Abstract
Biological sex (being female or male) significantly influences the course of disease. This simple fact must be considered in all cardiovascular diagnosis and therapy. However, major gaps in knowledge about and awareness of cardiovascular disease in women still impede the implementation of sex-specific strategies. Among the gaps are a lack of understanding of the pathophysiology of women-biased coronary artery disease syndromes (spasms, dissections, Takotsubo syndrome), sex differences in cardiomyopathies and heart failure, a higher prevalence of cardiomyopathies with sarcomeric mutations in men, a higher prevalence of heart failure with preserved ejection fraction in women, and sex-specific disease mechanisms, as well as sex differences in sudden cardiac arrest and long QT syndrome. Basic research strategies must do more to include female-specific aspects of disease such as the genetic imbalance of 2 versus one X chromosome and the effects of sex hormones. Drug therapy in women also needs more attention. Furthermore, pregnancy-associated cardiovascular disease must be considered a potential risk factor in women, including pregnancy-related coronary artery dissection, preeclampsia, and peripartum cardiomyopathy. Finally, the sociocultural dimension of gender should be included in research efforts. The organization of gender medicine must be established as a cross-sectional discipline but also as a centered structure with its own research resources, methods, and questions.
Keywords: Women, CVD, Gender, CAD, HF
INTRODUCTION
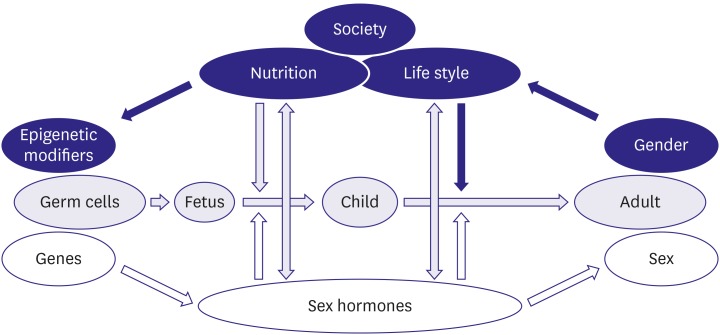
Both sex, the biological dimension of being female or male as transmitted by genes and hormones, and gender, the sociocultural dimension of being a woman or a man as determined by education, lifestyle, and socialization, significantly influence the course of disease and a great many other aspects of life (Figure 1).1) The resulting differences between women and men should be considered in all cardiovascular (CV) diagnoses and therapies, but they have frequently been neglected. To address that neglect, the discipline of sex- and gender-related medicine, frequently called gender medicine, has been developed to integrate the sex- and gender-related aspects of disease into clinical practice, research, and prevention efforts.
Figure 1. Complex interdependency of sex and gender in humans (reprinted with permission from Sex and gender aspects in clinical medicine, edited by Oertelt Prigione and Regitz-Zagrosek, Springer, 2011).1).
Gender medicine is a novel discipline that takes into account the effects of sex and gender on the health of women and men. Its major goal is to improve the health and health care of both males and females. It is based on the hypothesis that both sexes benefit from differentiated approaches and better understanding of their specific pathophysiology.
Gender medicine is not synonymous with women's health. However, much more is known about male aspects of cardiovascular disease (CVD) than female aspects. Therefore, this review focuses on unsettled CVD issues in women.
OPEN QUESTIONS ABOUT CORONARY ARTERY DISEASE IN WOMEN
Risk factors
Sex differences in cardiovascular risk factor profiles represent the first unsettled issue, as reviewed recently.2) The data reveal an advantage for young women and a higher cardiovascular risk factor profile at older ages, which led to the misconception that women are generally at lower risk for CVD than men, which they are not. Overall, women accumulate as many risk factors as men but at a higher age. In addition, the less obstructive pattern of coronary artery disease (CAD) in women added to the misperception that CAD is not that serious in women, resulting in suboptimal primary and secondary prevention efforts. In fact, prevention goals are often less well achieved in women than in men.3),4) In particular, younger women have taken up more bad smoking habits,5) even though it is well known that the risk of having an acute coronary syndrome before the age of 55 as a result of smoking is two times higher in women than in men.6) The reasons for that difference still need to be explored.
Premenopausal women often have less hypertension and lower lipid levels than similarly aged men, but that trend reverses in postmenopausal women.7) Systolic blood pressure rises more steeply in postmenopausal women than in similarly aged men.8) Hypertension causes a higher prevalence of strokes, left ventricular hypertrophy, and diastolic heart failure (HF) in women than in men.9) Even moderate or borderline hypertension (<140/90 mmHg) causes more endothelial dysfunction and cardiovascular complications in women than in men.10) As for the consequences of smoking, pathophysiological understanding is still lacking.
High lipid levels are an underestimated risk factor in women. Databases show that women with hyperlipidemia are less intensely treated than men.11) Because the risk attributed to increased lipid levels is similar in women and men, doctors and patients both need to be more aware of those risks in women.12)
Diabetes has emerged as a major risk factor that worsens CAD outcomes more in women than in men. In a meta-analysis of 37 prospective cohort studies, the increased risk of fatal ischemic heart disease with diabetes was 50% higher in women than in men.13) More comorbidities, including obesity and inflammation, as well as more unfavorable changes in coagulation and endothelial function, could contribute to the greater cardio-metabolic risk-factor load in diabetic women.14) Diabetes therefore reduces the female advantage of better CVD health at <60 years and should be considered a stronger risk factor for CVD in women than it is in men.15) Again, mechanistic studies are lacking.
Depression and various forms of sustained mental stress (anxiety, anger, marital conflict, work stress, depression, etc.) have been acknowledged as etiological and prognostic risk factors for CAD.7) They increase the risk of developing CAD similarly in women and men. However, the prevalence of those conditions is significantly higher in women, particularly young women, which leads to poor outcomes.16),17)
Increasing evidence suggests that air pollution, inhalation of dust, and traffic noise is associated with the degree of atherosclerosis in both men and women. This correlation needs much more attention in the field of infrastructure and environmental design, as well as consideration of gender-specific exposures.18)
Preeclampsia is an emerging CVD risk factor in women that is frequently unknown to women and their gynecologists. Only recently was it included in the 2014 American Heart Association (AHA) prevention guidelines for stroke.19) Again, mechanistic studies are lacking.
Pathophysiology
Ischemia, acute coronary syndrome, and myocardial infarction (MI) without epicardial CAD or structural heart disease occur more frequently in women than in men.20) In 20–30% of females with an acute MI, no angiographic obstructive CAD is seen, which is twice as often as in men.21),22) A sex difference in plaque morphology, i.e. a female pattern of CAD in acute coronary syndromes, has been identified but needs further clarification.23)
In particular, young women with acute coronary syndrome often present with open coronary arteries, displaying plaque erosions with distal embolization rather than plaque rupture with thrombus formation.24),25) The risk factors and mechanisms are still largely unknown. Not infrequently, angina and acute coronary syndrome in women can be caused by coronary microvascular disease, also called microvascular angina.26),27)
Women more frequently display components of pathological vasoreactivity, such as spasms and endothelial dysfunction.28),29) An underdiagnosed cause of acute coronary syndrome is spontaneous coronary artery dissection; it occurs predominantly in women, mostly between 45 and 60 years of age during pregnancy or in the immediate postpartum period and might be caused by hormonal changes.30) An estimated 8% of acute coronary syndrome in women (but less than 1% in men) is associated with the so-called Takotsubo syndrome (TTS) described below.31),32)
Studies at cellular, animal, and clinical levels on the sex-specific pathophysiology of acute MI and its predisposing risk factors are still badly needed.
Clinical symptoms and diagnosis
In acute coronary syndrome, patient delay before seeking medical help is longer in women than in men,33),34) which means that women arrive later in the emergency department than men. Reasons for this delay are manifold and depend on the cultural context as well as on some biological parameters, but they are incompletely understood.
The interpretation of non-invasive diagnostic testing is less reliable in women than in men, especially in women younger than 60 years, for whom the prevalence of obstructive CAD is still relatively low.35) Non-specific ECG changes at rest and a lower exercise capacity contribute to the lower sensitivity and specificity of non-invasive exercise testing in women.36),37) Also, most exercise testing scores were developed from populations composed primarily of men; only a few scores have been designed especially for women.38),39) The current European Society of Cardiology (ESC) guidelines advise stress imaging techniques (e.g. SPECT, stress echocardiography) when available as first test of choice, with a preference for non-radiation diagnostics in younger women.35),40)
It has recently been postulated that a sex-specific threshold for the highly sensitive biomarker Troponin I, which reflects myocardial damage, could improve the diagnostic accuracy of this most important laboratory test for acute coronary syndrome in women.41),42)
In the Swedish coronary angiography and angioplasty register, almost 80% of women younger than 60 with stable angina symptoms had no visible coronary obstructions on angiography, compared to only 40% of men.43) Thus, the primary diagnostic strategies, which search for the classical male pattern of obstructive CAD, may be suboptimal in women, increasing the risk of procedural complications and leaving vascular dysfunction and coronary microvessel disease in symptomatic women underdiagnosed.44) In 2014, the AHA published evidence-based gender-related guidelines that promote selective functional and anatomic testing using non-invasive imaging techniques in women at intermediate risk.35) Women at low risk for ischemic heart disease most often require no testing. Women with a low-intermediate or intermediate risk of ischemic heart disease who can exercise adequately should be referred to an exercise-first strategy. CAD imaging is indicated for women with an intermediate or high risk of ischemic heart disease risk and a functional disability or abnormal rest ECG. Diagnostic modalities for assessing coronary microvessel disease include measurement of coronary blood flow reserve by transthoracic echocardiography, PET-CT perfusion, and the calculation of microcirculatory resistance indexes during coronary catheterization (coronary flow reserve).26),45) Those guidelines could help reduce the number of unnecessary and inconclusive angiograms in this patient population, but it is not yet clear how they will be put into practice.
Women with recurrent chest pain syndromes and non-obstructive CAD need to be diagnosed and treated because they have a twofold increased risk of developing obstructive CAD events in the next 5–8 years, and they have a four times higher risk of re-hospitalization and recurrent angiograms than women without those symptoms. Studies have reported an expected consumption of nearly $750,000 in cardiovascular health care resources related to the burden of ongoing symptoms and medications.20),46),47)
Management
Treatment of stable CAD and acute coronary syndrome should be performed according to the current guidelines for both genders.40) It is now well accepted that women derive the same benefits from percutaneous coronary intervention as men. Previously, a worse prognosis in women was attributed to the smaller luminal diameters of the coronary arteries, risk factor profiles, more comorbidities, and referral bias. The Belgian Working Group on Interventional Cardiology analyzed a registry of 130,985 percutaneous coronary intervention procedures in Belgium from January 2006 to February 2011. Being female remained an independent predictor of mortality after multivariable adjustment.48) In most studies with second-generation, drug-eluting stents, sex and gender differences in long-term outcome after percutaneous coronary intervention are not supported, but more data are needed. So far, being female remains an independent predictor for peri-procedural MI and major bleeding after percutaneous coronary interventions, which are associated with increased short-term morbidity and mortality.49),50) Due to the relatively higher contribution of functional coronary abnormalities and a more diffuse pattern of atherosclerosis in female patients with CAD than in male patients, residual symptoms of angina are often present after acute coronary syndrome or coronary interventions in females.51),52)
Using the transradial access for coronary interventions reduces the incidence of peri-procedural bleeding complications and improves clinical outcomes.53) The use of fractional flow reserve-guided percutaneous coronary interventions has improved outcomes in both men and women.54) Fractional flow reserve values are found to be higher in women after correction for visually assessed coronary anatomic severity. Whether gender-related guidelines in interpreting fractional flow reserve measurements are warranted is currently under discussion.55),56)
Women have a higher mortality after elective coronary artery bypass surgery.57) Major risk factors for women's mortality are low physical function, respiratory dysfunction, renal failure, and old age.58) A recent study identified a significantly higher prevalence of diastolic dysfunction among females presenting for elective cardiac surgery and reported that women had prolonged hospital stays.59) Women have poorer health-related quality of life than men after coronary surgery.60) Depression is a significant predictor of worse outcomes in both women and men.61)
Therapeutic options for coronary microvascular dysfunction are less well investigated than those for epicardial CAD; the efficacy of anti-anginal medications for symptom reduction is relatively poor, and optimal treatment options are lacking.40),62) The presence of detectable peripheral coronary flow abnormalities in patients with microvascular dysfunction is associated with impaired prognosis in both men and women.63),64) Therefore this syndrome urgently needs more research.
Outcomes
Debate is ongoing about whether outcomes are identical in women and men. In general, less aggressive management of CAD leads to worse outcomes. Other factors, such as older age, more co-morbidities, and differences in the pathophysiology of underlying CAD, contribute to the current understanding of sex differences in the outcomes of CAD patients.
Thanks to the increased awareness of the prevalence of CVD in women, CAD in particular, hard outcomes (mortality, events) have improved in female patients during the past couple of decades. However, female patients <65 years old with acute coronary syndrome still face higher mortality than men, and an important difference in the gender-gap in outcomes remains between Eastern and Western European countries. Higher in-hospital mortality in women with acute coronary syndrome has been attributed to a longer patient delay before admission, older age, a higher clustering of CV risk factors, lower use of invasive and pharmacological treatments, and more bleeding complications after interventions.65) In younger women (<60 years) with STEMI, adjusted in-hospital mortality rates are nearly twice as high as in similarly aged men.66),67) More data are needed on gender differences in residual symptoms and softer endpoints related to quality of life. Evolving knowledge has led to the first initiatives to implement gender-related strategies in guidelines such as the NSTEMI guidelines.
In conclusion, important sex and gender differences exist in the early onset and progression of CAD, especially atherosclerosis and myocardial ischemia. Sex-related approaches are needed for women and men to identify their risk factors for CAD. In women, pregnancy-related issues need more attention, as well as rheumatic and autoimmune diseases. Socioeconomic conditions play an underestimated role in both sexes. Considering the pathophysiological basis for CAD, it is becoming clear that most research has focused on the male condition of atherosclerosis in the large coronary arteries. In contrast, women-characteristic conditions, e.g., dissections, spasms, and microvascular disease, have received less research attention. A different pattern of CAD translates into different symptoms of ischemic heart disease. Unfortunately, age- and sex-related diagnostic algorithms have not yet been sufficiently well developed. Under-treatment of female cardiac patients still occurs and can contribute to low quality of life and health problems in older women.
SEX DIFFERENCES IN CARDIOMYOPATHIES
Takotsubo syndrome
TTS is an acute and typically reversible HF syndrome that is being increasingly recognized since the first case was published in 1990, and it is particularly relevant for women, as reviewed recently.2) TTS causes up to 8% of acute coronary syndrome in women.31),32) It is often triggered by emotional or physical stress and occurs predominantly (in Europe in up to 90% of cases) in postmenopausal women. The reported in-hospital mortality is 1–5%, with a subsequent recurrence rate of 5–22%.68),69)
Many unsettled issues exist in TTS. In addition to the well-established list of research questions, such as the role of catecholamines, the effect of the location in the ventricle and medical therapy, and the reason for the female preponderance in presentation, the role of sex hormones in TTS is unclear. Also, no good explanation has been offered for the presentation post menopause.
Dilated cardiomyopathy and hypertrophic cardiomyopathy
As recently discussed, genetic cardiomyopathies (CMP) caused by autosomal gene variations are expected to occur with the same prevalence in women and men.70) However, dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) have a greater prevalence in men than in women and also more serious clinical manifestations.71),72),73),74) Thus, compensation for the genetic defect in those syndromes appears to be more efficient in women than in men. Presumably, the protective mechanisms active in women would also act positively in men. Based on the protective mechanisms in other forms of HF, I assume that sex differences in mitochondrial function and fibrosis play a role.
HCM, caused by sarcomeric protein mutations, has a much higher prevalence in men than in women for as yet unknown reasons. Sudden arrhythmic cardiac arrest (SCA) is a frequent threat in HCM, and sudden death in young male athletes, but not female athletes, is frequently attributed to undiagnosed HCM. Apparently, unknown protective mechanisms are active in women.
HEART FAILURE IN WOMEN
Epidemiology and pathophysiology
HF is a worldwide epidemic, affecting more than 20 million adults worldwide. It is consistently associated with older age. As recently discussed, given the longer life expectancy of women, the population of older women with HF is expected to grow.2),75)
HF can broadly be classified as HFpEF, HF with reduced ejection fraction (HFrEF), or HF with medium ejection fraction (HFmEF). As recently reviewed, compared to patients with HFrEF, patients with HFpEF are more likely to be women; in fact, women represent almost two-thirds of the HFpEF population. In reality, HFpEF is distinctly different from HFrEF, with a different set of risk factors. Hypertension is the highest risk for HF among women. The next best association is with diabetes.
In the Framingham Heart Study, women accounted for 40% of patients with HFrEF and 65% of patients with HFpEF. Women are less likely than men to experience MI, which could contribute to the lower prevalence of women in the HFrEF population.
HFrEF from non-ischemic etiologies deserves special attention because of risk factors specific to women, including cancer treatment for breast cancer. Breast cancer is the most common cancer among women, and the treatment is associated with cardiomyopathy with or without HF symptoms. At higher doses (≥700 mg/m2) of doxorubicin, HF incidence can be as high as 18%.
Diagnosis
For a comprehensive diagnosis of HF, sex- and gender-specific comorbidities and living conditions should be assessed, e.g., history of inflammatory or psychiatric disease, malignancies, depression and other potentially related psychological comorbidities, stress, socioeconomic and working conditions, and gynecological and andrological history, including number of children, still births, sexual activity, and hormone therapy.2),76) Detailed studies are needed to explore why women exhibit a worse quality of life and depression more frequently than men after an HF diagnosis.
When assessing ventricular size in HFrEF by echocardiography, ventricular diameters should be normalized to body size. Unfortunately, HF diagnoses in previous studies, e.g., in the Euro Heart Survey, used objective diagnostic tests in women less frequently than in men.2) Physicians should be informed about this potential bias so they can reduce it.
Treatment
HF treatment is better established for HFrEF than for HFpEF.2) The mainstays of HFrEF treatment include beta blockers and ACEIs. Randomized-controlled trials have compared beta blockers to placebo among patients with HFrEF, and a meta-analysis of those trials suggested a similar mortality benefit for men and women with HFrEF. The proportion of women included in first ACEI trials was low. Several of the original trial results did not include subgroup analyses, and only a subsequent meta-analysis suggested a similar mortality benefit for ACEI in men and women.
The original digoxin trial demonstrated a lack of mortality benefit in HFrEF, and subsequent analyses suggested that it increased mortality among women. In a post-hoc analysis, death from any cause was significantly higher in women, and digoxin was associated with a higher rate of death in women but not in men. Although those findings might have been due to higher drug levels in women, the results are still alarming and highlight the need for sex-specific attention to medical treatments.
With comparable treatment, women with HF have better clinical outcomes than men.2) This remains true even when HFrEF and HFpEF are analyzed separately. In most cases of HF due to inherited CMP, women appear to do better, independent of therapy. Thus it has been suggested that women have more efficient compensatory mechanisms that might be able to overcome an insult at the molecular or cellular level.
Invasive therapy, i.e., cardiac resynchronization therapy (CRT), appears to have greater efficacy in women than men with HFrEF. In a large registry, women had a greater mortality benefit than men. Recently, Biotronik launched the Biowomen trial, which might be the first HF trial that explicitly aims to enroll equal numbers of men and women, to examine sex-specific responses to CRT treatment.77)
Worldwide, fewer women than men undergo heart transplantation. In a large dataset of consecutive patients with idiopathic non-ischemic DCM referred to the German Heart Institute for heart transplantation, only 15.6% were women, suggesting a referral bias against women.2),78)
Women referred for heart transplantation frequently present in a more advanced HF state and have lower exercise tolerance, respiratory efficiency, and kidney function than male referrals. Women also have significantly less diabetes than men. Thus, women were referred at a more advanced disease state, and relevant contra-indications such as diabetes appear to have been taken more seriously in women. An international multicenter prospective study on referrals for heart transplantation, organ allocation, and survival is clearly needed.
ARRHYTHMIA AND SUDDEN CARDIAC ARREST IN WOMEN AND MEN
Sex differences in channelopathies
Women have a greater prevalence of developing QT prolongation and torsades de pointes arrhythmia than men (for a review, see 70)). This is true with both QT prolonging drugs and genetic defects.2) Genetic defects leading to long QT syndrome (LQTS) are located on autosomes. LQTS-induced tachycardia occurs with equal frequency in boys and girls. However, after puberty, arrhythmias are more frequent in women than men.79) Shortening of the QT interval by testosterone and lengthening of it by estrogen have been reported to contribute to that difference.
Sudden cardiac arrest in women
SCA is more than twice as frequent in men than in women and has different disease associations in the two sexes. In men, 80% is caused by coronary heart disease, whereas in women, less than half has that cause. In women who survive an SCA, CMP in structurally sound, apparently healthy hearts and genetic causes such as LQTS are more often found.
Sudden cardiac death in sports is overwhelmingly a men's disease: 95% of SCA victims in sports are men.80) Targeted examinations have shown that women are protected against sudden cardiac death when they are subjected to high stress levels. Sex-specific switches in adrenergic signaling under stress conditions could represent an endogenous protective mechanism in women. Furthermore, women might generate protective metabolites in the arachidonic acid pathways under the influence of estrogen, whereas men, under the influence of testosterone, generate more pro-arrhythmic and pro-hypertrophic metabolites. However, the overall mechanisms underlying protection in women are still unknown.81)
Treatment of patients at risk for SCA and SCA survivors depends on sex. Women and men are not equally treated with defibrillators. Women receive less treatment for the same indications in both primary care and secondary prevention, and the reasons for that difference are not yet clear.82),83),84)
SEX DIFFERENCES IN BASIC RESEARCH
The pathophysiology of women- and men-specific cardiovascular syndromes is still poorly understood (for a review, see 85)). However, estrogen and estrogen receptors play a significant role in cardiac fibrosis, energy metabolism, pressure overload, myocardial ischemia, and myocardial hypertrophy.86),87),88),89),90),91),92),93),94),95),96),97),98),99),100) Most interestingly, estrogen regulates microRNA expression and thereby has a role in the control of many genes.101) Estrogen and estrogen receptors (and testosterone in men) do their best to drive the cardiovascular system into specific directions in both sexes, making women and men sensitive to specific risks and stress factors. Activation of the coagulation system, the immune system, and myocardial and vascular function and growth all occur in a sex-specific manner. Stem-cell-derived cardiomyocytes are now increasingly used in CV research, but sex differences in those cells have not yet been studied. All these areas receive insufficient research attention and funding.
Furthermore, there is poor research coverage of pregnancy and its interaction with stress and the CV system.102),103),104)
Sex differences in drug therapy
Drugs are less well adapted for women than for men, and female-specific drugs might never be found because more than 80% of animal research in cardiology is done in male rodents.105) Sex differences in survival have been shown for experimental drugs, and several cardiovascular drugs have more adverse effects in women than in men2): more arrhythmia with QT prolongation, more bleeding complications, more adverse effects of digoxin, and more adverse effects of lipid lowering drugs have been reported. Furthermore, women receive less-effective drugs than men after an MI. They also receive less counseling, particularly for lifestyle, rehabilitation, and sexual life.
More and more-severe adverse effects of drugs in women than men led to drugs being withdrawn from the US market between 1997 and 2000 (US general accounting office 2011 Drug Safety). Indeed, new drugs often fail in phase 3 studies. Deficits in correspondence between animal models and human study settings, i.e., participant selection, could play a role.
As recently reported, most preclinical research used in drug development is done using male animals and cells with unidentified sex.85) However, significant differences exist in the outcomes for male and female mice in models of MI, pressure overload and genetic CVD, diabetes mellitus, multiple sclerosis, and other diseases, and those are rarely considered by researchers.106) As an extreme consequence, a drug might be effective in a male animal model and completely ineffective in females on some outcome parameters, or vice versa. For example, melusin overexpression reduced early mortality after MI in male mice but failed to do so in female animals,107) whereas it improved remodeling after MI in both sexes.
The different life phases of women and men are also insufficiently considered in drug development. The decline in the endogenous production of hormones, particularly estrogen at menopause, often leads to functional disorders. More generally, it is necessary to study the interaction between sex and age in both women and men.106) Finally, it is important to recall that pharmacodynamic aspects should be considered especially intensely in sex-specific drug design.108)
PREGNANCY RELATED CARDIOVASCULAR SYNDROMES
Peripartum cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a disease of unknown etiology leading to HF during the last months of pregnancy or within five months after delivery.109),110),111) The incidence is highest outside of Europe (Haiti, South Africa) and is lower in the US and European countries (range from 1–300:1–4,000). Predisposing factors are multiparity and multiple childbirths, family history, ethnicity, smoking, diabetes, hypertension, preeclampsia, malnutrition, advanced or very young age, and prolonged use of β-agonists.
The pathophysiology is largely unknown, but increased oxidative stress and activation of the protease cathepsin-D, which cleaves prolactin into pro-hypertrophic and anti-angiogenic fragments, might be involved.112),113) Alternative disease mechanisms, inflammation, virus infection, auto-immune mechanisms, or genetic mechanisms might also contribute. In some cases, unmasking familial dilated cardiomyopathy has been identified as the underlying cause, and circulating microparticles have been used as indicators of the disease.
Only one randomized-controlled trial for the treatment of PPCM has been published to date. In that study, the 21 women randomized to bromocriptine had greater recovery of left ventricular ejection fraction at six months and a lower rate of the composite end point (defined as death, New York Heart Association functional class III/IV, or left ventricular ejection fraction <35% at 6 months). A larger trial of bromocriptine treatment has been completed, and the results are forthcoming. Further studies on the pathophysiology of PPCM and related therapy are needed.
Pregnancy-related spontaneous coronary artery dissection
SCAD, as I discussed in a recent review, is a potentially life-threatening event that mainly occurs in young women and is particularly frequent in pregnancy and the peripartum period.75) Pregnancy-related SCAD (P-SCAD) predominantly occurs during the 3rd trimester of pregnancy and the postpartum period. P-SCAD can occur not only in the acute- (first 6–12 hours) and subacute phase (2–6 weeks) of the postpartum period but also up to 6 months after delivery.
Different possible causes of P-SCAD have been hypothesized, including estrogen and progesterone involvement. The increased progesterone levels, decreased collagen synthesis, and high media mucopolysaccharide content seen during pregnancy weaken the tunica media of the coronary wall, substantially increasing shear stress and the risk of media rupture.
The prevalence of P-SCAD still appears to be underestimated, accounting for 5–30% of all SCAD cases. Moreover, SCAD is diagnosed in approximately 25% of pregnant women who experience an acute MI.
Many SCAD cases may have been misdiagnosed due to technical imperfections in diagnostic coronary angiography. The etiology of SCAD remains unclear. Its occurrence in young and postpartum women suggests that hormonal changes and physiologic factors related to pregnancy could play a key role beyond other CV risk factors.112)
Pregnancy complications and later cardiovascular disease: vascular function
A woman's reproductive history serves as a predictor for her later risk of CVD, which needs more clinical attention.2) Women with a history of preeclampsia have a higher CVD risk than women with normal pregnancies. Women with preeclampsia who deliver preterm and mothers with recurrent preeclampsia carry even greater risks for later CVD and kidney failure. Being the mother of a growth-restricted baby or a preterm infant also increases the risk of CVD later in life. Preeclampsia and CVD share risk factors such as diabetes, obesity, and hypertension and pathogenetic mechanisms such as oxidative stress, endothelial dysfunction, and insulin resistance. The relevance of these mechanisms has recently been described by my group.102),104) In women who develop preeclampsia, the threshold for clinical CVD is breached during pregnancy and again later in life, as increasing age is added to already present and newly acquired CVD risk factors. In this way, adverse pregnancy outcomes can predict which women will be at increased risk of CVD in later life.
Impaired flow mediated dilatation is present in women with a history of preeclampsia as they age.114) Future challenges involve the elucidation of specific pathways through which pregnancy complications cause CVD using a battery of ex vivo and in vivo techniques that permit an assessment of cardiovascular structure and function, together with measurements of risk factors for CVD, including renal function.
INCLUSION OF SEX AND GENDER ASPECTS
Biological differences between female and male animals, including human beings, are called sex differences.115) These are based on differences in sexual hormones, sex chromosomes, and sex-specific gene expression from autosomes. They lead to obvious differences in body composition, metabolism, physiology, and the pathophysiology of many diseases. However, gender differences are equally important. Gender is the result of a sociocultural process and is associated with behavior, including stress and lifestyle-associated factors and diseases. Gender determines access to health care, help-seeking behavior, and individual use of the health care system. Gender thus reflects sociocultural processes influenced by society, education, environment, lifestyle, nutrition, and many other factors. It results in different attitudes, motivations, beliefs, and behaviors toward health and disease — encompassing both the attitudes of an individual facing disease and the health care system and the attitudes of the health care system toward that individual.
For example, strong gender differences exist in the awareness of CVD risk, leading to poor acceptance of CVD preventive measures in young men, older and poorly educated men, and women. Furthermore, referral for and acceptance of invasive therapeutic strategies such as pacemaker implantation or heart transplantation are largely determined by gender. In the medical field, sex and gender are closely related. On one hand, sex influences gender roles, i.e., testosterone plays a role in the aggressive behavior sometimes associated with risk seeking and the neglect of prevention. On the other hand, gender roles can influence sex, e.g., professional exposure to stress, poor nutrition, and environmental toxins or endocrine disrupters can lead to genetic or epigenetic modifications that affect gene expression differently in women and men and thereby modulate the biological phenotype.
Whereas biological sex (being female or male) is increasingly included in the analysis of biomedical trials, gender is rarely taken into account due to the lack of a quantifiable construct for gender. Recently, efforts have been made to develop a construct to quantify gender in CVD studies, and a recent study has indeed demonstrated that some risk factors for CVD are more closely associated with gender than with biological sex.116),117)
In conclusion, sex- and gender-dependent disease mechanisms are incompletely studied, hindering targeted therapy and personalized approaches. This gap will be narrowed by the GendAge project (BMBF, 2018–2021), which builds on the unique cohort of 1,600 women and men studied in BASE-II, a population-based cohort study with comprehensive CV, metabolic, socioeconomic, and quality of life assessments conducted in 2010–2014. Thus, GendAge will be able to analyze sex and gender effects both cross-sectionally and longitudinally.
ADVANCING GENDER MEDICINE
Lessons learned from the GenCAD project
The European Commission funded the Gender Differences in Coronary Artery Disease in Europe (GenCAD) project (www.gencad.eu) to improve understanding about and awareness of sex and gender differences in chronic diseases, using CAD as an example. GenCAD considered the existing knowledge on sex and gender differences in CAD and the inclusion of sex- and gender-related aspects in the databases and policies of EU member states.
In a state-of-the-art study, GenCAD first assembled existing knowledge on sex and gender differences in CAD using published literature from all over the world. The literature search covered prevention and health promotion, epidemiology, disease mechanisms, clinical symptoms and diagnosis, management, and outcomes of CAD. Almost 1,000 articles were reviewed in detail. Significant sex and gender differences that need consideration were found in all the fields just listed.
Even though many publications are available, the overall quality of gender-related analyses is often limited by retrospective, non-randomized, non-blinded study designs with few participants. Studies often include women in insufficient numbers to draw conclusions about them, sex- and gender-related research questions are rarely included in the protocols in a prospective manner, and sex- and gender-related confounders are generally not considered. Better studies must be planned, which will require training for the research community, which largely went untrained in sex- and gender-related research at universities. Researchers must thus be trained to identify both the right topics and the right methods.
Several sex- and gender-related indicators are included in public sociodemographic databases and major research databases, but those are not nearly enough to coherently analyze the effects of sex- and gender-related factors and covariates on CAD and its prevention. Sex- and gender-related covariates, such as the number of children, miscarriages, hormone therapy, andro- or menopause, and true gender variables, are incompletely covered by public and research databases, and therefore, sex- or gender-related analyses often fail.
Thus, more sex- and gender-disaggregated data are needed, along with more comprehensive inclusion of sex- and gender-related variables and increased age cut-offs to obtain a clear picture of CAD and its risk factors, prevention, and outcomes in women and men across Europe.
Researchers are only partially aware of the relevance of sex and gender and do not have the resources to include it in all studies. Therefore, awareness and funding to include sex and gender in studies and databases must be improved.
Gaps in knowledge
A lot of knowledge exists, but it is dispersed, difficult to find, and frequently not at the level required for inclusion in guidelines. Furthermore, in some areas, studies are lacking in one sex.
Gaps in knowledge still exist in risk factors and disease mechanisms that mainly affect women — mechanisms of greater CAD risk in diabetic women, cardiovascular risk associated with pregnancy, stress-induced cardiomyopathy, and microvascular and functional coronary disease. Furthermore, large state-of-the-art prospective studies on optimal diagnostic or therapeutic strategies for women and men (separately or in parallel) powered to reach valid diagnostic conclusions for one or both sexes based on their specific pathophysiology and studies on women-specific approaches simply haven't been done.
The lack of research on the relevance of gender on cardiovascular outcomes means that diagnostic and therapeutic recommendations specific to women and men are unavailable, leading to poor health quality and high costs.
Organization of gender medicine
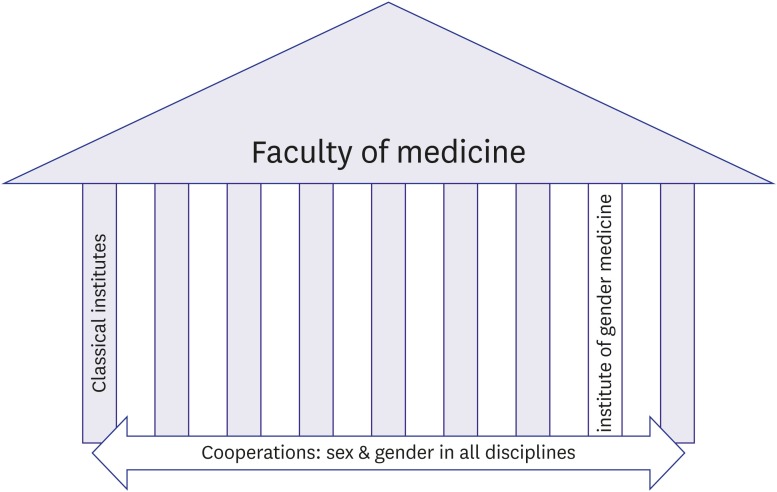
The organization of gender medicine (GM) is crucial to its success. In a faculty of medicine, GM should be organized both horizontally and vertically (Figure 2). Institutes of GM represent the vertical part of the organization: they should have scientific independence and be organized as independent institutes. They are needed to drive scientific progress in GM, i.e., the development of scientific aims, hypotheses, and methods. These institutes will also develop teaching programs to multiply their ideas and train fellows in the field.118),119) Once established, GM research ideas can than be exported to other disciplines in which gender research can function as a cross-sectional element. This is the horizontal aspect of integrating GM into a faculty. Without the mechanistic core elements developed in GM institutes, sex and gender research will be limited to head counting — comparing numbers of women and men without adequate consideration of sex- and gender-specific confounders or understanding of mechanisms, which will not bring the discipline and aims of GM to fruition.
Figure 2. Gender is needed as a cross-sectional element in all disciplines. However, major scientific achievements can only be expected if the discipline also develops its own scientific aims, hypotheses, and methods that can then be exported to other disciplines. This needs an own institute.
CONCLUSION
In spite of significant research on CVD, many issues about CVD in women remain unsettled, including female-specific mechanisms in CAD, spasms, dissections, endothelial dysfunction, HF, arrhythmia, SCA, and pregnancy-related syndromes. There is insufficient knowledge about drug therapy in women. Finally, including the sociocultural dimension of gender is only at its beginning. To improve the treatment of women and men, it is necessary to include sex and gender aspects in medical teaching and research organizations and health care. Success can only be achieved if the discipline develops its own scientific aims, hypotheses, and methods that can then be exported to other disciplines.
ACKNOWLEDGEMENTS
I thank Carola Schubert and Arne Kühne for editorial assistance. The work was supported by Margarete-Ammon Stiftung and DZHK, Standort Berlin.
Footnotes
Funding: This research was supported by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research) – partner site projects BER3.2HF (FKZ:81Z2100201) and BER I.1 (FKZ:81Z7100271).
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Oertelt-Prigione S, Regitz-Zagrosek V. Sex and gender aspects in clinical medicine. London: Springer Verlag; 2011. [Google Scholar]
- 2.EUGenMed Cardiovascular Clinical Study Group. Regitz-Zagrosek V, Oertelt-Prigione S, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Puymirat E, Simon T, Steg PG, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308:998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 5.Kotseva K, Wood D, De Backer G, et al. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137. doi: 10.1097/HJR.0b013e3283294b1d. [DOI] [PubMed] [Google Scholar]
- 6.Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–1047. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccarino V, Badimon L, Corti R, et al. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res. 2011;90:9–17. doi: 10.1093/cvr/cvq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358:1305–1315. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 9.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51:952–959. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 10.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 11.Silber TC, Tweet MS, Bowman MJ, Hayes SN, Squires RW. Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2015;35:328–333. doi: 10.1097/HCR.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- 14.Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M. Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care. 2007;30:354–359. doi: 10.2337/dc06-1772. [DOI] [PubMed] [Google Scholar]
- 15.Kalyani RR, Lazo M, Ouyang P, et al. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. 2014;37:830–838. doi: 10.2337/dc13-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parashar S, Rumsfeld JS, Reid KJ, et al. Impact of depression on sex differences in outcome after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:33–40. doi: 10.1161/CIRCOUTCOMES.108.818500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Bao H, Strait K, et al. Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation. 2015;131:614–623. doi: 10.1161/CIRCULATIONAHA.114.012826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann B, Moebus S, Möhlenkamp S, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 19.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochman JS, McCabe CH, Stone PH, et al. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. TIMI Investigators. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1997;30:141–148. doi: 10.1016/s0735-1097(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 22.Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2009;158:688–694. doi: 10.1016/j.ahj.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S62–S72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–2782b. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 29.Johnston N, Jönelid B, Christersson C, et al. Effect of gender on patients with ST-elevation and non-ST-elevation myocardial infarction without obstructive coronary artery disease. Am J Cardiol. 2015;115:1661–1666. doi: 10.1016/j.amjcard.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 31.Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602–609. doi: 10.4330/wjc.v6.i7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharkey SW, Maron BJ. Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circ J. 2014;78:2119–2128. doi: 10.1253/circj.cj-14-0770. [DOI] [PubMed] [Google Scholar]
- 33.Diercks DB, Owen KP, Kontos MC, et al. Gender differences in time to presentation for myocardial infarction before and after a national women's cardiovascular awareness campaign: a temporal analysis from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation (CRUSADE) and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network-Get with the Guidelines (NCDR ACTION Registry-GWTG) Am Heart J. 2010;160:80–87.e3. doi: 10.1016/j.ahj.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Kaul P, Armstrong PW, Sookram S, Leung BK, Brass N, Welsh RC. Temporal trends in patient and treatment delay among men and women presenting with ST-elevation myocardial infarction. Am Heart J. 2011;161:91–97. doi: 10.1016/j.ahj.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Mieres JH, Gulati M, Bairey Merz N, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130:350–379. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 36.Douglas PS, Ginsburg GS. The evaluation of chest pain in women. N Engl J Med. 1996;334:1311–1315. doi: 10.1056/NEJM199605163342007. [DOI] [PubMed] [Google Scholar]
- 37.Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 38.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 39.Morise AP, Lauer MS, Froelicher VF. Development and validation of a simple exercise test score for use in women with symptoms of suspected coronary artery disease. Am Heart J. 2002;144:818–825. doi: 10.1067/mhj.2002.125835. [DOI] [PubMed] [Google Scholar]
- 40.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 41.Huynh K. Biomarkers: high-sensitivity troponin assays for the diagnosis of AMI-sex-specific differences? Nat Rev Cardiol. 2015;12:129. doi: 10.1038/nrcardio.2015.15. [DOI] [PubMed] [Google Scholar]
- 42.Shah AS, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston N, Schenck-Gustafsson K, Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J. 2011;32:1331–1336. doi: 10.1093/eurheartj/ehr009. [DOI] [PubMed] [Google Scholar]
- 44.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knaapen P, Camici PG, Marques KM, et al. Coronary microvascular resistance: methods for its quantification in humans. Basic Res Cardiol. 2009;104:485–498. doi: 10.1007/s00395-009-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson JG, Wallace R, Limacher M, et al. Cardiovascular risk in women with non-specific chest pain (from the Women's Health Initiative Hormone Trials) Am J Cardiol. 2008;102:693–699. doi: 10.1016/j.amjcard.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 47.Jespersen L, Abildstrom SZ, Hvelplund A, et al. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PLoS One. 2014;9:e93170. doi: 10.1371/journal.pone.0093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lempereur M, Magne J, Cornelis K, et al. Impact of gender difference in hospital outcomes following percutaneous coronary intervention. Results of the Belgian Working Group on Interventional Cardiology (BWGIC) registry. EuroIntervention. 2014;12:e216–23. doi: 10.4244/EIJY14M12_11. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed B, Dauerman HL. Women, bleeding, and coronary intervention. Circulation. 2013;127:641–649. doi: 10.1161/CIRCULATIONAHA.112.108290. [DOI] [PubMed] [Google Scholar]
- 50.Park DW, Kim YH, Yun SC, et al. Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur Heart J. 2013;34:1662–1669. doi: 10.1093/eurheartj/eht048. [DOI] [PubMed] [Google Scholar]
- 51.Mega JL, Hochman JS, Scirica BM, et al. Clinical features and outcomes of women with unstable ischemic heart disease: observations from metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) Circulation. 2010;121:1809–1817. doi: 10.1161/CIRCULATIONAHA.109.897231. [DOI] [PubMed] [Google Scholar]
- 52.Tamis-Holland JE, Lu J, Korytkowski M, et al. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization: a report from the BARI 2D Trial (Bypass Angioplasty Revascularization Investigation 2 Diabetes) J Am Coll Cardiol. 2013;61:1767–1776. doi: 10.1016/j.jacc.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 53.Bertrand OF, Bélisle P, Joyal D, et al. Comparison of transradial and femoral approaches for percutaneous coronary interventions: a systematic review and hierarchical Bayesian meta-analysis. Am Heart J. 2012;163:632–648. doi: 10.1016/j.ahj.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 54.De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Rihal CS, Matsuo Y, et al. Sex-related differences in fractional flow reserve-guided treatment. Circ Cardiovasc Interv. 2013;6:662–670. doi: 10.1161/CIRCINTERVENTIONS.113.000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang SJ, Ahn JM, Han S, et al. Sex differences in the visual-functional mismatch between coronary angiography or intravascular ultrasound versus fractional flow reserve. JACC Cardiovasc Interv. 2013;6:562–568. doi: 10.1016/j.jcin.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Regitz-Zagrosek V, Lehmkuhl E, Hocher B, et al. Gender as a risk factor in young, not in old, women undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:2413–2414. doi: 10.1016/j.jacc.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 58.Lehmkuhl E, Kendel F, Gelbrich G, et al. Gender-specific predictors of early mortality after coronary artery bypass graft surgery. Clin Res Cardiol. 2012;101:745–751. doi: 10.1007/s00392-012-0454-0. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira RG, Worthington A, Huang CC, Aranki SF, Muehlschlegel JD. Sex differences in the prevalence of diastolic dysfunction in cardiac surgical patients. J Card Surg. 2015;30:238–245. doi: 10.1111/jocs.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kendel F, Dunkel A, Müller-Tasch T, et al. Gender differences in health-related quality of life after coronary bypass surgery: results from a 1-year follow-up in propensity-matched men and women. Psychosom Med. 2011;73:280–285. doi: 10.1097/PSY.0b013e3182114d35. [DOI] [PubMed] [Google Scholar]
- 61.Kendel F, Gelbrich G, Wirtz M, et al. Predictive relationship between depression and physical functioning after coronary surgery. Arch Intern Med. 2010;170:1717–1721. doi: 10.1001/archinternmed.2010.368. [DOI] [PubMed] [Google Scholar]
- 62.Kothawade K, Bairey Merz CN. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol. 2011;36:291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Champney KP, Frederick PD, Bueno H, et al. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart. 2009;95:895–899. doi: 10.1136/hrt.2008.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otten AM, Maas AH, Ottervanger JP, et al. Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent? Gender difference in STEMI stratified on age. Eur Heart J Acute Cardiovasc Care. 2013;2:334–341. doi: 10.1177/2048872612475270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 69.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 70.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97:1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 71.Arola A, Jokinen E, Ruuskanen O, et al. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am J Epidemiol. 1997;146:385–393. doi: 10.1093/oxfordjournals.aje.a009291. [DOI] [PubMed] [Google Scholar]
- 72.Bagger JP, Baandrup U, Rasmussen K, Møller M, Vesterlund T. Cardiomyopathy in western Denmark. Br Heart J. 1984;52:327–331. doi: 10.1136/hrt.52.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gillum RF. Idiopathic cardiomyopathy in the United States, 1970–1982. Am Heart J. 1986;111:752–755. doi: 10.1016/0002-8703(86)90111-0. [DOI] [PubMed] [Google Scholar]
- 74.Coughlin SS, Comstock GW, Baughman KL. Descriptive epidemiology of idiopathic dilated cardiomyopathy in Washington County, Maryland, 1975–1991. J Clin Epidemiol. 1993;46:1003–1008. doi: 10.1016/0895-4356(93)90167-y. [DOI] [PubMed] [Google Scholar]
- 75.Humphries KH, Izadnegahdar M, Sedlak T, et al. Sex differences in cardiovascular disease - impact on care and outcomes. Front Neuroendocrinol. 2017;46:46–70. doi: 10.1016/j.yfrne.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ouyang P, Wenger NK, Taylor D, et al. Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. doi: 10.1186/s13293-016-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clinical investigation on differences in the magnitude of CRT response in women versus men (BIOWOMEN) Bethesda, MD: National Library of Medicine; 2015. [Google Scholar]
- 78.Regitz-Zagrosek V, Petrov G, Lehmkuhl E, et al. Heart transplantation in women with dilated cardiomyopathy. Transplantation. 2010;89:236–244. doi: 10.1097/TP.0b013e3181c35255. [DOI] [PubMed] [Google Scholar]
- 79.Salama G, Bett GC. Sex differences in the mechanisms underlying long QT syndrome. Am J Physiol Heart Circ Physiol. 2014;307:H640–8. doi: 10.1152/ajpheart.00864.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marijon E, Uy-Evanado A, Reinier K, et al. Sudden cardiac arrest during sports activity in middle age. Circulation. 2015;131:1384–1391. doi: 10.1161/CIRCULATIONAHA.114.011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Westphal C, Spallek B, Konkel A, et al. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS One. 2013;8:e73490. doi: 10.1371/journal.pone.0073490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Khatib SM, Hellkamp AS, Hernandez AF, et al. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MacFadden DR, Tu JV, Chong A, Austin PC, Lee DS. Evaluating sex differences in population-based utilization of implantable cardioverter-defibrillators: role of cardiac conditions and noncardiac comorbidities. Heart Rhythm. 2009;6:1289–1296. doi: 10.1016/j.hrthm.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 84.Narasimha D, Curtis AB. Sex differences in utilisation and response to implantable device therapy. Arrhythm Electrophysiol Rev. 2015;4:129–135. doi: 10.15420/aer.2015.04.02.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ventura-Clapier R, Dworatzek E, Seeland U, et al. Sex in basic research: concepts in the cardiovascular field. Cardiovasc Res. 2017;113:711–724. doi: 10.1093/cvr/cvx066. [DOI] [PubMed] [Google Scholar]
- 86.Dworatzek E, Mahmoodzadeh S, Schriever C, et al. Sex-specific regulation of collagen I and III expression by 17β-Estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc Res. 2018 doi: 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dworatzek E, Mahmoodzadeh S, Schubert C, et al. Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovasc Res. 2014;102:418–428. doi: 10.1093/cvr/cvu065. [DOI] [PubMed] [Google Scholar]
- 88.Fliegner D, Schubert C, Penkalla A, et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1597–606. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- 89.Kararigas G, Bito V, Tinel H, et al. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol. 2012;59:410–417. doi: 10.1016/j.jacc.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 90.Kararigas G, Dworatzek E, Petrov G, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16:1160–1167. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 91.Kararigas G, Fliegner D, Forler S, et al. Comparative proteomic analysis reveals sex and estrogen receptor β effects in the pressure overloaded heart. J Proteome Res. 2014;13:5829–5836. doi: 10.1021/pr500749j. [DOI] [PubMed] [Google Scholar]
- 92.Kararigas G, Fliegner D, Gustafsson JA, Regitz-Zagrosek V. Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol Genomics. 2011;43:438–446. doi: 10.1152/physiolgenomics.00199.2010. [DOI] [PubMed] [Google Scholar]
- 93.Kararigas G, Nguyen BT, Zelarayan LC, et al. Genetic background defines the regulation of postnatal cardiac growth by 17β-estradiol through a β-catenin mechanism. Endocrinology. 2014;155:2667–2676. doi: 10.1210/en.2013-2180. [DOI] [PubMed] [Google Scholar]
- 94.Karlstädt A, Fliegner D, Kararigas G, Ruderisch HS, Regitz-Zagrosek V, Holzhütter HG. CardioNet: a human metabolic network suited for the study of cardiomyocyte metabolism. BMC Syst Biol. 2012;6:114. doi: 10.1186/1752-0509-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res. 2010;85:719–728. doi: 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahmoodzadeh S, Fritschka S, Dworatzek E, et al. Nuclear factor-kappaB regulates estrogen receptor-alpha transcription in the human heart. J Biol Chem. 2009;284:24705–24714. doi: 10.1074/jbc.M109.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahmoodzadeh S, Leber J, Zhang X, et al. Cardiomyocyte-specific Estrogen Receptor Alpha Increases Angiogenesis, Lymphangiogenesis and Reduces Fibrosis in the Female Mouse Heart Post-Myocardial Infarction. J Cell Sci Ther. 2014;5:153. doi: 10.4172/2157-7013.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahmoodzadeh S, Pham TH, Kuehne A, et al. 17β-Estradiol-induced interaction of ERα with NPPA regulates gene expression in cardiomyocytes. Cardiovasc Res. 2012;96:411–421. doi: 10.1093/cvr/cvs281. [DOI] [PubMed] [Google Scholar]
- 99.Schubert C, Raparelli V, Westphal C, et al. Reduction of apoptosis and preservation of mitochondrial integrity under ischemia/reperfusion injury is mediated by estrogen receptor β. Biol Sex Differ. 2016;7:53. doi: 10.1186/s13293-016-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schuster I, Mahmoodzadeh S, Dworatzek E, et al. Cardiomyocyte-specific overexpression of oestrogen receptor β improves survival and cardiac function after myocardial infarction in female and male mice. Clin Sci (Lond) 2016;130:365–376. doi: 10.1042/CS20150609. [DOI] [PubMed] [Google Scholar]
- 101.Queirós AM, Eschen C, Fliegner D, et al. Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int J Cardiol. 2013;169:331–338. doi: 10.1016/j.ijcard.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 102.Li J, Ruffenach G, Kararigas G, et al. Intralipid protects the heart in late pregnancy against ischemia/reperfusion injury via Caveolin2/STAT3/GSK-3β pathway. J Mol Cell Cardiol. 2017;102:108–116. doi: 10.1016/j.yjmcc.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, Umar S, Amjedi M, et al. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2:192–207. [PMC free article] [PubMed] [Google Scholar]
- 104.Li J, Umar S, Iorga A, et al. Cardiac vulnerability to ischemia/reperfusion injury drastically increases in late pregnancy. Basic Res Cardiol. 2012;107:271. doi: 10.1007/s00395-012-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
- 106.Franconi F, Rosano G, Campesi I. Need for gender-specific pre-analytical testing: the dark side of the moon in laboratory testing. Int J Cardiol. 2015;179:514–535. doi: 10.1016/j.ijcard.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Unsöld B, Kaul A, Sbroggiò M, et al. Melusin protects from cardiac rupture and improves functional remodelling after myocardial infarction. Cardiovasc Res. 2014;101:97–107. doi: 10.1093/cvr/cvt235. [DOI] [PubMed] [Google Scholar]
- 108.Regitz-Zagrosek V. Sex and gender differences in pharmacology. Heidelberg: Springer Verlag; 2012. [Google Scholar]
- 109.Sliwa K, Hilfiker-Kleiner D, Mebazaa A, et al. EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail. 2014;16:583–591. doi: 10.1002/ejhf.68. [DOI] [PubMed] [Google Scholar]
- 110.Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 111.Sliwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail. 2017;19:1131–1141. doi: 10.1002/ejhf.780. [DOI] [PubMed] [Google Scholar]
- 112.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171–180. doi: 10.1016/j.jacc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 113.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Evans CS, Gooch L, Flotta D, et al. Cardiovascular system during the postpartum state in women with a history of preeclampsia. Hypertension. 2011;58:57–62. doi: 10.1161/HYPERTENSIONAHA.111.173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012;13:596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pelletier R, Ditto B, Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom Med. 2015;77:517–526. doi: 10.1097/PSY.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 117.Pelletier R, Khan NA, Cox J, et al. Sex versus gender-related characteristics: which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. 2016;67:127–135. doi: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 118.Miller VM, Kararigas G, Seeland U, et al. Integrating topics of sex and gender into medical curricula-lessons from the international community. Biol Sex Differ. 2016;7:44. doi: 10.1186/s13293-016-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seeland U, Nauman AT, Cornelis A, et al. eGender-from e-learning to e-research: a web-based interactive knowledge-sharing platform for sex- and gender-specific medical education. Biol Sex Differ. 2016;7:39. doi: 10.1186/s13293-016-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]