Abstract
Steroid-induced maturation of Xenopus oocytes has long served as a model for studying meiosis. Progesterone has been considered the relevant steroid controlling maturation, perhaps through interactions with classical progesterone receptors. In this study, we provide evidence that androgens, rather than progesterone, are the physiologic mediators of Xenopus oocyte maturation. Androgens were equal or more potent activators of maturation in vitro relative to progesterone and were significantly more abundant in the serum and ovaries of β-human chorionic growth hormone-stimulated frogs. Androgen action appeared to be mediated by classical androgen receptors (ARs) expressed in oocytes, as androgen-induced maturation and signaling was specifically attenuated by AR antagonists. Interestingly, we found that progesterone was rapidly converted to the androgen androstenedione in isolated oocytes by the enzyme CYP17, suggesting that androgens may be promoting maturation even under conditions typical for “progesterone-mediated” maturation assays. Androgens are thought to play an important role in ovarian development as well as pathology, and signaling through the AR may prove to be a major regulatory mechanism mediating these processes.
The phenomenon of progesterone-induced maturation of Xenopus oocytes has served as an in vitro experimental model for studying meiosis and cell cycle regulation for over 30 years (1–3), with recent work implicating the classical nuclear/cytoplasmic progesterone receptor (PR) as the mediator of these processes (4, 5). Although progesterone is a potent promoter of Xenopus oocyte maturation in vitro, little is known about its role in mediating maturation in vivo. In other animals, progesterone does not appear to be the primary physiologic mediator of oocyte maturation. For example, oocyte maturation in fish is mediated by the progesterone metabolite 17α, 20β-dihydroxyprogesterone (6). In mice, ovaries from females lacking the PR gene still contain well-developed follicles with mature oocytes (7), suggesting that progesterone and its receptor are not necessary for oocyte maturation and that other factors are therefore important. Finally, mifepristone (RU486), a potent inhibitor of both mammalian and Xenopus PR-mediated transcription, does not block progesterone-mediated maturation (5), implying that, even in vitro, signaling via the PR may not be the only mechanism behind progesterone-induced maturation.
In an effort to clarify the physiologic importance of progesterone in Xenopus oocyte maturation, we measured the serum and ovarian steroid content of female frogs injected with β-human chronic growth hormone (β-hCG). We show that progesterone is barely detectable in the serum and ovaries of these animals but that androgen concentrations are >10 times higher than that of progesterone. Further, we demonstrate that oocytes are equally or more sensitive to signaling by androstenedione (AD) and testosterone than progesterone. We identify classical Xenopus androgen receptors (ARs) in oocytes and show that androgen-mediated induction of maturation and the mitogen-activated protein kinase (MAPK)-signaling cascade is specifically attenuated by the AR antagonist flutamide. Finally, we show that progesterone is rapidly metabolized to AD in isolated oocytes by endogenous CYP17. Together, these results argue that androgen-mediated signaling through the classical AR is the major pathway promoting maturation in Xenopus oocytes in vivo, and may play a role in “progesterone-mediated” maturation in vitro as well.
Experimental Procedures
Oocyte Preparation.
Ovaries were harvested from Xenopus laevis (Nasco, Fort Atkinson, WI) and follicular cells removed by incubation with 1 mg/ml collagenase A (Roche) in modified Barth's solution (MBSH) as described (8, 9). Oocytes were then washed and incubated overnight. After overnight incubation at 16°C in MBSH with 1 mg/ml BSA, 1 mg/ml ficoll, 100 units/ml penicillin, and 0.1 mg/ml streptomycin, Stage V–VI oocytes were selected and maturation assays performed on each preparation to determine steroid sensitivity. Because of variability in steroid sensitivity between preparations, experiments were done by using at least three different preparations.
Steroid Maturation Assay.
Oocytes were incubated with steroid (progesterone from Sigma and AD and testosterone from Steraloids, Wilton, NH) for 16 h with a constant ethanol concentration. Maturation was detected by visualizing germinal vesicle breakdown (2). Oocytes were pretreated with either ketoconazole (50 μM) or an equal amount of ethanol in MBSH for 4 h before and through the addition of steroid. Oocytes were pretreated with either flutamide (20 μM) or an equal amount of ethanol in MBSH for 1 h before and through the addition of steroid.
Cdc2 dephosphorylation was measured by treating oocytes with steroid at 500 nM for 8 h followed by solubilization, PAGE, and transfer to Immobilon-P membranes (Millipore; ref. 10). Membranes were probed with a rabbit anti-phospho-cdc2 Ab (NEB, Beverly, MA), stripped, and reprobed by using a rabbit anti-cdc2 Ab that binds all cdc2 regardless of phosphorylation status (NEB).
MAPK Assay.
Activation of the MAPK cascade was measured by examining p42 phosphorylation (10). Oocytes were incubated with steroid and solubilized in lysis buffer. Lysates were resolved by electrophoresis and proteins were transferred to Immobilon-P membranes. Membranes were probed with a rabbit anti-phospho-p44/42 MAPK Ab (NEB), stripped, and reprobed by using a rabbit anti-p44/42 MAPK polyclonal Ab that binds all p44/p42 regardless of phosphorylation status (NEB).
For Progesterone Receptor (PR) and AR Westerns, lysates were resolved on 6% polyacrylamide gels and probed with commercial anti-PR or anti-AR Abs (Santa Cruz Biotechnology).
Plasmid Construction, cRNA Synthesis, and cRNA Injection.
The Xenopus CYP17 sequence was found in the expressed sequence tags (EST) database and reported to GenBank (2). cDNAs encoding Xenopus CYP17, PR (4), and AR (U67129) were cloned by PCR by using Ex-Tak (Takara, Shuzo, Kyoto) from a Xenopus oocyte cDNA library. Reverse transcription–PCR of oocyte mRNA (Fast-Track, Invitrogen) was used to clone the 5′ portion of the AR-coding sequence. cDNAs were cloned into the Xenopus oocyte expression vector pGEM HE (L. Jan, Univ. of California, San Francisco) and the mammalian expression vector pcDNA3.1(+) (Invitrogen). pGEM constructs were linearized and transcribed in vitro with T7 RNA polymerase (Promega). Stage V–VI oocytes were injected with 50.6 nl of cRNA at a concentration of ≈200 ng/μl by using a Drummond automatic injector, and injected oocytes were incubated 36 h in MBSH before maturation or MAPK assays were performed.
Steroid Metabolism Assays.
Isolated oocytes were incubated with ≈500,000 cpm of [1,2,6,7-3H(N)]progesterone or dehydroepiandrosterone (DHEA) (NEN) in 1 ml of MBSH. Alternatively, 250 oocytes were injected with a total of ≈5,000 cpm of radiolabeled progesterone and incubated in 1 ml MBSH for 4 h. Medium was removed from the oocytes, and steroids were extracted with 3:2 ethyl acetate:hexane. Oocytes were washed with 20 ml of MBSH on glass microfiber filters (Millipore) with a vacuum filter holder (Millipore), placed in 5 ml of 3:2 ethyl acetate:hexane, and rocked for 1 h. Amounts of radioactivity were measured by using a scintillation counter, with >90% recovery from both medium and oocytes. Solvent was evaporated and TLC was performed (11).
Transfection, Binding, and Signaling Assays.
Cells were grown in complete medium consisting of DMEM, 10% FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin (GIBCO).
CYP17 experiments were performed in COS cells on 6-well plates. Cells were transfected by using FuGENE (Roche) with either pcDNA3.1 (mock) or pcDNA3.1 containing Xenopus or Human CYP17. After 24 h, cells were placed in fresh complete medium with 100 nM radiolabeled progesterone at 60,000 cpm/well. Medium was removed at the specified times, and steroids were extracted with 5 ml of 1:1 ethyl acetate:isooctane. The organic layer was concentrated and TLC was performed.
AR transfections were performed in CV1 cells on 12-well plates by using calcium phosphate precipitation (12). The transfection mixture consisted of 0.1 μg of Xenopus AR expression plasmid, 1.5 μg of mouse mammary tumor virus-luciferase plasmid, 50 ng of cytomegalovirus-β-galactosidase plasmid, and 0.9 μg of pcDNA3.1. Cells were incubated with the mixture for 6 h and placed in complete medium with 5% charcoal-filtered serum and the indicated steroids and inhibitors. Cells were lysed after 2 d and extracts analyzed by using the Promega luciferase assay system and Perkin–Elmer Galactostar kits.
PR transfections were performed in COS cells on 12-well plates by using lipofectamine (GIBCO). Cells were transfected with 0.4 μg of PR expression plasmid, 0.4 μg of mouse mammary tumor virus-luciferase plasmid, and 1 ng of cytomegalovirus-β-galactosidase plasmid and treated as above.
Binding assays were performed in COS cells on 24-well plates. Cells were transfected with either pcDNA3.1 or the Xenopus PR or AR in pcDNA3.1 by using Lipofectamine. After 48 h, cells were rinsed with chilled DMEM containing 1 mg/ml BSA and incubated with either 5 nM [1,2,6,7-3H(N)]progesterone or testosterone in chilled DMEM/BSA for 1 h. Cells were washed three times with DMEM/BSA and incubated with 500 ml of ethanol for 30 min. Radioactivity in the ethanol was then measured by using the scintillation counter.
RIAs.
Steroid RIAs were performed by using double Ab kits from ICN. Abs demonstrated <5% crossreactivity to all steroids tested, including 17α, 20α, and 20β-dihydroxyprogesterone. For the in vitro studies, 0.5-g pieces of ovary were removed from frogs and incubated in MBSH containing 1 mg/ml BSA with 10 units/ml β-hCG. Steroids were extracted from the medium at the indicated times with 3:2 ethyl acetate:hexane; solvent was evaporated; steroids were resuspended in reaction buffer from the kits, and RIAs were performed. Steroid recovery was >90% by this method. For the in vivo studies, frogs were injected with 500 units of β-hCG. At the indicated times, ≈0.3-g pieces of ovary were removed, placed in 5 ml of 3:2 ethyl acetate:hexane, and rocked for 1 h. The solvent was evaporated; steroids were resuspended in reaction buffer, and RIAs were performed. Serum also was recovered from these frogs and steroid concentrations measured by using the kit protocols.
Results
Androgens Are the Primary Steroids Produced by Xenopus Ovaries.
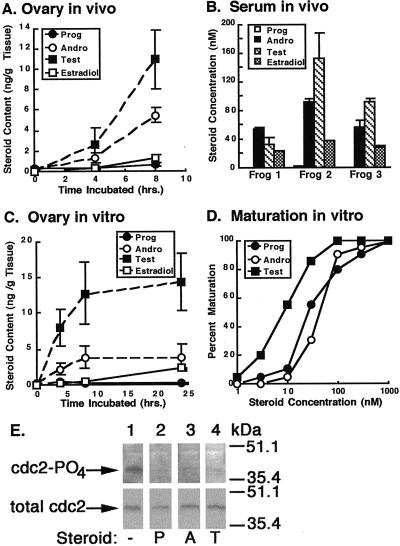
Serum and ovarian steroid levels in frogs were examined by RIA. Serum and ovarian concentrations of all steroids measured were <3 nM or 3 ng/g ovarian tissue, respectively, in normal and mock-injected female frogs (data not shown). Ovarian progesterone content remained <1 ng/g ovarian tissue over the course of 8 h after injection of 500 units of β-hCG. Surprisingly, ovarian androgen content rose rapidly after β-hCG injection, ranging from 10 (4 h) to 20 (8 h) times that of progesterone (Fig. 1A). Serum androgen concentrations were also high at 8 h, ranging from 50 to 90 nM for AD and from 30 to 150 nM for testosterone, whereas the progesterone concentration remained <3 nM (Fig. 1B). Estradiol, which does not promote maturation (data not shown), was also produced in β-hCG-stimulated frogs, confirming that aromatization of testosterone to estradiol was occurring normally. Oocyte maturation and ovulation occurred shortly after 8 h, obviating the need to measure steroid levels beyond this time. However, in vivo ovarian progesterone content remained <3 ng/g ovarian tissue and well below that of androgens at time points as late as 12 h (data not shown).
Figure 1.
Steroid production by β-hCG-stimulated ovaries and their effects on oocyte maturation. In vivo steroid levels were determined after injection of frogs with 500 units of β-hCG. (A) Steroids were extracted from ovaries at the indicated times, and RIAs were performed. Steroid levels are displayed as nanograms of steroid per gram of tissue ± SEM (n = 3). (B) Serum steroid concentrations 8 h after β-hCG injection of three β-hCG-injected frogs are displayed as nM ± SEM (n = 2). (C) In vitro ovarian steroid production was measured by incubating pieces of ovary in MBSH with 10 units of β-hCG. Buffer was removed at the indicated times and steroid concentrations determined. Steroid production is displayed as nanograms of steroid per gram of ovarian tissue ± SEM (n = 2). (D) Maturation responses of oocytes to androgens and progesterone were determined (n = 20 oocytes). (E) Progesterone (P), AD (A), and testosterone (T) at 500 nM promote dephosphorylation of cdc2 at 8 h relative to cells treated with ethanol alone (−) (Upper). Total cdc2 is equal in all samples (Lower). All experiments were performed at least three times with nearly identical results.
Direct stimulation of Xenopus ovaries in vitro with 10 units/ml β-hCG resulted in nearly undetectable progesterone production over the course of 24 h. In contrast, AD and testosterone levels reached steady–states of >10-times that of progesterone by 8 h (Fig. 1C). These results indicate that the steroids detected in the frog serum and ovaries were likely being produced primarily by the ovaries. Similar ratios of steroid production were measured when ovaries were stimulated with β-hCG at concentrations ranging from 10 to 100 units/ml, as well as with 10 units/ml of pregnant mares' serum (data not shown), arguing that the high ovarian androgen production relative to progesterone was not due solely to supraphysiologic dosing with β-hCG.
Androgens Are Potent Promoters of Maturation in Xenopus Oocytes.
The high androgen levels in β-hCG-stimulated ovaries suggested they may be playing a role in oocyte maturation. Maturation responses of oocytes to progesterone, AD, and testosterone were compared (Fig. 1D). The EC50 for AD-induced maturation was almost identical to that of progesterone (≈50 nM), whereas the EC50 for testosterone-induced maturation was even lower (5–10 nM). Notably, the serum androgen concentrations in β-hCG-stimulated frogs are at or higher than these EC50s. In contrast, the serum progesterone concentration is >20 times lower than the EC50 for progesterone-mediated maturation. Progesterone (P), AD (A), and testosterone (T) all mediated dephosphorylation of cdc2 at 8 h, providing biochemical confirmation of androgen-induced maturation (Fig. 1E).
The observation that AD and testosterone are equal or more potent activators of oocyte maturation than progesterone, combined with the dramatically higher concentrations of androgens relative to progesterone both in the serum and ovaries of β-hCG-injected frogs, strongly suggests that androgens, rather than progesterone, are the principle physiologic mediators of Xenopus oocyte maturation.
Androgen-Induced Maturation of Oocytes Is Mediated by the Classical Xenopus Androgen Receptor.
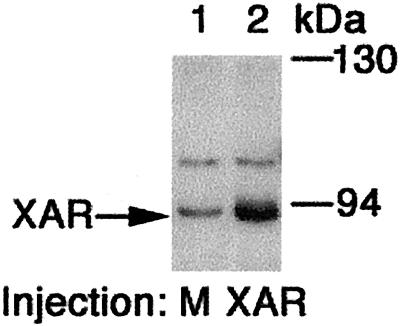
Because androgens were strong promoters of maturation, the role of the classical AR in these processes was examined. A cDNA encoding the Xenopus AR was isolated from a Xenopus oocyte cDNA library, and Western blot analysis of oocyte extracts by using an anti-AR Ab identified the presence endogenous AR protein (Fig. 2, lane 1). The identity of the AR protein on the blot was confirmed by comparison with an extract from oocytes overexpressing the cloned AR (Fig. 2, lane 2).
Figure 2.
The AR is expressed in Xenopus oocytes. Western blots were performed on extracts of oocytes injected with buffer (mock) or cRNA encoding the Xenopus AR (XAR).
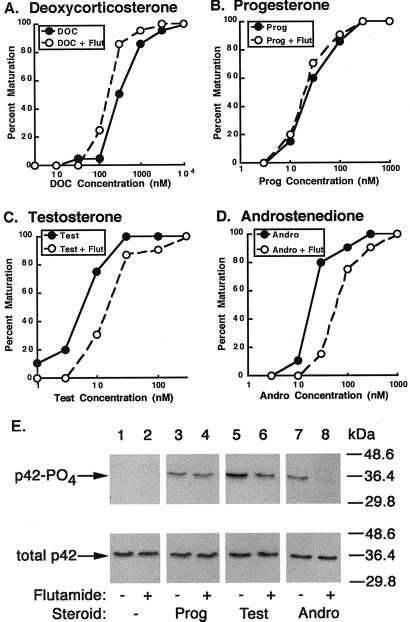
Addition of the AR antagonist flutamide at 20 μM significantly reduced testosterone (Fig. 3C) and AD (Fig. 3D)-mediated maturation, increasing their EC50 values by fivefold. In contrast, flutamide did not impair deoxycorticosterone or progesterone-mediated maturation (Fig. 3 A and B), suggesting that its effects were specific for androgens. Flutamide also attenuated androgen-mediated activation of MAPK (a relatively rapid signal induced by steroids) when oocytes were stimulated with steroid concentrations at or near their EC50s for maturation (50 nM). At 4 h, flutamide reduced testosterone-induced phosphorylation of p42 by 50% and AD-induced phosphorylation of p42 by 90% (Fig. 3D). Again, flutamide had no effect on progesterone-mediated phosphorylation of p42 (Fig. 3D). The inhibitory effects of flutamide on maturation and MAPK signaling in oocytes closely mirrored its inhibitory effects on androgen-induced transcription through the AR (Table 1) when by using similar steroid concentrations (5–100 nM). This result suggests that the incomplete blockade of maturation and MAPK activation may be due to the inability of flutamide to completely antagonize steroid binding at the higher steroid concentrations (relative to those needed to promote transcription; ref. 13) necessary to mediate maturation. Increased concentrations of flutamide inhibited maturation in a fashion that could not be overcome by very high levels of steroids, suggesting that these concentrations of flutamide were toxic to cells (data not shown).
Figure 3.
Flutamide inhibition of androgen-induced maturation and p42 phosphorylation. Oocytes were incubated in MBSH in the presence or absence of flutamide with deoxycorticosterone (DOC) (A), progesterone (B), testosterone (C), or AD (D). Similar experiments were performed 3, 5, 5, and 3 times, respectively, with nearly identical results. (E) Ooocytes were treated as above except incubated with the indicated steroid at 50 nM for 4 h. Blots were probed with antiserum against phosphorylated p42 protein (Upper) followed by antiserum against total p42 protein (Lower). Twenty oocytes were used for each sample, and similar experiments were performed three times with equivalent results.
Table 1.
Steroid binding and transcriptional activation of the Xenopus AR and PR
| Transcription
|
Binding
|
||||
|---|---|---|---|---|---|
| Steroid | Fold induction | Percent inhibition | Steroid | fmol bound | |
| AR | Ethanol | 1.0 ± 0.1 | — | — | — |
| 100 nM prog | 1.0 ± 0.02 | — | 5 nM prog | 30 ± 19 | |
| 5 nM test | 37 ± 6.0 | 0 | 5 nM test | 58 ± 14 | |
| 5 nM test + 20 μM flut | 16 ± 8.0 | 60 | — | — | |
| 100 nM AD | 18 ± 12 | 0 | — | — | |
| 100 nM AD + 20 μM flut | 3 ± 1.0 | 83 | — | — | |
| PR | Ethanol | 1 ± 0.1 | — | — | — |
| 100 nM prog | 23 ± 7 | — | 5 nM prog | 65 ± 4 | |
| 100 nM test | 1 ± 0.3 | — | 5 nM test | 8 ± 0.5 | |
| 100 nM AD | 1 ± 0.4 | — | — | — | |
Transcriptional activation of AR was measured in CV1 cells transfected with the Xenopus AR, whereas PR activation was measured in COS cells transfected with the Xenopus PR. Cells were incubated with the indicated steroids and inhibitors for 48 h and results are displayed as fold-induction ±SEM (n = 3) over the signal from cells treated with ethanol alone. Values were corrected for β-galactosidase expression. Similar experiments were performed three times with similar results. Binding activity was measured by using COS cells transfected with either AR or PR and incubated with the indicated radiolabeled steroid. Binding is displayed as fmol of steroid specifically bound per well (24-well plate) ±SEM (n = 3). Background (counts bound to mock-transfected cells) has been subtracted. prog, progesterone; test, testosterone; flut, flutamide.
Notably, testosterone did not significantly activate or bind to the Xenopus PR at the concentrations tested (Table 1), indicating that its actions are not likely to be mediated via the PR. Although progesterone demonstrated some specific binding to the AR, it was unable to promote AR-mediated transcription at a concentration of 100 nM (above the EC50 for maturation) (Table 1). This suggests that progesterone is not mediating its effects via direct binding to the AR
Progesterone Is Rapidly Metabolized to AD by CYP17 in Isolated Oocytes.
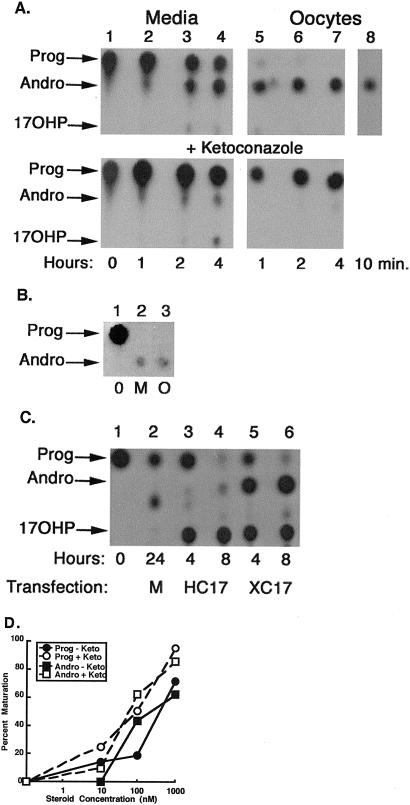
Because progesterone and/or pregnenolone is rapidly converted to AD and subsequently testosterone in β-hCG-stimulated ovaries, we determined whether steroid metabolism might also be occurring in isolated oocytes. Progesterone metabolism by oocytes was measured by separating oocytes from stromal cells with collagenase and incubating them with radiolabeled progesterone at concentrations near the EC50 for the progesterone-mediated maturation response (10–100 nM). Surprisingly, we detected very little progesterone associated with oocytes at any time ranging from 10 min to 4 h (Fig. 4A Upper). Instead, AD was the most significant oocyte-associated steroid, suggesting that progesterone was rapidly converted to AD by the oocytes. More than 50% of progesterone in the incubation medium was converted to AD by 4 h (Fig. 4A Upper), and nearly all by 24 h (data not shown). Injected progesterone was also nearly completely converted to AD by 4 h (Fig. 4B), suggesting that the conversion might be occurring within the oocytes themselves.
Figure 4.
Progesterone metabolism by isolated Xenopus oocytes and its effects on maturation. (A) Progesterone metabolism was examined by incubating 40 oocytes for the times indicated with 10 nM (lane 8) or 100 nM (lanes 1–7) radiolabeled progesterone. Oocytes in the lower panel were preincubated with ketoconazole followed by addition of 100 nM radiolabeled progesterone. Steroids were extracted from the media or oocytes and examined by TLC. Locations of progesterone (Prog), AD (Andro), and 17OHP are indicated. The identities of these steroids were confirmed by HPLC. The other minor components were <5% of the total counts. (B) Oocytes (250) were injected with radiolabeled progesterone and incubated in MBSH for 4 h. Steroids were extracted from the oocytes (O) or medium (M) and treated as above. Lane 1 is radiolabeled progesterone (0). (C) Autoradiograms of progesterone metabolites in COS cells transfected with pcDNA3.1 (M) or cDNA encoding Xenopus CYP17 (XC17) or Human CYP17 (HC17). (D) Maturation responses to progesterone (Prog, circles) and AD (Andro, squares) in the presence (open symbols) or absence (closed symbols) of ketoconazole (n = 20 oocytes). Each experiment was performed at least three times with equivalent results.
How are oocytes converting progesterone to AD? CYP17, a member of the cytochrome P450 enzyme family, converts progesterone to AD by catalyzing two reactions: metabolism of progesterone to 17α-hydroxyprogesterone (17OHP) by 17α-hydroxylase activity, followed by conversion of 17OHP to AD via 17, 20-lyase activity (14, 15). We confirmed the presence of CYP17 mRNA in oocytes by cloning the cDNA encoding the Xenopus CYP17 protein from an oocyte library. Xenopus CYP17 demonstrated both 17α-hydroxylase and 17, 20-lyase activities when expressed in COS cells, as radiolabeled progesterone was almost completely converted to either 17OHP or AD by 8 h (Fig. 4C, lanes 5 and 6). Human CYP17 demonstrated very little 17, 20-lyase activity relative to that of the Xenopus CYP17, producing little AD relative to 17OHP (Fig. 4C, lanes 3 and 4).
Intracellular Metabolism of Steroids Can Regulate the Oocyte Maturation Response to Steroids.
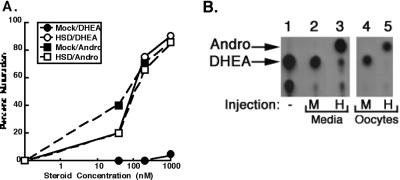
The observation that AD and progesterone were equally potent promoters of maturation, and that progesterone was metabolized to AD in vitro, suggested that AD may in part be mediating “progesterone-induced” maturation. To determine whether intracellular conversion of a steroid to AD can cause maturation similarly to exogenous addition of AD, we asked whether oocytes could be engineered to respond to a poor promoter of maturation, DHEA, by acquiring the capacity to convert DHEA to AD. Oocytes were injected with either buffer or cRNA encoding human 3β-hydroxysteroid dehydrogenase/isomerase type II (HSDII), which converts DHEA to AD (16). As expected, mock-injected oocytes matured in response to AD; however, they were virtually unresponsive to DHEA at concentrations up to 1 μM (Fig. 5A). In contrast, oocytes over-expressing HSDII were equally sensitive to both AD and DHEA (Fig. 5A), with the DHEA-induced maturation being accompanied by rapid conversion of DHEA to AD (Fig. 5B). This experiment provided proof-of-concept that AD generated by intracellular conversion from another steroid can effectively promote maturation.
Figure 5.
Metabolism of and responses to DHEA in oocytes with and without 3βHSDII. (A) DHEA (circles) and AD- (squares) induced maturation of oocytes injected with either buffer (mock, closed symbols) of cRNA encoding 3βHSDII (HSDII, open symbols) was measured (n = 20 oocytes). (B) DHEA metabolism in the injected oocytes was examined 48 h after injection by incubating oocytes in MBSH containing 100 nM radiolabeled DHEA for 4 h. Steroids were extracted from the media or oocytes and resolved by TLC (30). The remaining spots represent <10% of the total counts. Each of these experiments was performed twice with equivalent results.
Inhibition of CYP17 Does Not Affect Progesterone-Mediated Maturation.
We next asked whether intracellular conversion of progesterone to AD was necessary for maturation. CYP17 was inhibited in oocytes by treatment with the cytochrome P450 inhibitor ketoconazole. High concentrations of ketoconazole (50 μM) markedly inhibited conversion of progesterone to AD, with very little AD detected in association with oocytes at 4 h (Fig. 4A Lower). The amount of AD in the incubation medium of ketoconazole-treated oocytes was also significantly reduced, but not completely absent (Fig. 4A Lower), when compared to medium from untreated cells (Fig. 4A Upper). Inhibition of the conversion of progesterone to AD did not attenuate progesterone-mediated maturation (Fig. 4D, circles), and ketoconazole similarly did not attenuate progesterone-mediated activation of the MAPK cascade (data not shown).
Unaltered progesterone sensitivity at a time when CYP17 activity was almost completely abrogated argues that, although rapid metabolism of progesterone normally occurs during “progesterone-induced” maturation assays, conversion of progesterone to AD is not necessary for maturation.
Discussion
We provide evidence that androgens, rather than progesterone, are the dominant physiologic mediators of maturation in oocytes. AD and testosterone are equal or more potent mediators of MAPK activation and maturation than progesterone, and androgen concentrations are considerably higher than progesterone in the serum and ovaries of β-hCG-stimulated frogs at all time points measured up to ovulation (Fig. 1). These results are consistent with earlier reports that androgens are secreted by Xenopus ovarian follicles in vitro (17, 18) and are capable of promoting oocyte maturation (3, 19); however, our studies provide a detailed analysis of their potency relative to progesterone and of their concentrations in the serum and ovaries of living frogs.
Surprisingly, we found that progesterone was rapidly converted to AD by isolated oocytes (Fig. 4). These results elaborate on earlier studies describing the presence of steroid metabolism in ovarian follicles (20–23) by looking specifically at the metabolism of progesterone in isolated oocytes and by identifying CYP17 as the primary enzyme mediating this process. The rapid metabolism of progesterone injected directly into oocytes suggests that CYP17 might be expressed in the oocytes themselves, rather than in contaminating stromal cells; however, the ability of progesterone to rapidly enter and leave oocytes qualifies this conclusion. Regardless of the location of CYP17, the excess of AD relative to progesterone under conditions typical for “progesterone-mediated” maturation assay implies that AD may be a mediator of “progesterone-induced” maturation. Progesterone itself still appears capable of inducing maturation, however, as substantial inhibition of CYP17 with ketoconazole (Fig. 4) or with another inhibitor, SU-1063 (24) (data not shown), does not attenuate progesterone-mediated maturation.
The presence of functional ARs in oocytes, combined with the ability of the AR antagonist flutamide to specifically attenuate androgen-mediated maturation and activation of MAPK (Fig. 3), implies that the classical AR may in part be mediating androgen-induced signaling and maturation. The inability of flutamide to block deoxycorticosterone or progesterone-mediated maturation confirms the specificity of its inhibitory effects for androgen-mediated signaling, as does our observation that AR antagonists 17-hydroxyflutamide and bicalutamide also reduce androgen, but not progesterone-mediated, signaling (data not shown).
Details as to how the classical AR mediates maturation remain obscure. Progesterone-induced maturation is considered to occur independent of transcription because oocytes that have been enucleated or treated with transcription inhibitors still respond to progesterone (3, 19, 25). We find that androgen-mediated maturation or activation of the MAPK-signaling cascade is likewise unaffected by actinomycin D (data not shown), implying that androgen action through the AR may also be transcription-independent.
The small amount of endogenous AR in the Xenopus oocyte appears sufficient to elicit maximal responses to steroid-induced maturation because androgen-induced maturation was not enhanced by over-expression of either the Xenopus or human ARs (data not shown). This result is consistent with the “all or none” nature of MAPK activation (26). Similarly, we could not enhance progesterone-induced maturation by over-expression of one of the Xenopus PRs, nor could we convincingly attenuate steroid-induced maturation by injection of anti-sense oligonucleotides to mRNAs encoding AR or one of the two PRs (data not shown). Although these results conflict with published reports (4, 5), the potential for nonspecific effects in anti-sense experiments likely accounts for this variation.
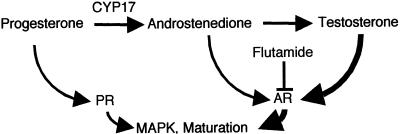
To reconcile the available data, we propose the model outlined in Fig. 6 to describe steroid-induced maturation of oocytes. Androgens appear to be the major promoters of maturation in vivo. Testosterone, which may be derived in vivo from progesterone or via the Δ5 pathway through DHEA, is likely the primary physiologic androgen, as it is both the most abundant and potent steroid in the serum and ovaries of β-hCG-stimulated frogs. Androgen signaling seems to be mediated at least in part via the classical AR, although our data do not formally preclude the role of other androgen-binding proteins in androgen-mediated processes. In vitro, both progesterone and AD appear capable of mediating “progesterone-induced” maturation. If AD action through the AR is attenuated by flutamide, or if its production is blocked by inhibition of CYP17, then progesterone signaling through the PR is still adequate to promote maturation. Our results emphasize that caution must be taken in interpreting experiments using “progesterone-mediated” maturation, as more than one steroid are likely signaling during these experiments. The notion that multiple steroids can mediate similar signaling events through their classical receptors is not new, as both estrogen and androgens have been reported to mediate apoptosis in osteoblasts and osteocytes via transcription-independent signaling by their classical receptors (27).
Figure 6.
Model for steroid-induced maturation of oocytes.
Androgens may be important mediators of oocyte and follicle development in mammals as well as frogs. Mice lacking the PR gene failed to ovulate in response to gonadotropins; yet their ovaries contained well-developed follicles with mature oocytes (7), suggesting that some factor(s) other than progesterone, perhaps androgens, are important. Androgens also appear to modulate human oocyte development. Hyperandrogen disease states, such as polycystic ovarian syndrome, are manifested by abnormal follicular development (28), and women taking exogenous androgens have a high incidence of polycystic ovaries (†, 29). Although their exact physiologic effects have yet to be completely addressed, androgens clearly play an important role in oocyte development, and nongenomic signaling via the classical AR may prove to be a major regulatory mechanism. † Toorians, A. W., Gooren, L. J. & Assecheman, H. (2001) Endocrinology 142, Supp., 110 (abstr.).
Acknowledgments
We thank Andre Gilep for help in cloning the Xenopus CYP17 cDNA and Ron Estabrook and Mike Brown for their critical reading of the manuscript. We are grateful to Michael McPhaul for his advice and generosity regarding the AR experiments. R.J.A. was supported by National Institutes of Health Grants K08DK02387 and R03DK56641. S.R.H. was supported by the Endowed Scholars Program at University of Texas Southwestern Medical Center.
Abbreviations
- PR
progesterone receptor
- β-hCG
β-human chorionic growth hormone
- AD
androstenedione
- AR
androgen receptor
- MAPK
mitogen-activated protein kinase
- DHEA
dehydroepiandrosterone
- HSDII
3β-hydroxysteroid dehydrogenase/isomerase type II
- 17OHP
17α-hydroxyprogesterone
- MBSH
modified Barth's solution
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF325435).
References
- 1.Newport J W, Kirschner M W. Cell. 1984;37:731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- 2.Maller J L, Krebs E G. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- 3.Smith L D, Ecker R E. Dev Biol. 1971;25:232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Kim S, Heilig E, Ruderman J V. Proc Natl Acad Sci USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. . (First Published December 12, 2000; 10.1073/pnas.250492197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayaa M, Booth R A, Sheng Y, Liu X J. Proc Natl Acad Sci USA. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. . (First Published October 24, 2000; 10.1073/pnas.220302597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagahama Y. Steroids. 1997;62:190–196. doi: 10.1016/s0039-128x(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 7.Lydon J P, DeMayo F J, Conneely O M, O'Malley B W. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 8.Vu T-K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 9.Williams J A, McChesney D J, Calayag M C, Lingappa V R, Logsdon C D. Proc Natl Acad Sci USA. 1988;85:4939–4943. doi: 10.1073/pnas.85.13.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz L B, Kim B, Jahani D, Hammes S R. J Biol Chem. 2000;275:41512–41520. doi: 10.1074/jbc.M006757200. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Black S M, Nagahama Y, Miller W L. Endocrinology. 1993;132:2498–2506. doi: 10.1210/endo.132.6.8504753. [DOI] [PubMed] [Google Scholar]
- 12.Gao T, Marcelli M, McPhaul M J. J Steroid Biochem Mol Biol. 1996;59:9–20. doi: 10.1016/s0960-0760(96)00097-0. [DOI] [PubMed] [Google Scholar]
- 13.Deslypere J P, Young M, Wilson J D, McPhaul M J. Mol Cell Endocrinol. 1992;88:15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 14.Nakajin S, Shively J E, Yuan P M, Hall P F. Biochemistry. 1981;20:4037–4042. doi: 10.1021/bi00517a014. [DOI] [PubMed] [Google Scholar]
- 15.Zuber M X, Simpson E R, Waterman M R. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 16.Rheaume E, Lachance Y, Zhao H F, Breton N, Dumont M, de Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F. Mol Endocrinol. 1991;5:1147–1157. doi: 10.1210/mend-5-8-1147. [DOI] [PubMed] [Google Scholar]
- 17.Fortune J E. Dev Biol. 1983;99:502–509. doi: 10.1016/0012-1606(83)90299-3. [DOI] [PubMed] [Google Scholar]
- 18.el-Zein G, Boujard D, Garnier D H, Joly J. Gen Comp Endocrinol. 1988;71:132–140. doi: 10.1016/0016-6480(88)90304-8. [DOI] [PubMed] [Google Scholar]
- 19.Smith L D, Ecker R E. Dev Biol. 1969;19:281–309. doi: 10.1016/0012-1606(69)90065-7. [DOI] [PubMed] [Google Scholar]
- 20.Mulner O, Thibier C, Ozon R. Gen Comp Endocrinol. 1978;34:287–295. doi: 10.1016/0016-6480(78)90250-2. [DOI] [PubMed] [Google Scholar]
- 21.Reynhout J K, Smith L D. Dev Biol. 1973;30:392–402. doi: 10.1016/0012-1606(73)90096-1. [DOI] [PubMed] [Google Scholar]
- 22.Schatz F, Morrill G A. Biol Reprod. 1975;13:408–414. doi: 10.1095/biolreprod13.4.408. [DOI] [PubMed] [Google Scholar]
- 23.Thibier-Fouchet C, Mulner O, Ozon R. Biol Reprod. 1976;14:317–326. doi: 10.1095/biolreprod14.3.317. [DOI] [PubMed] [Google Scholar]
- 24.Gower D B. J Steroid Biochem. 1974;5:501–523. doi: 10.1016/0022-4731(74)90051-x. [DOI] [PubMed] [Google Scholar]
- 25.Masui Y, Markert C L. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 26.Ferrell J E, Jr, Machleder E M. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 27.Kousteni S, Bellido T, Plotkin L I, O'Brien C A, Bodenner D L, Han L, Han K, DiGregorio G B, Katzenellenbogen J A, Katzenellenbogen B S, et al. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 28.Nelson V L, Legro R S, Strauss J F, III, McAllister J M. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 29.Franke W W, Berendonk B. Clin Chem. 1997;43:1262–1279. [PubMed] [Google Scholar]
- 30.Lee T C, Miller W L, Auchus R J. J Clin Endocrinol Metab. 1999;84:2104–2110. doi: 10.1210/jcem.84.6.5646. [DOI] [PubMed] [Google Scholar]