doi: 10.1097/IAE.0b013e31818ba9ca. [DOI] [PubMed] [Google Scholar]
- 5.Koreen L, Yoshida N, Escariao P, et al. Incidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomy. Retina. 2012;32(1):160–167. doi: 10.1097/IAE.0b013e318217fffb. [DOI] [PubMed] [Google Scholar]
- 6.Lalezary M, Kim SJ, Jiramongkolchai K, Recchia FM, Agarwal A, Sternberg PJ. Long-term trends in intraocular pressure after pars plana vitrectomy. Retina. 2011;31(4):679–685. doi: 10.1097/IAE.0b013e3181ff0d5a. [DOI] [PubMed] [Google Scholar]
- 7.Fujikawa M, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ohji M. Long-term intraocular pressure changes after vitrectomy for epiretinal membrane and macular hole. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):389–393. doi: 10.1007/s00417-013-2475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu S, Tanabe T, Yoshimura N. A rat model of glaucoma induced by episcleral vein ligation. Exp Eye Res. 2006;83(4):758–770. doi: 10.1016/j.exer.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Pinninti UR, McPherson AR, Carvounis PE. Long-term risk of glaucoma after encircling scleral buckle. Retina. 2015;35(6):1084–1086. doi: 10.1097/IAE.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 10.Wubben TJ, Talwar N, Blachley TS, et al. Rates of Vitrectomy among Enrollees in a United States Managed Care Network, 2001-2012. Ophthalmology. 2016;123(3):590–598. doi: 10.1016/j.ophtha.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prum BEJ, Rosenberg LF, Gedde SJ, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 2016;123(1):P41–P111. doi: 10.1016/j.ophtha.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 13.Prum BEJ, Lim MC, Mansberger SL, et al. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 2016;123(1):P112–51. doi: 10.1016/j.ophtha.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 15.Malihi M, Moura Filho ER, Hodge DO, Sit AJ. Long-term trends in glaucoma-related blindness in Olmsted County, Minnesota. Ophthalmology. 2014;121(1):134–141. doi: 10.1016/j.ophtha.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therneau T, Sicks JD, Bergstralh EJ, Offord JR. Expected Survival Based on Hazard Rates. Section of Biostatistics, Mayo Clinic; Rochester, MN: 1994. (Technical Rep Ser No 52). [Google Scholar]
- 17.Yu AL, Brummeisl W, Schaumberger M, Kampik A, Welge-Lussen U. Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension–a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1407–1414. doi: 10.1007/s00417-010-1409-7. [DOI] [PubMed] [Google Scholar]
- 18.Chang S, Smith SD. Comment Re: Yu AL, Brummeisl W, Schaumberger M, Kampik A, Welge-Lussen U (2010) Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension - a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol 248:1407-1414. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):461–462. doi: 10.1007/s00417-010-1409-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Berrocal MH, Rodriguez FJ, et al. Intraocular pressure elevation after uncomplicated pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina. 2014;34(10):1985–1989. doi: 10.1097/IAE.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 20.Lalezary M, Shah RJ, Reddy RK, et al. Prospective Retinal and Optic Nerve Vitrectomy Evaluation (PROVE) study: twelve-month findings. Ophthalmology. 2014;121(10):1983–1989. doi: 10.1016/j.ophtha.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee YW, Kim JM, Shim SH, Kim DY, Bae JH, Park KH. The Influence of a Vitrectomy on the Diurnal Intraocular Pressure. J Ophthalmol. 2015;2015:427808. doi: 10.1155/2015/427808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura A, Namekata K, Guo X, Noro T, Harada C, Harada T. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid Med Cell Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holekamp NM, Shui Y-B, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 24.Almasieh M, Lieven CJ, Levin LA, Di Polo A. A cell-permeable phosphine-borane complex delays retinal ganglion cell death after axonal injury through activation of the pro-survival extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2011;118(6):1075–1086. doi: 10.1111/j.1471-4159.2011.07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


